Abstract
Adhesive interactions between endothelial cells and leukocytes contribute to atherosclerotic plaque growth. However, mechanism(s) responsible for endothelial priming and deactivation in inflammatory diseases such as atherosclerosis are not clear. Apolipoprotein E deficient mice were generated with deficiency of P-selectin glycoprotein ligand-1 (Psgl-1−/−, ApoE−/−). On both standard chow and Western diet, Psgl-1−/−, ApoE−/− mice were protected against atherosclerosis compared to Psgl-1+/+, ApoE−/− controls. Psgl-1−/−, ApoE−/− mice also showed reduced leukocyte rolling and firm attachment on endothelial cells, however, adoptively transferred Psgl-1+/+, ApoE−/− leukocytes into Psgl-1−/−, ApoE−/− hosts displayed similar reduced rolling as Psgl-1−/−, ApoE−/− leukocytes. Hematopoietic deficiency of Psgl-1 conferred resistance to the effects of interleukin-1β (IL-1β) on leukocyte rolling along with reduced circulating levels of sP-sel and sE-sel. Antibody blockade of Psgl-1 also reduced endothelial activation in response to IL-1β, eliminated leukocyte rolling, and was protective against atherosclerosis in ApoE−/− mice. Monocyte depletion with clodronate restored the endothelial response to IL-1β in Psgl-1−/− mice. This study suggests that Psgl-1 deficiency leads to reduced atherosclerosis and adhesive interactions between endothelial cells and leukocytes by indirectly regulating endothelial responses to cytokine stimulation.
Keywords: atherosclerosis, apolipoprotein E, monocyte, interleukin-1, 4RA10
Introduction
Complications of atherosclerosis remain the leading cause of mortality in the US and many other countries1. Since the majority of cardiac events are not prevented with conventional treatment 2, new therapeutic targets need to be identified and tested to reduce this residual vascular risk.
Leukocytes are major contributors to the initiation and growth of atherosclerotic plaques3,4. Leukocyte infiltration into the vessel wall is preceded by leukocyte-endothelial (L-E) interactions such as leukocyte rolling and firm attachment 4,5. L-E interactions are increased in atherosclerosis, although specific factors responsible for mediating these increased interactions are unclear. P-selectin glycoprotein ligand-1 (Psgl-1) is an adhesive ligand expressed on leukocytes and endothelial cells, and Psgl-1 deficiency is associated with reduced leukocyte rolling and firm endothelial attachment 6,7. Since selectins may affect the phenotype of the leukocyte by inititating signaling cascades following binding to leukocyte Psgl-18, it is possible that the effects of Psgl-1 on adhesive characteristics of the endothelium may be via mechanisms other than direct binding of leukocyte Psgl-1 to endothelial selectins.
In the current study, we explored a novel mechanism by which Psgl-1 regulates L-E interactions in atherosclerosis. We also determined the effect of Psgl-1 deficiency on atherosclerosis and tested the effects of pharmacologic inhibition of Psgl-1 on the progression of atherosclerosis.
Material and Method
Mice
Apolipoprotein E deficient (ApoE−/−), and Psgl-1−/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME) on the C57BL6/J strain background. Mice were housed under specific pathogen-free conditions in static microisolator cages. Psgl-1−/− mice were crossed to ApoE−/− mice to generate Psgl-1+/−, ApoE+/− mice, which were then mated to generate Psgl-1−/−, ApoE−/− breeding pairs. From these breedings, Psgl-1−/−, ApoE−/− mice and Psgl-1+/+, ApoE−/− offspring were studied for analysis of atherosclerosis. One group of experimental animals was fed a standard laboratory rodent chow (#5001, TestDiet, Richmond, IN) ad libitum for 30 weeks of age and then sacrificed. Another group of mice was fed a Western diet (TD88137, Harlan, WI) from 4 to 13 weeks of age. For passive transfer experiments, leukocytes were prepared from 10 week old Psgl-1+/+, ApoE−/− mice and injected to either Psgl-1+/+, ApoE−/− or Psgl-1−/−, ApoE−/− recipients that were 24 weeks of age and had been fed a Western diet for 8 weeks. Anti- Psgl-1 antibody (4RA10) 9 was administered to 15 week old Psgl-1+/+, ApoE−/− mice for analysis of cytokines and to 20 week old Psgl-1+/+, ApoE−/− mice with 2 weeks of Western diet for intravital microscopy studies. For monocyte depletion experiments, clodronate-containing liposomes were administrated to 8 week old Psgl-1+/+ or Psgl-1−/− mice. All procedures conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and were approved by the University of Michigan Committee on Use and Care of Animals.
Bone marrow transplantation (BMT)
At 10 weeks of age, ApoE−/− mice were irradiated and BMT was performed as previously described 10. Mice were given a Western diet beginning 5 weeks following the BMT for an additional 7 weeks, after which mice were euthanized.
Atherosclerosis quantification
For quantification of atherosclerosis, animals were euthanized under intraperitoneal pentobarbital anesthesia (100mg/kg) and analyzed as previously reported11. Briefly, animals were perfused with saline at physiological pressure and then fixed using formalin with a 25-gauge needle inserted into the left ventricle at a rate of 1 ml/min. The carcass was fixed in formalin >24 hours, and the arterial tree was then meticulously dissected and placed in 70% ethanol. Arterial trees were stained with oil-red-O, pinned on wax trays, and the surface area occupied by oil-red-O staining lesion was quantified throughout the arterial tree including the aortic arch, brachiocephalic trunk, right and left common carotid arteries, and right and left subclavian arteries with Image-Pro Plus software (Media Cybernetics, Bethesda, MD). The lesion area was expressed as a percentage of total surface area examined.
Plasma measurements
Plasma samples were collected by retro-orbital bleeding with heparinized capillary tubes (Fisher Scientific, Pittsburgh, PA) or by ventricular puncture at time of euthanasia. Circulating levels of soluble E-selectin (sE-sel) and P-selectin (sP-sel) were assayed with respective murine ELISA kits (R&D systems, Minneapolis, MN) according to manufacturers’ instructions.
Intravital microscopy
The intravital microscopy model consisted of a Nikon FN1 fixed stage microscopy system with X-cite for epi-fluorescence, Photometrics Coolsnap Cacade 512B color digital camera system, and MetaMorph premier software package and computer system 12. For analysis of cremaster vessels, male mice were anesthetized with pentobarbital (50 mg/kg) and positioned supine securely with tape. An incision was made in the scrotal skin to expose the left cremaster muscle, which was then removed from the surrounding fascia. A lengthwise incision was made on the ventral surface of the cremaster muscle, and the testicle and epididymis were separated from the underlying muscle and reintroduced into the abdominal cavity. The muscle was then spread over an optically clear viewing pedestal and secured along the edges with 3-0 suture. The exposed tissue was superfused with warm bicarbonate-buffered saline (pH 7.4). The cremaster microcirculation was observed through the intravital microscope with a 10x eyepiece and 40x objective len. To visualize white blood cells, rhodamine 6G (0.67mg/kg) (Sigma Chemical, St Louis, MO) was injected into the tail vein immediately prior to visualization. At this dose, rhodamine 6G labels leukocytes and allows detection of all rolling leukocytes. Rhodamine 6G-associated fluorescence was visualized by epi-illumination with a 510–560 nm emission filter. Single unbranched venules (35–50 µm in diameter) were selected for study and images of the microcirculation were digitally recorded. Rolling leukocytes were defined as leukocytes that rolled at a velocity slower than red blood cells. Firm leukocyte adhesion was detected if leukocytes remained stationary for 30 seconds or longer. The number of rolling and firmly adherent leukocytes during each 35 second video was counted and expressed as cells/mm length of vessel. Three venules were analyzed from each mouse.
For adoptive transfer experiments donor leukocytes were obtained from ApoE−/− mice after injection of pentobarbital (100mg/kg). Leukocytes were isolated from whole blood using Histopaque-1077 (Sigma-Aldrich, St. Louis, MO) according to manufacturer’s instructions, then incubated with rhodamine 6G (0.067mg/ml) (Thermo Fisher Scientific, New Jersey).
Drug administration
Recombinant interleukin-1β (IL-1β) (Peprotech, Rocky Hill, NJ) was injected every other day for 2 weeks via tail vein (500ng in 200µl PBS) into mice beginning 8 weeks post-BMT and 4 weeks after starting a Western diet. Serum was collected 6 hours after the first injection and 2 weeks later after injections were completed.
Rat anti-mouse Psgl-1 antibody (rat anti-mouse CD162 (4RA10)) or control antibody (rat anti-mouse IgG k, BD Pharmingen, Franklin Lakes, NJ)9 were injected via tail vein (50µg in 200ul PBS) into mice either 16 hours before or 2.5 hours following IL-1β injection.
Clodronate (dichloromethylene diphosphonate) encapsulated liposomes (Encapsula Nanosciences, Nashville, TN) have been previously shown to cause monocyte depletion after intravenous administration13. Mice were anesthetized with isoflurane and clodronate-containing liposomes (50µl) or PBS was injected via tail vein. Complete blood cells counting (CBC) were measured in 20µl of whole blood obtained by retro-orbital bleeding into Microtainer® blood collection tubes (BD Biosciences, San Jose, CA and enumerated using an automatic cell counter (HEMAVET; Drew Scientific).
Statistical analyses
Values are expressed as mean ± SEM. The statistical significance of differences between groups was determined by Student’s t test. Values of p<0.05 were considered significant.
Results
Effect of Psgl-1 on leukocyte-endothelial interactions
Psgl-1 may affect L-E interactions via direct interactions with selectins6 or by indirect regulatory effects on endothelial transcription12. Furthermore, both endothelial and hematopoietic pools of Psgl-1 have been shown to promote leukocyte adhesion12.
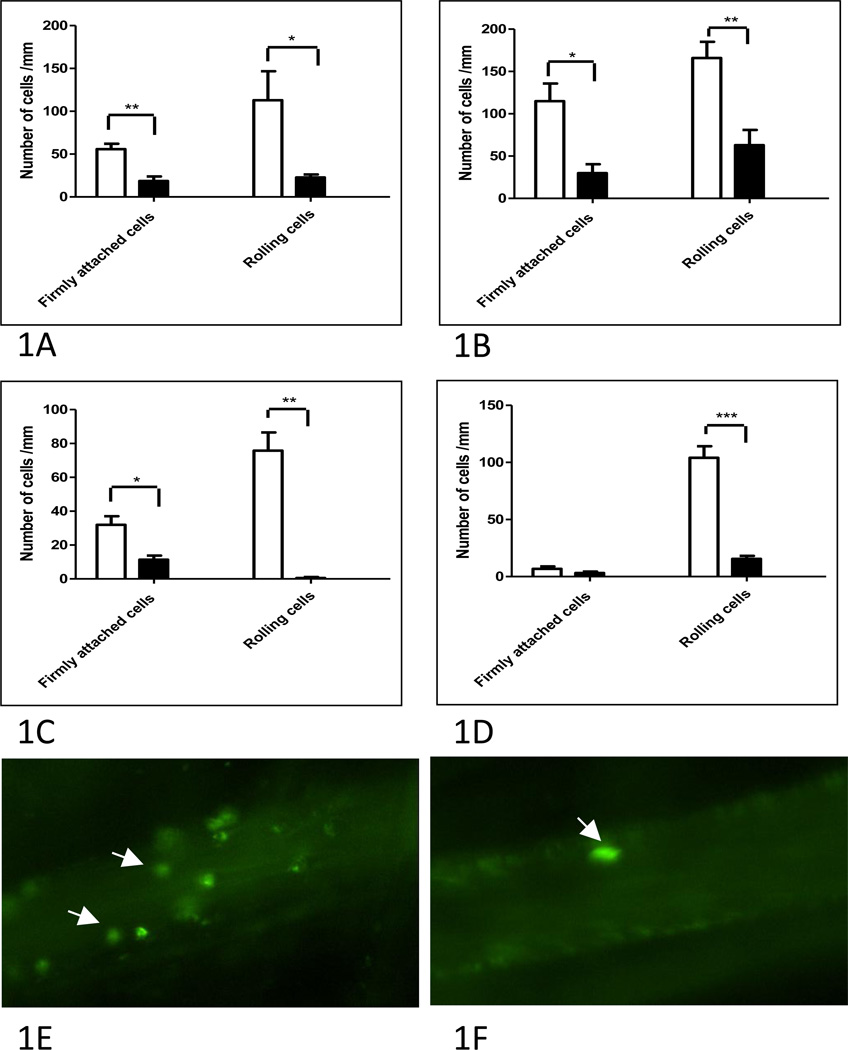
To determine whether deficiency of Psgl-1 limited to the hematopoietic-cellular pool was sufficient to reduce L-E interactions in ApoE−/− mice following an 8 week challenge of a Western diet, BMT was performed from Psgl-1−/−, ApoE−/− and Psgl-1+/+, ApoE−/− donors to Psgl-1+/+, ApoE−/− recipients. 12 weeks following the BMT, leukocyte rolling and firm attachment were both markedly reduced in Psgl-1+/+, ApoE−/− mice that received Psgl-1−/−, ApoE−/− bone marrow compared to Psgl-1+/+, ApoE−/− mice that received Psgl-1+/+, ApoE−/− marrow (Figure 1A). To test the hypothesis that the hematopoietic Psgl-1 deficiency state was capable of limiting the enhanced L-E interactions in response to inflammatory cytokine stimulation, we challenged Psgl-1+/+, ApoE−/− mice (standard chow) that had received Psgl-1−/−, ApoE−/− or control Psgl-1+/+, ApoE−/− bone marrow with intravenous recombinant IL-1β injection. Six hours following cytokine stimulation, L-E interactions (rolling and firm attachment) were markedly reduced in Psgl-1+/+, ApoE−/− mice that had received Psgl-1−/−, ApoE−/− bone marrow compared to those that received Psgl-1+/+, ApoE−/− marrow (Figure 1B). Thus, these studies demonstrate the importance of hematopoietic Psgl-1 in mediation L-E interactions induced by diet or cytokines.
Figure 1. Effect of Psgl-1 status on leukocyte-endothelial (L-E) interactions.
A) L-E interactions in Psgl-1+/+, ApoE−/− mice that received either Psgl-1+/+, ApoE−/− (white bar) or Psgl-1−/−, ApoE−/− bone marrow transplants (black bar) at baseline and B) 6 hours post IL-1β challenge. C) Adoptively transferred leukocytes from Psgl-1+/+, ApoE−/− donors into Psgl-1+/+, ApoE−/− (white bar) or Psgl-1−/−, ApoE−/− recipients (black bar). D) Psgl-1+/+, ApoE−/− mice with control antibody (white bar) or 4RA10 (black bar). Representative intravital microscopy image of venule from Psgl-1+/+, ApoE−/− mouse treated with E) control antibody or with F) 4RA10. Arrows point to rhodamine labeled leukocytes. *P<0.05. **P<0.001. ***P<0.0001. N= 5 per group.
To investigate whether the effects of hematopoietic Psgl-1 were due to acute interactions with endothelial selectins or to other indirect effects of hematopoietic Psgl-1 on endothelial adhesive characteristics, adoptive transfer experiments were performed with rhodamine-labeled leukocytes isolated from Psgl-1+/+, ApoE−/− donors infused into either Psgl-1+/+, ApoE−/− or Psgl-1−/−, ApoE−/− recipients. Despite the presence of Psgl-1, transferred labeled Psgl-1+/+, ApoE−/− leukocytes still displayed reduced rolling and firm attachment if the recipient was Psgl-1−/−, ApoE−/− compared to Psgl-1+/+, ApoE−/− (Figure 1C).
To investigate potential therapeutic applications of Psgl-1 inhibition, a Psgl-1 antibody (4RA10) previously shown to block interactions between Psgl-1 and selectins was given 16 hours prior to IVM and found to reproduce the Psgl-1 deficiency state as Psgl-1+/+, ApoE−/− mice treated with 4RA10 showed reduced L-E interactions compared to control antibody-treated mice (Figure 1D).
Effect of Psgl-1 Deficiency on circulating levels of sP-sel and sE-sel
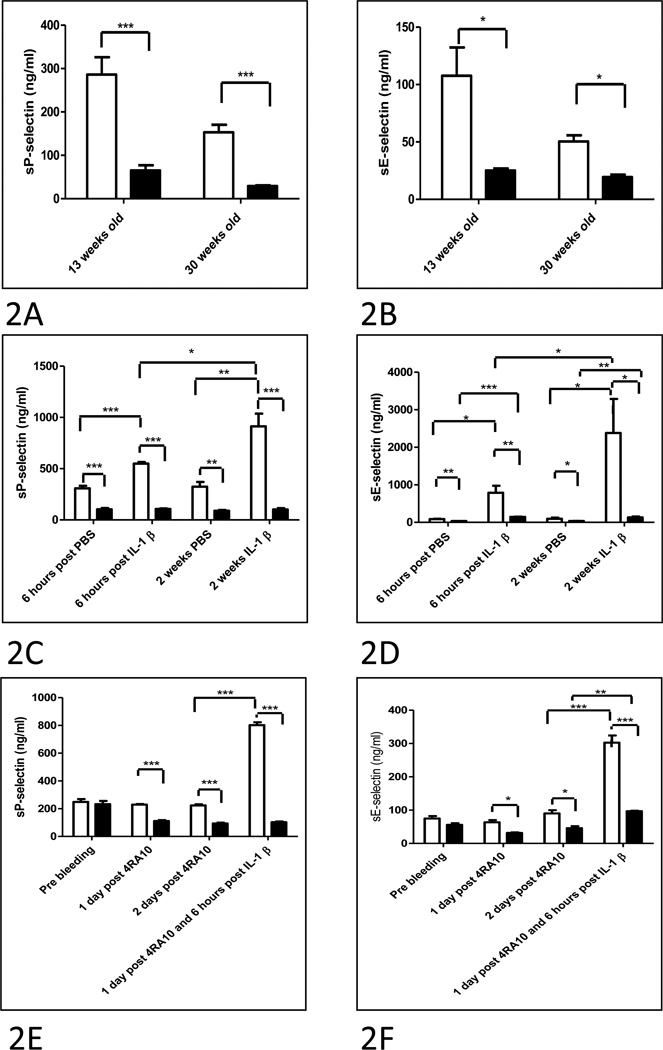
Since the experiment described above using adoptive transfer indicated that the mechanism by which Psgl-1 deficiency affects L-E interactions may be indirect, the effects of Psgl-1 deficiency on circulating markers of endothelial adhesive properties were determined. Levels of sP-sel and sE-sel were measured from plasma of Psgl-1+/+, ApoE−/− and Psgl-1−/−, ApoE−/− mice. Circulating levels of sP-sel and sE-sel were markedly decreased in Psgl-1−/−, ApoE−/− mice compared to Psgl-1+/+, ApoE−/− mice on both Western and standard diets (Figure 2A and B). This regulatory effect of Psgl-1 deficiency on generation of these circulating molecules was due to the hematopoietic pool as plasma from Psgl-1+/+, ApoE−/− mice that were transplanted with Psgl-1−/−, ApoE−/− marrow contained markedly reduced levels of sP-sel and sE-sel compared to the Psgl-1+/+, ApoE−/− mice that received the Psgl-1+/+, ApoE−/− bone marrow. This was evident in the basal state, and after challenge with both acute (6 hours post) and chronic (2 weeks of injections every 48 hours) administration of IL-1 β (Figure 2C and D).
Figure 2. Effect of Psgl-1 status on circulating levels of sP-sel and sE-sel.
A) sP-sel and B) sE-sel levels from 13 week old mice on Western diet for 9 weeks, and 30 week old mice maintained on standard diet; Psgl-1+/+, ApoE−/− (white bar) and Psgl-1−/−, ApoE−/− (black bar). C) sP-sel and D) sE-sel levels in Psgl-1+/+, ApoE−/− mice after either Psgl-1+/+, ApoE−/− (white bar) or Psgl-1−/−, ApoE−/− bone marrow transplantation (black bar). E) sP-sel and F) sE-sel levels in Psgl-1+/+, ApoE−/− mice treated with control antibody (white bar) or 4RA10 (black bar). *P<0.05. **P<0.001. ***P<0.0001. N=9 per genotype.
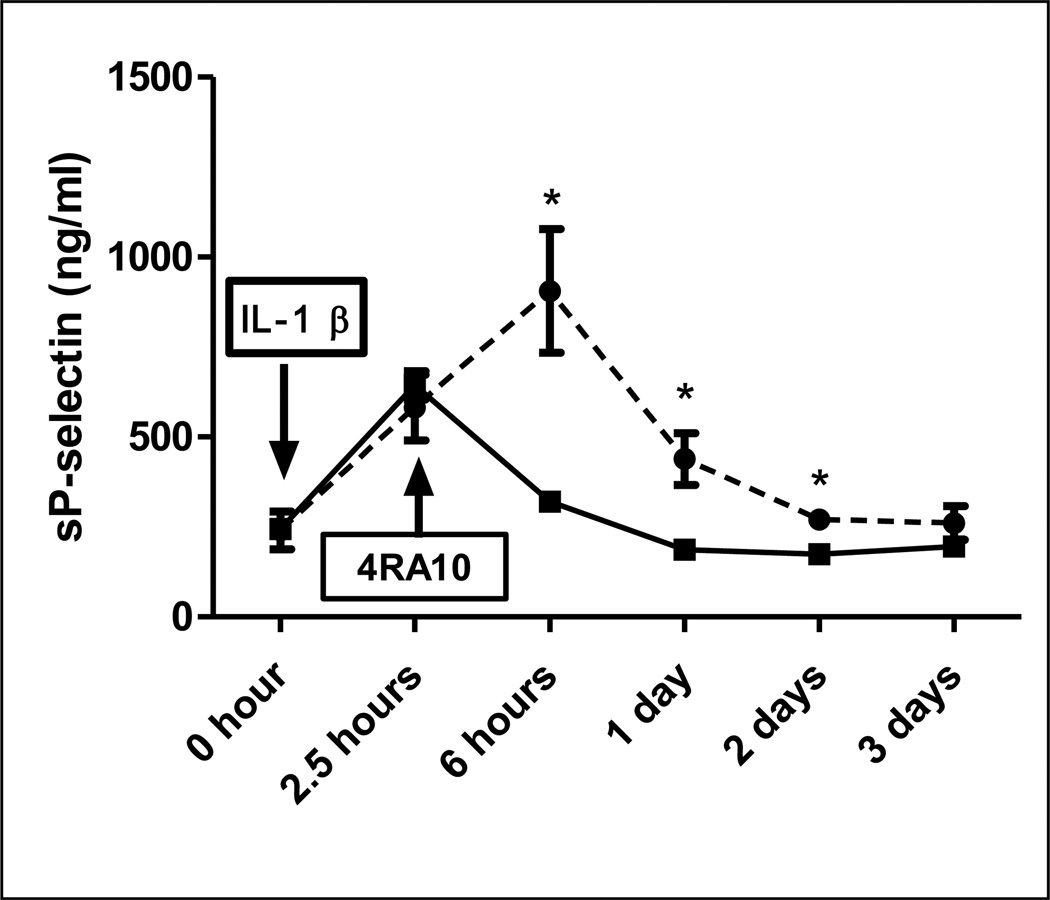
Psgl-1 antibody blockade with 4RA10 mimicked the effect of the Psgl-1 deficiency state on generation of sP-sel and sE-sel. A single injection of 4RA10 reduced levels of sP-sel and sE-sel for at least 2 days (Figure 2E and F). Psgl-1+/+, ApoE−/− mice treated with 4RA10 also showed reduced sP-sel and sE-sel levels in response to IL-1β compared to mice receiving control antibody (Figure 2E and F). To further determine whether this anti-adhesive effect of Psgl-1 blockade could deactivate endothelium already stimulated with IL-1β, 4RA10 was administered 2.5 hours after IL-1β stimulation at a time point when sP-sel levels were already rising in plasma. The antibody was capable of reversing the rising concentrations of sP-sel in plasma indicating deactivation of the endothelium occurs with Psgl-1 blockade (Figure 3).
Figure 3. Effect of Psgl-1 antibody blockade given after IL-1β on sP-sel levels.
Circulating levels of sP-sel in Psgl-1+/+, ApoE−/− mice in which IV control antibody (dashed line) or 4RA10 (solid line) was administered (arrow) 2.5 hours after IL-1β stimulation at a time point when sP-sel levels were rising in plasma. *P<0.02. N=4 per genotype.
Effect monocyte depletion with clodronate on endothelial response to IL-1β in Psgl-1 deficient mice
To determine the potential role of monocyte Psgl-1 deficiency in mediating endothelial deactivation, Psgl-1+/+ and Psgl-1−/− mice were given clodronate to reduce circulating monocytes and then challenged with IL-1β. Monocytes were reduced from 0.7 ± 0.03 ×103/µL to 0.2 ±0.04 ×103/µL following clodronate (p<0.003). There were no significant changes in total WBC (10.1 ± 1.2 ×103/µL to 9.02 ± 0.33 ×103/µL, p=NS), neutrophils (1.8 ± 0.2 ×103/µL to 2.1 ± 0.2 ×103/µL, p=NS), or lymphocytes (7.6 ± 1.01 ×103/µL to 6.7 ± 0.22 ×103/µL, p=NS) following clodronate and no differences in response to control injection between Psgl-1+/+ and Psgl-1−/− mice (data not shown). Control Psgl-1−/− mice treated with PBS were completely resistant to the effects of IL-1β on sP-sel levels, however Psgl-1−/− mice became responsive to IL-1β after monocytes were depleted with clodronate (Figure 4). Psgl-1+/+ mice were similarly responsive to IL-1β challenge with and without clodronate (Figure 4). This indicates that Psgl-1 deficient monocytes are capable of blocking the endothelial effects of IL-1β.
Figure 4. Effect of monocyte depletion on sP-sel levels in Psgl-1+/+, ApoE−/− and Psgl-1−/−, ApoE−/− mice.
Circulating levels of sP-sel in Psgl-1+/+, ApoE−/− and Psgl-1−/−, ApoE−/− mice treated with PBS or clodronate liposome before (white bar) and after IL-1β (black bar). *P<0.05. **P<0.001. N=3 per genotype.
Effect of Psgl-1 deficiency on atherosclerosis
To determine the effect of Psgl-1 deficiency on progression of atherosclerosis in ApoE−/− mice on both a standard chow and Western diet, quantitation of atherosclerosis by surface oil-red-O staining was performed and revealed reduced aortic surface area covered by atherosclerosis in Psgl-1−/−, ApoE−/− mice compared to Psgl-1+/+, ApoE−/− mice on both standard chow and Western diet protocols (Figure 5A–E). There were no differences in body weight or cholesterol E levels between Psgl-1−/−, ApoE−/− and Psgl-1+/+, ApoE−/− mice on either dietary protocol (data not shown).
Figure 5. Effect of Psgl-1 deficiency on atherosclerosis.
Representative en face view of aortic trees stained with oil-red-O from A) Psgl-1+/+, ApoE−/− and B) Psgl-1−/−, ApoE−/− 13 week old mice on Western diet for 9 weeks; and C) Psgl-1+/+, ApoE−/− and D) Psgl-1−/−, ApoE−/− 30 week old mice on standard chow. E) Mean % surface area of aortic arch covered with lipid-rich atherosclerotic lesions. *P<0.05. **P<0.001. N=11–15 per genotype.
Effect of Psgl-1 antibody blockade on atherosclerosis
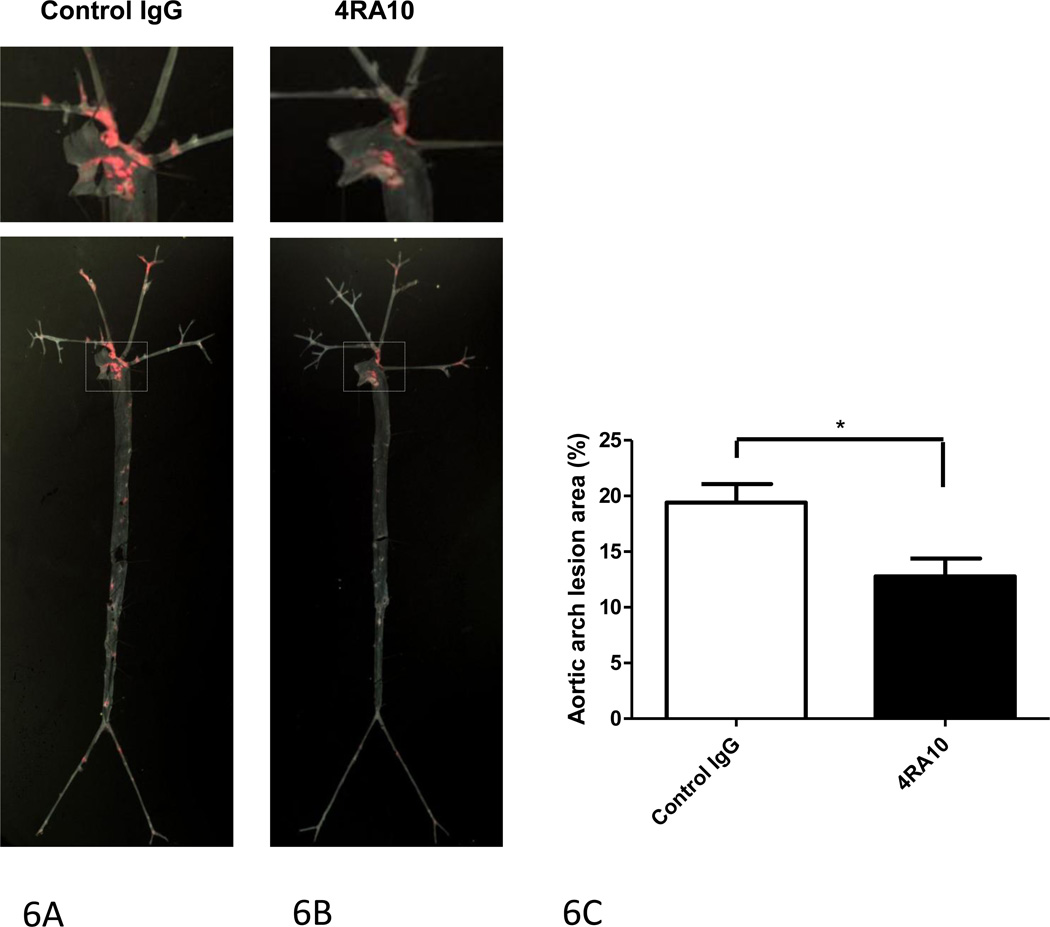
Since Psgl-1 deficiency demonstrated a protective effect on atherosclerosis, a chronic pharmacologic intervention with the anti-Psgl-1 antibody, 4RA10, was tested. Weekly administration of 4RA10 to 14 week old Psgl-1+/+, ApoE−/− mice up to 20 weeks of age was associated with reduced atherosclerosis compared to mice receiving control antibody injections (Figure 6).
Figure 6. Effect of Psgl-1 antibody blockade with 4RA10 on atherosclerosis.
Representative en face view of aortic trees stained with oil-red-O from mice treated with 9 weekly injections of A) Control IgG k antibody or B) 4RA10. C) Mean % surface area of aortic arch covered with lipid-rich atherosclerotic lesions. *P<0.03. N=8 per genotype.
Discussion
Leukocyte recruitment to the arterial wall plays a prominent role in atherogenesis and complications of advanced atherosclerosis 3,4. Factors involved in promoting leukocyte recruitment to atherosclerotic plaques may therefore be useful therapeutic targets. However, the precise mechanisms by which leukocytes are recruited to growing atherosclerotic plaques remain unclear. For example, it is unknown how endothelial adhesive properties are switched on and off. While many cytokines and signaling pathway components have shown to be involved in endothelial activation, the mechanism by which the endothelium is turned off is not known. Elucidation of the molecular mechanism(s) by which the endothelium is deactivated could lead to development of agents designed to turn off inflammatory processes after they have been initiated.
Psgl-1 is a ligand for P, E, and L-selectin, and has been shown to directly mediate rolling of leukocytes on surfaces expressing P-selectin in vitro 6. In addition, mice deficient in Psgl-1 have reduced rolling in vivo as assessed using intravital microscopy 15. In ApoE−/− mice on a Western diet, deficiency of Psgl-1 has been shown to be associated with reduced atherosclerosis and reduced neointima formation following carotid injury 16. An et al showed that Psgl-1 was highly expressed on Ly6-Chi monocytes and that these cells exhibited more interactions with immobilized selectins under flow conditions and more rolling on carotid arteries in an ex vivo flow model 16. The increased rolling in the ex vivo model by Ly6-Chi monocytes was attenuated if the monocytes were taken from a Psgl-1 deficient mouse 16. Previous studies had shown that Western diet triggered an increase of circulating Ly6-Chi monocytes and that these cells were the subtype found trafficking to atherosclerotic plaques 17 Taken together, these previous studies suggest that the increased atherosclerosis triggered by Western diet in ApoE−/− mice is due to increased L-E interactions mediated by Ly6-Chi monocyte Psgl-1.
In the current study, the effect of Psgl-1 was examined on the development of atherosclerosis in ApoE−/− mice on both standard and Western diets. Consistent with the previous study 16, deficiency of Psgl-1 was associated with reduced atherosclerosis burden in mice challenged with Western diet. However, even on a standard chow, which doesn’t induce the Ly6-Chi monocytosis observed in Western diet-fed mice 17, protection was noted in Psgl-1−/−, ApoE−/− compared to Psgl-1+/+, ApoE−/− mice. This athero-protection was associated with reduced L-E interactions in Psgl-1−/−, ApoE−/− mice compared to Psgl-1+/+, ApoE−/− mice. These results are consistent with Psgl-1-dependent leukocyte trafficking to sites of developing atheroma on both Western and standard diets.
To determine whether changes in the leukocyte versus the endothelium were directly responsible for driving the increased L-E interactions observed in Western diet-fed ApoE−/− mice, adoptive transfer experiments were performed with rhodamine-labeled leukocytes obtained from Psgl-1+/+, ApoE−/− mice on standard chow diet and then analyzed using intravital microscopy in Psgl-1+/+, ApoE−/− or Psgl-1−/−, ApoE−/− mice on the Western diet. When these cells were infused into control Psgl-1+/+, ApoE−/− mice, they were found to exhibit a similar increased rolling and firm attachment as observed in the endogenous Western diet-fed leukocyte population. When these Psgl-1-bearing labeled leukocytes were injected into recipient Psgl-1−/−, ApoE−/− mice, the leukocytes demonstrated reduced rolling similar to that observed in the endogenous Psgl-1 deficient leukocyte population. This finding suggested to us that in the setting of host Psgl-1 deficiency, adhesive properties of the endothelium are down-regulated and that changes in the endothelium are primarily responsible for the reduced L-E interactions observed in the Psgl-1 deficient state, in the setting of hyperlipidemia and atherosclerosis.
We have previously demonstrated in lean and obese mice that hematopoietic Psgl-1 deficiency leads to reduced generation of sP-sel, sE-sel, and Mcp-112,14. This unexpected regulatory role of hematopoietic Psgl-1 deficiency appears to involve transcriptional repression of endothelial adhesive and chemotactic factors12. To determine the relevance of this mechanism in the setting of atherosclerosis, we measured levels of sP-sel, sE-sel in ApoE−/− mice with variable Psgl-1 expression. Levels of sP-sel and sE-sel were markedly reduced in Psgl-1−/−, ApoE−/− mice compared to Psgl-1+/+, ApoE−/− mice on both standard and Western diets. Experiments involving the generation of chimeric mice using bone marrow transplantation into ApoE−/− mice revealed that the effect of Psgl-1 on the generation of sP-sel and sE-sel was due to deficiency of the hematopoietic pool of Psgl-1. Thus, Psgl-1+/+, ApoE−/− recipient mice receiving Psgl-1−/−, ApoE−/− marrow had reduced levels of circulating selectins along with reduced L-E interactions.
Since IL-1β has been used to induce L-E interactions and may contribute to increased L-E interactions in inflammatory states through effects on the endothelium18,19, we hypothesized that the state of hematopoietic Psgl-1 deficiency might impair the ability of cytokines, such as IL-1β, to stimulate the endothelium. Consistent with this hypothesis, mice with hematopoietic deficiency of Psgl-1 were resistant to the effects of systemic administration of IL-1β on generation of sP-sel, sE-sel and on L-E interactions. Monocyte depletion with clodronate restored the endothelial response to IL-1β in Psgl-1 deficient mice, indicating that the Psgl-1 deficient monocyte is responsible for attenuating the IL-1β stimulatory effects on the endothelium.
The results with Psgl-1 on atherosclerosis indicate that therapeutic blockade of Psgl-1 may prevent endothelial activation and slow progression of atherosclerosis. To pursue the therapeutic potential of Psgl-1 blockade, an antibody was used that has previously been shown to be protective against neointima formation in a model of acute arterial injury 9. Because of our observation that the Psgl-1 deficiency state is associated with reduced levels of sP-sel and sE-sel, we have an excellent means by which to determine efficacy of treatment with Psgl-1 antagonists. Using sP-sel as a biomarker of Psgl-1 expression, we found that a single dose of antibody reduced baseline levels of sP-sel within 24 hours of administration and levels remained significantly lower than control antibody-treated mice for 2 days after treatment. The antibody was effective in completely blocking the effects of IL-1β on generation of sP-sel, sE-sel, and on L-E interactions. Even when given 2.5 hours following IL-1β, after IL-1β had initiated the increased generation of circulating sP-sel, the antibody was capable of reversing the rise in sP-sel levels. These findings indicate that induction of the Psgl-1 deficient state leads to rapid deactivation of the endothelium and support the hypothesis that the Psgl-1 inhibited leukocyte rapidly assumes potent anti-inflammatory properties. This effect could be mediated by secretion of an anti-inflammatory soluble factor or by leukocyte-endothelial cell-mediated contact inhibition of post IL-1 receptor signaling pathways. Adhesive interactions mediated via other molecules that were not measured in this study are likely also be affected by the Psgl-1 deficiency state. Although precise details of the downstream mechanism(s) responsible for this effect are unknown at this time, these findings suggest a promising therapeutic potential for Psgl-1 inhibition in vascular diseases. To determine the potential for Psgl-1 inhibition in atherosclerosis, ApoE−/− mice were treated with weekly injections of the anti-Psgl-1 antibody for 6 weeks. Compared to mice treated with the control antibody, anti-Psgl-1 treated mice demonstrated protection from atherosclerosis.
In conclusion, hematopoietic Psgl-1 deficiency prevents endothelial activation and reduces atherosclerosis in ApoE−/− mice. These athero-protective effects can be achieved with antibody blockade of Psgl-1. Although additional mechanistic studies related to Psgl-1 deficiency may uncover other downstream targets, the current study supports consideration of anti-Psgl-1 therapy for patients at high risk of vascular complications.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health (HL57346, HL073150 to D.T.E.) and a VA Merit Award (BX000353 to DTE).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship Contributions
Contribution: D.T.E. designed research and wrote the paper; W.L. designed research, performed experiments, analyzed data, and wrote the paper; H.W, M.K.O., C.G., K.S. and J.W. performed experiments.
DISCLOSURES
None.
References
- 1.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115(5):e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. Prepublished on 2006/12/30 as DOI CIRCULATIONAHA.106.179918 [pii] 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM, Glynn RJ. JUPITER, rosuvastatin, and the European Medicines Agency. Lancet. 375(9731):2071. doi: 10.1016/S0140-6736(10)60760-X. Prepublished on 2010/05/25 as DOI S0140-6736(10)60760-X [pii] 10.1016/S0140-6736(10)60760-X. [DOI] [PubMed] [Google Scholar]
- 3.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–1143. doi: 10.1161/hc0902.104353. Prepublished on 2002/03/06 as DOI. [DOI] [PubMed] [Google Scholar]
- 4.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi: 10.1038/nature01323. Prepublished on 2002/12/20 as DOI 10.1038/nature01323 nature01323 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Rao RM, Yang L, Garcia-Cardena G, Luscinskas FW. Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circ Res. 2007;101(3):234–247. doi: 10.1161/CIRCRESAHA.107.151860b. Prepublished on 2007/08/04 as DOI 101/3/234 [pii] 10.1161/CIRCRESAHA.107.151860b. [DOI] [PubMed] [Google Scholar]
- 6.Moore KL, Patel KD, Bruehl RE, et al. P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J Cell Biol. 1995;128(4):661–671. doi: 10.1083/jcb.128.4.661. Prepublished on 1995/02/01 as DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J, Hirata T, Croce K, et al. Targeted gene disruption demonstrates that P-selectin glycoprotein ligand 1 (PSGL-1) is required for P-selectin-mediated but not E-selectin- mediated neutrophil rolling and migration. J Exp Med. 1999;190(12):1769–1782. doi: 10.1084/jem.190.12.1769. Prepublished on 1999/12/22 as DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox R, Nhan TQ, Law GL, Morris DR, Liles WC, Schwartz SM. PSGL-1 and mTOR regulate translation of ROCK-1 and physiological functions of macrophages. EMBO J. 2007;26(2):505–515. doi: 10.1038/sj.emboj.7601522. Prepublished on 2007/01/25 as DOI 7601522 [pii] 10.1038/sj.emboj.7601522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phillips JW, Barringhaus KG, Sanders JM, et al. Single injection of P-selectin or P-selectin glycoprotein ligand-1 monoclonal antibody blocks neointima formation after arterial injury in apolipoprotein E-deficient mice. Circulation. 2003;107(17):2244–2249. doi: 10.1161/01.CIR.0000065604.56839.18. [DOI] [PubMed] [Google Scholar]
- 10.Bodary PF, Westrick RJ, Wickenheiser KJ, Shen Y, Eitzman DT. Effect of leptin on arterial thrombosis following vascular injury in mice. Jama. 2002;287(13):1706–1709. doi: 10.1001/jama.287.13.1706. [DOI] [PubMed] [Google Scholar]
- 11.Bodary PF, Gu S, Shen Y, Hasty AH, Buckler JM, Eitzman DT. Recombinant leptin promotes atherosclerosis and thrombosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25(8):e119–e122. doi: 10.1161/01.ATV.0000173306.47722.ec. [DOI] [PubMed] [Google Scholar]
- 12.Russo HM, Wickenheiser KJ, Luo W, et al. P-selectin glycoprotein ligand-1 regulates adhesive properties of the endothelium and leukocyte trafficking into adipose tissue. Circ Res. 2010;107(3):388–397. doi: 10.1161/CIRCRESAHA.110.218651. Prepublished on 2010/06/19 as DOI CIRCRESAHA.110.218651 [pii] 10.1161/CIRCRESAHA.110.218651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sunderkotter C, Nikolic T, Dillon MJ, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172(7):4410–4417. doi: 10.4049/jimmunol.172.7.4410. Prepublished on 2004/03/23 as DOI. [DOI] [PubMed] [Google Scholar]
- 14.Bodary PF, Homeister JW, Vargas FB, et al. Generation of soluble P- and Eselectins in vivo is dependent on expression of P-selectin glycoprotein ligand-1. J Thromb Haemost. 2007;5(3):599–603. doi: 10.1111/j.1538-7836.2007.02388.x. Prepublished on 2007/01/19 as DOI JTH2388 [pii] 10.1111/j.1538-7836.2007.02388.x. [DOI] [PubMed] [Google Scholar]
- 15.Norman KE, Moore KL, McEver RP, Ley K. Leukocyte rolling in vivo is mediated by P-selectin glycoprotein ligand-1. Blood. 1995;86(12):4417–4421. Prepublished on 1995/12/15 as DOI. [PubMed] [Google Scholar]
- 16.An G, Wang H, Tang R, et al. P-Selectin Glycoprotein Ligand-1 Is Highly Expressed on Ly-6Chi Monocytes and a Major Determinant for Ly-6Chi Monocyte Recruitment to Sites of Atherosclerosis in Mice. Circulation. 2008 doi: 10.1161/CIRCULATIONAHA.108.771048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swirski FK, Libby P, Aikawa E, et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117(1):195–205. doi: 10.1172/JCI29950. Prepublished on 2007/01/04 as DOI 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorlacius H, Lindbom L, Raud J. Cytokine-induced leukocyte rolling in mouse cremaster muscle arterioles in P-selectin dependent. Am J Physiol. 1997;272(4 Pt 2):H1725–H1729. doi: 10.1152/ajpheart.1997.272.4.H1725. Prepublished on 1997/04/01 as DOI. [DOI] [PubMed] [Google Scholar]
- 19.Harari OA, McHale JF, Marshall D, et al. Endothelial cell E- and P-selectin up-regulation in murine contact sensitivity is prolonged by distinct mechanisms occurring in sequence. J Immunol. 1999;163(12):6860–6866. Prepublished on 1999/12/10 as DOI ji_v163n12p6860 [pii] [PubMed] [Google Scholar]