Abstract
Background
Exacerbations are responsible for a substantial burden of morbidity and health care utilization in children with asthma. Most asthma exacerbations are triggered by viral infections, however, the underlying mechanisms have not been systematically investigated.
Objective
The objective of this study was to elucidate the molecular networks that underpin virus-induced exacerbations in asthmatic children in vivo.
Methods
We followed exacerbation-prone asthmatic children prospectively, and profiled global patterns of gene expression in nasal lavage samples obtained during an acute, moderate, Picornavirus-induced exacerbation, and 7–14 days later. Coexpression network analysis and prior knowledge was employed to reconstruct the underlying gene networks.
Results
The data showed that an intricate, modular program consisting of more than one thousand genes was upregulated during acute exacerbations, in comparison to 7–14 days later. The modules were enriched for coherent cellular processes, including interferon-induced antiviral responses, innate pathogen sensing, response to wounding, small nucleolar RNAs, and the ubiquitin-proteosome and lysosome degradation pathways. Reconstruction of the wiring diagram of the modules revealed the presence of hyper-connected hub nodes, most notably IRF7, which was identified as a major hub linking interferon-mediated antiviral responses.
Conclusions
This study provides an integrated view of the inflammatory networks that are upregulated during virus-induced asthma exacerbations in vivo. A series of innate signalling hubs were identified that could be novel therapeutic targets for asthma attacks.
Keywords: Asthma, exacerbation, Picornavirus, rhinovirus, gene expression, gene networks, innate immunity, interferons, systems biology
Introduction
Asthma exacerbations are acute episodes of wheezing, shortness of breath, cough, and/or chest tightness, and these illnesses are responsible for millions of emergency room visits and thousands of fatalities annually in the Unites States.1 Up to 80 % of asthma exacerbations are triggered by viral infections, especially with the single stranded RNA virus rhinovirus (Picornavirus family), which causes the common cold.2, 3 However, the molecular mechanisms that underpin virus-host interactions and provoke asthma attacks are not well understood. Systematic studies are urgently needed to characterize the underlying biology.
Deciphering cellular function and behaviour is a challenging task because cellular processes are carried out by networks of interacting genes. These networks are likely to be highly complex in acute asthma, because rhinovirus infections and asthma attacks are associated with alterations in expression of thousands of genes.4–9 A significant advance in the field was the discovery that gene networks are governed by universal organizing principles.10 Gene network structures are scale-free and modular. In scale-free networks the vast majority of genes have few connections, whereas a few genes have many connections, behaving as hubs that “hold” the network together.10 The modular property refers to the organization of gene networks into smaller functional modules, which contain sets of genes that work in concert to carry out cellular processes.11–13 The development of molecular profiling technologies and computational algorithms, which work backwards from the observed molecular profiling data to reconstruct the underlying gene networks, now enables the systems-level study of biology.14 In this study, we employed coexpression network analysis and bioinformatics, to provide an integrated view of the inflammatory modules and hubs that underpin virus-induced exacerbations in asthmatic children in vivo.
Methods
Study population
The study population consisted of 16 children from a larger prospective study of 218 children with mild/moderate asthma, who were followed for 18 months or until they had an exacerbation. The protocol design and follow-up has been described previously.9 Briefly, at enrolment subjects were assessed by a study physician and if necessary adjustments were made to achieve national guideline recommendations for asthma control. When a child experienced symptoms of an exacerbation (cough, dyspnea, chest tightness, and/or wheeze), they were instructed to use albuterol (2 puffs, 90 mcg/puff) by MDI every 20 minutes for up to 1 hour, and then every 4 hours if necessary. A moderate exacerbation was defined as lack of symptom relief after 3 treatments, and/or low peak flow readings (< 80 % of personal best), and it is noteworthy that these criteria are equivalent to the ATS/ERS consensus.15 Participants who met these criteria were scheduled to visit the clinic within 24 h for collection of nasal lavage samples. This research was approved by the Institutional Review Board of the University of Arizona.
Collection of nasal lavage samples
The subjects were instructed to hold their breath and tilt their head back. 5 ml of sterile, warm 3 % saline was instilled into one nostril, and ten seconds later the subject tilted their head forward and allowed the saline to drip from the nostril into a sterile cup. The subject was then instructed to blow slightly to maximize saline recovery. The procedure was then repeated in the opposite nostril, and the sample was stored at 4 °C. After 30 minutes, the procedure was repeated once more. The samples were processed within 1 hour by vigorous pipetting to release cells, followed by centrifugation at 800xg for 10 minutes. The supernatant was removed and the cell pellet was immediately stabilized in RNALater (QIAgen). Evidence of a Picornavirus infection was tested by RT-PCR (primers: OL27-5′-CGGACACCCAAAGTAG-3′; OL26-5′-GCACTTCTGTTTCCCC-3′) as described previously.9
Expression profiling studies
Total RNA was extracted from nasal lavage cells employing TRIzol (Invitrogen) followed by RNeasy (QIAgen). The RNA samples were labelled and hybridized to Human Gene ST1.0 microarrays (Affymetrix) at the Genomics Core facility, the University of Arizona. The quality of the microarray data was assessed with the RMA algorithm (pos v neg AUC mean±sd = 0.74±0.04, all probeset mean 6.49±0.03, all probe set RLE mean 0.33±0.16). The microarray data was preprocessed in Expression Console software (Affymetrix) employing the PLIER+16 algorithm (gc background, quantile normalization, iterPLIER).9, 16 The raw microarray data are available from the Gene Expression Omnibus repository (GSE30326).
Coexpression network analysis
To discriminate between relevant signals and noise, gene expression levels were compared in paired samples from 16 subjects obtained during an acute, virus-induced exacerbation, and 7–14 days later. Differentially expressed genes were identified employing moderated t-statistics, and genes which were significantly modulated at a False Discovery Rate (FDR) adjusted p-value < 0.05 were selected for further analysis.17 A coexpression network was constructed employing the weighted gene coexpression network analysis algorithm (WGCNA).14, 16 The WGCNA algorithm employs a stepwise analytical process that begins by calculating absolute Pearson correlations for each gene pair across the samples. The correlations were raised to a power (power=12), to emphasize stronger over weaker correlations. The topological overlap was calculated to quantify the extent at which genes have similar overall correlation patterns with other genes. The topological overlap similarity measure was subtracted from 1 to convert it into a distance measure, and analysed by hierarchical clustering. Modules were defined from the output of the clustering analysis employing an automated algorithm.14
Module reconstruction
The list of constituent genes in each module was submitted to the Ingenuity Systems Pathway analysis tool (www.ingenuity.com). The Ingenuity Systems knowledgebase is the most comprehensive database available of molecular interactions that have been manually extracted and curated from the literature. The build/connect tool was employed to identify all documented molecular relationships between genes (eg. activation, inhibition, modulates expression of, protein-DNA interactions, protein-protein interactions). Genes with no known molecular interactions were removed from the analysis. Hubs were defined as genes that had 10 or more molecular interactions with other genes in the same module.
Bioinformatics
Gene Ontology terms, Swiss-Prot keywords, and canonical pathways from the Kyoto Encyclopedia of Genes and Genomes (KEGG) that were associated with the differentially expressed genes and modules were investigated employing the Database for Annotation Visualization and Integrated Discovery (DAVID).18 DAVID employs a modified Fisher Exact test (EASE score) to identify specific biological/functional categories that are overrepresented in the set of genes identified in the microarray study in comparison to a reference set (all genes represented on the Human Gene ST1.0 microarray). As a complimentary analysis, canonical pathways from the Ingenuity Systems database were also tested for association, and this analysis was based on the Fisher Exact test. The data are presented as unadjusted and Benjamini-Hochberg corrected p-values.
Results
Gene expression profiling of virus-induced asthma exacerbations in nasal lavage cells
To investigate the mechanisms underlying asthma exacerbations, children with mild/moderate asthma (n=16) were followed prospectively, and nasal lavage samples were obtained during an acute, Picornavirus-induced exacerbation, and 7–14 days later. The characteristics of the study population are presented in Table 1, and it is noteworthy that these were moderate exacerbations defined with criteria equivalent to the ATS/ERS consensus.15 The cellular composition of the acute samples was predominantly macrophages (mean ± sem, 83.9 % ± 2.7), followed by neutrophils (12.3 % ± 2.5), epithelial cells (2.2 % ± 1.0), and eosinophils (1.6 % ± 0.5). The follow-up samples contained a lower proportion of macrophages (72.1 % ± 4.8, p-value = 0.006), a higher proportion of epithelial cells (16.6 % ± 4.7, p-value = 0.005), and comparable proportions of neutrophils (9.1 % ± 1.2, p-value = 0.6) and eosinophils (1.7 % ± 0.8, p-value = 0.9).
Table 1.
Characteristics of the study population.
| Characteristics | Study population |
|---|---|
| Ethnicity, % | |
| Hispanic white | 87.5 (14/16) |
| Non-Hispanic white | 12.5 (2/16) |
| Gender, % | |
| Male | 62.5 (10/16) |
| Female | 37.5 (6/16) |
| Age, years, mean±sd | 9±3.1 |
| Aeroallergen skin test positive, % | 68.7 (11/16) |
| Ever hospitalized for asthma, % | 37.5 (6/16) |
| FEV1 at enrolment, % predicted, mean±sd | 103.4±10.9 |
| FEV1 at exacerbation, % predicted, mean±sd | 95.4±14.2 |
| Time to exacerbation, days, mean±sd | 151.8±126.6 |
| Medications at enrolment, % | |
| Inhaled corticosteroids | 31.25 (5/16) |
| Combination therapy | 25 (4/16) |
| Leukotriene receptor antagonist | 31.25 (5/16) |
To investigate global changes in the patterns of gene expression, the samples were labelled and hybridized to microarrays. Employing Bayesian statistical analyses with FDR adjustment for multiple testing,17 1,577 probes sets, representing 1,198 unique, annotated genes were differentially expressed during the acute illness, in comparison to 7–14 days later (FDR < 0.05, see Figure E1 online). The vast majority of these genes (1,121 genes) were upregulated during the responses, and a smaller subset was downregulated (77 genes). A series of bioinformatics resources was employed to interrogate the biological functions associated with the upregulated genes. The functional category “immune response” was the most prominent signature in the data (Gene Ontology, Swis-Prot databases, see Tables E1a, E1b online), and this accounted for 10.3 % of the gene expression program. Additional signatures that were prominent in the data included genes involved in the regulation of apoptosis (7.26 % of genes), defence response (7.44 %), response to wounding (6.28 %), inflammatory response (4.3 %), host-virus interaction (3.77 %), proteins found in lysosomes (3.14 %), response to virus (2.78 %), positive regulation of protein modification (2.6 %), innate immune response (2.24 %), and chemotaxis (2.15 %).
Biological pathways that were upregulated in the responses were investigated utilizing the KEGG and Ingenuity Systems canonical pathway databases (Tables E1c, E1d online). Of note, the pathway coverage is different in the KEGG and Ingenuity databases, thus they provide complementary information about the data. This analysis identified multiple pathways involved in innate pathogen sensing, including the TLR pathway (eg. MD2, TLR2, TLR5, TLR8), the NLR/inflammasome pathway (AIM2, CASP1, CASP5, HSP90, NAIP5, NLRC4, NOD2), and the cytosolic RNA helicases (MDA-5, RIG-I, RIG-I-like receptor LGP2). Additional immune-related pathways that were identified included the interferon-induced antiviral pathway (eg. IRF7, IRF9, ISG15, Mx1, OAS1, PKR, STAT1, STAT2), NFκB signalling (eg. CHUK, IRAK3, NFKB1, NFKBIB, RELB, RIPK1, TANK, TBK1, TRAF3), death receptor/apoptosis signalling (eg. BID, FAS, granzyme B, perforin-1, TRAIL, XIAP), antigen processing and presentation (CTSB, CTSL, HSP90, LGMN, LMP2, LMP7, LMP10, PDIA3, TAP1, TAP2), and the complement system (C1QC, C1QA, C1QB, C2, C3AR1, CD59, SERPING1). The primary intracellular protein degradation systems (ubiquitin-proteasome and lysosome pathways) were also strongly overrepresented in the data. The downregulated genes were not significantly enriched for coherent biological functions or pathways, however multiple olfactory receptors (OR2A1, -A4, -A7, -A9P, -M5, OR7E5P) and speedy homologs (SPDYE1, -E2, -E5, -E7P, -E8P) were identified.
Gene coexpression networks underlying virus-induced asthma exacerbations
To obtain a holistic view of the exacerbation responses, a coexpression network was constructed employing the weighted gene coexpression network analysis (WGCNA) algorithm, as described in Methods.14, 16 Briefly, WGCNA utilizes information derived from the patterns of gene-gene correlations across the samples, to reveal the structure of the underlying gene network. As illustrated in Fig. 1, the correlation structure of the coexpression network was characterized by a block-like pattern. This demonstrates that the exacerbation responses had a modular architecture.11, 12 The WGCNA algorithm identified eight coexpression modules (M1–M8, Fig. 1); seven of these modules were upregulated during exacerbations, and one module (M5) was downregulated (Figure E2 online).
Figure 1. Coexpression network underlying virus-induced asthma exacerbations in vivo.
Nasal lavage samples were obtained from asthmatic children (n=16) during an acute, virus-induced exacerbation, and 7–14 days later. Gene expression was profiled on microarrays, and a coexpression network was constructed employing the WGCNA algorithm. The figure illustrates the strength of connections between genes (derived from pairwise gene-gene correlations across the samples), and stronger correlations are indicated by the increasing red intensity. The genes were arranged by hierarchical clustering, and the red, block-like structures represent clusters/modules of highly coexpressed genes; eight modules were identified (M1–M8).
The first module contained 158 genes, and this module was significantly enriched for genes involved in the response to wounding, immune response, endocytosis, chemotaxis, cell adhesion, lysosome proteins, and the complement system (Table E2a, online). The second module contained 197 genes, and dominant biological signatures in this module included metallothioneins, interferon signalling, antiviral defence, antigen processing and presentation, and the protein ubiquitination pathway (Table E2b, online). The third module consisted of 120 genes, including hydrolases, proteasome subunits, and genes involved in virus-host interactions (Table E2c, online). The fourth module contained 98 genes, which were mainly involved lysosome degradation pathways and lipid metabolism (Table E2d, online). Modules M5 and M6 contained 46 and 29 genes respectively, and although these modules were not significantly enriched for known biological processes (data not shown), module M5 contained the olfactory receptors and speedy homologs receptors mentioned above, and module M6 contained a series of small nucleolar RNAs (SNORA20, -A22, -A23, -A28, -A36A, -A40, -A49, -D32A, -D32B, -D35A, -D45A, -D57, -D80, -D82, P-value = 2.4 × 10−6). Module M7 contained 76 genes, and this module was enriched with genes involved in immune responses (Table E2e, online). Module M8 contained 475 genes, and this module was enriched for genes involved innate pathogen sensing (TLR pathway, NLR pathway, RNA helicases, inflammasomes), NFκB signalling, and cell death (Table E2f, online).
Reconstruction of the network modules
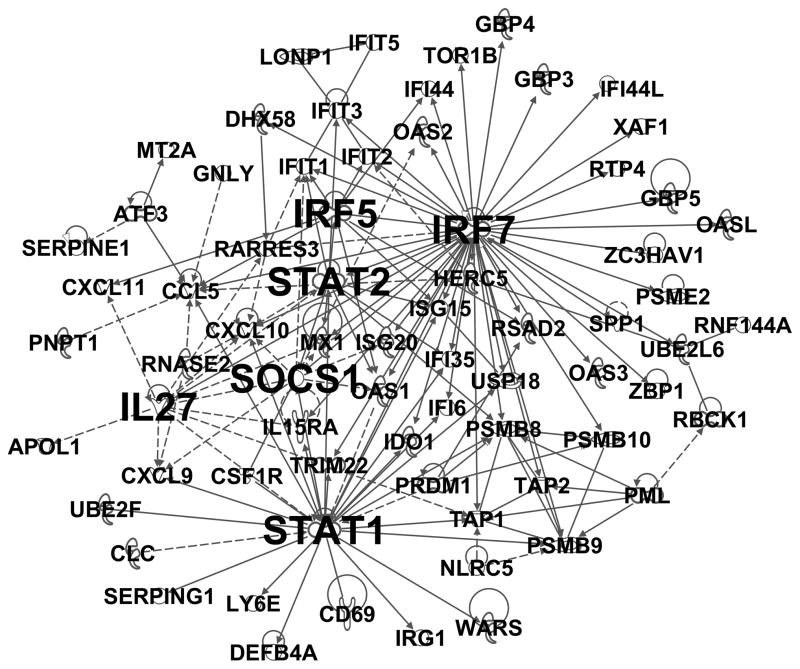
The above analyses suggested that exacerbation responses are modular, and additionally that coherent immunological processes are enriched to a certain extent within specific modules. However, the mechanistic insight obtained above is limited, because coexpression networks are based on correlations and not functional data. To obtain more detailed information in this regard, we utilized molecular interaction data from the Ingenuity Systems Knowledgebase, to reconstruct the “wiring diagram” of the modules. The result for module M2, which contained the interferon-induced antiviral genes and is illustrated in Fig. 2 (see Table E3 online for annotated gene list), revealed that interferon-regulatory factor 7 (IRF7) was the most highly interactive gene, harbouring 45 functional interactions with other genes. Signal transducer and activator of transcription 1 (STAT1) was also a major hub within this module, harbouring 27 functional interactions. Additional hubs in this module included STAT2, suppressor of cytokine signaling 1 (SOCS1), interleukin-27 (IL27), and IRF5 (13, 12, 12 and 10 interactions respectively). Of note, these hubs are all known regulators of interferon-induced antiviral responses (Table 2).
Figure 2. Reconstruction of module M2 identifies IRF7 as a major regulator of interferon-mediated antiviral responses.
The wiring diagram of module M2 from Figure 1 was reconstructed employing molecular interaction data from prior studies. See supplementary Figure E3 for the definition of the of the line types connecting the genes, and Table E3 for the list of component genes in the module.
Table 2.
Biological functions of the hubs identified in modules M2 and M8
| Symbol | Function/pathway |
|---|---|
| Module M2 | |
| IRF7 | Master regulator and amplifier of type I interferon-induced antiviral responses.45 |
| STAT1 | Mediates signalling by type I, type II, and type III interferons, and induces antiviral gene expression program.29, 39 |
| STAT2 | Mediates signalling by type I, type II, and type III interferons, and induces antiviral gene expression program.29, 39 |
| SOCS1 | Negative regulator of IFNAR1/STAT1 signalling. Limits duration of interferon-induced antiviral program.40 |
| IL-27 | Promotes Th1 differentiation and type I interferon-induced antiviral responses.41 |
| IRF5 | Knockout mice have deficient type I interferon responses and are susceptible to infections with DNA and RNA viruses.42 |
| Module M8 | |
| NFκB1 | Encodes NFκB p50 subunit. Knockdown of NFκB1 attenuates TLR-induced inflammatory responses.29 NFκB1 has both pro and anti-inflammatory functions.32, 33 Regulation of apoptosis. |
| STAT3 | Mutations in STAT3 underlie hyper-IgE syndrome, which is associated with recurrent pulmonary infections.30 Protects epithelial cells from apoptosis during pulmonary viral infections.31 |
| XBP1 | Transcription factor that is activated by the endoplasmic reticulum stress sensing kinase IRE1. Knockdown of XBP1 attenuates TLR2/TLR4-induced (PAM3CSK4/LPS) inflammatory responses,34 and poly-IC-induced antiviral responses.35 |
| CHUK | Component of the IκB kinase complex, which activates NFκB signalling by modulating inhibitory proteins (IκB) that sequester NκB in the cytoplasm.36 |
| CASP1 | Caspase-1 activation is triggered by inflammasomes and is essential for processing pro-IL-1β and pro-IL-18 into bioactive forms.37 Regulation of apoptosis. |
| RB1 | Cell cycle checkpoint/arrest; tumour suppressor activity, regulation of apoptosis. |
| TBK1 | IKK-related kinase that couples viral detection via pathogen recognition receptors to activation of IRF3 and IRF7.36 |
| FAS | Central role in regulation of programmed cell death. |
A reconstruction of module M8 is shown in Fig. 3 (see Table E4 online for annotated gene list). This module contained genes involved in innate pathogen sensing, NFκB signalling, and cell death. NFκB1 and STAT3 were the dominant hubs in this module, with 20 functional interactions each. Additional hubs in this module included X-box binding protein 1 (XBP1), IκB kinase-α (CHUK), caspase-1 (CASP1), Retinoblastoma-1 (RB1), TANK-binding kinase 1 (TBK1), and FAS (15, 13, 12, 12, 11, 10 interactions respectively). These hubs were mainly involved in pathogen recognition receptor mediated signalling pathways and/or regulation of apoptosis (Table 2).
Figure 3. Reconstruction of the innate pathogen sensing module M8.
The wiring diagram of module M8 from Figure 1 was reconstructed employing molecular interaction data from prior studies. See supplementary Figure E3 for the definition of the line types connecting the genes, and Table E4 for the list of component genes in the module.
Discussion
Acute exacerbations have a substantial impact on health care utilization and treatment costs for children with asthma. Although inhaled corticosteroids can reduce the frequency of exacerbations, they cannot entirely prevent them, and they are not effective at controlling neutrophilic inflammation, which is increasingly recognized as playing a major role in the pathogenesis.19 New drugs are urgently needed. However, selection of drug targets based on oversimplified, gene/factor-centric paradigms of inflammatory mechanisms, which focus on the role of individual effector molecules without taking into account the broader molecular context (ie local network topology and interaction partners), is likely to be suboptimal.20–22 In contrast to previous studies in this area that have identified differentially expressed genes and/or pathways,4–8 this is the first study in which the gene networks associated with Picornavirus-induced asthma exacerbations have been identified. Our findings illustrate several important principles, which may stimulate further research in this area. First, we demonstrated that an intricate, modular,9, 16, 23–25 inflammatory program consisting of more than one thousand genes was upregulated during asthma exacerbations, in comparison to 7–14 days later. Second, we showed that the modules were enriched with coherent cellular and immunological processes. Finally, reconstruction of the modules employing molecular interaction data from prior studies,26 revealed the presence of hyper-connected hub nodes. This suggests a network structure that is tolerant to random perturbations from variations in genes and the environment, but vulnerable to the targeted removal of hubs.16, 27 These hubs therefore represent treatment targets for asthma attacks.
Innate immune responses to viruses are initiated when viral proteins and nucleic acids are detected by pattern recognition receptors. This activates intracellular signalling cascades that converge on the NFκB and IRF families of transcription factors, which translocate to the nucleus and switch on inflammatory and antiviral programs.28, 29 Our findings provide a modular view of this paradigm, and in particular show that modules involved in “innate pathogen sensing” (module M8, Fig. 3) and “interferon-induced antiviral responses” (module M2, Fig. 2) are upregulated during virus-induced asthma exacerbations. The innate pathogen sensing module contained a diverse set of pathogen recognition receptors (toll-like receptors, nod-like receptors/inflammasomes, retinoic acid inducible gene-I-like receptors, IFI200 family) arranged around a series of innate signalling hubs. Previous studies have shown that the dominant hubs in this module (NFκB1, STAT3) are essential for mounting effective innate immune responses whilst limiting collateral damage.30–33 It is also noteworthy that the other hubs in this module have established roles in innate immunity (Table 2).34–37
The interferon-induced antiviral module contained multiple genes downstream of interferon signalling, including archetypal antiviral effectors (ISG15, OAS1-3, Mx1, PML), chemokines (CXCL9, CXCL10, CXCL11, RANTES), genes involved in antigen processing and presentation (TAP1, TAP2, LMP2, LMP7, LMP10), and an extended cohort of genes (DDX60, IFI44L, IFI6, OASL, RTP4, MB21D1, MOV10, RTP4, SLC25A28, TRIM14, UNC93B1) that inhibit viral replication in high throughput assays.38 The hubs that were identified in this module are all known regulators of interferon-induced antiviral responses (Table 2).39–42 IRF7 was by far the most dominant hub identified in this module and in the entire analysis. IRF7 is potently induced by rhinovirus infections and is a master regulator of the antiviral response.43–45 IRF7 normally resides in the cytoplasm at low levels in an inactive form, but during viral infections, signalling via pattern recognition receptors triggers phosphorylation and translocation of IRF7 to the nucleus, where it activates expression of type-I interferons. Type-I interferon signalling via STAT1 and STAT2 in turn activates IRF7 transcription, which further amplifies interferon expression, thus IRF7 mediates positive feedback amplification of antiviral responses.46
This study has limitations that should be acknowledged. The study population consisted of asthmatic children experiencing symptoms of a moderate, virus-induced exacerbation, but did not include a control group of healthy children experiencing symptoms of a cold. Therefore the analyses cannot differentiate between variations in gene network patterns that are associated with viral infection, as opposed to those that are specific to exacerbations. Follow-up studies in a much larger sample will be required to determine how variations in gene network patterns underpin phenotypic patterns, and to examine how disease cofactors such as atopy modify exacerbation responses and disease severity. The expression profiling studies were based on cells obtained from nasal lavage and not the airways, and distinct cellular and molecular mechanisms may be operating in these compartments. The nasal lavage samples comprised a mixed cell population, variations in which may potentially confound the analyses. The modules were reconstructed utilizing molecular interaction data from prior studies, which may have limited relevance to the current study. Therefore detailed mechanistic studies will be required to define the precise function of the hubs in the direct content of virus-induced exacerbations. Finally, because the analytical strategy incorporated prior knowledge, genes for which there are no functional interaction data available cannot be interpreted. Notwithstanding these limitations, this study provides a modular view of the inflammatory networks that are upregulated during virus-induced exacerbations in asthmatic children in vivo. Moreover, a cohort of innate signalling hubs exemplified by IRF7 were identified which are logical therapeutic targets for asthma exacerbations.
Supplementary Material
Key messages.
This study provides a modular view of the inflammatory networks that are upregulated during virus-induced asthma exacerbations in vivo. IRF7 was identified as a major hub connecting interferon-mediated antiviral responses.
Acknowledgments
Declaration of funding sources. This research was supported by National Institutes of Health Grant HL080083. AB is the recipient of a Medical Research Fellowship from the Faculty of Medicine, Dentistry and Health Sciences, the University of Western Australia.
List of abbreviations
- AIM2
absent in melanoma 2
- ATS/ERS
American Thoracic Society/European Respiratory Society
- AUC
area under curve
- BID
BH3 interacting domain death agonist
- CASP1, -5
caspase-1, -5
- CHUK
conserved helix-loop-helix ubiquitous kinase
- C1QA, -B, -C
complement component 1, q subcomponent, chain A, -B, -C
- C2
complement component 2
- C3AR1
complement component 3a receptor 1
- CTSB
cathepsin B
- CTSL
cathepsin L1
- CXCL9-11
chemokine (C-X-C motif) ligand 9-11
- DAVID
Database for Annotation Visualization and Integrated Discovery
- DDX60
DEAD (Asp-Glu-Ala-Asp) box polypeptide 60
- EASE
Expression Analysis Systematic Explorer
- FDR
false discovery rate
- HSP90
heat shock protein 90
- IFI44L
interferon-induced protein 44-like
- IFI6
interferon, alpha-inducible protein 6
- IFNAR1
interferon alpha/beta receptor 1
- IKK
IkappaB kinases
- ISG15
Interferon stimulated gene 15 kDa protein
- IRE1
endoplasmic reticulum-to-nucleus signaling 1
- IRF5, -7
interferon regulatory factor 5, -7
- IRAK3
interleukin-1 receptor-associated kinase 3
- iterPLIER
iterative probe logarithmic intensity error
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LGMN
legumain
- LGP2
laboratory of genetics and physiology 2
- LMP2, -7, -10
low molecular mass protein-2, -7, -10
- LPS
Lipopolysaccharide
- M1-8
module 1-8
- MB21D1
Mab-21 domain containing 1
- MDA-5
Melanoma differentiation-associated gene-5
- MD2
myeloid differentiation factor 2
- MDI
metered dose inhaler
- MOV10
Moloney leukemia virus 10 homolog
- Mx1
myxovirus resistance 1
- NAIP5
NLR family apoptosis inhibitory protein
- NFKB1
nuclear factor of kappa light polypeptide gene enhancer in B-cells 1
- NFKBIB
NKFB inhibitor, beta
- NLR
Nucleotide Oligomerization Domain like receptors
- NLRC4
NLR family, CARD domain containing 4
- NOD2
nucleotide-binding oligomerization domain containing 2
- OAS1-3
2′-5′-oligoadenylate synthetase 1-3
- OASL
2′-5′-oligoadenylate synthetase-like OR-, olfactory receptor
- PDIA3
protein disulfide isomerase family A, member 3
- PKR
Protein kinase R
- PML
promyelocytic leukemia
- RANTES
Regulated upon Activation, Normal T-cell Expressed, and Secreted
- RELB
v-rel reticuloendotheliosis viral oncogene homolog B
- RIG-I
retinoic acid-inducible gene I
- RIPK1
receptor (TNFRSF)-interacting serine-threonine kinase 1
- RTP4
receptor transporter protein 4
- SERPING1
serpin peptidase inhibitor, clade G, member 1
- SLC25A28
solute carrier family 25, member 28
- SNOR
small nucleolar RNA
- SPDY-
speedy homolog
- STAT1, -2
Signal transducer and activator of transcription-1, -2
- TANK
TRAF family member-associated NFKB activator
- TAP1, -2
transporter-1, -2
- TBK1
TANK-binding kinase 1
- TRIM14
tripartite motif containing 14
- TLR2, -7, -8
Toll-like receptor-2, -7, -8
- TRAF3
TNF receptor-associated factor 3
- TRAIL
TNF-related apoptosis-inducing ligand
- UNC93B1
unc-93 homolog B
- WGCNA
weighted gene coexpression network analysis algorithm
- XBP-1
X-box binding protein 1
- XIAP
X-linked inhibitor of apoptosis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dougherty RH, Fahy JV. Acute exacerbations of asthma: epidemiology, biology and the exacerbation-prone phenotype. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2009;39:193–202. doi: 10.1111/j.1365-2222.2008.03157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–6. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ. 1995;310:1225–9. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Proud D, Turner RB, Winther B, Wiehler S, Tiesman JP, Reichling TD, et al. Gene expression profiles during in vivo human rhinovirus infection: insights into the host response. American journal of respiratory and critical care medicine. 2008;178:962–8. doi: 10.1164/rccm.200805-670OC. [DOI] [PubMed] [Google Scholar]
- 5.Zaas AK, Chen M, Varkey J, Veldman T, Hero AO, 3rd, Lucas J, et al. Gene expression signatures diagnose influenza and other symptomatic respiratory viral infections in humans. Cell host & microbe. 2009;6:207–17. doi: 10.1016/j.chom.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guajardo JR, Schleifer KW, Daines MO, Ruddy RM, Aronow BJ, Wills-Karp M, et al. Altered gene expression profiles in nasal respiratory epithelium reflect stable versus acute childhood asthma. The Journal of allergy and clinical immunology. 2005;115:243–51. doi: 10.1016/j.jaci.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 7.Subrata LS, Bizzintino J, Mamessier E, Bosco A, McKenna KL, Wikstrom ME, et al. Interactions between innate antiviral and atopic immunoinflammatory pathways precipitate and sustain asthma exacerbations in children. Journal of immunology. 2009;183:2793–800. doi: 10.4049/jimmunol.0900695. [DOI] [PubMed] [Google Scholar]
- 8.Bochkov YA, Hanson KM, Keles S, Brockman-Schneider RA, Jarjour NN, Gern JE. Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal immunology. 2010;3:69–80. doi: 10.1038/mi.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosco A, Ehteshami S, Stern DA, Martinez FD. Decreased activation of inflammatory networks during acute asthma exacerbations is associated with chronic airflow obstruction. Mucosal immunology. 2010;3:399–409. doi: 10.1038/mi.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barabasi AL, Oltvai ZN. Network biology: understanding the cell’s functional organization. Nature reviews Genetics. 2004;5:101–13. doi: 10.1038/nrg1272. [DOI] [PubMed] [Google Scholar]
- 11.Hartwell LH, Hopfield JJ, Leibler S, Murray AW. From molecular to modular cell biology. Nature. 1999;402:C47–52. doi: 10.1038/35011540. [DOI] [PubMed] [Google Scholar]
- 12.Ravasz E, Somera AL, Mongru DA, Oltvai ZN, Barabasi AL. Hierarchical organization of modularity in metabolic networks. Science. 2002;297:1551–5. doi: 10.1126/science.1073374. [DOI] [PubMed] [Google Scholar]
- 13.Schadt EE. Molecular networks as sensors and drivers of common human diseases. Nature. 2009;461:218–23. doi: 10.1038/nature08454. [DOI] [PubMed] [Google Scholar]
- 14.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC bioinformatics. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. American journal of respiratory and critical care medicine. 2009;180:59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 16.Bosco A, McKenna KL, Firth MJ, Sly PD, Holt PG. A network modeling approach to analysis of the Th2 memory responses underlying human atopic disease. Journal of immunology. 2009;182:6011–21. doi: 10.4049/jimmunol.0804125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Statistical applications in genetics and molecular biology. 2004;3:Article3. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 18.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 19.Martinez FD. Managing childhood asthma: challenge of preventing exacerbations. Pediatrics. 2009;123 (Suppl 3):S146–50. doi: 10.1542/peds.2008-2233D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schadt EE, Sachs A, Friend S. Embracing complexity, inching closer to reality. Science’s STKE : signal transduction knowledge environment. 2005;2005:pe40. doi: 10.1126/stke.2952005pe40. [DOI] [PubMed] [Google Scholar]
- 21.Benoist C, Germain RN, Mathis D. A plaidoyer for ‘systems immunology’. Immunological reviews. 2006;210:229–34. doi: 10.1111/j.0105-2896.2006.00374.x. [DOI] [PubMed] [Google Scholar]
- 22.Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nature chemical biology. 2008;4:682–90. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- 23.Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, et al. A network-based analysis of systemic inflammation in humans. Nature. 2005;437:1032–7. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- 24.Chaussabel D, Quinn C, Shen J, Patel P, Glaser C, Baldwin N, et al. A modular analysis framework for blood genomics studies: application to systemic lupus erythematosus. Immunity. 2008;29:150–64. doi: 10.1016/j.immuni.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mobini R, Andersson BA, Erjefalt J, Hahn-Zoric M, Langston MA, Perkins AD, et al. A module- based analytical strategy to identify novel disease-associated genes shows an inhibitory role for interleukin 7 Receptor in allergic inflammation. BMC systems biology. 2009;3:19. doi: 10.1186/1752-0509-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ideker T, Dutkowski J, Hood L. Boosting Signal-to-Noise in Complex Biology: Prior Knowledge Is Power. Cell. 2011;144:860–3. doi: 10.1016/j.cell.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albert R, Jeong H, Barabasi AL. Error and attack tolerance of complex networks. Nature. 2000;406:378–82. doi: 10.1038/35019019. [DOI] [PubMed] [Google Scholar]
- 28.Katze MG, Fornek JL, Palermo RE, Walters KA, Korth MJ. Innate immune modulation by RNA viruses: emerging insights from functional genomics. Nature reviews Immunology. 2008;8:644–54. doi: 10.1038/nri2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amit I, Garber M, Chevrier N, Leite AP, Donner Y, Eisenhaure T, et al. Unbiased reconstruction of a mammalian transcriptional network mediating pathogen responses. Science. 2009;326:257–63. doi: 10.1126/science.1179050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, et al. STAT3 mutations in the hyper-IgE syndrome. The New England journal of medicine. 2007;357:1608–19. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 31.Matsuzaki Y, Xu Y, Ikegami M, Besnard V, Park KS, Hull WM, et al. Stat3 is required for cytoprotection of the respiratory epithelium during adenoviral infection. Journal of immunology. 2006;177:527–37. doi: 10.4049/jimmunol.177.1.527. [DOI] [PubMed] [Google Scholar]
- 32.Ishikawa H, Claudio E, Dambach D, Raventos-Suarez C, Ryan C, Bravo R. Chronic inflammation and susceptibility to bacterial infections in mice lacking the polypeptide (p)105 precursor (NF-kappaB1) but expressing p50. The Journal of experimental medicine. 1998;187:985–96. doi: 10.1084/jem.187.7.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quinton LJ, Mizgerd JP. NF-kappaB and STAT3 signaling hubs for lung innate immunity. Cell and tissue research. 2011;343:153–65. doi: 10.1007/s00441-010-1044-y. [DOI] [PubMed] [Google Scholar]
- 34.Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nature immunology. 2010;11:411–8. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu F, Yu X, Wang H, Zuo D, Guo C, Yi H, et al. ER stress and its regulator X-box-binding protein-1 enhance polyIC-induced innate immune response in dendritic cells. European journal of immunology. 2011;41:1086–97. doi: 10.1002/eji.201040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hiscott J. Convergence of the NF-kappaB and IRF pathways in the regulation of the innate antiviral response. Cytokine & growth factor reviews. 2007;18:483–90. doi: 10.1016/j.cytogfr.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Kanneganti TD. Central roles of NLRs and inflammasomes in viral infection. Nature reviews Immunology. 2010;10:688–98. doi: 10.1038/nri2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472:481–5. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takaoka A, Yanai H. Interferon signalling network in innate defence. Cellular microbiology. 2006;8:907–22. doi: 10.1111/j.1462-5822.2006.00716.x. [DOI] [PubMed] [Google Scholar]
- 40.Fenner JE, Starr R, Cornish AL, Zhang JG, Metcalf D, Schreiber RD, et al. Suppressor of cytokine signaling 1 regulates the immune response to infection by a unique inhibition of type I interferon activity. Nature immunology. 2006;7:33–9. doi: 10.1038/ni1287. [DOI] [PubMed] [Google Scholar]
- 41.Greenwell-Wild T, Vazquez N, Jin W, Rangel Z, Munson PJ, Wahl SM. Interleukin-27 inhibition of HIV-1 involves an intermediate induction of type I interferon. Blood. 2009;114:1864–74. doi: 10.1182/blood-2009-03-211540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yanai H, Chen HM, Inuzuka T, Kondo S, Mak TW, Takaoka A, et al. Role of IFN regulatory factor 5 transcription factor in antiviral immunity and tumor suppression. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3402–7. doi: 10.1073/pnas.0611559104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y, Hamati E, Lee PK, Lee WM, Wachi S, Schnurr D, et al. Rhinovirus induces airway epithelial gene expression through double-stranded RNA and IFN-dependent pathways. American journal of respiratory cell and molecular biology. 2006;34:192–203. doi: 10.1165/rcmb.2004-0417OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Q, Nagarkar DR, Bowman ER, Schneider D, Gosangi B, Lei J, et al. Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses. Journal of immunology. 2009;183:6989–97. doi: 10.4049/jimmunol.0901386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–7. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 46.Honda K, Takaoka A, Taniguchi T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349–60. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.