Abstract
Epac is a guanine nucleotide exchange protein that is directly activated by cAMP, but whose cardiac cellular functions remain unclear. It is important to understand cardiac Epac signaling, because it is activated in parallel to classical cAMP-dependent signaling via protein kinase A. In addition to activating contraction, Ca2+ is a key cardiac transcription regulator (excitation-transcription coupling). It is unknown how myocyte Ca2+ signals are decoded in cardiac myocytes to control nuclear transcription. We examine Epac actions on cytosolic ([Ca2+]i) and intranuclear ([Ca2+]n) Ca2+ homeostasis, focusing on whether Epac alters [Ca2+]n and activates a prohypertrophic program in cardiomyocytes. Adult rat cardiomyocytes, loaded with fluo-3 were viewed by confocal microscopy during electrical field stimulation at 1 Hz. Acute Epac activation by 8-pCPT increased Ca2+ sparks and diastolic [Ca2+]i, but decreased systolic [Ca2+]i. The effects on diastolic [Ca2+]i and Ca2+ spark frequency were dependent on phospholipase C (PLC), inositol 1,3,5 triphosphate receptor (IP3R) and CaMKII activation. Interestingly, Epac preferentially increased [Ca2+]n during both diastole and systole, correlating with the perinuclear expression pattern of Epac. Moreover, Epac activation induced histone deacetylase 5 (HDAC5) nuclear export, with consequent activation of the prohypertrophic transcription factor MEF2. These data provide the first evidence that the cAMP-binding protein Epac modulates cardiac nuclear Ca2+ signaling by increasing [Ca2+]n through PLC, IP3R and CaMKII activation, and initiates a prohypertrophic program via HDAC5 nuclear export and subsequent activation of the transcription factor MEF2.
Keywords: Calcium signaling, excitation-contraction coupling, Epac, excitation-transcription coupling, ventricular myocyte
1. Introduction
Ca2+ is a key element in cardiac excitation-contraction (EC) coupling. In each heartbeat, membrane depolarization during an action potential activates L-type Ca2+ channels located in the sarcolemma. Ca2+ entry through these channels activates intracellular Ca2+ release channels, named ryanodine receptors (RyRs), which are located in the membrane of the sarcoplasmic reticulum (SR). RyRs amplify the initial Ca2+ signal via Ca2+-induced Ca2+ release (CICR), providing enough Ca2+ to activate contractile myofibrils. Relaxation then occurs when intracellular Ca2+ concentration ([Ca2+]i) returns to diastolic values, due mainly to Ca2+ pumped back into the SR by the Ca2+-ATPase (SERCA) and extrusion from the cell via Na+/Ca2+ exchange (NCX).[1] While Ca2+ in EC coupling is physiologically and pathophysiologically relevant, new roles for cardiac myocyte Ca2+ are being elucidated. For instance, prohypertrophic signaling seems to be activated by perinuclear activation of CaMKII promoted by local elevation of nuclear [Ca2+] ([Ca2+]n).[2] By analogy to EC coupling, this process has been named excitation-transcription (ET) coupling. However, it is still not fully understood how [Ca2+]n variations may be dissociated from bulk [Ca2+]i oscillations during contraction-relaxation cycles. We recently showed that Epac (exchange protein directly activated by cAMP) could activate SR Ca2+ release in ventricular myocytes, via a CaMKII-dependent phosphorylation of the RyR[3], and we hypothesized that Epac-dependent Ca2+ signaling may also be implicated in cardiac hypertrophy.[4, 5]
Epac induces cardiomyocyte hypertrophy, both in cultured neonatal ventricular myocytes[4] and in adult myocytes.[5] Epac is a guanylyl exchange protein (GEF)[6, 7] widely distributed in the organism, including the heart, whose functional roles are just beginning to be defined.[8, 9] The hypertrophic effects of Epac are independent of the classical effector of cAMP, protein kinase A (PKA), but rather involve the Ca2+/calmodulin-dependent protein kinase II (CaMKII).[5] In addition, Epac expression is increased in experimental animal models of cardiac hypertrophy[10] and contributes to the hypertrophic effect of β-adrenergic receptor.[5] CaMKII is also known to activate nuclear export of class II histone deacetylases (e.g. HDAC4 and 5),[2, 11] an effect which derepresses myocyte enhancer factor 2 (MEF2) driven transcription, and contributes to hypertrophic remodeling.
Here we dissected the signaling pathway linking Epac activation to Ca2+ mobilization, paying a special attention to [Ca2+]n, and activation of HDAC5 nuclear export. By simultaneously analyzing cytoplasmic and [Ca2+]n during selective Epac activation with 8-pCPT-2′-O-Me-cAMP (8-pCPT), we found that Epac preferentially and differently increases [Ca2+]n (more than bulk [Ca2+]i). This action involves phospholipase C (PLC)/Inositol 1,4,5-trisphosphate (IP3) signaling and CaMKII activation. In addition, we found that Epac induced HDAC5 export via activation of IP3R and CaMKII, and results in activation of the hypertrophic transcription factor MEF2.
2. Methods
All experiments were carried out according to the ethical principles laid down by the French (Ministry of Agriculture) and European Union Council Directives for the care of laboratory animals.
2.1 Cell isolation and Ca2+ imaging
Ventricular cardiomyocytes from Wistar rats (250–300 g) were isolated using a standard enzymatic digestion as previously described.[12] Only rod-shaped cells, quiescent when unstimulated and excitable were used for the Ca2+ experiments.
[Ca2+]i transients and Ca2+ sparks were recorded in intact myocytes loaded with fluorescent Ca2+ dye (Fluo-3 AM)[13] and under control Tyrode perfusion (in mmol/L): 140 NaCl, 4 KCl, 1.1 MgCl2, 10 HEPES, 10 glucose, 1.8 CaCl2; pH=7.4, with NaOH. To record [Ca2+]i transients, cells were excited at 1 Hz by field stimulation using two parallel Pt electrodes. Spontaneous Ca2+ sparks were obtained in quiescent cells after [Ca2+]i transients recordings.
To record Ca2+ fluorescence in the Ca2+ stores (SR and nuclear envelope) cells were loaded with the low-affinity Ca2+ dye Mag-Fluo-4 AM (6μMol/L) at 37°C for 60 min allowing the compartmentalization of the dye for intra store [Ca2+]i measurements.
Images were obtained with confocal microscopy (Meta Zeiss LSM 510, objective w.i. 63x, n.a. 1.2) by scanning the cell with an Argon laser every 1.54 ms; fluorescence was excited at 488 nm and emissions were collected at >505 nm. Image analyses were performed by homemade routines using IDL software (Research System Inc.). Images were corrected for the background fluorescence. Ca2+ sparks were detected using an automated detection system and a criterion that limited the detection of false events while detecting most Ca2+ sparks.[14]
2.2 Cardiac myocytes transfection
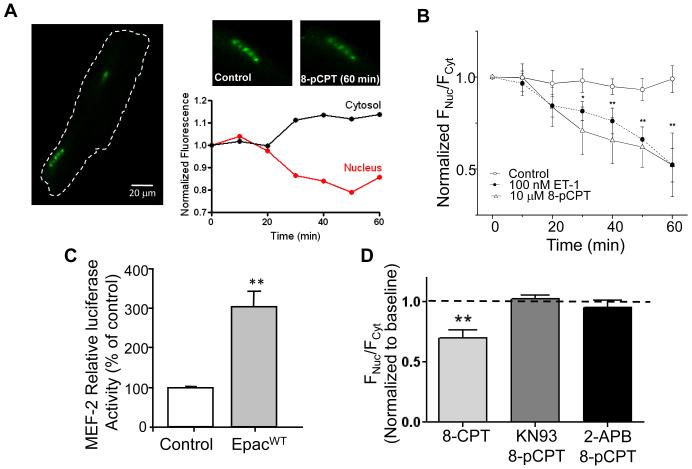
For HDAC5 transfection, isolated adult rat cardiomyocytes were seeded on laminin-coated glass coverslips and cultured in a PC-1™ complete serum-free medium (Lonza) completed with penicillin (5%) and streptomycin (5%). Nonadherent cells were removed after 30-45 min. The myocytes were then exposed overnight to recombinant replication-deficient adenovirus expressing HDAC5-GFP (100 multiplicities of infection). HDAC5-GFP signal was studied in control condition, under endothelin-1 (ET-1) (100 nmol/L) and upon 8-pCPT (10 μmol/L) exposure. HDAC5-GFP signals were assessed every 10 minutes for 60 minutes by confocal microscopy (Zeiss LSM 5 Live, objective o.i. 40x). Image-J software was used for image analysis.
For MEF2 activity, neonatal rat ventricular myocytes were isolated.[15] Transient transfection experiments were performed with Lipofectamine 2000 (Invitrogen Life Technologies, France) in optimum medium in the presence of 1 μg of the plasmid constructs according to the manufacturers’ instructions. The Epac1 plasmid construct and 3xMEF2-luciferase reporter gene (MEF2-Luc) constructs were provided by Dr. J. Bos (University of Utrecht, The Netherlands) and Dr K.C. Wollert (Hanover Medical School, Germany), respectively.
2.3 Immunolabeling
Freshly isolated adult cadiomyocytes were fixed in 4% paraformaldehyde for 10 min and washed two times in phosphate buffered saline (PBS) solution for 15 min. Myocytes were incubated in a solution of 1% bovine serum albumin (BSA) and 5 mg/mL saponin in PBS for 1h at room temperature (RT). Myocytes were incubated with anti-Epac antibody (1:100, Santa Cruz Biotechnology, Inc) in PBS solution with 1% BSA overnight at 4°C. Then myocytes were washed 4 times every 30 min with 1% BSA in PBS solution and incubated with the secondary antibody goat anti-rabbit Alexa 488 in the same solution for 2 hours at RT. 4′,6′-diamidino-2′-phenylindoladihydrochloride (DAPI) was used as nuclear counterstain and was added to the secondary antibody solution in a final concentration of 1 μg/mL. After a serial of washes (first every 30 min for 2 times with 1% BSA in PBS solution, and later 2 times 15 min each in PBS), samples were mounted on glass slides in Slow Fade Lite (Invitrogen Corp.). For negative control we used the same protocol omitting the primary antibody in the solution. Images were recorded using confocal microscopy (Meta Zeiss LSM 510, objective w.i. 63x). Alexa fluorescence was excited by the 488nm line of an Argon laser and emission collected through a BP filter (505-530 nm). DAPI fluorescence was excited by the Hg lamp (BP 365/12) and emission collected through a BP filter (480-520 nm).
2.4 Immunoblot
Adult cardiac myocytes treated or not with 8-pCPT were lysed in a buffer containing 50 mmol/L Tris pH 7.5, 500 mmol/L NaCl, 20 mmol/L MgCl2, 1% Triton X-100, 0.5% deoxycholic acid, 0.1 % sodium dodecyl sulphate (SDS), and protease inhibitors (1 mmol/L Phenylmethylsulphonylfluoride, 10 μg/L aprotinine, 10 μg/L leupeptin). After centrifugation for 20 min at 15000 g, proteins were denaturated in Laemmli’s buffer (10 min, 37°C), separated on SDS-polyacrylamide gel electrophoresis, and transferred onto a polyvinylidene difluoride membrane (Hybond-P; GE Healthcare). Membranes were hybridized overnight at 4°C with the primary antibody against phosphorylated RyR at Ser2815 site (gift from Dr. A.R. Marks[16]) or RyR (Affinity BioReagents).
2.5 Chemical products
8-pCPT was purchased from Sigma and used at a final concentration of 10 μmol/L. CaMKII inhibitor KN93 (1 μmol/L) was purchased from Calbiochem and PLC inhibitor U73122 (2 μmol/L) from Tocris. IP3R antagonist 2-aminoethoxydiphenyl borate (2-APB) (2 μmol/L) and PKC inhibitor chelerythrine (2 μM) were purchased from Sigma.
2.6 Statistical analysis
Data are presented as means±SEM. Statistical significance was evaluated by analysis of Student́s t-test paired or unpaired whenever appropriate. Differences with values of p<0.05 were considered statistically significant.
3. Results
3.1 Epac effect on intracellular Ca2+
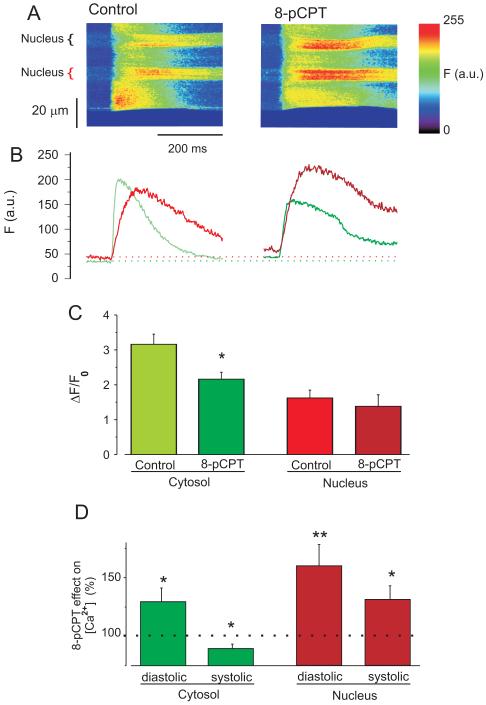
To simultaneously analyze Epac effects on cytosolic ([Ca2+]i) and [Ca2+]n, fluo-3 loaded isolated adult ventricular myocytes field-stimulated at 1 Hz were subjected to line scans including the nucleus, and normalized to the diastolic fluorescence in each compartment (F0)[17]. We are not comparing [Ca2+]n vs. [Ca2+]i in absolute terms, but separately assessing how Epac alters [Ca2+]n and [Ca2+]i. Figure 1A shows representative line scan images from a cardiomyocyte paced (1 Hz) before (left) and during (right) 8-pCPT exposure to selectively activate Epac.[18] Figure 1B shows that [Ca2+]n transients (red) are delayed with respect to [Ca2+]i transients (green) and have much slower decay (Supplemental Fig. S1). Moreover, the amplitudes (ΔF/F0) of nuclear [Ca2+]n transients are smaller than those in the cytosol (Fig. 1C).
Figure 1. Epac differently modulates intranuclear and cytosolic [Ca2+].
A. Line scan images (including nuclei) of rat ventricular myocytes before (left) and during (right) 10 μM 8-pCPT application during field stimulation at 1 Hz. B. Ca2+ fluorescence (F) traces of the images above. Green is [Ca2+]i and the red is [Ca2+]n. C. [Ca2+] transient amplitudes (ΔF/F0). Green bars are cytosol (n=14) and red bars nucleus (n=13). Lighter colors are control condition, and darker colors are with 8-pCPT. D. 8-pCPT effect on [Ca2+] as percentage of the values before the drug application in cytosol (green bars) and in the nucleus (red bars). *p<0.05, **p<0.01
Application of 8-pCPT increased both diastolic [Ca2+]n and [Ca2+]i, with a larger increase in [Ca2+]n (as %; Fig. 1D). Exposure to 8-pCPT caused reduction of peak systolic [Ca2+]i and [Ca2+]i transient amplitude (Fig. 1C&D), but enhanced peak systolic [Ca2+]n (Fig. 1D). In the nucleus, this combination of effects (increase of both, diastolic and systolic [Ca2+]n) resulted in no significant change in the amplitude of the [Ca2+]n transient (Fig. 1C). The overall elevation of [Ca2+]n in response to 8-pCPT (both diastolic and peak) (Fig. 1D) suggests a preferential effect of 8-pCPT on nuclear vs. cytoplasmic [Ca2+], which may influence nuclear Ca2+-dependent signaling. Similar data were observed with 10 fold lower 8-pCPT concentration (supplementary Fig. S2)
3.2 Mechanism of Epac effect on Diastolic and Systolic [Ca2+]i
In assessing the mechanism of Epac induced alterations in Ca2+ signaling, we monitored both diastolic and systolic [Ca2+]i simultaneously at 1Hz stimulation (Fig. 2). Although fluo-3 is non-ratiometric, data were collected in the same cell before and during 8-pCPT application, allowing assessment of Epac effects on [Ca2+]i (i.e. normalization vs. the same cell before Epac activation). A time control without Epac activation shows that the [Ca2+]i signals were stable during the 5-7 min recording period (Fig. 2A-B, light grey bars). Epac can activate phospholipase C (PLC) in mouse cardiomyocytes[19], and that could mediate these effects via consequent PKC and IP3 activation. Moreover, IP3R are concentrated at the nuclear envelope[20] and associated with CaMKII in ventricular myocytes.[21] These pathways were tested.
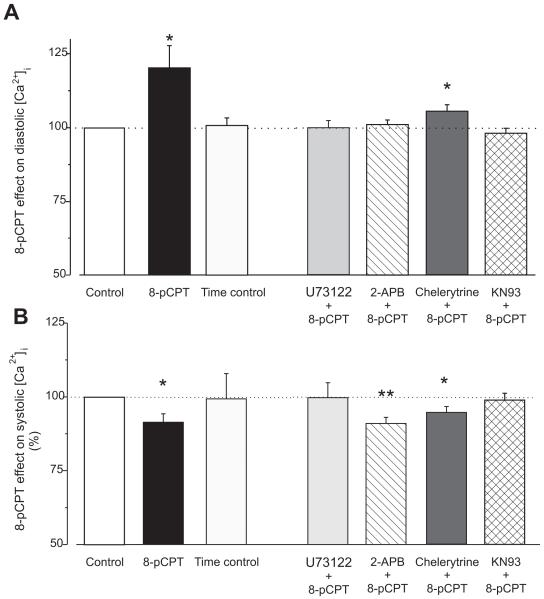
Figure 2. Epac differently modulates diastolic and systolic [Ca2+]i in the cytosol.
A. Epac-induced increase in diastolic [Ca2+]i during field stimulation at 1 Hz. Fluorescence value in each cell during 8-pCPT perfusion was normalized with the value in the same cell before 8-pCPT perfusion. White bar indicate the control in every conditions (n=59), black bar indicates 8-pCPT alone (n=13). Next to the right are time controls (same perfusion time than 8-pCPT but with control solution, n=8). Further to the right, 8-pCPT effect on diastolic [Ca2+]i in the presence of various inhibitors: Light grey bar indicates PLC inhibitor (2 μmol/L U73122, n=9), hatched bar are IP3R blocker by 2 μmol/L 2-APB (n=16), dark are PKC inhibitor 2 μmol/L chelerytrine (n=14), crosshatched are CaMKII inhibitor 1 μmol/L KN93 (n=22). B. The same than in A but for the peak [Ca2+]i fluorescence during the twitch. Bar colors and n numbers as in A. *p<0.05, **p<0.001.
We observed that Epac activation significantly increased diastolic [Ca2+]i (Fig. 2A), and this effect was completely prevented by inhibition of either phospholipase C (PLC; by 2 μM U73122), the IP3R (by 2 μM 2-aminoethoxydiphenyl borate, 2-APB), or CaMKII (by 1 μM KN-93). Inhibition of protein kinase C (PKC by 2 μmol/L chelerytrine[22]) partially, but incompletely inhibited the Epac-induced elevation of diastolic [Ca2+]i (Fig. 2A). These results suggest that Epac-induced elevation of diastolic [Ca2+]i may be attributable to activation of PLC, IP3R and CaMKII (with possible involvement of PKC). Similar results were obtained in quiescent cells: 8-pCPT treatment induced an increase on resting Ca2+ fluorescence by 23.25% (p<0.05, n=14) that was inhibited in the presence of PLC blocker (5.63% increase, N.S., n=9), or in the presence of IP3R blocker (1.23% increase, N.S., n=14), and CaMKII blocker (0.72% increase, N.S., n=13). This increase in resting Ca2+ was maintained in the presence of the PKC blocker (29.8%, p<0.05, n=9). The increase in diastolic [Ca2+]i is likely not due to an increase in Ca2+ entry. The main Ca2+ entry pathway in cardiac myocytes are the L-type Ca2+ channels and we [3] and others [23] previously showed that 8-pCPT did not modified the L type Ca2+ current. Although not prominent in these cells, store-operated Ca2+ entry (SOCE) may exist in cardiac myocytes and could contribute to Epac effects. This hypothesis was tested but, under our conditions, 8-pCPT failed to induce Ca2+ entry through SOCE (data not shown).
Epac has the opposite effect on systolic [Ca2+]i, showing a reduction within 2-3 min of 8-pCPT application (Fig. 2B). Both PLC and CaMKII inhibition prevented the 8-pCPT-induced decrease in systolic [Ca2+]i (Fig. 2B), while PKC and IP3R inhibition did not prevent the Epac-dependent reduction of systolic [Ca2+]i. Taken together, these data suggest that Epac-induced activation of PLC and CaMKII seem to be important in both the diastolic and systolic [Ca2+]i modulation, while the IP3R are more centrally involved in the diastolic [Ca2+]i increase.
3.3 Ca2+ sparks
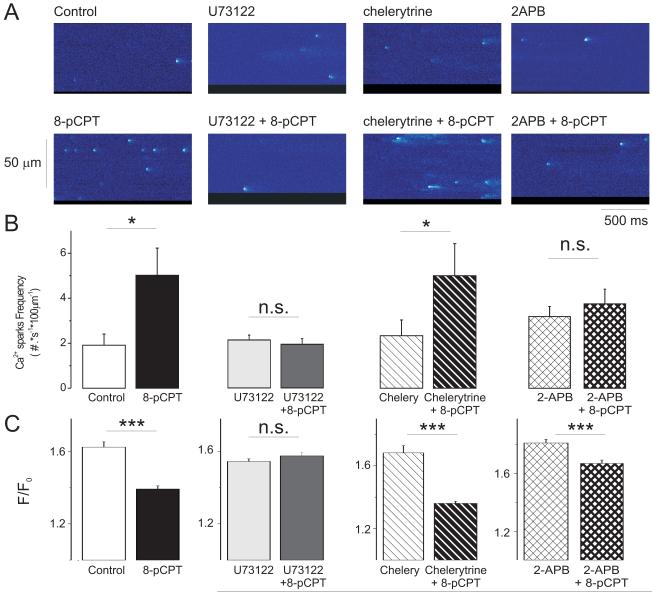
The 8-pCPT-induced increase of diastolic [Ca2+]i may be due to increased SR Ca2+ leak through RyRs, but could also be secondary to slowed [Ca2+]i decline[3] (Supplemental Fig. S1) during the [Ca2+]i transient or alterations in trans-sarcolemmal Ca2+ fluxes. Epac activation did not alter the rate of Ca2+ extrusion via Na+/Ca2+ exchange or Ca2+ influx via Ca2+ current, but can increase diastolic Ca2+ sparks in a CaMKII-dependent manner.[3] Figure 3A shows line scan images of Ca2+ sparks recorded in control conditions after drug preincubation (top) and in the same cells during application of 10 μmol/L 8-pCPT (bottom). The expected increase in Ca2+ spark frequency[3] was completely prevented by PLC inhibition (Fig. 3B), but not by PKC inhibition, consistent with the effects on diastolic [Ca2+]i in Figure 2. In the continuous presence of 2-APB, Epac activation was unable to significantly increase Ca2+ spark frequency. These results also rule out a possible direct effect of 8-pCPT on RyR2. Thus Ca2+ spark frequency data were consistent with those of diastolic [Ca2+]i. Similar to [Ca2+]i transient amplitude, Ca2+ spark amplitudes were decreased by 8-pCPT ([3], Fig. 3C), and its effect was blocked by either PLC inhibition (Fig. 3C) or CaMKII inhibition [3], thus being consistent with systolic [Ca2+]i.
Figure 3. Epac increases Ca2+ sparks frequency by PLC and IP3R activation.
A. Line scan images of cardiac myocytes showing Ca2+ sparks in several conditions: From left to right: Control, 2μmol/L U73122 (PLC inhibitor), 2μ mol/L chelerytrine (PKC inhibitor) and 2 μ mol/L 2-APB (IP3R inhibitor). Below each image it is shown another image from each cell, recorded in the presence of 10 μ mol/L 8-pCPT (Epac activator) and in the continuous presence of each inhibitor. B. Bar graph showing the measured Ca2+ spark frequency in: control cells (white bar, n=21) and cells during 8-pCPT alone (black bar, n=14), cells in the presence of the PLC inhibitor U73122 (n=10) before (light grey bar) and during (dark grey bar) 8-pCPT application, cells in the presence of the PKC inhibitor chelerytrine (n=8) before (hatched white bar) and during 8-pCPT application (black hatched bar), and cells in the presence of the IP3R inhibitor 2-APB (n=15) before (cross hatched bar) and during 8-pCPT application (thick crosshatched bar). *p<0.05 with respect to their own control. C. As in B but for the Ca2+ spark amplitudes (peak F/F0). N of Ca2+ sparks was: 201 in control, 251 in 8-pCPT, 207 in U73122, 155 in U73122 + 8pCPT, 228 in chelerytrine, 422 in chelerytrine + 8-pCPT, 298 in 2-APB and 290 in 2-APB + 8pCPT. ***p<0.001
Taken together, these data show that PLC, CaMKII and possibly IP3/IP3R, but not PKC signaling are involved in the Epac-induced increase of Ca2+ spark occurrence. This is similar to the conclusions taken from Epac effects on diastolic [Ca2+]i. A working hypothesis could be that Epac activates PLC and IP3 production, which causes release of Ca2+ via IP3R and consequent activation of local CaMKII to induce RyR phosphorylation thereby increasing Ca2+ spark frequency. In favor of this hypothesis, 8-pCPT induced RyR phosphorylation at the CaMKII site (Ser2815) which was blocked by the PLC inhibitor, U73122 (Supplemental Fig. S3).
3.4 Nuclear Epac and [Ca2+]n
To further analyze the effects of Epac activation on [Ca2+]n during continuous field stimulation at 1Hz, we measured diastolic, systolic and time-averaged [Ca2+]n during 8-pCPT exposure (Fig. 4A). The Epac-dependent increase in [Ca2+]n was prevented by the IP3R blocker, 2APB, and by the CaMKII inhibitor, KN93.
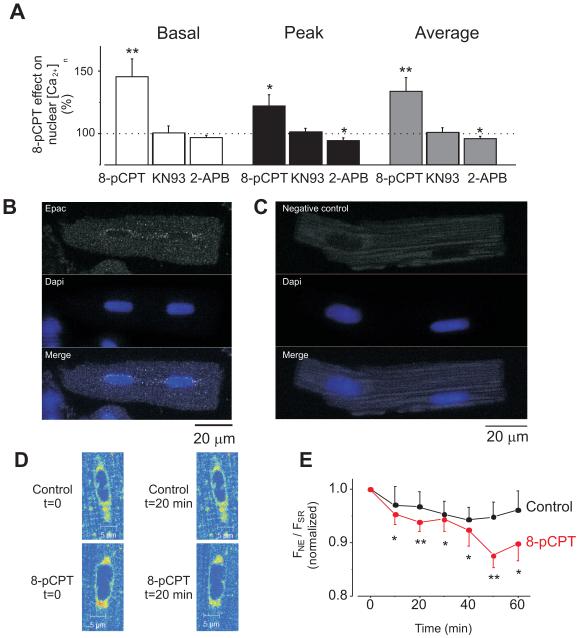
Figure 4. Endogenous Epac preferentially localizes at perinuclear area and its activation induces [Ca2+]n increment via CaMKII and IP3Rs pathways.
A. Percentage variations in the [Ca2+]n induced by 10 μmol/L 8-pCPT (n=13) and in the presence of 1 μmol/L KN-93 (CaMKII inhibitor; n=8) or 2 μmol/L 2-APB (IP3R inhibitor; n=13) during constant field stimulation at 1 Hz. Increase in basal Ca2+ fluorescence (during diastolic period) is represented in the white bar, the maximum Ca2+ fluorescence (peak) is represented by the black bar and the average Ca2+ fluorescence (average) is represented by the grey bar. B. Image of a rat ventricular cardiomyocyte immunolabeled with Epac antibody and DAPI to localize the nuclei. Epac fluorescence is shown in the top, nuclei in the middle and merged image in the bottom. C. Negative control: cells were treated as in A but primary antibody was omitted. D. Details of Mag-Fluo-4-AM loaded myocytes to differentially visualize sarcoplasmic reticulum (SR) from nuclear envelope (NE) Ca2+ stores at control conditions (t=0) and following 20 minutes (t=20) of perfusion with control solution (top) or 10 μmol/L 8-pCPT (bottom). E. Averaged traces of nuclear and cytosolic fluorescence (FNE/FSR) in perfused with 10 μmol/L 8-pCPT (red, n=8) and control (black, n=5) myocytes. The afluorescence observed was normalized to the initial FNE/FSR ratio for each cell. *p<0.05, **p<0.01.
As shown in Figure 1, Epac effects [Ca2+]n more than [Ca2+]i, and this seems to involve IP3R activation (see above). Moreover, 8-pCPT promoted IP3 production (Supplementary Fig. S4). Since IP3Rs in adult ventricular cells are concentrated in the nuclear envelope and/or perinuclear regions[20, 21, 24], we tested whether Epac localization is also perinuclear, as previously suggested.[4, 5, 25] We assessed endogenous Epac localization in freshly isolated adult ventricular cardiomyocytes by immunolabeling. Figure 4B shows fluorescence images obtained from an adult cardiac myocyte double labeled with Epac antibody (top) and DAPI to localize the nuclei (middle). Epac fluorescence is seen over the background as bright dots, which were absent in negative controls (Fig. 4C). The signal showed a preferential nuclear/perinuclear localization. The nuclear envelope may act as a Ca2+ reservoir and it is enriched in IP3Rs. We monitored Ca2+ stored in the nuclear envelope during 8-pCPT application (Fig 4D-E). Although Mag-fluo is both a Ca2+ and Mg2+ indicator, it is currently used as low sensitivity Ca2+ dye to image Ca2+ in intracellular stores. We observed that the nuclear envelope [Ca2+] decreased with respect to the SR [Ca2+] during Epac activation, indicating proportionally higher Ca2+ release from the nuclear envelope than from the SR.
The Epac-induced elevation of [Ca2+]n and CaMKII, and prohypertrophic effect of Epac[5] raises the question whether this IP3 and CaMKII-dependent nuclear signaling is involved in regulating Ca2+ -dependent transcription factors.
3.5 Effect of Epac on transcription factors
We tested whether Epac could induce HDAC5 nuclear export in an IP3- and CaMKII-dependent manner. Rat myocytes were infected with an adenovirus coding for GFP-HDAC5. Figure 5A-B shows that 8-pCPT induced HDAC5 translocation out of the nucleus (seen as a reduction in nuclear to cytosolic fluorescence). The extent and time course were similar to that seen for endothelin 1 (ET-1) used as positive control (Fig. 5B). Epac also increased MEF2 transcriptional activity in primary neonatal cardiac myocytes (Fig. 5C). Finally, we found that inhibiting either CaMKII or IP3R blocked 8-pCPT-induced HDAC5 nuclear translocation (Fig. 5D). These data show that Epac activates translocation of HDAC5 in an IP3R and CaMKII dependent manner.
Figure 5. Epac activation induces nuclear export of HDAC5 via IP3R and CaMKII.
A. Rat ventricular cardiomyocyte expressing the fusion protein HDAC5-GFP before and after 60 minutes 8-pCPT (10 μmol/L) exposure and the corresponding 8-pCPT time-dependent HDAC5-GFP fluorescence changes (normalized to t=0 min) for both cytosol and nucleus B. Time dependence of HDAC5 nuclear export represented as FNuc/FCyto, normalized to the initial ratio. Cells were treated by 10 μmol/L 8-pCPT (open triangles, n=6) or 100 nmol/L Endothelin-1 (black circles, n=4) during 60 minutes. The control group (open circles) was treated the same, except without 8-pCPT or ET-1 application (n=5). C. Neonatal rat ventricular myocytes transfected with either an empty vector (control) or Epac1 (EpacWT) and MEF2-Luc. MEF2-luciferase activity was measured 48h later. Values represent the average of 4 independent experiments performed in triplicates and are normalized to total protein and the relative level of luciferase activity in the control transfected cells was assigned a value of 100. D. Average of nuclear and cytosolic fluorescence ratio (FNuc/FCyto) in presence of 8-pCPT without (light grey bar, n=10) or with KN93 (dark grey bar, n=10) or 2-APB (black bar, n=9) exposure. * p<0.05, **p<0.01.
4. Discussion
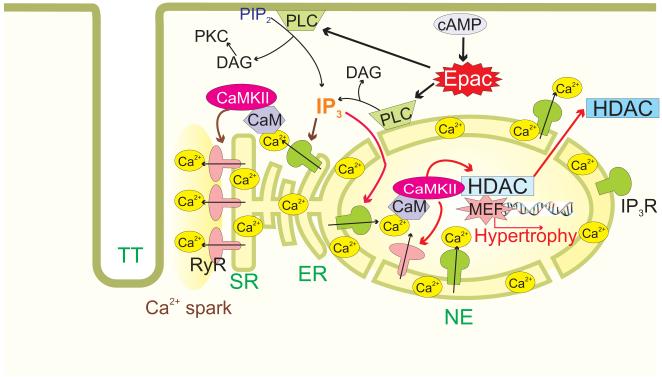
The present work demonstrates a novel role for endogenous Epac in cardiac Ca2+ and hypertrophic signaling. Moreover, the activation of Epac by cAMP in the physiological context, likely coexists with and parallels the classical cAMP-dependent signaling via PKA, but may have very divergent downstream targets. We find that acute activation of endogenous Epac elevates [Ca2+]n and Ca2+ sparks in a manner that depends on the activation of PLC, IP3R and CaMKII. Epac is preferentially located near the nucleus, and acute Epac activation also drives HDAC5 nuclear export (and MEF2 activation) in a manner that is IP3R and CaMKII-dependent, paralleling the [Ca2+]n elevation. We propose a working hypothesis for diastolic [Ca2+] schematized in Figure 6 whereby Epac activation can activate PLC, causing DAG and IP3 production, that the IP3 activates Ca2+ release via IP3R which result in local activation of CaMKII. This CaMKII activation may then be responsible for the phosphorylation of both RyR (enhancing Ca2+ sparks and unloading the SR) and HDAC5 (resulting in nuclear HDAC5 export and derepression of MEF2-dependent hypertrophic transcription). Not all of the details of these two Epac-dependent pathways are fully worked out yet (see below), but this represents a novel paradigm in cAMP-dependent Ca2+ signaling in cardiac myocytes that influences both EC coupling and ET coupling.
Figure 6. Proposed scheme of Epac activated hypertrophic signaling in cardiac myocytes.
Epac leads to PLC activation and IP3 production. Activation of IP3Rs induces Ca2+ leak from stores, which 1) sensitizes RyRs, 2) activates CaMKII and 3) induces increase in intranuclear Ca2+. The increase in [Ca2+]n and CaMKII activation translocates HDAC out of the nucleus releasing the repression against hypertrophy development.
4.1 Epac activates Ca2+ release from the SR
We previously showed in rat cardiac myocytes that Epac enhances diastolic SR Ca2+ release by increasing Ca2+ spark frequency, and that this required CaMKII activation and RyR phosphorylation at Ser2815, without significant effects on L-type Ca2+ current (ICa) or Ca2+ removal via Na+/Ca2+ exchange.[3] Others have also seen that β-adrenergic agonists can activate RyR-mediated leak by CaMKII-dependent and PKA-independent pathways.[26] We also used this strategy to activate native Epac with isoproterenol in the absence of PKA activity. Data presented in Supplementary Fig. S5 shows that β-adrenergic stimulation with isoproterenol in the presence of a PKA inhibitor, KT5722 is able to significantly increase cytosolic and nuclear [Ca2+]. The Epac-induced CaMKII activation[27], RyR phosphorylation and lack of ICa modulation were also confirmed by other authors in mouse cardiomyocytes.[23] In adult rat ventricular myocytes, systolic [Ca2+]i transient amplitude is reduced by 8-pCPT, but this did not translate into a negative inotropic effect [3], because of a parallel increase in myofilament Ca2+ sensitivity.[28] Moreover, the reduction in the Ca2+ transient may result from the reduced SR Ca2+ content (secondary to increased diastolic SR Ca2+ leak).
Oestreich et al.[19] found that Epac activation by 8-pCPT enhanced twitch [Ca2+]i transients in mouse (the opposite of our result), but they did not assess SR Ca2+ leak, Ca2+ sparks or SR Ca2+ content. This leaves open the possibility that in their mouse experiments, the SR Ca2+ content may not have been decreased (perhaps diastolic Ca2+ leak was less), allowing higher fractional SR Ca2+ release and [Ca2+]i transients. Despite this [Ca2+]i transient amplitude discrepancy (which may have plausible explanations) a central concurrent finding of both groups is that Epac activation enhances RyR activity.
Here we found that the 8-pCPT-induced increase in both diastolic [Ca2+]i and Ca2+ sparks depend on PLC, IP3Rs and CaMKII. While ventricular IP3R type 2 are concentrated at the nuclear envelope, there are some in the SR (especially in atria), where they are known to be able to facilitate the activation RyR-mediated Ca2+ sparks.[29, 30] The PLC- and CaMKII-dependence we observe here, agrees with results from Oestreich et al.[19, 23] where Epac-dependent changes in Ca2+ handling were also abolished in PLCε knockout mice. While their 8-pCPT effects on Ca2+ handling were sensitive to PKCε inhibition, our effects reflect activation of the PLC product IP3.
This allows a working hypothesis in which Epac-dependent PLC activation causes IP3-induced Ca2+ release that can activate CaMKII, which is known to associate with the IP3R. CaMKII can then phosphorylate the RyR to facilitate Ca2+ release. Indeed, CaMKII can enhance RyR activation both at rest and during EC coupling.[31, 32] An important remaining question is how and where Epac activation causes PLC activation in adult rat cardiac myocytes.
4.2 Nuclear Ca2+ signaling activated by Epac
The Epac effects on Ca2+ signaling were more prominent on diastolic [Ca2+]n than [Ca2+]i (a 45 vs 28% increase with respect to control). In addition, Epac activation also increased the peak systolic [Ca2+]n by 22%, whereas peak systolic [Ca2+]i was reduced by 9%. The simplest interpretation of this result is that the very much higher density of IP3R at the nuclear envelope vs. SR in ventricular myocytes allows the Epac-dependent IP3 signal to directly raise [Ca2+]n (potentially aided by CaMKII-dependent activation of sparse nuclear envelope RyRs). This may be further accentuated by the preferential perinuclear location of endogenous Epac (Fig. 4). These localization data confirm previous findings with Epac overexpression in cardiomyocytes[5], and also the finding that Epac is part of a muscle-specific A-Kinase Anchoring Protein (m-AKAP) complex localized in the nuclear envelope of neonatal cardiac myocytes.[33]
The higher diastolic [Ca2+]n may also partly explain why peak systolic [Ca2+]n is increased despite the reduction in peak systolic [Ca2+]i. That is, the bolstered baseline allows even a slightly reduced SR Ca2+ release signal to raise [Ca2+]n to a higher level. In addition, the sensitization of nuclear IP3R upon Epac stimulation could amplify the RyR dependent release. Additionally, in the presence of IP3, the IP3R2 is activated by increased [Ca2+][34]. This sort of enhancement of nuclear Ca2+ transients has been reported before during agonist stimulation that increases IP3 production.[24]. Although the 2-APB is not a completely specific IP3R blocker, the involvement of IP3R is further supported by the fact that PLC is identified as an upstream element in the Epac signaling cascade (effects are blocked by U73122) and by the finding that 8-pCPT treatment increases IP3 content.
Nuclear Ca2+ in the vicinity of the IP3R has been shown to modulate transcription factors, dependent at least in part by CaMKII-dependent HDAC5 phosphorylation in ET coupling.[2] Our results here would be consistent with Epac feeding in to the same nuclear IP3R-CaMKII pathway to activate HDAC5 nuclear export and the prohypertrophic transcription factor MEF2. Indeed, the 8-pCPT-induced HDAC5 nuclear export is prevented by either IP3R or CaMKII inhibition (Fig. 5). This pathway may also provide a mechanistic basis for our previous findings that Epac induces cardiomyocyte hypertrophy.[4, 5] Epac has also been reported to be part of a perinuclear m-AKAP complex, which might also be an important locus for Epac induced cardiac myocyte hypertrophy.[35] Taking together, these data demonstrate that Epac has a role on ET coupling.
The proposed signaling cascade is schematized in Figure 6. Epac activation of PLC induces IP3 production, which activates IP3R, releasing Ca2+ into the nucleus and also activating RyRs in the SR. The Ca2+ released by IP3Rs activates CaMKII, which can phosphorylate RyRs to increase Ca2+ spark occurrence, and also HDAC5 to induce its translocation out of the nucleus derepressing MEF2 transcriptional activity and thereby initiating a prohypertrophic signaling.
In summary, we have identified a role of Epac on both EC and ET coupling, and involving the activation of PLC, IP3Rs and CaMKII with effects on RyR gating and nuclear transcription. In this way, the well-known second messenger cAMP may exert actions that are parallel to, but independent of the traditional cAMP-PKA pathway. This alternative pathway must be considered in future studies of cAMP-dependent effects on both EC and ET coupling, and cardiac pathophysiological mechanisms.
Supplementary Material
Acknowledgements
We thank Mathieu Ruiz and Florence Lefebvre for cell isolation.
Sources of funding
This wok was funded by Agence National de la Recherche (Physio-06-Epac and Geno-09-HyperEpac) to FL and AMG. Inserm U-769 is a member of the Laboratory of excellence LERMIT, supported by a grant ANR “Investissements d́avenir”. SL was fellow of the program CAPES-COFECUB (Coordenação de Aperfeiçoamento do Pessoal de Nível Superior - Comité Français d’Évaluation de la Coopération Universitaire et Scientifique avec le Brésil) and the National Institutes of Health (P01-HL80101) to DMB.
Abbreviations
- [Ca2+]
calcium concentration
- [Ca2+]i
intracytoplasmic calcium concentration
- [Ca2+]n
intranuclear calcium concentration
- 2-APB
2-aminoethoxydiphenyl borate
- 8-pCPT
8-(4-chlorophenylthio)-2_-O-methyladenosine-3_,5_-cyclic monophosphate
- BP
band Pass
- BSA
bovine serum albumin
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- CICR
Ca2+ -induced Ca2+ release
- DAPI
4′,6′-diamidino-2′-phenylindoladihydrochloride
- EC
excitation-contraction
- ET
excitation-transcription
- ET-1
endothelin-1
- GEF
guanylyl exchange protein
- GFP
green fluorescence protein.
- HDAC5
histone deacetylase 5
- IP3
Inositol 1,4,5 triphosphate
- IP3R
inositol 1,4,5 triphosphate receptor
- MEF2
myocyte enhancer factor 2
- MEF2-Luc
3xMEF2-luciferase reporter gene
- n.a.
numeric aperture
- NCX
Na+/Ca2+ exchange
- o.i.
oil inmersion
- PBS
phosphate buffered saline
- PKA
protein kinase A
- PKC
Protein Kinase C
- PLC
phospholipase C
- RT
room temperature
- RyR
ryanodine receptor
- SDS
sodium dodecyl sulphate
- SERCA
Ca2+-ATPase
- SR
sarcoplasmic reticulum
- w.i.
water immersion
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- [1].Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- [2].Wu X, Zhang T, Bossuyt J, Li X, McKinsey TA, Dedman JR, et al. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J Clin Invest. 2006;116:675–82. doi: 10.1172/JCI27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pereira L, Metrich M, Fernandez-Velasco M, Lucas A, Leroy J, Perrier R, et al. The cAMP binding protein Epac modulates Ca2+ sparks by a Ca2+/calmodulin kinase signalling pathway in rat cardiac myocytes. J Physiol. 2007;583:685–94. doi: 10.1113/jphysiol.2007.133066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Morel E, Marcantoni A, Gastineau M, Birkedal R, Rochais F, Garnier A, et al. cAMP-binding protein Epac induces cardiomyocyte hypertrophy. Circ Res. 2005;97:1296–304. doi: 10.1161/01.RES.0000194325.31359.86. [DOI] [PubMed] [Google Scholar]
- [5].Metrich M, Lucas A, Gastineau M, Samuel JL, Heymes C, Morel E, et al. Epac mediates beta-adrenergic receptor-induced cardiomyocyte hypertrophy. Circ Res. 2008;102:959–65. doi: 10.1161/CIRCRESAHA.107.164947. [DOI] [PubMed] [Google Scholar]
- [6].de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, et al. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–7. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- [7].Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, et al. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282:2275–9. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- [8].Roscioni SS, Elzinga CR, Schmidt M. Epac: effectors and biological functions. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:345–57. doi: 10.1007/s00210-007-0246-7. [DOI] [PubMed] [Google Scholar]
- [9].Metrich M, Berthouze M, Morel E, Crozatier B, Gomez AM, Lezoualc’h F. Role of the cAMP-binding protein Epac in cardiovascular physiology and pathophysiology. Pflugers Arch. 2010;459(4):535–46. doi: 10.1007/s00424-009-0747-y. [DOI] [PubMed] [Google Scholar]
- [10].Ulucan C, Wang X, Baljinnyam E, Bai Y, Okumura S, Sato M, et al. Developmental changes in gene expression of Epac and its upregulation in myocardial hypertrophy. Am J Physiol Heart Circ Physiol. 2007;293:H1662–72. doi: 10.1152/ajpheart.00159.2007. [DOI] [PubMed] [Google Scholar]
- [11].Backs J, Song K, Bezprozvannaya S, Chang S, Olson EN. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J Clin Invest. 2006;116:1853–64. doi: 10.1172/JCI27438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bénitah J-P, Perrier E, Gómez AM, Vassort G. Effects of aldosterone on transient outward K+ current density in rat ventricular myocytes. J Physiol (Lond) 2001;537:151–26. doi: 10.1111/j.1469-7793.2001.0151k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fernandez-Velasco M, Rueda A, Rizzi N, Benitah JP, Colombi B, Napolitano C, et al. Increased Ca2+ Sensitivity of the Ryanodine Receptor Mutant RyR2R4496C Underlies Catecholaminergic Polymorphic Ventricular Tachycardia. Circ Res. 2009;104:201–9. doi: 10.1161/CIRCRESAHA.108.177493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gómez AM, Schuster I, Fauconnier J, Prestle J, Hasenfuss G, Richard S. FKBP12.6 overexpression decreases Ca2+ spark amplitude but enhances [Ca2+ ]i transient in rat cardiac myocytes. Am J Physiol Heart Circ Physiol. 2004;287:H1987–H93. doi: 10.1152/ajpheart.00409.2004. [DOI] [PubMed] [Google Scholar]
- [15].Wollert KC, Taga T, Saito M, Narazaki M, Kishimoto T, Glembotski CC, et al. Cardiotrophin-1 activates a distinct form of cardiac muscle cell hypertrophy. Assembly of sarcomeric units in series VIA gp130/leukemia inhibitory factor receptor-dependent pathways. J Biol Chem. 1996;271:9535–45. doi: 10.1074/jbc.271.16.9535. [DOI] [PubMed] [Google Scholar]
- [16].Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004;94:e61–70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- [17].Luo D, Yang D, Lan X, Li K, Li X, Chen J, et al. Nuclear Ca2+ sparks and waves mediated by inositol 1,4,5-trisphosphate receptors in neonatal rat cardiomyocytes. Cell Calcium. 2008;43:165–74. doi: 10.1016/j.ceca.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, et al. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol. 2002;4:901–6. doi: 10.1038/ncb874. [DOI] [PubMed] [Google Scholar]
- [19].Oestreich EA, Wang H, Malik S, Kaproth-Joslin KA, Blaxall BC, Kelley GG, et al. EPAC-mediated activation of phospholipase C plays a critical role in -adrenergic receptor dependent enhancement of Ca2+ mobilization in cardiac myocytes. Journal of Biological Chemistry. 2007;282:5488–95. doi: 10.1074/jbc.M608495200. [DOI] [PubMed] [Google Scholar]
- [20].Escobar M, Cardenas C, Colavita K, Petrenko NB, Franzini-Armstrong C. Structural evidence for perinuclear calcium microdomains in cardiac myocytes. J Mol Cell Cardiol. 2011;50:451–9. doi: 10.1016/j.yjmcc.2010.11.021. [DOI] [PubMed] [Google Scholar]
- [21].Bare DJ, Kettlun CS, Liang M, Bers DM, Mignery GA. Cardiac type 2 inositol 1,4,5-trisphosphate receptor: interaction and modulation by calcium/calmodulin-dependent protein kinase II. J Biol Chem. 2005;280:15912–20. doi: 10.1074/jbc.M414212200. [DOI] [PubMed] [Google Scholar]
- [22].Miyamae M, Rodriguez MM, Camacho SA, Diamond I, Mochly-Rosen D, Figueredo VM. Activation of epsilon protein kinase C correlates with a cardioprotective effect of regular ethanol consumption. Proc Natl Acad Sci U S A. 1998;95:8262–7. doi: 10.1073/pnas.95.14.8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Oestreich EA, Malik S, Goonasekera SA, Blaxall BC, Kelley GG, Dirksen RT, et al. Epac and phospholipase Cepsilon regulate Ca2+ release in the heart by activation of protein kinase Cepsilon and calcium-calmodulin kinase II. J Biol Chem. 2009;284:1514–22. doi: 10.1074/jbc.M806994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kockskamper J, Zima AV, Roderick HL, Pieske B, Blatter LA, Bootman MD. Emerging roles of inositol 1,4,5-trisphosphate signaling in cardiac myocytes. J Mol Cell Cardiol. 2008;45:128–47. doi: 10.1016/j.yjmcc.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dodge-Kafka KL, Soughayer J, Pare GC, Carlisle Michel JJ, Langeberg LK, Kapiloff MS, et al. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature. 2005;437:574–8. doi: 10.1038/nature03966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Curran J, Hinton MJ, Rios E, Bers DM, Shannon TR. Beta-adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ Res. 2007;100:391–8. doi: 10.1161/01.RES.0000258172.74570.e6. [DOI] [PubMed] [Google Scholar]
- [27].Hothi SS, Gurung IS, Heathcote JC, Zhang Y, Booth SW, Skepper JN, et al. Epac activation, altered calcium homeostasis and ventricular arrhythmogenesis in the murine heart. Pflugers Arch. 2008;457:253–70. doi: 10.1007/s00424-008-0508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cazorla O, Lucas A, Poirier F, Lacampagne A, Lezoualc’h F. The cAMP binding protein Epac regulates cardiac myofilament function. Proc Natl Acad Sci U S A. 2009;106:14144–9. doi: 10.1073/pnas.0812536106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lipp P, Laine M, Tovey SC, Burrell KM, Berridge MJ, Li W, et al. Functional InsP3 receptors that may modulate excitation-contraction coupling in the heart. Curr Biol. 2000;10:939–42. doi: 10.1016/s0960-9822(00)00624-2. [DOI] [PubMed] [Google Scholar]
- [30].Harzheim D, Movassagh M, Foo RS, Ritter O, Tashfeen A, Conway SJ, et al. Increased InsP3Rs in the junctional sarcoplasmic reticulum augment Ca2+ transients and arrhythmias associated with cardiac hypertrophy. Proc Natl Acad Sci U S A. 2009;106:11406–11. doi: 10.1073/pnas.0905485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li L, Satoh H, Ginsburg KS, Bers DM. The effect of Ca(2+)-calmodulin-dependent protein kinase II on cardiac excitation-contraction coupling in ferret ventricular myocytes. J Physiol (Lond) 1997;501:17–31. doi: 10.1111/j.1469-7793.1997.017bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Guo T, Zhang T, Mestril R, Bers DM. Ca2+/Calmodulin-dependent protein kinase II phosphorylation of ryanodine receptor does affect calcium sparks in mouse ventricular myocytes. Circ Res. 2006;99:398–406. doi: 10.1161/01.RES.0000236756.06252.13. [DOI] [PubMed] [Google Scholar]
- [33].McConnachie G, Langeberg LK, Scott JD. AKAP signaling complexes: getting to the heart of the matter. Trends Mol Med. 2006;12:317–23. doi: 10.1016/j.molmed.2006.05.008. [DOI] [PubMed] [Google Scholar]
- [34].Ramos-Franco J, Fill M, Mignery GA. Isoform-specific function of single inositol 1,4,5-trisphosphate receptor channels. Biophys J. 1998;75:834–9. doi: 10.1016/S0006-3495(98)77572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Pare GC, Bauman AL, McHenry M, Michel JJ, Dodge-Kafka KL, Kapiloff MS. The mAKAP complex participates in the induction of cardiac myocyte hypertrophy by adrenergic receptor signaling. J Cell Sci. 2005;118:5637–46. doi: 10.1242/jcs.02675. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.