Abstract
Objective
Ospemifene, a new drug indicated for the treatment of vulvovaginal atrophy, has completed Phase III clinical trials. A condition affecting millions of women worldwide, vulvovaginal atrophy has long been treated with estrogen therapy. Estrogen treatment carries with it risks of thromboembolism, endometrial proliferative effects, and breast cancer promotion. In this study, we test the effects of three dosing levels of ospemifene in both the prevention and treatment of breast cancer in the MTag.Tg mouse model.
Methods
The polyomavirus middle-T transgenic mouse model (MTag.Tg), which produces synchronized, multifocal mammary tumors in the immunologically intact C57BL/6 background, was used to examine the impact of ospemifene treatment. First, a cell line derived from an MTag.Tg mouse tumor (Mtag 34) was treated in vitro with ospemifene and its major metabolite, 4-OH ospemifene. MTag.Tg mice were treated daily by gavage with three different doses of ospemifene (5, 25, and 50 mg/kg) before or after the development of mammary tumors. Survival and tumor development results were used to determine the effect of ospemifene treatment on mammary tumors in both the preventive and treatment settings.
Results
Tumors and the MTag 34 cell line were positive for estrogen receptor expression. The MTag 34 line was not stimulated by ospemifene or its major, active metabolite 4-OH ospemifene in vitro. Ospemifene increased survival time and exerted an antitumor effect on the development and growth of estrogen receptor positive mammary tumors in the MTag.Tg mouse model at the 50 mg/kg dose. Levels of ospemifene and 4-OH ospemifene in both the tumors and plasma of mice confirmed dosing. Ospemifene did not exert an estrogenic effect in the breast tissue at doses equivalent to human dosing.
Conclusions
Ospemifene prevents and treats estrogen receptor positive MTag.Tg mammary tumors in this immune intact mouse model in a dose-dependent fashion. Ospemifene drug levels in the plasma of treated mice were comparable to those found in humans. Combined with our previous data, ospemifene does not appear to pose a breast cancer risk in animals, and slows cancer development and progression in the MTag.Tg model.
Keywords: Ospemifene, Vulvovaginal Atrophy, Osteoporosis, Breast Cancer, Hormone Therapy, Chemoprevention
Introduction
Vulvovaginal atrophy (VVA) manifests as vaginal dryness, itching, and dyspareunia. It affects millions of postmenopausal women worldwide. Treatment options include lubricants, moisturizers, or estrogen, administered locally or systemically. Estrogen products for the treatment of VVA include vaginal rings, creams, tablets and transdermal patches. Common side effects of oral estrogen therapy include increased breast density (which may lead to abnormal mammograms and biopsies) and tenderness, nausea [1], weight gain [2], fluid retention and vaginal bleeding [3]. Additionally, estrogen usage may expose women to health risks including gallbladder and thromboembolic diseases [4–12], coronary heart disease, breast cancer, and stroke [13, 14]. The safety of hormone therapy (HT) use in women with a history of cancer is controversial [15, 16], and estrogen treatments address only the symptoms of VVA, not the underlying cause of the disease.
Selective estrogen receptor modulators (SERMs) are non-steroidal compounds that bind to the estrogen receptor (ER), and can exert both agonistic and antagonistic effects in tissue-specific settings. Sites expressing ER include breast, bone, brain, uterine and genitourinary tissues [17]. SERMs such as tamoxifen have been useful in the treatment and prevention of breast cancer, leading to reduced rates of recurrence and death, but produce undesirable side effects including VVA.
The development of new SERMs which do not induce vasomotor and VVA side effects but are still efficacious would provide an advance in therapies and enhance the quality of life for breast cancer patients and menopausal women as a whole. Ospemifene, a triphenylethylene derivative, was originally developed as an osteoporosis drug, but has been prepared for submission to the Food and Drug Administration (FDA) as a treatment for VVA. Unlike estrogen, which carries endometrial proliferation and thromboembolic risks, ospemifene treatment is not associated with such events [1, 2, 14, 18–21]. The impact of ospemifene treatment on breast cancer development, however, has not been fully examined. Ospemifene has been tested in several animal models, but none with intact immune systems or without reliance on the chemical induction of cancer. In this study, we use the immunologically intact MTag.Tg transgenic mouse model, which produces synchronized, multifocal mammary tumors, to test the effect of ospemifene on ER-positive breast tumors in vivo.
Methods
Chemicals
Ospemifene, Ophena™ (chemical name: Z-2-[4-(4-chloro-1,2-diphenyl-but-1- enyl)phenoxy]ethanol) was manufactured by Lipomed AG (Arlesheim, Switzerland). 4-hydroxyospemifene (4-OH ospemifene) and toremifene were provided by the Orion Corporation, Orion-Pharma, Finland. All reagents including hexane, methanol, butanol and water were HPLC grade and purchased from Fisher Scientific (Pittsburgh, PA, USA). Triethylamine and 17β-estradiol was obtained through Sigma Chemical Company (St. Louis, MO, USA). All drugs and reagents were stored as indicated by the manufacturer.
Tissue Culture
MCF-7 cells were purchased from the American Type Culture Collection (Mannassas, VA, USA). The MTag-34 cell line was derived from a mammary tumor excised from an MTag.Tg animal. All cells were grown in Improved Minimum Essential Medium (Invitrogen, Carlsbad, CA, USA) without antibiotics and supplemented with 10% fetal bovine serum. Cells were maintained in an atmosphere of 5% CO2/95% air at 37°C. Cells were propagated in 75-cm2 tissue culture treated flasks (Corning, Corning, NY, USA) and passaged at least once a week.
In vitro Drug Treatments
Stock solutions of ospemifene and 4-OH ospemifene were prepared by dissolution in DMSO followed by ethanol (EtOH) and brought up to volume in sterile normal saline immediately before use. MTag-34 cells were treated with four concentrations of ospemifene or 4-OH ospemifene (0.01, 0.1, 1.0, and 6.6 μM). For the control, sterile saline (drug diluent) was added to the cell cultures in equivalent volumes. The final concentration of dimethylsulfoxide (DMSO) and ethanol in all flasks was 0.0083% and 0.15% respectively. Cells were harvested when controls reached approximately 70% confluency and trypsinized for the trypan blue dye exclusion assay. Trypsinized cells from each treatment condition were diluted in trypan blue and counted on a T4 Auto Cellometer (Nexcelom Bioscience, Lawrence, MA, USA). Growth inhibition/stimulation was calculated as a percent of the control.
Flow Cytometry
Cells were trypsinized, pelleted, and resuspended in 1X phosphate buffered saline (PBS). Cells were transferred dropwise into a polystyrene tube containing ice cold 70% EtOH. Tubes were stored at −20°C for at least two hours. Next, cells were pelleted, washed once in PBS, and resuspended in PBS and 10μg/mL DNase-free RNaseA. Tubes were incubated in a 37°C waterbath for 45 minutes. Propidium iodide was added to a concentration of 10 mg/mL followed by a 10 minute incubation at room temperature protected from light. Samples were analyzed on a Becton Dickenson Fortessa (BD, San Jose, CA, USA) flow cytometer, and data was analyzed using ModFit software (Verity Software House, Topsham, ME, USA).
Western Blotting
Cells were lysed immediately in IP lysis buffer (Pierce IP Lysis Buffer, Thermo Scientific #8778), and stored until protein quantitation and Western blotting was performed. Protein lysates (45 μg) were separated in pre-cast 4–15% gradient SDS-PAGE gels (BioRad 456-1083) and transferred onto 0.2-μm PVDF membranes (BioRad 162-0174). The following antibodies were used to assess protein expression: ER-α (E115; Novus Biologicals NB110-56961 lot#YF112101C, 1:1500), ER-β (Abcam, ab3576, lot#GR14900-1, 1:1500) and β-actin (AC-15; Sigma A5441, 1:50,000). All images were obtained on a FluorChem E System with AlphaView (SA) software, version 3.2.2 (Cell Biosciences, Santa Clara, CA.).
HPLC
Ospemifene and 4-OH ospemifene levels in the tumors and plasma of MTag.Tg mice were quantified as previously described [22]. Briefly, a Beckman (Fullerton, CA) Model 320 gradient liquid chromatograph, a Model 420 controller, two Model 110A pumps along with a Whatman (Clifton, NJ, USA) 5-μm reverse phase C18 Partisphere RTF 4.6 × 250 mm column, 100-μl injection loop, and a Rheodyne injector were used to analyze samples. Photochemically activated ospemifene, 4-OH ospemifene, and an internal standard (toremifene) were detected with a Linear Instruments Model LC305 (ThermoFinnigan, San Jose, CA, USA) fluorescence detector set at an excitation wavelength of 266 nm and an emission wavelength of 370 nm. Peak height ratios of ospemifene and 4-OH ospemifene to internal standard were then calculated, and linear regression analysis of the data yielded the slope, y-intercept, and correlation coefficient of each standard curve.
Immunohistochemistry
Hematoxylin and eosin (H&E) and ER-α staining were carried out using standard protocols. For H&E staining, slides were cleared with xylene three times for 2 minutes each, hydrated with 100-70% EtOH in 5 steps at 2 minutes each and rinsed with tap water for at least 2 minutes. Staining was carried out with hematoxylin for three minutes and eosin for 2 minutes. Slides were then dehydrated in 95 to 100% EtOH for 20 dips followed by three, 2-minute incubations. Finally, slides were cleared with three 2-minute washes in xylene and coverslipped.
For ER staining, ER-α antibody from Santa Cruz (MC 20; Cat #sc542) was used. Slides were deparaffinized in three changes of xylene for 5 minutes each, followed by 3 changes of 100% EtOH for 2 minutes each. Endogenous peroxidase was quenched with hydrogen peroxide:methanol 20 ml:180 ml for 30 minutes. Two changes of 100% EtOH, one 95% EtOH and one 70% EtOH for 2 minutes each followed. Slides were hydrated for 5 minutes in running tap water and rinsed in distilled water for 2 minutes. Antigen retrieval was performed using freshly made citrate buffer, pH 6.0 in a de-cloaker. Slides were next rinsed in warm tap water for 5 minutes. Two PBS rinses for 5 minutes each preceded a blocking step for 20 minutes with 10% normal horse serum, diluted in PBS. Two PBS rinses for 5 minutes each followed the secondary antibody incubation, which was diluted in PBS + ovalbumin and incubated for 1 hour at room temperature. Two PBS rinses for 5 minutes each followed. Next, slides were incubated with Avidin Biotin Complex (ABC) diluted in PBS + ovalbumin for 30 minutes at room temperature followed by two PBS rinses for 5 minutes each. Slides were exposed to DAB (made in distilled water) for 1–5 minutes depending on development and rinsed in tap water for 5 minutes. Slides were counterstained for 10–20 seconds if antigen was nuclear; 30 seconds if antigen was cytoplasmic or on cell membrane. Slides were rinsed in tap water for 5 minutes to “blue” them. Finally, slides were dehydrated in 70% to 100% EtOH for 2 minutes each followed by 3 changes of xylene for 5 minutes each and coverslipped.
Animal Studies
Polyomavirus middle-T transgenic male founder mice were obtained from Mayo Clinic (Scottsdale, AZ). All mice were supplied by our breeding colony maintained by the UC Davis Mouse Biology Program and housed at the UC Davis Center for Laboratory Animal Science vivarium. Mice were kept in rooms maintained at constant temperature and humidity with a 12-h light/12-h dark cycle. All mice had free access to water and Purina Laboratory Rodent Diet (LabDiet® 5001, PMI® Nutrition International, St. Louis, MO). Animal studies were conducted under a protocol approved by the University of California Davis Institutional Animal Care and Use Administrative Advisory Committee. UC Davis is an Association for Assessment and Accreditation of Laboratory Animal Care accredited institution. For genotyping, toe clippings from two-week old mice were placed in a 96-well plate. Next, 40 μl of 50 mM NaOH was added to each well, and plates were heated to 94°C for 10 minutes. Extracted DNA was neutralized with 20 μl of 1 M Tris (pH 8) per well. Plates were sealed, vortexed briefly, centrifuged (6000 rpm × 2 minutes), and stored at −20°C until use. Polymerase chain reaction was used to identify MTag.Tg mice using an ABI Prism 7900HT Sequence Detection System (AB Applied Biosystems, Carlsbad, CA). MTag forward and reverse primers were 5′-TTGGAGAATGTTTTTGTCTTGAATG and 3′-CAGCACATCTCGGGTTGGT. The MTag TaqMan probe (ACATGCAATGGTTTGGAA) carrying a 6′ FAM reporter label and a 3′ MGBNFQ quencher group was used. The amplification program consisted of one cycle of 2 minutes at 95°C and 40 cycles of 15 seconds each at 60°C and 95°C.
In vivo Drug Treatments
Prevention Study
Female 4–6 week old MTag.Tg C57BL/6 mice weighing approximately 12 grams were assigned to four treatment groups: Control (n=7), and ospemifene 5 mg/kg (n=7), 25 mg/kg (n=8) and 50 mg/kg (n=8). Ospemifene doses were calculated weekly using weight data. Ospemifene was dissolved in DMSO and then brought up to volume in peanut oil yielding final concentrations of ospemifene that would deliver 5, 25, and 50 mg/kg doses in a volume of 100μl. Control solution consisted of 2% DMSO in peanut oil. The final concentration of DMSO in all solutions was 2%. Ospemifene and control solutions were orally administered daily by gavage according to treatment group. Mice were evaluated weekly for changes in weight and the development of mammary tumors. Tumors were palpated and when possible, measured using calipers to assess tumor volume. Approximate tumor volumes were calculated using the formula v = l × w × h where v is volume, w is width, h is height and l was length of the tumor. Once tumors reached 1.5 cm3, or the mouse had an abscessed tumor, was visibly in pain or unable to ambulate, animals were sacrificed in a CO2 chamber. Upon euthanization, whole blood was collected by cardiac puncture into heparinized tubes, and tissues were harvested for HPLC analysis. A portion of each tumor was preserved in buffered 4% formalin, followed by 70% EtOH storage and paraffin embedding for histological analyses.
Treatment Study
4-week old MTag.Tg C57BL/6 mice that weighed approximately 16 grams were allowed to develop breast cancer for 16 weeks unperturbed. At this time, mice were sorted to achieve equal average weights and tumor volumes in each treatment group. The average weight of all groups was approximately 22 grams. Ospemifene doses were calculated using weekly weight data, and dosing solutions were prepared and administered as described for the Prevention Study. As in the Prevention Study, the treatment groups consisted of control and ospemifene 5, 25, and 50 mg/kg (n=10, all groups). Mice were evaluated weekly for changes in weight and the development of mammary tumors. Tumors were palpated and when possible, measured using calipers to assess tumor volume. Approximate tumor volumes were calculated as described for the Prevention Study. As in the Prevention Study, once tumors reached 1.5 cm3, or the mouse had an abscessed tumor, was visibly in pain or unable to ambulate, animals were sacrificed in a CO2 chamber. Upon euthanization, whole blood and tissues were collected and processed as described for the Prevention Study.
Statistical Analysis
Differences in average tumor volume between experimental groups were evaluated for statistical significance using a one-way ANOVA test. A p-value of ≤ 0.05 was considered significant. Kaplan-Meier survival curves and long-rank test for significance were generated by the GraphPad (La Jolla, CA, USA) Prism® 5 software.
Results
MTag.Tg tumors and a derived cell line are ER positive
The MTag.Tg mouse breast cancer model involves the directed expression of the polyomavirus middle-T antigen fusion gene in the mammary tissue of C57BL/6 mice [23]. This expression leads to rapid and early transformation of the mammary tissue in all ten fat pads of an animal [23]. This model’s progression of cancer has been shown to parallel the development of human mammary cancers, both histologically and biologically [23, 24].
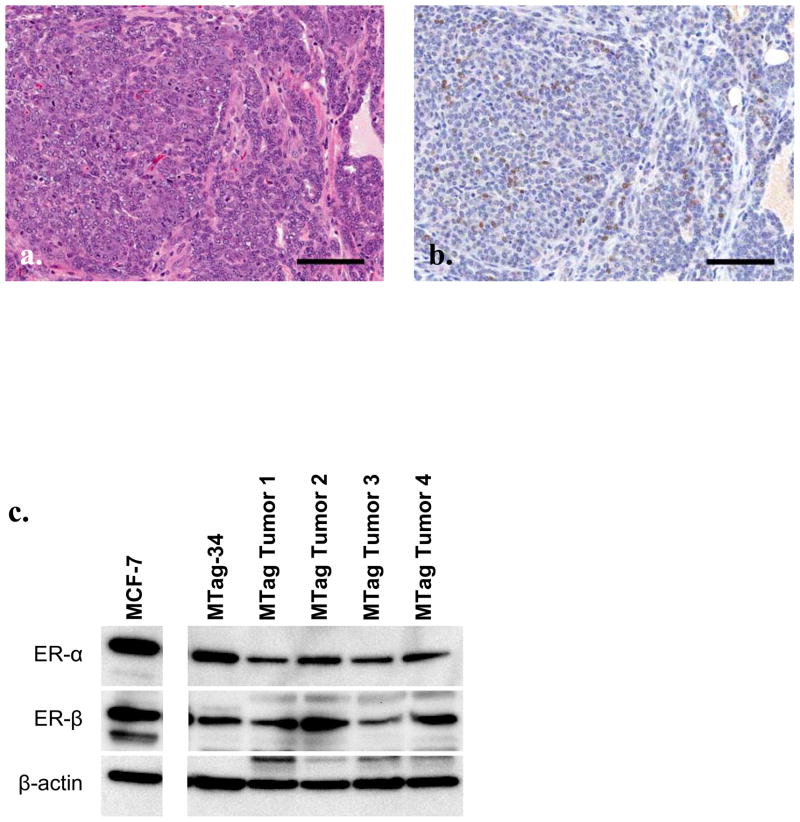
In order to examine the ER status and potential antiestrogen sensitivity of the MTag.Tg tumors, mice were allowed to develop tumors, which were then prepared for immunohistochemical staining and Western blotting. Figure 1a shows representative H&E staining, while Figure 1b shows ER-α expression patterns in an MTag.Tg tumor. A cell line established from an MTag.Tg tumor (MTag-34) shows preservation of the same protein expression patterns of ER-α and ER-β in vitro (see Figure 1c for Western blotting data).
Figure 1.
MTag.Tg tumors and cell line express estrogen receptor. Representative Mtag.Tg tumor stained for H&E (a) and ER-α (b). Black bar indicates 100 μm. (c) Western blot for ER-α and β in MTag.Tg tumors and derived cell line lysates. MCF-7 cell lysates serves as a positive control, β actin as loading control.
Ospemifene and 4-OH ospemifene treatments do not stimulate growth of the MTag.Tg cell line in vitro
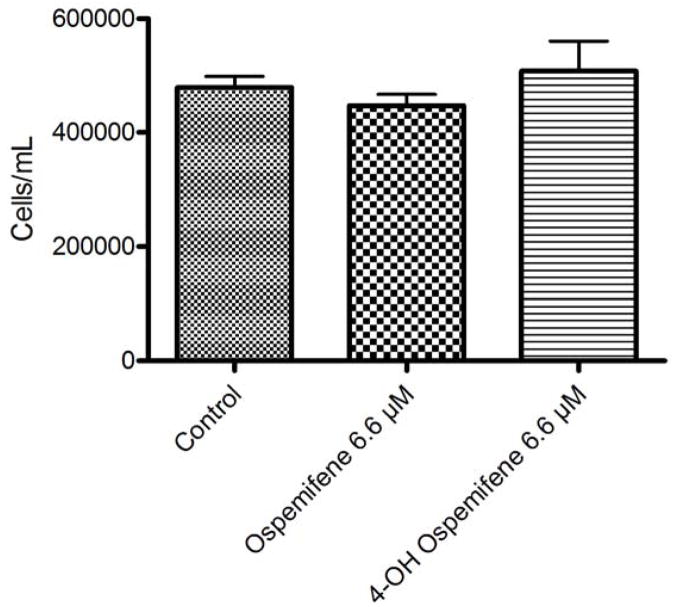
In order to test the in vitro effect of ospemifene on the MTag-34 cell line, cells were treated with four concentrations of ospemifene or its active metabolite 4-OH ospemifene and harvested for the trypan blue dye exclusion assay and cell counts when controls reached approximately 70% confluence. The results shown in Figure 2a demonstrate that ospemifene did not significantly affect cell growth over four days, as it previously did in the MCF-7 human breast cancer cell line, which is known to be sensitive to antiestrogens [25]. When MTag-34 cells were treated with 4-OH ospemifene, similar results were observed. These treated cells were analyzed for DNA content, and flow cytometric data did not indicate a cell cycle arrest (data not shown). When cells were plated at a lower confluency and continuously exposed for 7 days, the same results were observed.
Figure 2.
Ospemifene and its major metabolite 4-OH ospemifene do not stimulate Mtag-34 cells in vitro. Cells were continuously exposed to ospemifene or 4-Hydroxyospemifene at 6.6 μM for 4 days. Viable cell counts assessed by trypan blue exclusion demonstrated that the MTag-34 cell line is not stimulated by these SERMs.
These data led us to conclude that neither ospemifene nor its major metabolite stimulated the growth of the MTag.Tg derived tumor cell line, and that these compounds may indeed delay the development or progression of breast cancer in the context of the MTag.Tg background given continuous exposure.
Ospemifene delays the development of breast cancer in vivo
In order examine the effects of ospemifene in vivo, 31 four-week old mice were separated into groups and administered control, 5, 25, or 50 mg/kg doses of ospemifene daily, by oral gavage. These doses were chosen based on previous work [22, 25]. Mice were weighed weekly and palpated for the presence of mammary tumors. Mice weights were not significantly affected in any treatment group. Tumors were measured in three dimensions and animals were sacrificed when maximum tumor burden was reached. Ospemifene and its metabolite 4-OH ospemifene were quantified in the plasma and tumors of each animal to confirm dosing levels in this model. Table 1a shows the average plasma and tumor concentrations of ospemifene and 4-OH ospemifene in the tumors and plasma of all animals in the Prevention Study. Observed plasma concentrations were similar to those found in humans at equivalent dose levels [26]. In the higher dosing groups, average tumor concentrations of ospemifene were elevated compared to those observed in plasma, as previously observed [25].
Table 1.
Average concentrations of ospemifene and 4-OH ospemifene in the tumors and plasma of mice in each cohort of the prevention and treatment experiments.
| a.
| |||||
|---|---|---|---|---|---|
| Prevention | 4-OH OSP | OSP | n | ||
| Group | Plasma (nM) | Tumor (nmol/g) | Plasma (nM) | Tumor (nmol/g) | |
| Osp 5 mg/kg | 96.5 | 0.05 | 72.6 | 0.07 | 7 |
|
|
|||||
| Osp 25 mg/kg | 760.2 | 0.35 | 446.3 | 1.71 | 8 |
|
|
|||||
| Osp 50 mg/kg | 2505.2 | 0.92 | 2013.2 | 4.04 | 8 |
|
| |||||
| Control animals (n=10) had no detectible peaks | |||||
| b.
| |||||
|---|---|---|---|---|---|
| Treatment | 4-OH OSP | OSP | n | ||
| Group | Plasma (nM) | Tumor (nmol/g) | Plasma (nM) | Tumor (nmol/g) | |
| Osp 5 mg/kg | 53.2 | 0.05 | 42.2 | 0.07 | 5 |
|
|
|||||
| Osp 25 mg/kg | 397.1 | 0.26 | 181.8 | 0.66 | 2 |
|
|
|||||
| Osp 50 mg/kg | 1015.4 | 0.99 | 1947.2 | 3.43 | 3 |
|
| |||||
| Control animals (n=10) had no detectible peaks | |||||
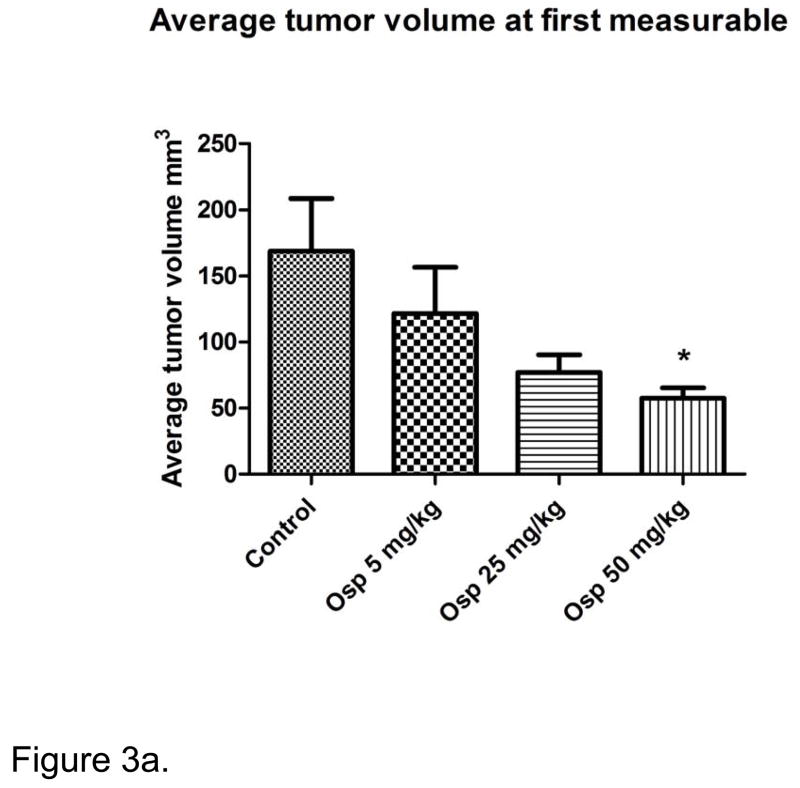
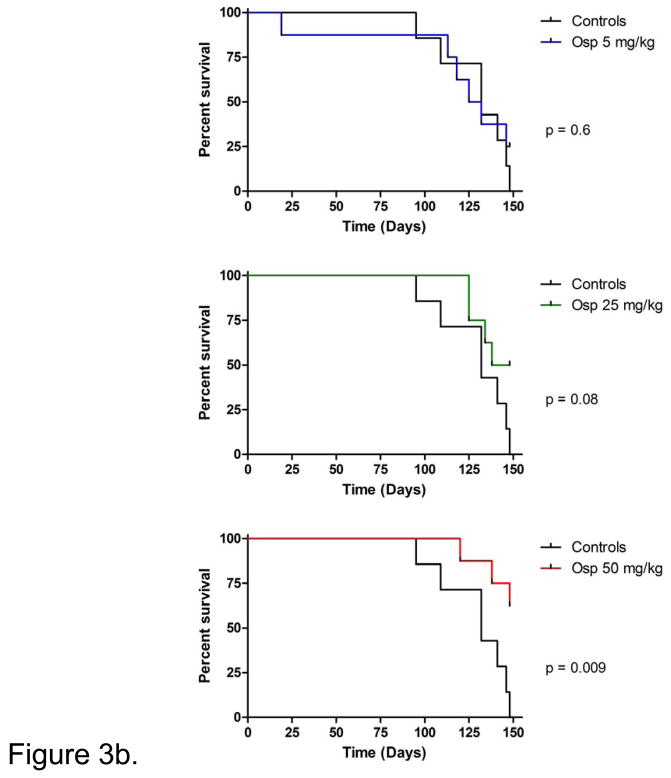
The effects of ospemifene on tumor volumes were observed early in this study. The average tumor volumes at the emergence of the first measurable tumor are shown in Figure 3a. Average tumor volumes from the control mice were significantly larger than those of the 50 mg/kg group at this intermediate point. Figure 3b shows the survival curves produced from the different dose groups. No difference was observed between the control and 5 mg/kg treatment groups. The 25 mg/kg group did not reach significance compared to the control, (p=0.0787) but a trend was evident. In contrast, animals in the highest dose group did gain a clear and significant survival advantage over controls (p=0.0085).
Figure 3.
Figure 3a. Tumors in the control group were significantly larger at first measurable than those in the highest dose of ospemifene. Tumor volumes at first palpable were averaged by group. No difference in the time to first palpable or measurable was observed. Asterisk indicates statistical significance of p <0.05.
Figure 3b. Ospemifene slows the progression of tumor growth in young MTag.Tg mice. Thirty-one MTag.Tg mice were grouped and treated with either control or three increasing doses of ospemifene (5, 25, or 50 mg/kg, respectively) and palpated weekly for the presence of tumors. Mice were euthanized when tumor burden maximum was reached. The study was ended when 80% of the controls were lost.
Ospemifene treats breast cancer
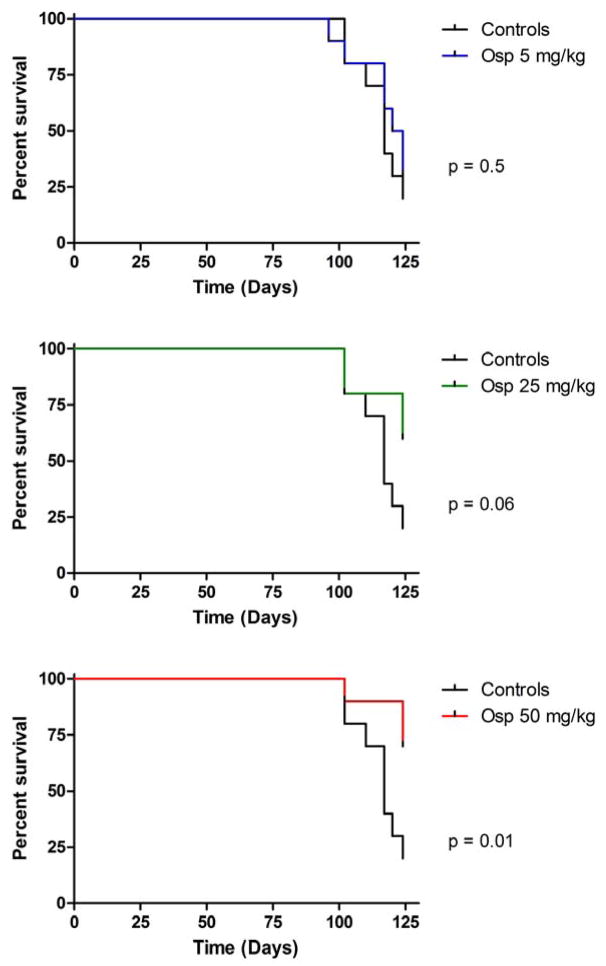
In order to examine the impact of ospemifene in the treatment setting, 40 MTag.Tg mice were allowed to develop tumors for 16 weeks. At this time, mice were sorted into 4 groups with equal weights and tumor burdens. The average tumor burden including all 10 tumor sites was approximately 300 mm3 (297.3, 295.2, 295.1, and 301.8 mm3, respectively) in each group. Mice underwent daily gavage at the same four doses: control, 5, 25, or 50 mg/kg ospemifene until maximum tumor volumes were reached. Table 1b shows the concentration of ospemifene and 4-OH ospemifene in the tumors and plasma from each animal in this study. Again, observed plasma concentrations were similar to those found in humans at equivalent dose levels [26]. Survival curves from this study are shown in Figure 4. As seen previously, ospemifene treatment prolonged the average lifespan of mice in the 25 and 50 mg/kg treatment groups, but as in the Prevention Study, only significant at the 50 mg/kg dose (p=0.0586 and 0.0143 respectively).
Figure 4.
Effect of ospemifene treatment on MTag.Tg breast cancer. Forty MTag.Tg mice were allowed to develop tumors for 16 weeks. At this time, mice were grouped to achieve equal average weights and tumor volumes, and treated with either control or three increasing doses of ospemifene (5, 25, or 50 mg/kg, respectively). Mice were palpated weekly for the presence of tumors. Mice were euthanized when tumor burden maximum was reached. The study was ended when 80% of the controls were lost.
Discussion
Ospemifene, an investigational SERM, was originally developed as an osteoporosis drug. It reduces bone turnover, increases bone strength [40–42], and has a positive effect on serum lipids [41, 43] while cardiovascular surrogate markers remain neutral [41]. These bone effects are thought to be ER mediated [44]. Ospemifene exerts antiestrogenic effects on breast tissue, and estrogen-like effects on bone, serum lipids (lowering LDL) [41, 43] and the genitourinary tract [45, 46].
Ospemifene recently completed two phase III clinical trials, in addition to a long-term safety study [47], and is a well-tolerated treatment in development for VVA. Treatment with ospemifene results in relief of VVA symptoms compared to vaginal lubricants alone, and improves most bothersome symptom and decreases vaginal pH [48]. Ospemifene does not cause breast tenderness or aggravate vasomotor symptoms associated with menopause, such as hot flashes [47]. Its positive effects on VVA symptoms were sustained during a 1-year follow-up study [49]. Ospemifene does not carry with it the risks of thromboembolism or endometrial proliferative effects as estrogen does, but concerns regarding the treatment of women with a SERM demand the examination of its effects on the breast.
In previous studies, ospemifene prevented the growth of premalignant lesions and the progression to invasive carcinoma in the adenoma/mammary intraepithelial neoplasia (MIN-O) mouse model [52], and slowed the tumor growth of MCF-7 xenografts [25]. These models are not optimal to study drug treatment as the implantation of the MIN-O tissue is a surgical procedure, and the MCF-7 studies were carried out in ovariectomized nude mice. Neither group possessed intact, or otherwise unperturbed immune systems. Ospemifene has also been shown to be effective in preventing the development of dimethylbenzanthracene (DMBA) chemically-induced breast carcinomas in the Sencar mouse model, but in this case, the cancer was induced rather than arising without intervention [22]. An examination of the impact of ospemifene on breast cancer in an immune intact, non-chemically-induced model was lacking.
The MTag.Tg model utilizes directed expression of the polyomavirus middle-T protein in mammary tissue to produce synchronized, multifocal tumors which are pathologically very similar to the development of breast cancer in humans [23, 24]. This model has a short latency period and is pregnancy independent. Four distinct stages of disease can be observed. By 4 weeks, hyperplasia is already present, followed by the development of adenoma/mammary intraepithelial neoplasia (MIN) at 8 weeks, with early and late carcinoma following (8–9 and 8–12 weeks, respectively). Tumors are initially ER-positive and lose some expression over time. We show here that the MTag.Tg tumors and a derived cell line remain ER positive and respond to antiestrogen therapy. This is the first time such an antiestrogen study has been carried out in this model. MTag-34 cells treated with ospemifene and 4-OH ospemifene were not stimulated in vitro and no impact on cell cycle was seen by flow cytometric analysis (data not shown). This data warranted further testing in our animal model, despite the lack of an anti-tumor effect in vitro. Ospemifene is a good example of how important in vivo testing is regardless of outcomes in vitro. Many effective hormonal therapies may be overlooked during the process of drug development due to their lack of short-term toxicity in vitro, while proving to be effective in vivo after metabolism and prolonged exposure at low doses.
Antiestrogenic therapies used in the treatment of women with breast cancer include tamoxifen, anastrozole, letrozole, and exemestane, which can cause sexual dysfunction and increased vasomotor symptoms [56–58]. Tamoxifen use increased reported rates of vaginal dryness and dyspareunia [59] which were more severe than those experienced by untreated women [10, 60], and these symptoms correlated with decreased libido, arousal, and ability to experience orgasm [59, 61]. Most women are still sexually active while being treated for breast cancer [62, 63], and high non-compliance rates for women with breast cancer taking adjuvant tamoxifen and other antiestrogen therapy (up to 50% for tamoxifen use) may be partially attributed to the side effects including VVA [64–66]. In this study, ospemifene effectively treated and prevented breast cancer development in mice, suggesting that ospemifene could be developed as a breast cancer agent that does not carry the risks of VVA and other side effects associated with currently approved antiestrogenic therapies.
Conclusions
Ospemifene, a late-stage drug in development for the treatment of women suffering from vulvovaginal atrophy, does not appear to carry the risks of thromboembolism or clinically significant endometrial proliferative effects, which have been associated with estrogen-based treatments. In the present study, we confirmed the results of previous studies showing that ospemifene does not exert an estrogenic effect in the breast and provides some protection against breast cancer in several animal models. Because of its pathological and physiological similarity to human breast cancer, the mouse model employed in this study provides the best evidence yet that ospemifene may be an effective agent in treating women with breast cancer in both the adjuvant and preventive settings. These findings are even more important given the strength of the MTag.Tg model’s oncogene. In a post menopausal woman, ospemifene may have an even greater impact on breast cancer risk.
Acknowledgments
The authors would like to thank Julia Tsai for her helpful discussions and critical reading of the manuscript.
Financial Support: This work was supported in part by the UC Davis Cancer Center Support Grant P30 CA93373-01
List of Abbreviations
- VVA
Vulvovaginal atrophy
- HT
Hormone therapy
- DMBA
Dimethylbenzanthracene
- DMSO
Dimethyl Sulfoxide
- ER
Estrogen receptor
- EtOH
ethanol
- FDA
Food and Drug Administration
- LDL
Low Density Lipoprotein
- HPLC
High-performance liquid chromatography
- NDA
New Drug Application
- PBS
Phosphate buffered saline
- WHI
Women’s Health Initiative
- SERM
Selective Estrogen Receptor Modulator
Footnotes
Conflict of Interest: MW DeGregorio is one of the original inventors of ospemifene and has an inventor’s royalty agreement with the manufacturer. R.A. Burich, J.L. McCall, N. R. Mehta, G.T. Wurz, B. E. Greenberg, K. E. Bell, and S. Griffey declare no conflicts of interest.
References
- 1.Cody JD, et al. Oestrogen therapy for urinary incontinence in post-menopausal women. Cochrane Database Syst Rev. 2009;(4):CD001405. doi: 10.1002/14651858.CD001405.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Bennink HJ. Reprint of Are all estrogens the same? Maturitas. 2008;61(1–2):195–201. doi: 10.1016/j.maturitas.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Beral V, et al. Hormone replacement therapy and high incidence of breast cancer between mammographic screens. Lancet. 1997;349(9058):1103–4. doi: 10.1016/S0140-6736(05)62328-8. [DOI] [PubMed] [Google Scholar]
- 4.Rossouw JE, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 5.Hulley S, et al. Noncardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) JAMA. 2002;288(1):58–66. doi: 10.1001/jama.288.1.58. [DOI] [PubMed] [Google Scholar]
- 6.Daly E, et al. Risk of venous thromboembolism in users of hormone replacement therapy. Lancet. 1996;348(9033):977–80. doi: 10.1016/S0140-6736(96)07113-9. [DOI] [PubMed] [Google Scholar]
- 7.Jick H, et al. Risk of hospital admission for idiopathic venous thromboembolism among users of postmenopausal oestrogens. Lancet. 1996;348(9033):981–3. doi: 10.1016/S0140-6736(96)07114-0. [DOI] [PubMed] [Google Scholar]
- 8.Grodstein F, et al. Prospective study of exogenous hormones and risk of pulmonary embolism in women. Lancet. 1996;348(9033):983–7. doi: 10.1016/S0140-6736(96)07308-4. [DOI] [PubMed] [Google Scholar]
- 9.Grodstein F, Colditz GA, Stampfer MJ. Postmenopausal hormone use and cholecystectomy in a large prospective study. Obstet Gynecol. 1994;83(1):5–11. [PubMed] [Google Scholar]
- 10.Mac Bride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc. 2010;85(1):87–94. doi: 10.4065/mcp.2009.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet. 1997;350(9084):1047–59. [PubMed] [Google Scholar]
- 12.Grady D, et al. Hormone replacement therapy and endometrial cancer risk: a meta-analysis. Obstet Gynecol. 1995;85(2):304–13. doi: 10.1016/0029-7844(94)00383-O. [DOI] [PubMed] [Google Scholar]
- 13.Martin KA, Manson JE. Approach to the patient with menopausal symptoms. J Clin Endocrinol Metab. 2008;93(12):4567–75. doi: 10.1210/jc.2008-1272. [DOI] [PubMed] [Google Scholar]
- 14.Chlebowski RT, Anderson, Garnet L, Gass, Margery, Lane, Dorothy S, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304(15):1684–1692. doi: 10.1001/jama.2010.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh P, Oehler MK. Hormone replacement after gynaecological cancer. Maturitas. 2010;65(3):190–7. doi: 10.1016/j.maturitas.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 16.Holmberg L, Anderson H. HABITS (hormonal replacement therapy after breast cancer--is it safe?), a randomised comparison: trial stopped. Lancet. 2004;363(9407):453–5. doi: 10.1016/S0140-6736(04)15493-7. [DOI] [PubMed] [Google Scholar]
- 17.Shelly W, et al. Selective estrogen receptor modulators: an update on recent clinical findings. Obstet Gynecol Surv. 2008;63(3):163–81. doi: 10.1097/OGX.0b013e31816400d7. [DOI] [PubMed] [Google Scholar]
- 18.Chlebowski RT, et al. Estrogen alone in postmenopausal women and breast cancer detection by means of mammography and breast biopsy. J Clin Oncol. 2010;28(16):2690–7. doi: 10.1200/JCO.2009.24.8799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson GL, et al. Prior hormone therapy and breast cancer risk in the Women’s Health Initiative randomized trial of estrogen plus progestin. Maturitas. 2006;55(2):103–15. doi: 10.1016/j.maturitas.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Calleja-Agius J, Brincat MP. Urogenital atrophy. Climacteric. 2009;12(4):279–85. doi: 10.1080/13697130902814751. [DOI] [PubMed] [Google Scholar]
- 21.Beral V, Bull D, Reeves G. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2005;365(9470):1543–51. doi: 10.1016/S0140-6736(05)66455-0. [DOI] [PubMed] [Google Scholar]
- 22.Wurz GT, et al. Ospemifene inhibits the growth of dimethylbenzanthracene-induced mammary tumors in Sencar mice. J Steroid Biochem Mol Biol. 2005;97(3):230–40. doi: 10.1016/j.jsbmb.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 23.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12(3):954–61. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin EY, et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 2003;163(5):2113–26. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taras TL, Wurz GT, DeGregorio MW. In vitro and in vivo biologic effects of Ospemifene (FC-1271a) in breast cancer. J Steroid Biochem Mol Biol. 2001;77(4–5):271–9. doi: 10.1016/s0960-0760(01)00066-8. [DOI] [PubMed] [Google Scholar]
- 26.DeGregorio MW, et al. Pharmacokinetics of (deaminohydroxy)toremifene in humans: a new, selective estrogen-receptor modulator. Eur J Clin Pharmacol. 2000;56(6–7):469–75. doi: 10.1007/s002280000176. [DOI] [PubMed] [Google Scholar]
- 27.Altman A. Postmenopausal dyspareunia- a problem for the 21st century. OBG management. 2009;21(3):37–44. [Google Scholar]
- 28.Simon JaKJ. Vulvovaginal atrophy (VVA) negatively impacts sexual function, psychosocial well-being, and partner relationships. Poster presented at the North American Menopause Association Annual Meeting; October 3–6, 2007; Dallas, Texas. 2007. [Google Scholar]
- 29.Lewis V. Undertreatment of menopausal symptoms and novel options for comprehensive management. Curr Med Res Opin. 2009;25(11):2689–98. doi: 10.1185/03007990903240519. [DOI] [PubMed] [Google Scholar]
- 30.The role of local vaginal estrogen for treatment of vaginal atrophy in postmenopausal women: 2007 position statement of The North American Menopause Society. Menopause. 2007;14(3 Pt 1):355–69. doi: 10.1097/gme.0b013e31805170eb. quiz 370–1. [DOI] [PubMed] [Google Scholar]
- 31.Labrie F, et al. Effect of one-week treatment with vaginal estrogen preparations on serum estrogen levels in postmenopausal women. Menopause. 2009;16(1):30–6. doi: 10.1097/gme.0b013e31817b6132. [DOI] [PubMed] [Google Scholar]
- 32.Paganini-Hill A, V, Henderson W. Estrogen replacement therapy and risk of Alzheimer disease. Arch Intern Med. 1996;156(19):2213–7. [PubMed] [Google Scholar]
- 33.Krause MM, Wheeler II, Thomas L, Richter Holly E, Snyder Thomas E. Systemic Effects of vaginally Administered Estrogen Therapy. Female Pelvic Medicine and Reconstructive Surgery. 2010 May/June 16;:188–195. doi: 10.1097/SPV.0b013e3181d7e86e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurst BS, et al. Absorption of vaginal estrogen cream during sexual intercourse: a prospective, randomized, controlled trial. J Reprod Med. 2008;53(1):29–32. [PubMed] [Google Scholar]
- 35.Rigg LA, Hermann H, Yen SS. Absorption of estrogens from vaginal creams. N Engl J Med. 1978;298(4):195–7. doi: 10.1056/NEJM197801262980406. [DOI] [PubMed] [Google Scholar]
- 36.Door M, Ranganath RP, et al. Plasma estradiol and esterone concentrations after oral and vaginal administration of conjugated estrogens in postmenopausal women with atrophic vaginitis. Poster presented at the 55th American College of Obstetrics and Gynecology; May 5th–9th 2007.2007. [Google Scholar]
- 37.Pastore LM, et al. Self-reported urogenital symptoms in postmenopausal women: Women’s Health Initiative. Maturitas. 2004;49(4):292–303. doi: 10.1016/j.maturitas.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 38.Indhavivadhana S, et al. Vaginal atrophy and sexual dysfunction in current users of systemic postmenopausal hormone therapy. J Med Assoc Thai. 2010;93(6):667–75. [PubMed] [Google Scholar]
- 39.Notelovitz M. Urogenital aging: solutions in clinical practice. Int J Gynaecol Obstet. 1997;59(Suppl 1):S35–9. doi: 10.1016/s0020-7292(97)90197-1. [DOI] [PubMed] [Google Scholar]
- 40.Komi J, et al. Effects of ospemifene, a novel SERM, on biochemical markers of bone turnover in healthy postmenopausal women. Gynecol Endocrinol. 2004;18(3):152–8. doi: 10.1080/09513590410001672197. [DOI] [PubMed] [Google Scholar]
- 41.Ylikorkala O, et al. Effects of ospemifene, a novel SERM, on vascular markers and function in healthy, postmenopausal women. Menopause. 2003;10(5):440–7. doi: 10.1097/01.GME.0000063566.84134.98. [DOI] [PubMed] [Google Scholar]
- 42.Qu Q, et al. Selective estrogenic effects of a novel triphenylethylene compound, FC1271a, on bone, cholesterol level, and reproductive tissues in intact and ovariectomized rats. Endocrinology. 2000;141(2):809–20. doi: 10.1210/endo.141.2.7342. [DOI] [PubMed] [Google Scholar]
- 43.Komi J, et al. Effects of ospemifene and raloxifene on hormonal status, lipids, genital tract, and tolerability in postmenopausal women. Menopause. 2005;12(2):202–9. doi: 10.1097/00042192-200512020-00015. [DOI] [PubMed] [Google Scholar]
- 44.Qu Q, Harkonen PL, Vaananen HK. Comparative effects of estrogen and antiestrogens on differentiation of osteoblasts in mouse bone marrow culture. J Cell Biochem. 1999;73(4):500–7. [PubMed] [Google Scholar]
- 45.Gennari L. Ospemifene Hormos. Curr Opin Investig Drugs. 2004;5(4):448–55. [PubMed] [Google Scholar]
- 46.Gennari L, et al. Selective estrogen receptor modulators for postmenopausal osteoporosis: current state of development. Drugs Aging. 2007;24(5):361–79. doi: 10.2165/00002512-200724050-00002. [DOI] [PubMed] [Google Scholar]
- 47.Gennari L, et al. Ospemifene use in postmenopausal women. Expert Opin Investig Drugs. 2009;18(6):839–49. doi: 10.1517/13543780902953715. [DOI] [PubMed] [Google Scholar]
- 48.Rutanen EM, et al. Effects of ospemifene, a novel SERM, on hormones, genital tract, climacteric symptoms, and quality of life in postmenopausal women: a double-blind, randomized trial. Menopause. 2003;10(5):433–9. doi: 10.1097/01.GME.0000063609.62485.27. [DOI] [PubMed] [Google Scholar]
- 49.Portman DKJ. Long-term effects of ospemifene on the clinical signs of vaginal atrophy. Abstract/poster; 20th Annual Meeting of the North American Menopause Society; 30 Sept-3 October 2009; San Diego CA. 2009. p. 42. [Google Scholar]
- 50.Hellmann-Blumberg U, et al. Genotoxic effects of the novel mixed antiestrogen FC-1271a in comparison to tamoxifen and toremifene. Breast Cancer Res Treat. 2000;60(1):63–70. doi: 10.1023/a:1006311214152. [DOI] [PubMed] [Google Scholar]
- 51.Wurz GT, Hellmann-Blumberg U, DeGregorio MW. Pharmacologic effects of ospemifene in rhesus macaques: a pilot study. Basic Clin Pharmacol Toxicol. 2008;102(6):552–8. doi: 10.1111/j.1742-7843.2008.00235.x. [DOI] [PubMed] [Google Scholar]
- 52.Namba R, et al. Selective estrogen receptor modulators inhibit growth and progression of premalignant lesions in a mouse model of ductal carcinoma in situ. Breast Cancer Res. 2005;7(6):R881–9. doi: 10.1186/bcr1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Voipio SK, et al. Effects of ospemifene (FC-1271a) on uterine endometrium, vaginal maturation index, and hormonal status in healthy postmenopausal women. Maturitas. 2002;43(3):207–14. doi: 10.1016/s0378-5122(02)00206-2. [DOI] [PubMed] [Google Scholar]
- 54.Komi J, et al. Effects of ospemifene and raloxifene on biochemical markers of bone turnover in postmenopausal women. J Bone Miner Metab. 2006;24(4):314–8. doi: 10.1007/s00774-006-0689-9. [DOI] [PubMed] [Google Scholar]
- 55.Bachmann GA, Komi JO. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17(3):480–6. doi: 10.1097/gme.0b013e3181c1ac01. [DOI] [PubMed] [Google Scholar]
- 56.Derzko C, Elliott S, Lam W. Management of sexual dysfunction in postmenopausal breast cancer patients taking adjuvant aromatase inhibitor therapy. Curr Oncol. 2007;14(Suppl 1):S20–40. doi: 10.3747/co.2007.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Day R, et al. Health-related quality of life and tamoxifen in breast cancer prevention: a report from the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Clin Oncol. 1999;17(9):2659–69. doi: 10.1200/JCO.1999.17.9.2659. [DOI] [PubMed] [Google Scholar]
- 58.Kwan KW, Chlebowski RT. Sexual dysfunction and aromatase inhibitor use in survivors of breast cancer. Clin Breast Cancer. 2009;9(4):219–24. doi: 10.3816/CBC.2009.n.037. [DOI] [PubMed] [Google Scholar]
- 59.Mortimer JE, et al. Effect of tamoxifen on sexual functioning in patients with breast cancer. J Clin Oncol. 1999;17(5):1488–92. doi: 10.1200/JCO.1999.17.5.1488. [DOI] [PubMed] [Google Scholar]
- 60.Lester JL, Bernhard LA. Urogenital atrophy in breast cancer survivors. Oncol Nurs Forum. 2009;36(6):693–8. doi: 10.1188/09.ONF.693-698. [DOI] [PubMed] [Google Scholar]
- 61.Al-Azzawi F, et al. Continuous combined hormone replacement therapy compared with tibolone. Obstet Gynecol. 1999;93(2):258–64. doi: 10.1016/s0029-7844(98)00403-7. [DOI] [PubMed] [Google Scholar]
- 62.Ganz PA, et al. Base-line quality-of-life assessment in the National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial. J Natl Cancer Inst. 1995;87(18):1372–82. doi: 10.1093/jnci/87.18.1372. [DOI] [PubMed] [Google Scholar]
- 63.Ganz PA, et al. Life after breast cancer: understanding women’s health-related quality of life and sexual functioning. J Clin Oncol. 1998;16(2):501–14. doi: 10.1200/JCO.1998.16.2.501. [DOI] [PubMed] [Google Scholar]
- 64.Partridge AH, et al. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21(4):602–6. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 65.Partridge AH, et al. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26(4):556–62. doi: 10.1200/JCO.2007.11.5451. [DOI] [PubMed] [Google Scholar]
- 66.Mortimer JE. Managing the toxicities of the aromatase inhibitors. Curr Opin Obstet Gynecol. 2010;22(1):56–60. doi: 10.1097/GCO.0b013e328334e44e. [DOI] [PubMed] [Google Scholar]