Abstract
Cardiac myosin binding protein-C (cMyBP-C) is a thick filament assembly protein that stabilizes sarcomeric structure and regulates cardiac function; however, the profile of cMyBP-C degradation after myocardial infarction (MI) is unknown. We hypothesized that cMyBP-C is sensitive to proteolysis and is specifically increased in the bloodstream post-MI in rats and humans. Under these circumstances, elevated levels of degraded cMyBP-C could be used as a diagnostic tool to confirm MI. To test this hypothesis, we first established that cMyBP-C dephosphorylation is directly associated with increased degradation of this myofilament protein, leading to its release in vitro. Using neonatal rat ventricular cardiomyocytes in vitro, we were able to correlate the induction of hypoxic stress with increased cMyBP-C dephosphorylation, degradation, and the specific release of N′-fragments. Next, to define the proteolytic pattern of cMyBP-C post-MI, the left anterior descending coronary artery was ligated in adult male rats. Degradation of cMyBP-C was confirmed by a reduction in total cMyBP-C and the presence of degradation products in the infarct tissue. Phosphorylation levels of cMyBP-C were greatly reduced in ischemic areas of the MI heart compared to non-ischemic regions and sham control hearts. Post-MI plasma samples from these rats, as well as humans, were assayed for cMyBP-C and its fragments by sandwich ELISA and immunoprecipitation analyses. Results showed significantly elevated levels of cMyBP-C in the plasma of all post-MI samples. Overall, this study suggests that cMyBP-C is an easily releasable myofilament protein that is dephosphorylated, degraded and released into the circulation post-MI. The presence of elevated levels of cMyBP-C in the blood provides a promising novel biomarker able to accurately rule in MI, thus aiding in the further assessment of ischemic heart disease.
Keywords: Myosin binding protein-C, Phosphorylation, Cardiac troponin I, Cardiac biomarker
1. Introduction
Coronary heart disease is the most common cause of myocardial infarction (MI), which afflicts approximately five million people in the U.S. each year [1]. The infarct is associated with altered Ca2+ handling and myofilament protein phosphorylation that leads to lower cross-bridge cycling rates, and a loss of cardiac contractility [2, 3]. Changes in calcium sensitivity have been found to be associated with development of heart failure [4, 5]. Furthermore, increased intracellular Ca2+ activates the protease calpain, which leads to proteolytic degradation of contractile proteins [6, 7]. These changes correspond to the detection of degraded contractile proteins in the blood [8–11], such as cardiac troponin (cTn) I and T, which are used clinically to determine the severity of myocardial injury [12]. Recent studies have also shown that alterations in the state of contractile proteins, such as changes in phosphorylation status, might also contribute to cardiac dysfunction after an ischemic period [13–15]. The cardiac sarcomere consists of thick and thin filament proteins in which thick filaments are composed of titin, myosin, cardiac myosin binding protein-C (cMyBP-C) and myosin light chains, whereas thin filaments consist of actin, cTnI, cTnT, cTnC, and α-tropomyosin (α-TM). During MI, degradation of the thin filament proteins, such as cTnI and cTnT, has been extensively studied [6–12]. However, the degradation profile of thick filament proteins, in particular, cMyBP-C, and the resulting contractile dysfunction have not been systematically characterized.
cMyBP-C is an assembly protein and myosin stabilizer that is involved in regulating sarcomeric structure and function in the heart [16–19]. Increasing evidence suggests that cMyBP-C plays a critical role in regulating myosin function and cardiac contraction [14, 19, 20]. Given that mutations in this protein have been linked to familial hypertrophic cardiomyopathy in more than 60 million people worldwide [21], elucidation of its function is clinically imperative [19]. cMyBP-C comprises 2% of the total contractile proteins in the heart, belongs to the intracellular immunoglobulin super family, and is highly soluble due to its hydrophilic properties [22]. Previous literature has shown that cMyBP-C phosphorylation at Ser-273, Ser-282 and Ser-302 regulates myocardial function [14, 23, 24] and confers resistance to proteolysis and protection against MI [14, 20]. On the other hand, the degradation of cMyBP-C during MI correlates with contractile dysfunction [14, 25]. We previously demonstrated that cMyBP-C is a substrate for calpains and that calpains cleave cMyBP-C during MI [26]. This event results in the release of 40 kDa fragments, polypeptides that could potentially cause pathogenic cardiac muscle damage by interacting with myosin and inhibiting its function. At this time, the interrelationship of cMyBP-C phosphorylation, degradation, and contractile dysfunction is poorly understood. Therefore, it is necessary to identify mechanisms for cMyBP-C phosphorylation and degradation post-MI to better understand the pathological processes [8].
The objectives of this study were to determine whether cMyBP-C is an easily releasable protein, whether degradation is associated with its phosphorylation status, and whether cMyBP-C is released into the circulation post-MI. To accomplish this, we utilized an in vitro system, an in vivo rat model of MI, and samples from post-MI patients. Our data show that cMyBP-C is rapidly degraded and released in a time-dependent manner from myocardial tissue in vitro and that its degraded products appear in the circulation post-MI in vivo. In addition, our data associate cMyBP-C degradation with its dephosphorylation, which establishes a firm rationale for using elevated plasma cMyBP-C titers as a novel biomarker of myocardial injury.
2. Materials and Methods
An expanded methods section is available in the online-only Supplement.
2.1 Determining cMyBP-C degradation, phosphorylation and plasma levels
To determine whether cMyBP-C is an easily releasable myofilament in vitro, left ventricular (LV) tissue from normal male Sprague Dawley rat hearts was incubated in phosphate buffered saline (PBS) for different durations at 37°C. The released proteins in the effluent were analyzed by Western blot using rabbit polyclonal antibodies against cMyBP-C residues 2–14 (cMyBP-C2–14) [27], domains C0 (Santa Cruz), C0–C1 [23, 25], C5 [28] and C8–C9 [28], and a custom made rabbit polyclonal antibody against C-terminal 1119–1212 residues (ProSci, Inc.). Release of other sarcomeric proteins, such as myosin, actin, cTnI, and α-TM, was determined by Western blot, as described previously [20]. To determine the proteolytic profile of cMyBP-C degradation, hypoxia was induced in 3-day-old neonatal rat ventricular myocytes (NRVMs) for 12 hrs, and total proteins were analyzed by Western blot using rabbit anti-cMyBP-C2–14 antibodies [27]. To investigate cMyBP-C degradation in MI heart tissue, total proteins were separated on 4–15% precast Tris-HCl gels (Bio-Rad Laboratories), followed by Western blot analyses using rabbit anti-cMyBP-C2–14 antibodies [20]. Site-specific phospho antibodies against Ser-273, Ser-282, Ser-302 (number refers to mouse Uniport O70468) were used to determine cMyBP-C phosphorylation levels [29]. Plasma levels of cMyBP-C were quantified by sandwich ELISA using capture antibody (monoclonal anti-cMyBP-C antibody, E-7, Santa Cruz) and detection antibody (rabbit polyclonal anti-cMyBP-CC0–C1) [23]. Plasma cTnI levels were measured by sandwich ELISA according to the manufacturer’s recommendations (rat, Life Diagnostics; human, Calbiotech).
2.2 Rat model of myocardial infarction
Ten-week old male Sprague Dawley rats were used to induce MI as described [30]. Three days post-MI, LV structure and function was measured in MI, sham, and naïve animals by non-invasive M-mode echocardiography, and blood and tissue samples were collected for analyses [30]. Animals were handled in accordance with the principles and procedures of the Guide for the Care and Use of Laboratory Animals. The Institutional Animal Care and Use Committee at Loyola University Medical Center approved all experimental procedures.
2.3 Human samples
Echocardiography and blood samples were collected from normal controls and patients admitted to the Scott & White Hospital, Temple, Texas, who were diagnosed with MI based on ECG findings and elevations in cTnI levels (cutoff >5 ng/ml). Plasma was separated immediately after blood collection, and plasma cTnI, glucose and creatine levels were measured within 1 hour in a clinical lab; aliquoted and stored at −80°C prior to determining cMyBP-C levels. In addition, explanted heart samples (non-ischemic, viable region) were obtained from patients with ischemic cardiomyopathy undergoing heart transplantation and non-failing donor hearts from the Gift of Hope from the tissue repository of the Cardiovascular Institute at Loyola University Medical Center, Maywood, IL. These samples were used to determine the phosphorylation status of cMyBP-C and its degradation in human hearts. All human samples were obtained with informed consent and de-identified with Institutional Review Board approval.
2.4 Statistical analysis
Results are presented as mean ± SEM. Comparisons between groups (Sham vs. MI) were made using a Student’s t-test. Differences within and between groups were analyzed with Two-Way Repeated-Measures ANOVA, followed by a Tukey post-hoc test using SigmaPlot V11. P < 0.05 was considered significant.
3. Results
3.1 cMyBP-C is an easily releasable sarcomeric protein in vitro, and cMyBP-C dephosphorylation is associated with its degradation
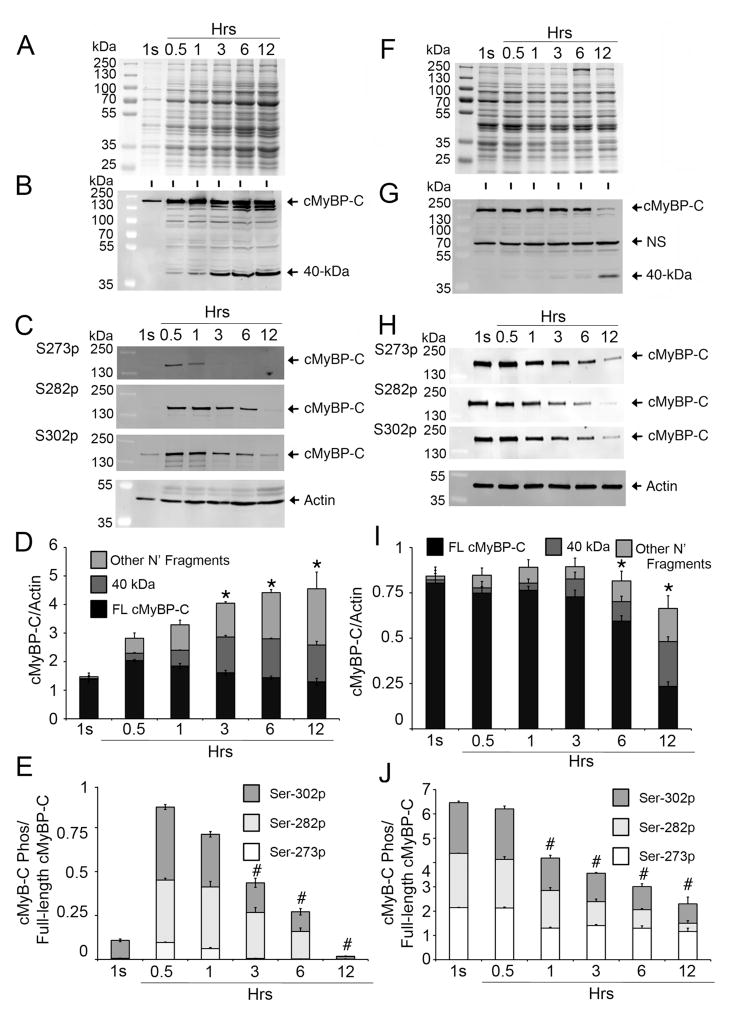
To determine whether cMyBP-C is an easily releasable myofilament protein, LV heart tissue from wild-type rats was incubated in PBS at 37°C for 1 second, 0.5, 1, 3, 6 and 12 hrs. During these incubations, releasable proteins from the LV tissue were allowed to freely diffuse into the PBS effluent. SDS-PAGE analyses showed that the release of total cardiac protein increased over time (Fig. 1A). To determine whether full-length cMyBP-C and its proteolytic fragments were released into the PBS effluent, Western blot analysis was performed with N′-specific rabbit anti-cMyBP-C2–14 antibodies which recognize full-length and all N′-fragments of cMyBP-C (Fig. 1B, 1D). Results show that cMyBP-C and its fragments are released into the effluent as early as 1 second with a time-dependent increase in release up to 12 hrs. After 0.5 hours, the predominant cMyBP-C fragments (40 kDa) were also released in a time-dependent manner. For comparison, other cardiac sarcomeric proteins, such as actin (Fig. 1C), myosin, cTnI and α-TM (Online Supplemental Fig. 1), were measured in the effluent. Results show the presence of myosin and actin, and a time-dependent increase in α-TM and cTnI. Antibodies generated against different cMyBP-C domains (C5, C8–C9 and C10) were used to further demonstrate the release of cMyBP-C fragments into the effluent (Online Supplemental Fig. 2). Briefly, these antibodies verified the release of full-length cMyBP-C and other fragments. Western blot analyses of the effluent with site-specific phospho antibodies for cMyBP-C at Ser-273, Ser-282 and Ser-302 demonstrate a time-dependent decrease in the phosphorylation of these sites (Fig. 1C, 1E), which also corresponds to an increase in the 40 kDa fragments (Fig. 1D). Next, we determined cMyBP-C concentrations in the leftover tissue at each time point with Western blot analysis (Fig. 1F–J). Data show that full-length cMyBP-C was present at all time points, but was reduced at 12 hrs, which corresponded with the emergence of 40 kDa fragments (Fig. 1G, 1I). Compared to released intact cMyBP-C phosphorylation status, the tissue cMyBP-C phosphorylation levels at Ser-273, Ser-282 and Ser-302 were significantly reduced at later time points (Fig. 1H, 1J), demonstrating that the dephosphorylation status of cMyBP-C is directly proportional to the release of cMyBP-C from cardiac sarcomere and its increased fragmentation (Online Supplemental Fig. 3).
Fig 1. cMyBP-C is an easily releasable myofilament.
Rat LV tissue (100 mg) was incubated in 500 μl of PBS (effluent) at 37°C for 1 second (1s), and 0.5, 1, 3, 6 and 12 hrs. Effluent (10 μl) was used for SDS-PAGE (A), followed by Western blot analyses with rabbit anti-cMyBP-C2–14 antibody (B) and site-specific Ser-273 (S273p), Ser-282 (S282p) and Ser-302 (S302p) phospho antibodies (C). Sarcomeric α-actin was used as a loading control. Quantitative analyses (D) show the increase of full-length (FL) cMyBP-C, 40 kDa and other N′-fragments, either in total cMyBP-C or individually as FL, 40 kDa and other N′-fragments, over time (n=4, P<0.01 vs. 0.5 hr time point), but, at the same time, gradual reduction of cMyBP-C phosphorylation at Ser-273, Ser-282 and Ser-302 sites individually or in total (E, P<0.01 vs. 0.5 hr time point), suggesting an inverse relationship between the degradation and phosphorylation status of cMyBP-C. Ten μg of total protein from the tissue homogenates of the leftover rat heart tissue at different time points were resolved on SDS-PAGE (F) and analyzed by Western blot analyses with rabbit anti-cMyBP-C2–14 antibodies (G) and site-specific Ser-273 (S273p), Ser-282 (S282p) and Ser-302 (S302p) phospho antibodies (H). Quantitative analyses show (I) reduced total cMyBP-C with increased release of 40 kDa and other N′-fragments at 6- and 12-hr time points (n=4, P<0.01 vs. 1s time point). Phosphorylation status at the three sites was decreased in total or individually (J), starting from the 1-hr time point (n=4, P<0.01 vs. 1s time point). A nonspecific (NS) protein, which was not released into the effluent, was identified (Online Supplement Fig. 5).
3.2 A predominant N′-fragment, 40 kDa, is specific to cMyBP-C and increased post-hypoxia in vitro
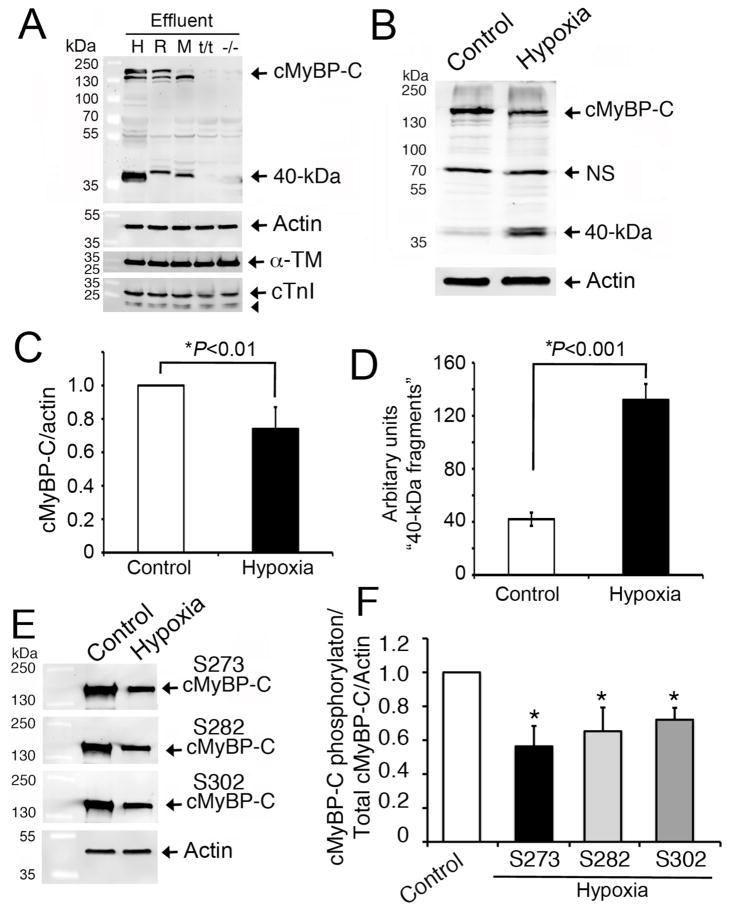
To determine whether the 40 kDa fragments were exclusively from cMyBP-C, we used LV tissue from wild-type mouse, rat, and donor human hearts. These tissue samples were incubated in PBS at 37°C for 6 hrs and the release and degradation of cMyBP-C was analyzed by Western blot. As negative controls, homozygous cMyBP-C null mice (t/t) [31] and cMyBP-C knockout (−/−) [17] mouse hearts were used in which full-length cMyBP-C is completely absent. As expected, full-length cMyBP-C and 40 kDa fragments were released into the PBS effluent at 6 hrs in the wild-type human, rat, and mouse heart samples in contrast to cMyBP-C null and knockout hearts (Fig. 2A). These data suggest that the 40 kDa fragments are derived from cMyBP-C across several species. Finally, to determine whether the 40 kDa fragments can be released by hypoxia, NRVMs were used in vitro and the levels of cMyBP-C and its fragments were determined by Western blot analyses (Fig. 2B). Compared to controls, three-day old hypoxic cardiomyocytes show a significant decrease in full-length cMyBP-C (P<0.01, Fig. 2C) and a corresponding increase in 40 kDa fragments (P<0.0001, Fig. 2D). In addition, the phosphorylation status of cMyBP-C at Ser-273, Ser-282 and Ser-302 was significantly reduced in the hypoxia-induced NRVMs (Fig 2E–F). Taken together, these data suggest that cMyBP-C is an easily releasable sarcomeric protein. In addition, the decrease in phosphorylation status coupled with the decrease in intact cMyBP-C, and a corresponding increase in 40 kDa fragments, supports the role played by phosphorylation in the degradation process of cMyBP-C.
Fig 2. Release of the 40 kDa fragment is specific to cMyBP-C.
Representative Western blot analyses (n=3) show that the cleavage of 40 kDa N-terminal cMyBP-C fragments was consistent in human (H), rat (R) and mouse (M) heart effluents at 6 hrs and was absent in negative controls, such as cMyBP-C null (t/t) and systemic knockout (−/−) mouse hearts (A). LV tissue (100 mg) from the respective species was incubated in 500 μl of PBS (effluent) at 37°C for 6 hrs. Effluent (10 μl) was used for SDS-PAGE, followed by Western blot analyses with rabbit anti-cMyBP-C2–14 antibody. Sarcomeric α-actin, α-TM and cTnI (arrowhead shows its degraded fragments) were determined for controls. Three-day-old NRVMs exposed to hypoxia were compared to controls for cMyBP-C degradation. Representative Western blot analyses using 10 μg of total cell lysates with rabbit anti-cMyBP-C2–14 antibodies (B) show a significant decrease in full-length cMyBP-C (C) and an increase in 40 kDa fragments (D) post-hypoxia. Phosphorylation status of cMyBP-C at Ser-273, Ser-282 and Ser-302 was determined by Western blot analyses using site-specific antibodies (E). Data, which are normalized with full-length cMyBP-C and actin, are summarized for phosphorylation status (F). *P<0.01, hypoxia vs control (n=4).
3.3 Decreased phosphorylation and increased degradation of cMyBP-C in post-MI rats in vivo
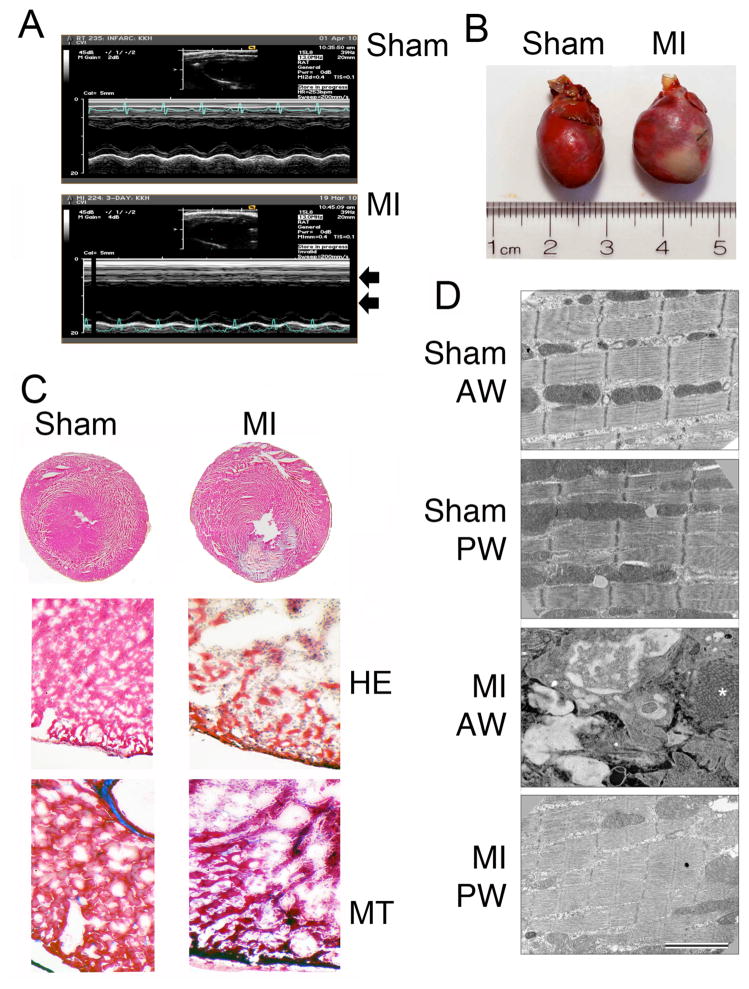
During ischemia, the thick and thin sarcomeric proteins are modified, disorganized and degraded, thereby altering contractile function [25]. In order to study the phosphorylation and degradation pattern of cMyBP-C in the ischemic and non-ischemic regions of MI hearts, a rat model with 3-day permanent coronary artery ligation was used. LV structure and function were significantly altered 3 days post-MI as measured by echocardiography (Table 1). Data show that MI led to significant LV chamber dilation and loss of function as measured by ejection fraction, stroke volume and cardiac output. Echocardiography further demonstrated a lack of mid-papillary anterior wall motion in infarcted animals (Fig. 3A). Gross morphological analysis of pressure perfused fixed hearts demonstrated ventricular dilation (Fig. 3B), and histopathological analyses revealed the presence of infarcted regions and necrosis in MI hearts, compared to sham rat hearts (Fig. 3C). Electron microscopic analyses show a disorganized myofibrillar structure in the infarcted anterior wall (AW) versus the posterior wall (PW) of MI rats and sham control hearts (Fig. 3D).
Table 1.
MI leads to decreased LV function as determined by Echocardiography
| Baseline | Sham | MI | |
|---|---|---|---|
| Number of rats | 19 | 8 | 8 |
| HR (beat/min) | 352±5 | 373±5* | 383±9* |
| BW (grm) | 330±3 | 320±5 | 314±5* |
| IVSd (mm) | 1.59±0.05 | 1.74±0.08 | 1.61±0.22 |
| LVd (mm) | 7.38±0.10 | 7.39±0.13 | 7.78±0.13*$ |
| LV-PWd (mm) | 1.51±0.05 | 1.54±0.06 | 1.50±0.10 |
| IVSs (mm) | 2.54±0.06 | 2.60±0.07 | 2.05±0.30 |
| LVs (mm) | 4.89±0.14 | 4.95±0.16 | 6.01±0.28*$ |
| LV-PWs (mm) | 2.26±0.09 | 2.28±0.09 | 2.13±0.09 |
| IVS (%) | 60±3 | 51±5 | 27±9*$ |
| LV-PW (%) | 50±4 | 48±4 | 44±7 |
| LV EDV(μl) | 371±10 | 371±13 | 411±14*$ |
| LV ESV (μl) | 162±9 | 165±10 | 247±23*$ |
| LV SV (ml) | 208±4 | 206±6 | 164±17*$ |
| LV CO (ml) | 73±1 | 77±2 | 62±6*$ |
| EF (%) | 57±2 | 56±2 | 40±4*$ |
Myocardial function was determined by transthoracic M-mode and 2D echocardiography in adult male Sprague-Dawley rats (~290g) under isoflurane anesthesia (4%) at 3 days post-MI. Baseline values were determined before the surgery. LV indicates left ventricular; d, diastole; s, systole; AW, anterior wall thickness; PW, posterior wall thickness; IVS, intraventricular septum; EDV, end diastolic volume; ESV, end systolic volume; SV, Stroke volume; CO, cardiac output; FS%, fractional shortening; EF%, ejection fraction. Data are mean±SEM. Significant differences:
versus Baseline and
versus sham, P<0.05.
Fig 3. A rat model of myocardial infarction.
Representative M-mode echocardiograms show loss of anterior wall motion and LV dilation (arrows) in post-MI rat hearts, compared to sham controls (A). Representative rat heart shows the presence of a severe anterior LV infarct, compared to sham heart (B). Representative cross-sections of sham and MI rat hearts stained with hematoxylin and eosin (HE) and masson-trichrome (MT) show gross histopathological changes (C). Electron microscopic analyses of rat hearts show the disarrangements of sarcomeres in the infarct regions of the MI rat hearts (D). Sham anterior wall (AW) and posterior wall (PW) of MI show normal fine structure and ordered myofibrils. In contrast, the AW of the MI regions shows the absence of intact myofibrils. The region is filled with inflammatory cells (neutrophils and macrophages). The EM shows detail of the invading cells and large regions of new collagen (*).
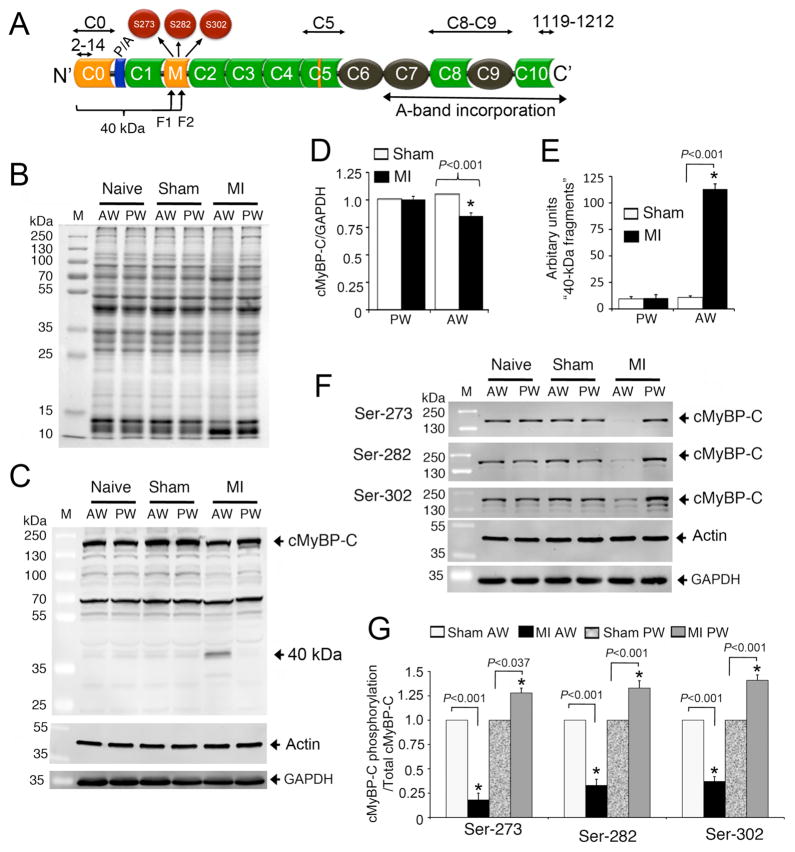
cMyBP-C domain structures, interacting regions and antibodies used in this study are shown in Figure 4A. We hypothesized that the degradation of cMyBP-C would be significantly increased in the ischemic region of MI hearts, resulting in decreased cMyBP-C and its phosphorylation status and increased levels of 40 kDa fragments. To test this hypothesis, we used samples from the AW and PW of naïve, sham and MI hearts. SDS-PAGE shows the altered total protein profile in the AW of MI hearts (Fig. 4B), compared to the PW of MI hearts and controls. Western blot analyses using cMyBP-C2–14 antibodies demonstrated that full-length cMyBP-C was significantly reduced in AW of the MI hearts, compared to PW of the control hearts (Fig. 4C–D), and these findings were confirmed using C5, C8–C9, and C10 domain-specific antibodies (Online Supplemental Fig. 4). Importantly, the 40 kDa fragments were only present in the AW of MI hearts (Fig. 4C, 4E). This supports our hypothesis that the release of 40 kDa fragments is specific to ischemic LV tissue. We then partially purified the 40 kDa fragments to determine the site of cleavage using mass spectrometry. Results show that there are two fragments at the 40 kDa position (Fig. 4A) and that both were identified as rat cMyBP-C (Online Supplemental Fig. 6). Strikingly, both fragments were cleaved within the phosphorylation motifs (M domain) of cMyBP-C, adding to the evidence that phosphorylation of cMyBP-C is cardioprotective [14]. We then hypothesized that decreased phosphorylation of cMyBP-C in the AW region of the MI heart is associated with cMyBP-C degradation and the release of its 40 kDa fragments. To test the hypothesis, we measured phosphorylation levels of Ser-273, Ser-282 and Ser-302 in naïve, sham and infarcted hearts in the AW and PW regions (Fig. 4F). In the AW of the MI heart, there was a significant decrease in phosphorylation levels of all sites (Fig. 4G). As a possible compensatory mechanism resulting from MI in the AW region, the PW region of MI hearts showed a significant increase in cMyBP-C phosphorylation levels at all sites. These changes in cMyBP-C phosphorylation in the AW and PW may contribute to LV remodeling and dysfunction after MI.
Fig 4. Proteolysis of cMyBP-C post-MI in rat hearts.
A schematic diagram illustrates the cMyBP-C domain structure (A). Immunoglobulin (Ig)-like and fibronectin domains are shown in green and black, respectively. Ig-like C0 and M domains and a 28-residue insertion in C5 domain are specific to cMyBP-C (yellow). Proline-Alanine (P/A)-rich region is shown at the N′-region. The three phosphorylatable serines, S273, S282 and S302, are shown in the M domain. The region of the 40 kDa fragments (fragment 1 (F1) and 2 (F2), Online Supplemental Fig. 6) is shown in which C0 and C1 domains and P/A region are included. The antigenic region of cMyBP-C2–14, C0–C1, C5, C8–C9, and cMyBP-C1119–1212 antibodies that were used in this study are marked. Representative SDS-PAGE analyses of tissue homogenate from naïve, sham and infarct rat hearts show the protein profile of anterior wall (AW) and posterior wall (PW) of MI hearts, compared to naïve and sham controls (B). Ten micrograms of total proteins were resolved on SDS-PAGE, stained in SYPRO RUBY overnight, and scanned on a Typhoon TRIO+ scanner (GE Healthcare, Piscataway, NJ). Representative Western blot analyses of tissue homogenates from naïve, sham and MI rat hearts show the proteolytic pattern of cMyBP-C using rabbit anti-cMyBP-C2–14 antibodies in the AW and PW; Sarcomeric α-actin and GAPDH used as loading controls (C). Data were quantified, and a significant reduction (15±3%) of total cMyBP-C was observed in the AW of MI rat hearts compared to shams (D). Proteolytic release of the 40 kDa N-terminal fragment in the AW of MI rat hearts was significantly higher than controls (E). Phosphorylation levels of Ser-273, Ser-282 and Ser-302 were determined in naïve, sham and MI rat heart tissue by Western blots using site-specific phospho antibodies (F); Sarcomeric α-actin and GAPDH were used as loading controls. Data are summarized (G). *P< 0.001, MI (n=6) vs. sham (n=6).
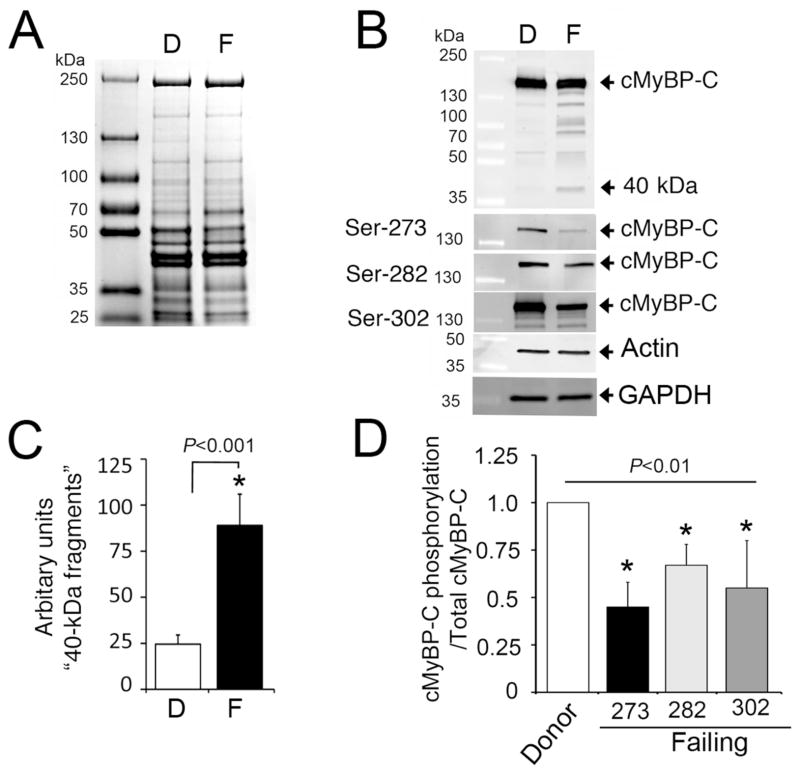
3.4 Decreased cMyBP-C phosphorylation in patients with failing myocardium
We then extended our investigation to determine whether decreased cMyBP-C phosphorylation is associated with the presence of HF in human hearts and the presence of 40 kDa fragments. We used left ventricular non-ischemic (non-failing) donor samples (n=10) obtained from the Gift of Hope, and explanted (failing) samples from patients with ischemic cardiomyopathy (ICM; n=10). The average age and gender of donors from the Gift of Hope and ICM patients were 53.7±4.4 (30% female) and 60.4±2.2 (20% female), respectively. ICM patients were undergoing heart transplantation with near end-stage heart failure. Hearts from Gift of Hope donors were primarily rejected for transplantation based on the following: 20% hypertension, 20% atrial fibrillation, 30% CAD, and 30% CDC high-risk donor. Total cardiac proteins of donors and patients with HF were analyzed by SDS-PAGE (Fig. 5A). Western data from failing human hearts showed a trend (P = 0.07) in the reduction of total cMyBP-C and the presence of 40 kDa fragments (Fig. 5B–C). Importantly, failing samples showed a significant (P< 0.001) decrease in phosphorylation levels of Ser-273, Ser-282 and Ser-302 (Fig 5B, 5D), compared to donor samples. Altogether, our data demonstrate that cMyBP-C undergoes severe degradation during MI and in failing human hearts and that the release of 40 kDa fragments is directly associated with cMyBP-C dephosphorylation.
Fig 5. Decreased cMyBP-C phosphorylation in patients with HF.
Ten micrograms of total proteins from donor (D) and failing (F) human heart samples were used for SDS-PAGE (A), followed by Western blot analyses with rabbit anti-cMyBP-C2–14 antibody and site-specific Ser-273 (S273p), Ser-282 (S282p) and Ser-302 (S302p) phospho antibodies (B). Sarcomeric α-actin and GAPDH were used as loading controls. Data are summarized for the 40 kDa fragments (C) and phosphorylation status (D). *P< 0.001, failing (n=10) vs. donor (n=10).
3.5 Elevated levels of plasma cMyBP-C post-MI in rats and patients
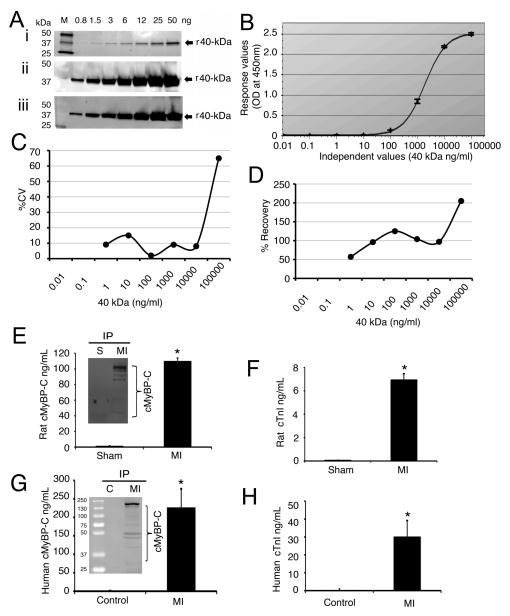
After MI, proteases such as calpains are activated which target myofilament proteins, such as cardiac troponin T and cTnI, leading to their appearance in the circulation [8–11]. We therefore hypothesized that cMyBP-C and its fragments are released into the circulatory system from 3 days post-MI in rats. To quantify plasma cMyBP-C concentrations, we first established a sandwich ELISA assay using capture (monoclonal) and detection (polyclonal) anti-cMyBP-C antibodies. We used recombinant 40 kDa fragments (F1, residues 1–271) for the standard calibrations that were spiked in naïve plasma samples (Fig. 6A–B). To evaluate the quality of the fit at each concentration and sensitivity and accuracy of the assay, we determined the percentage coefficient of variation (CV, Fig. 6C) and percentage recovery (Fig. 6D) using the actual and expected values. The CV is less than 20% and recovery is 10% across the working range (0.1–10,000 ng/ml) of the assay. To determine the sensitivity of the sandwich ELISA, we then defined the limit of detection values and quantification for lower and upper limits (Online Supplemental Table 1). The values of lower limit of quantification and upper limit of quantification are 0.02 and 2053 ng/ml, respectively.
Fig 6. cMyBP-C is released into the circulation post-MI in rats and patients.
To standardize the sandwich ELISA, a sensitivity analysis of both rabbit and mouse N′-specific antibodies was performed (A). Recombinant 40 kDa peptides (F1, residues 1–271) of cMyBP-C were loaded in increasing concentration; SYPRO Ruby-stained gel (i), Western with rabbit polyclonal anti-cMyBP-CC0–C1 antibody (ii) and mouse monoclonal anti-cMyBP-CC0 antibody (iii). A calibration curve of the sandwich ELISA assay is shown (B) to demonstrate absorbance and accuracy of the assay (average mean value of n=3). Corresponding plot of % CV vs. 40 kDa concentration (C). Plot of percentage calibrator recovery vs. 40 kDa concentration (D). Plasma levels of cMyBP-C in sham and MI rats by sandwich ELISA (E). Insert is an IP assay of sham and MI rat plasma. Plasma levels of cTnI in sham and MI rats (F). *P< 0.001, MI (n=11) vs. sham (n=7). Plasma levels of cMyBP-C in human controls and patients with MI (G). Insert shows the presence of cMyBP-C by IP assay. Plasma levels of human cTnI in control and patients with MI (H). *P< 0.001, control (n=11) vs. patients (n=15).
Results show that cMyBP-C titers in the MI rat plasma samples were significantly increased to 109±4 ng/ml, compared to 1.22±0.4 ng/ml in sham rats (Fig. 6E). The presence of intact cMyBP-C and its fragments in the plasma samples of rats was further confirmed by an immunoprecipitation (IP) assay (Fig. 6E insert). For a standard, we measured cTnI levels, which were also significantly higher in the MI plasma samples (7.0±0.5 ng/ml), compared to 0.079±0.02 ng/ml in sham controls (Fig. 6F). We next extended the analyses of cMyBP-C levels to the plasma samples of patients with MI. Plasma samples were collected from 16 MI patients (Table 2) and 11 normal healthy controls (Online Supplemental Table 2). Importantly, cMyBP-C and cTnI levels in the plasma samples of healthy controls were very low, 0.95±0.34 ng/ml and 0.238±0.07 ng/ml, respectively. Strikingly, the concentration of cMyBP-C in the plasma of MI patients was significantly increased to 227±50 ng/ml, compared to controls (Fig. 6G), and greater than plasma cTnI levels at 30±9 ng/ml (Fig. 6H). IP analyses confirmed the presence of cMyBP-C in the plasma samples of these patients (Fig. 6G, insert). Summarized values in molar concentration of these data are shown in Table 3. Blood glucose and creatine concentrations were significantly higher in patients with MI in addition to reduced ejection fraction (Online Supplemental Table 3). Based on our data, it can be concluded that (1) cMyBP-C and its fragments can be released into the circulatory system after MI and (2) the level of plasma cMyBP-C and its N′-fragments is higher than plasma cTnI in humans and rats post-MI. These data strongly suggest that plasma concentrations of cMyBP-C could be a potential biomarker for MI.
Table 2.
Clinical and biochemical profiles of the 15 patients who were diagnosed with MI and positive for cTnI plasma levels
| ID | AGE (Year) | SEX | Time of sample collection after AMI (Hrs) | Glucose (mg/dl) | Creatine (mg/dl) | cTnI (ng/ml) | cMyBP-C (ng/ml) | LVEF (%) | Regional Wall Motion Abnormalities |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 56 | M | 27 | 108 | 1.25 | 6.05 | 310 | 28% | Apex is dyskinetic, AW and septum are hypokinetic |
| 2 | 51 | M | 6 | 254 | 1 | 37.5 | 26.9 | 45% | Apex is akinetic with a mobile thrombus measuring 1.2 by 1.2 cm in diameter |
| 3 | 58 | M | 41 | 153 | 1.13 | 31.6 | 557.9 | 40% | Severe hypokinesis of the apical anteriorseptum and LV apex |
| 4 | 48 | M | 52 | 95 | 1.1 | 9.09 | 146 | 28% | Severe anteroseptal hypokinesis |
| 5 | 51 | M | 62 | 125 | 0.86 | 7.77 | 36.4 | 51% | Hypokinesis of the mid to apical anteroseptum |
| 6 | 73 | F | 42 | 101 | 0.76 | 5.58 | 89 | 40% | The entire septum and AW were severely hypokinetic; the interior wall was hyperdynamic |
| 7 | 79 | M | 60 | 453 | 1.6 | 28.1 | 115.9 | 54% | Anteroseptal/apical region of LV is akinetic |
| 8 | 85 | F | 72 | 134 | 0.98 | 7.7 | 24.7 | 32% | Akinesis or severe hypokinesis of mid and apical anterior, anterior septal and lateral segments |
| 9 | 59 | M | 72 | 400 | 0.74 | 123 | 650.8 | 25% | Dyskinesis of the LV inferolateral wall |
| 10 | 85 | F | 47 | 111 | 1.28 | 55.9 | 364 | 40% | Akinesis or severe hypokinesis of mid and apical anterior, anterior septal and lateral segments |
| 11 | 91 | F | 38 | 129 | 1.66 | 6.83 | 365.1 | 30% | Dyskinesis of the LV inferolateral wall |
| 12 | 55 | M | 20 | 108 | 0.7 | 17.8 | 330 | 50% | Severe septal hypokinesis with akinetic apical anteroseptal/apical region of LV |
| 13 | 74 | F | 8 | 359 | 2.38 | 18.7 | 172.2 | 40% | Severe inferolateral hypokinesis (base to apex) |
| 14 | 86 | F | 60 | 111 | 1 | 8.4 | 90.42 | 45% | Akinetic apical anteroseptal/apical region of LV |
| 15 | 48 | M | 17 | 140 | 0.99 | 89.8 | 127.4 | 39% | Akinetic LV inferolateral wall |
Echocardiographic and clinical data of the patients were obtained using deidentified numbers. M/F indicates male/female; LVEF, LV ejection fraction, calculated as [(LVEDD-LVESD)/LVEDD*100]. Data for eleven normal healthy controls are shown in Online Supplemental Table 2. Summarized data for glucose, creatine and LVEF are available in Online Supplemental Table 3. Time of sample collection after acute myocardial infarction was calculated that interval between the time of chest pain and time of blood sample collection.
Table 3.
Sandwich ELISA values for plasma cTnI and cMyBP-C levels
| Rat | Human | |||||
|---|---|---|---|---|---|---|
| Sham (n=7) | MI (n=11) | Fold of change | Normal (n=11) | MI (n=15) | Fold of change | |
| cTnI | 0.79±0.02 (ng/ml) | 7.0±0.5* (ng/ml) | 0.24±0.07 (ng/ml) | 30±9* (ng/ml) | ||
| 33 (fmoles/ml) | 292 (fmoles/ml) | 9 | 11 (fmoles/ml) | 1250 (fmoles/ml) | 114 | |
|
| ||||||
| cMyBP-C | 1.22±0.4 (ng/ml) | 109±4* (ng/ml) | 0.95±0.34 (ng/ml) | 227±50* (ng/ml) | ||
| 9 (fmoles/ml) | 775 (fmoles/ml) | 86 | 7 (fmoles/ml) | 1613 (fmoles/ml) | 230 | |
Values are expressed as mean ± SEM.
P< 0.001, control vs. MI. The mean values (ng/ml) were used to convert into fmoles/ml in order to demonstrate the number of molecules per ml. Data show that plasma levels of cMyBP-C in MI are significantly higher, compared to sham and controls. However, because ELISA antibodies only recognize the full-length and N′-fragments, cMyBP-C quantitation based on this method may not represent the exact amount of cMyBP-C present in the plasma samples. Femtomole, fmole (10−15 of a mole).
4. Discussion
cMyBP-C is a key assembly and structural protein which plays an important functional role in the heart [16, 17, 19]. Three isoforms of MyBP-C exist: fast skeletal, slow skeletal and cardiac, which are encoded by specific genes [22]. cMyBP-C is exclusively expressed in the heart, and the N′-regions (C0 domain and phosphorylation motifs in the M domain) are specific to the cardiac isoform. cMyBP-C modulates myosin assembly and stabilizes the thick filaments [16], both of which are important for the precise arrangement of actin-myosin filaments in the sarcomere. During MI, it has been demonstrated that the N′-regions of cMyBP-C are severely degraded [14, 25]. These data prompted us to ask whether the proteolysis and release of the cMyBP-C could be measured in the blood and correlated to the extent of MI. In this report, we evaluated cMyBP-C degradation, correlated the degradation profile with its phosphorylation status, and demonstrated its release into the blood post-MI.
4.1 Decreased cMyBP-C phosphorylation is associated with its degradation
cMyBP-C phosphorylation regulates myocardial function [14, 20, 23] and confers cardioprotection against injury [14, 26]. The mechanism by which cMyBP-C is cardioprotective has not been fully elucidated. cMyBP-C phosphorylation has been shown to be reduced in the failing heart [32], suggesting an association with the development of HF. cMyBP-C phosphorylation at Ser-273, Ser-282, and Ser-302 sites is differentially targeted by PKA [33], PKC [33], PKD [24], CaMKII [34] and RSK [24]. These sites are located in the cardiac-specific M domain (Fig. 4A) and are key targets for several kinases [29] regulating myosin and actin function during muscle contraction [19]. Previously, we demonstrated that cMyBP-C is severely degraded during MI, resulting in the production of 40 kDa fragments that are associated with post-ischemic contractile dysfunction [14, 25, 26]. In the present study, we confirmed that the 40 kDa fragments are released when cMyBP-C is cleaved within the phosphorylation motifs. We hypothesized that dephosphorylation of the M domain at Ser-273, Ser-282 and Ser-302 sites is directly associated with the release of the 40 kDa fragment. During MI, the necrotic region of the myocardium becomes impaired which causes a shift in metabolic regulation in response to the low oxygen environment and alters cellular energetics. As a consequence, the myocyte becomes unable to handle Ca2+ properly. As cytosolic Ca2+ levels rise, Ca2+-dependent proteases, such as calpains, may become activated and degrade its substrates, including cTnI, cTnT and cMyBP-C [6, 26, 35]. Dephosphorylation of cTnI and cTnT enhances their degradation, whereas phosphorylation protects them from degradation [36]. It is therefore possible that dephosphorylation of cMyBP-C during ischemia enhances its proteolysis, whereas cMyBP-C phosphorylation modifies its sensitivity to degradation [26]. We hypothesize that dephosphorylation may dissociate protein binding, thus providing access for calpain binding on the targeted site. When this occurs, cMyBP-C could be cleaved at the M domain, causing the release of 40 kDa N′-fragments (F1 and F2, Fig. 4A) into the cytosol, while the C′ end of cMyBP-C may be further cleaved or remain anchored to the A-band. We further hypothesize that N′-fragment cleavage within the M-domain will alter the interaction between myosin and actin, including interaction with thick and thin filament proteins, Mg2+-ATPase activity and contractility. Our present data demonstrate that 1) cMyBP-C phosphorylation is correlated with the reduction in full-length cMyBP-C and the release of 40 kDa fragments in vitro and in vivo in necrotic tissue, hypoxic conditions, and ischemic regions of the post-MI heart; and 2) the cleavage site for the 40 kDa fragments occurs at the phosphorylation motifs, as verified by mass spectrometry. The identification of specific proteases and cleavage sites for cMyBP-C degradation advances our understanding of its cardioprotective function and expands the potential for therapeutic interventions.
4.2 Elevated level of plasma cMyBP-C as a potential biomarker for MI
The diagnosis of MI is made by a variety of assays and methods. However, MI represents only a small number of total patients presenting with chest pain. In fact, studies have shown that 60–70% of all patients with chest pain do not have cardiac-related issues, even though many of these patients are admitted at a very high level of hospital care [37]. This is mostly because current diagnostic methods of assessing MI are not completely reliable [37]. While electrocardiography is practical for detecting major heart attacks, it is not sensitive enough for diagnosing mild or false-positive cases. This is where plasma biomarkers play a major role. According to the American College of Cardiology and American Heart Association guidelines, cTnI and creatine kinase-MB (CK-MB) should be used as the main biomarkers for diagnosing heart attacks [38]. The cardiac sarcomeric proteins, cTnI [11] and cTnT [39], have become the gold standard biomarkers for the diagnosis of MI. Unlike cytosolic proteins, such as CK-MB and myoglobin, the measure of cardiac sarcomeric proteins in the circulatory system is specific (96%) to MI [40, 41]. However, these cardiac biomarkers do not become elevated in the blood until 4–6 hours at the earliest after MI onset. This delay results that low-risk and false-positive patients are unnecessarily reserved in the emergency room, when they could be diagnosed and discharged much earlier, saving considerable time and expense. A combination of cTnI, CK-MB, myoglobin and fatty acid binding protein may offer a 98% accurate rule-out test for MI [42], and such signature assays have been shown to be 20% more sensitive than a single cTnI assay [43]. In addition, cTnI is not specific for just MI, but is also elevated in a large number of clinical situations, suggesting a need for developing a diagnostic assay that specifically detects MI.
cMyBP-C degradation and the release of its fragments into the blood after myocardial stress have thus far not been established. Since cMyBP-C is severely degraded post-MI, we hypothesized that cMyBP-C is released into the circulation post-MI. Our present studies show that intact cMyBP-C and its cleaved fragments are released with increased levels into the effluent (in vitro) and circulation post-MI in a rat model (in vivo). We further demonstrated elevated levels of cMyBP-C in the blood of patients with MI. Future studies would determine the time course of release, peak concentrations, and half-life in the circulatory system. cMyBP-C may be readily detectable by its larger molecular size and higher concentration, thus providing an alternative clinical measurement of myocardial injury. A systematic analysis of cMyBP-C release post-MI has yet to be performed to demonstrate that the release of cMyBP-C occurs from ischemic, necrotic and/or injured cells. Additionally, post-translational modification of plasma cMyBP-C and its degradation in the circulatory system remains to be determined.
4.3 Conclusion
In summary, we investigated the proteolysis and release of cMyBP-C into the circulatory system using in vitro and in vivo techniques based on animal models and human samples. Our data suggest a direct correlation between cMyBP-C dephosphorylation and its degradation. Importantly, the use of different monoclonal and polyclonal anti-cMyBP-C domain-specific antibodies and mass spectrophotometery identified the sites of cleavage, and a sandwich ELISA assay was used to determine the plasma concentrations of cMyBP-C. Because full-length cMyBP-C is susceptible to proteolysis and may undergo further degradation, we propose the need to use a mixture of different domain-specific antibodies to fully measure and characterize cMyBP-C and its fragments in post-MI blood samples. Importantly, the onset of cMyBP-C release and its correlation to myocardial function have yet to be elucidated. These future studies would determine whether cMyBP-C could be used as a true biomarker of MI injury.
Supplementary Material
Highlights.
Cardiac myosin binding protein-C (cMyBP-C) is an easily releasable sarcomeric protein.
Dephosphorylation of cMyBP-C is directly correlated with its degradation in vitro and in vivo.
cMyBP-C and N-terminal fragments are released into the blood post-myocardial infarction.
Established a sandwich ELISA to quantify concentration of plasma cMyBP-C.
Plasma concentrations of cMyBP-C could be a potential biomarker for myocardial infarction.
Acknowledgments
Funding Sources
This work was supported by National Institutes of Health grants 5P30HL101297 and R01HL105826 to Dr. Sadayappan and American Heart Association Grants (11PRE7240022 to Mr. Barefield, 10SDG-2640219 to Dr. Henderson, 0830311N-SDG to Dr. Sadayappan).
We thank Dr. Richard L. Moss, University of Wisconsin at Madison, for providing us with cMyBP-C knockout mouse hearts.
Abbreviations
- cTnI
cardiac troponin I
- cTnT
cardiac troponin T
- cMyBP-C
cardiac myosin binding protein-C
- LV
left ventricular
- MI
myocardial infarction
- kDa
kilo Dalton
- PBS
phosphate buffered saline
- ELISA
enzyme-linked immunosorbent assay
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- AW
anterior wall
- PW
posterior wall
- IP
immunoprecipitation
- ng
nanogram
- ml
milliliter
- α-TM
α-tropomyosin
- ICM
ischemic cardiomyopathy
Footnotes
Disclosures
Dr. Sadayappan developed the sandwich ELISA assay for detecting cMyBP-C levels using human body fluids and holds a provisional patent to determine the risk factors associated with cMyBP-C degradation and release into human body fluid.
References
- 1.Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, et al. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113(6):e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 2.Bodor GS, Oakeley AE, Allen PD, Crimmins DL, Ladenson JH, Anderson PA. Troponin I phosphorylation in the normal and failing adult human heart. Circulation. 1997;96(5):1495–500. doi: 10.1161/01.cir.96.5.1495. [DOI] [PubMed] [Google Scholar]
- 3.Belin RJ, Sumandea MP, Kobayashi T, Walker LA, Rundell VL, Urboniene D, et al. Left ventricular myofilament dysfunction in rat experimental hypertrophy and congestive heart failure. Am J Physiol Heart Circ Physiol. 2006;291(5):H2344–53. doi: 10.1152/ajpheart.00541.2006. [DOI] [PubMed] [Google Scholar]
- 4.Wolff MR, Buck SH, Stoker SW, Greaser ML, Mentzer RM. Myofibrillar calcium sensitivity of isometric tension is increased in human dilated cardiomyopathies: role of altered beta-adrenergically mediated protein phosphorylation. J Clin Invest. 1996;98(1):167–76. doi: 10.1172/JCI118762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Velden J, Boontje NM, Papp Z, Klein LJ, Visser FC, de Jong JW, et al. Calcium sensitivity of force in human ventricular cardiomyocytes from donor and failing hearts. Basic Res Cardiol. 2002;97(Suppl 1I):118–26. doi: 10.1007/s003950200040. [DOI] [PubMed] [Google Scholar]
- 6.Gao WD, Atar D, Liu Y, Perez NG, Murphy AM, Marban E. Role of troponin I proteolysis in the pathogenesis of stunned myocardium. Circ Res. 1997;80(3):393–9. [PubMed] [Google Scholar]
- 7.Patterson C, Portbury AL, Schisler JC, Willis MS. Tear me down: role of calpain in the development of cardiac ventricular hypertrophy. Circ Res. 2011;109(4):453–62. doi: 10.1161/CIRCRESAHA.110.239749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Eyk JE, Powers F, Law W, Larue C, Hodges RS, Solaro RJ. Breakdown and release of myofilament proteins during ischemia and ischemia/reperfusion in rat hearts: identification of degradation products and effects on the pCa-force relation. Circ Res. 1998;82(2):261–71. doi: 10.1161/01.res.82.2.261. [DOI] [PubMed] [Google Scholar]
- 9.Labugger R, Organ L, Collier C, Atar D, Van Eyk JE. Extensive troponin I and T modification detected in serum from patients with acute myocardial infarction. Circulation. 2000;102(11):1221–6. doi: 10.1161/01.cir.102.11.1221. [DOI] [PubMed] [Google Scholar]
- 10.Colantonio DA, Pickett W, Brison RJ, Collier CE, Van Eyk JE. Detection of cardiac troponin I early after onset of chest pain in six patients. Clin Chem. 2002;48(4):668–71. [PubMed] [Google Scholar]
- 11.Keller T, Zeller T, Peetz D, Tzikas S, Roth A, Czyz E, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361(9):868–77. doi: 10.1056/NEJMoa0903515. [DOI] [PubMed] [Google Scholar]
- 12.Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361(9):858–67. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 13.Marston SB, de Tombe PP. Troponin phosphorylation and myofilament Ca2+-sensitivity in heart failure: increased or decreased? J Mol Cell Cardiol. 2008;45(5):603–7. doi: 10.1016/j.yjmcc.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sadayappan S, Osinska H, Klevitsky R, Lorenz JN, Sargent M, Molkentin JD, et al. Cardiac myosin binding protein-C phosphorylation is cardioprotective. Proc Natl Acad Sci U S A. 2006;103(45):16918–23. doi: 10.1073/pnas.0607069103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scruggs SB, Hinken AC, Thawornkaiwong A, Robbins J, Walker LA, de Tombe PP, et al. Ablation of ventricular myosin regulatory light chain phosphorylation in mice causes cardiac dysfunction in situ and affects neighboring myofilament protein phosphorylation. J Biol Chem. 2009;284(8):5097–106. doi: 10.1074/jbc.M807414200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winegrad S. Cardiac myosin binding protein-C. Circ Res. 1999;84(10):1117–26. doi: 10.1161/01.res.84.10.1117. [DOI] [PubMed] [Google Scholar]
- 17.Harris SP, Bartley CR, Hacker TA, McDonald KS, Douglas PS, Greaser ML, et al. Hypertrophic cardiomyopathy in cardiac myosin binding protein-C knockout mice. Circ Res. 2002;90(5):594–601. doi: 10.1161/01.res.0000012222.70819.64. [DOI] [PubMed] [Google Scholar]
- 18.Flashman E, Redwood C, Moolman-Smook J, Watkins H. Cardiac myosin binding protein C: its role in physiology and disease. Circ Res. 2004;94(10):1279–89. doi: 10.1161/01.RES.0000127175.21818.C2. [DOI] [PubMed] [Google Scholar]
- 19.Barefield D, Sadayappan S. Phosphorylation and function of cardiac myosin binding protein-C in health and disease. J Mol Cell Cardiol. 2010;48(5):866–75. doi: 10.1016/j.yjmcc.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadayappan S, Gulick J, Klevitsky R, Lorenz JN, Sargent M, Molkentin JD, et al. Cardiac myosin binding protein-C phosphorylation in a {beta}-myosin heavy chain background. Circulation. 2009;119(9):1253–62. doi: 10.1161/CIRCULATIONAHA.108.798983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhandapany PS, Sadayappan S, Xue Y, Powell GT, Rani DS, Nallari P, et al. A common MYBPC3 (cardiac myosin binding protein-C) variant associated with cardiomyopathies in South Asia. Nat Genet. 2009;41(2):187–91. doi: 10.1038/ng.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Offer G, Moos C, Starr R. A new protein of the thick filaments of vertebrate skeletal myofibrils. Extractions, purification and characterization. J Mol Biol. 1973;74(4):653–76. doi: 10.1016/0022-2836(73)90055-7. [DOI] [PubMed] [Google Scholar]
- 23.Sadayappan S, Gulick J, Osinska H, Martin LA, Hahn HS, Dorn GW, 2nd, et al. Cardiac myosin binding protein-C phosphorylation and cardiac function. Circ Res. 2005;97(11):1156–63. doi: 10.1161/01.RES.0000190605.79013.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bardswell SC, Cuello F, Rowland AJ, Sadayappan S, Robbins J, Gautel M, et al. Distinct sarcomeric substrates are responsible for protein kinase D-mediated regulation of cardiac myofilament Ca2+ sensitivity and cross-bridge cycling. J Biol Chem. 2010;285(8):5674–82. doi: 10.1074/jbc.M109.066456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Decker RS, Decker ML, Kulikovskaya I, Nakamura S, Lee DC, Harris K, et al. Myosin-binding protein C phosphorylation, myofibril structure, and contractile function during low-flow ischemia. Circulation. 2005;111(7):906–12. doi: 10.1161/01.CIR.0000155609.95618.75. [DOI] [PubMed] [Google Scholar]
- 26.Sadayappan S, Greis K, Robbins J. Phosphorylation-dependent proteolysis and pathogenesis of cardiac myosin binding protein–C. J Mol Cell Cardiol. 2008:44S44. [Google Scholar]
- 27.Copeland O, Sadayappan S, Messer AE, Steinen GJ, van der Velden J, Marston SB. Analysis of cardiac myosin binding protein-C phosphorylation in human heart muscle. J Mol Cell Cardiol. 2010;49(6):1003–11. doi: 10.1016/j.yjmcc.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Kulikovskaya I, McClellan G, Flavigny J, Carrier L, Winegrad S. Effect of MyBP-C binding to actin on contractility in heart muscle. J Gen Physiol. 2003;122(6):761–74. doi: 10.1085/jgp.200308941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadayappan S, Gulick J, Osinska H, Barefield D, Cuello F, Avkiran M, et al. A critical function for ser-282 in cardiac Myosin binding protein-C phosphorylation and cardiac function. Circ Res. 2011;109(2):141–50. doi: 10.1161/CIRCRESAHA.111.242560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henderson KK, Danzi S, Paul JT, Leya G, Klein I, Samarel AM. Physiological replacement of T3 improves left ventricular function in an animal model of myocardial infarction-induced congestive heart failure. Circ Heart Fail. 2009;2(3):243–52. doi: 10.1161/CIRCHEARTFAILURE.108.810747. [DOI] [PubMed] [Google Scholar]
- 31.McConnell BK, Jones KA, Fatkin D, Arroyo LH, Lee RT, Aristizabal O, et al. Dilated cardiomyopathy in homozygous myosin-binding protein-C mutant mice. J Clin Invest. 1999;104(9):1235–44. doi: 10.1172/JCI7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Dijk SJ, Dooijes D, dos Remedios C, Michels M, Lamers JM, Winegrad S, et al. Cardiac myosin-binding protein C mutations and hypertrophic cardiomyopathy: haploinsufficiency, deranged phosphorylation, and cardiomyocyte dysfunction. Circulation. 2009;119(11):1473–83. doi: 10.1161/CIRCULATIONAHA.108.838672. [DOI] [PubMed] [Google Scholar]
- 33.Mohamed AS, Dignam JD, Schlender KK. Cardiac myosin-binding protein C (MyBP-C): identification of protein kinase A and protein kinase C phosphorylation sites. Arch Biochem Biophys. 1998;358(2):313–9. doi: 10.1006/abbi.1998.0857. [DOI] [PubMed] [Google Scholar]
- 34.Schlender KK, Bean LJ. Phosphorylation of chicken cardiac C-protein by calcium/calmodulin-dependent protein kinase II. J Biol Chem. 1991;266(5):2811–7. [PubMed] [Google Scholar]
- 35.Galvez AS, Diwan A, Odley AM, Hahn HS, Osinska H, Melendez JG, et al. Cardiomyocyte degeneration with calpain deficiency reveals a critical role in protein homeostasis. Circ Res. 2007;100(7):1071–8. doi: 10.1161/01.RES.0000261938.28365.11. [DOI] [PubMed] [Google Scholar]
- 36.Di Lisa F, De Tullio R, Salamino F, Barbato R, Melloni E, Siliprandi N, et al. Specific degradation of troponin T and I by mu-calpain and its modulation by substrate phosphorylation. Biochem J. 1995;308(Pt 1):57–61. doi: 10.1042/bj3080057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ekelund U, Forberg JL. New methods for improved evaluation of patients with suspected acute coronary syndrome in the emergency department. Emerg Med J. 2007;24(12):811–4. doi: 10.1136/emj.2007.048249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kushner FG, Hand M, Smith SC, Jr, King SB, 3rd, Anderson JL, Antman EM, et al. 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction (updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (updating the 2005 Guideline and 2007 Focused Update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120(22):2271–306. doi: 10.1161/CIRCULATIONAHA.109.192663. [DOI] [PubMed] [Google Scholar]
- 39.Katus HA, Remppis A, Neumann FJ, Scheffold T, Diederich KW, Vinar G, et al. Diagnostic efficiency of troponin T measurements in acute myocardial infarction. Circulation. 1991;83(3):902–12. doi: 10.1161/01.cir.83.3.902. [DOI] [PubMed] [Google Scholar]
- 40.Babuin L, Jaffe AS. Troponin: the biomarker of choice for the detection of cardiac injury. CMAJ. 2005;173(10):1191–202. doi: 10.1503/cmaj.050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrow DA. Clinical application of sensitive troponin assays. N Engl J Med. 2009;361(9):913–5. doi: 10.1056/NEJMe0905790. [DOI] [PubMed] [Google Scholar]
- 42.Vasatová M, Tichý M, Horácek JM, Pudil R, Horáková L, Palicka V. Multi-marker approach in the diagnostics of cardiac diseases by protein biochip technology. Cas Lek Cesk. 2009;148(2):591–6. [PubMed] [Google Scholar]
- 43.Viswanathan K, Kilcullen N, Morrell C, Thistlethwaite SJ, Sivananthan MU, Hassan TB, et al. Heart-type fatty acid-binding protein predicts long-term mortality and re-infarction in consecutive patients with suspected acute coronary syndrome who are troponin-negative. J Am Coll Cardiol. 2010;55(23):2590–8. doi: 10.1016/j.jacc.2009.12.062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.