Abstract
A Brain-Computer Interface (BCI) is a device that enables severely disabled people to communicate and interact with their environments using their brain waves. Most research investigating BCI in humans have used scalp-recorded electroencephalography (EEG). We have recently demonstrated that signals from intracranial electrocorticography (ECoG) and stereotactic depth electrodes (SDE) in the hippocampus can be used to control a BCI P300 Speller paradigm. We report a case in which stereotactic depth electrodes positioned in the ventricle were able to obtain viable signals for a BCI. Our results demonstrate that event-related potentials from intraventricular electrodes can be used to reliably control the P300 Speller BCI paradigm.
Keywords: Brain-Computer Interface, Brain Ventricle, Hippocampus, Intracranial Electrodes, P300 Speller
1. Introduction
A brain-computer interface (BCI) is a device that uses brain signals to provide a non-muscular communication channel (Wolpaw, Birbaumer et al. 2002), particularly for individuals with severe neuromuscular disabilities. One of the most promising signals for controlling a BCI are event-related potentials (ERPs) such as the P300. The P300 event-related potential is an evoked response to an external stimulus that has been traditionally observed in scalp-recorded electroencephalography (EEG). The scalp-recorded P300 response has proven to be a reliable signal for controlling a BCI using the P300 Speller paradigm (Farwell and Donchin 1988). Based on multiple studies in healthy and disabled volunteers (Serby, Yom-Tov et al. 2005; Vaughan, McFarland et al. 2006; Krusienski, Sellers et al. 2008; Lenhardt, Kaper et al. 2008; Nijboer, Sellers et al. 2008; Sellers, Vaughan et al. 2010), the P300 Speller has the potential to serve as an effective communication device for persons who have lost or are losing the ability to write and speak.
Intracranial surface grid arrays and depth electrodes are routinely implanted in humans for localizing epileptic seizure foci because they offer superior spatial resolution and the recorded brain signals are not attenuated by dura, bone or skin (Akhtari, Bryant et al. 2000). Both styles of electrodes record local field potentials, with the surface grid array recordings referred to as the electrocorticogram (ECoG). Whether intracranial electrode recordings ultimately prove to be superior to scalp electrode recordings in controlling a BCI system remains to be seen. We have recently shown that humans can effectively control the P300 Speller using ECoG (Krusienski and Shih 2011) and ERPs recorded from stereotactic depth electrodes (SDE) in the hippocampus (Krusienski and Shih 2011). Others have also used intracranial electrode recordings to control a computer cursor (Vansteensel, Hermes et al. 2010; Leuthardt, Gaona et al. 2011). The location of the recording electrodes and the types of responses obtained are areas of active research. We report a proof of concept case in which ERPs from intraventricular electrodes were used to control the P300 Speller BCI paradigm.
2. Materials and Methods
The subject is a 34 year-old female with medically intractable epilepsy who underwent a clinical evaluation for epilepsy surgery with temporary placement of bilateral hippocampal depth electrodes to localize her seizure focus prior to surgical resection. The plan was to insert SDEs into both hippocampal bodies to record and determine the epileptic focus. The patient also consented to participate in an ongoing BCI study approved by the Institutional Review Board of both Mayo Clinic and the University of North Florida.
2.1 Electrode Locations and Data Acquisition
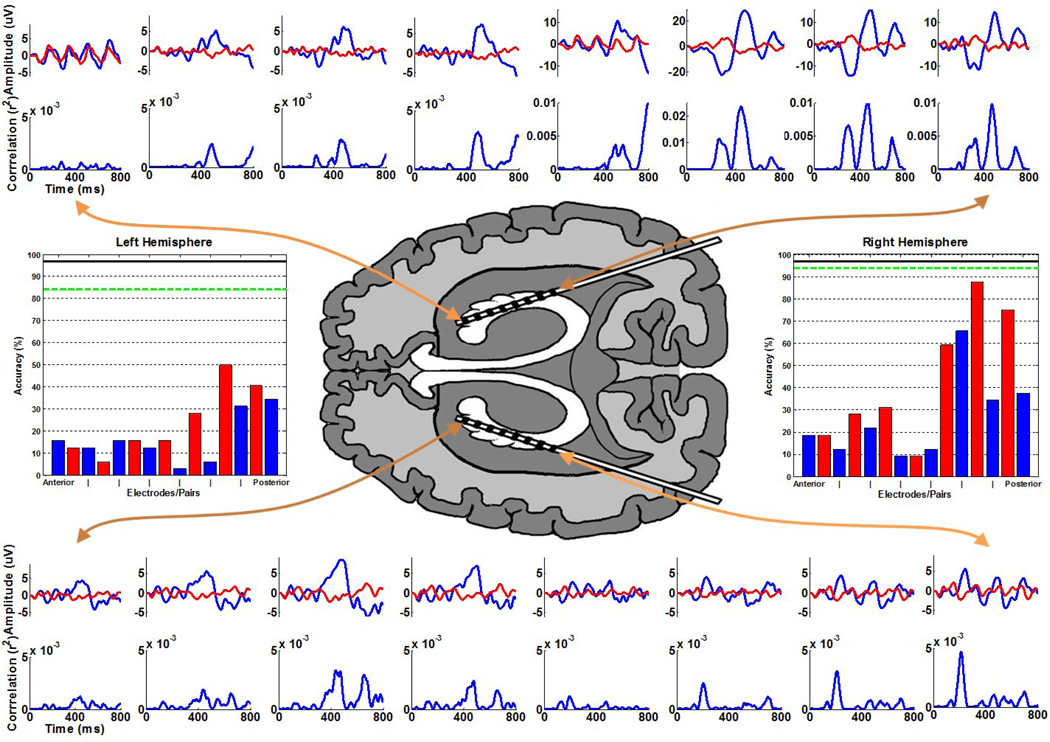
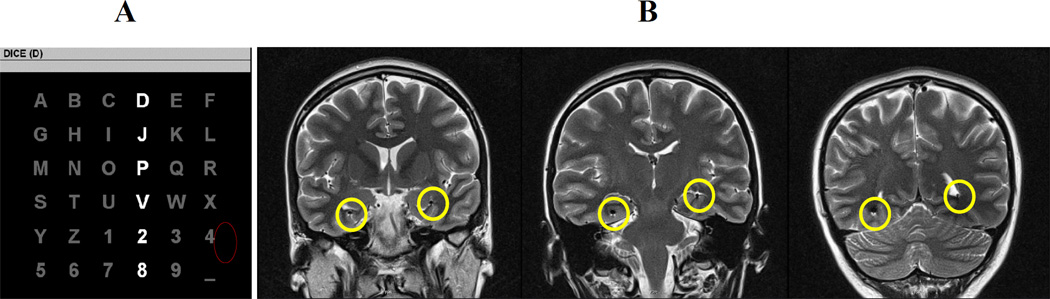
Electrode placements and duration of intracranial monitoring were based solely on the requirements of the clinical evaluation, without any consideration of the BCI study. Two eight-contact stereotactic depth electrodes with 5mm spacing between contacts were inserted in a longitudinal fashion along the plane of the hippocampus through an occipital burr hole and guided intra-operatively by an MRI neuronavigational system. After electrode implantation, the subject was admitted to the epilepsy monitoring unit to record her typical seizures. EEG data in the epilepsy monitoring unit demonstrated a significant asymmetry in signal amplitude and power between the left and right stereotactic electrodes in the range of 30–50%. This amplitude discrepancy was also observed during the BCI sessions as shown by the averaged responses provided in Figure 2. A MRI of the brain was then performed and showed the contacts of the left stereotactic depth electrode to lie in the atrium and inferior horn of the left lateral ventricle. The distal contacts lie in contact with the ventricular surface of the left hippocampal formation (Figure 1B). This confirmed the unanticipated finding that left depth recording electrodes were not positioned in brain tissue, but in the ventricular space.
Fig. 2.
The ERPs and the respective r2 correlations with the task are plotted in the periphery, ordered corresponding to the provided axial viewpoint representing the approximate relative electrode positions (note that for the purposes of the illustration the left hemisphere electrodes are not shown in the ventricle). These waveforms represent the average responses to the target (blue) and non-target (red) stimuli, with 0 ms indicating the stimulus onset. Note that the four most posterior responses in the right hemisphere are plotted on different scales for emphasis. The classification accuracy after 15 flash sequences for subsets of electrodes in the left and right hemispheres is provided in the bar graphs. The blue bars indicate the accuracy using individual common referenced electrodes ordered from anterior to posterior. The intermediate red bars indicate the accuracy using adjacent electrode pairs ordered in the same fashion. The green dashed line indicates the accuracy using all 8 electrodes within a given hemisphere (left: 84.4%, right: 93.8%), and the solid black line indicates the accuracy using all 16 electrodes (96.9%), which also represents the accuracy achieved during the online experiments. Chance accuracy for the task is 2.8%.
Fig. 1.
A) The 6×6 matrix used in the current study. A row or column flashes for 100 ms every 175 ms. The letter in parentheses at the top of the window is the current target character “D.” A P300 should be elicited when the fourth column or first row is flashed. After 15 flash sequences, the collected brain responses are processed, classified, and online feedback is provided directly below the character to be copied. The process is then repeated for the subsequent target characters. B) Post-implant brain MRI. T2-weighted coronal sections through anterior, middle and posterior temporal lobe (from left to right) demonstrating the intraventricular location of stereotactic depth electrode in the left temporal lobe and the intraparenchymal location of the stereotactic depth electrode in the right temporal lobe (encircled in yellow).
The subject performed BCI testing 24 hours after electrode implantation. Testing was performed when the subject was clinically judged to be at cognitive baseline and free of physical discomfort that would affect attention and concentration. Testing was performed at least six hours after a clinical seizure. Stimuli were presented and the data were recorded using the general-purpose BCI system BCI2000 (Schalk, McFarland et al. 2004). All electrodes were referenced to a scalp vertex electrode, amplified, band pass filtered (0.5–500 Hz), digitized at 1200 Hz using a 16-channel Guger Technologies g.USBamp, and stored. The signals for the BCI experiments were acquired concurrent with the clinical monitoring via a 32-channel electrode splitter box.
2.2 Task, Procedure, & Design
The experimental protocol was based on the protocol used in an EEG-based P300 Speller study (Krusienski, Sellers et al. 2008). The subject sat in a hospital bed about 75 cm from a video monitor and viewed the matrix display. The task was to focus attention on a specified letter of the matrix and silently count the number of times the target character flashed, until a new character was specified for selection. All data were collected in the copy speller mode: words were presented on the top left of the video monitor and the character currently specified for selection was listed in parentheses at the end of the letter string as shown in Figure 1A. Each session consisted of 8 experimental runs of the P300 Speller paradigm; each run was composed of a word or series of characters chosen by the investigator. The first four runs (16 characters) were used to train a linear classifier using stepwise linear discriminant analysis (SWLDA) and online feedback of the selected character was provided to the subject for all subsequent runs (Krusienski and Shih 2011). Two sessions were conducted on consecutive days. Each session consisted of 32 character epochs and lasted approximately one hour.
2.3 Offline Response Classification and Visualization
Classifiers were generated using the following combinations of electrodes: all 16, 8 right hemisphere, 8 left hemisphere, each adjacent bipolar pair along each strip, and each individually using a common reference. For each channel used in the analysis, 700-ms segments of data beginning 50-ms after each flash were extracted. The data segments were lowpass filtered and decimated to 20 Hz and concatenated by channel for each flash, creating a single feature vector corresponding to each stimulus. The features from the first session were used to generate a linear classifier using linear discriminant analysis. The performance of the classifier for selecting the attended character was tested on the data from the subsequent session.
The ERPs from all electrodes and their r2 correlations (i.e. the proportion of the variance of the instantaneous signal amplitude accounted for by the stimulus type, i.e., target or non-target) with the task are presented in Figure 2. The waveforms were generated using the average of all training data used for classification. The averaged waveforms were smoothed for visualization using a 0–30 Hz lowpass filter.
3. Results
The results of the offline analysis are provided in Figure 2. The classification accuracy after 15 flash sequences for subsets of electrodes in the left and right hemispheres is provided in the bar graphs. The blue bars indicate the accuracy using individual common referenced electrodes ordered from anterior to posterior. The intermediate red bars indicate the accuracy using adjacent electrode pairs ordered in the same fashion. The green dashed line indicates the accuracy using all 8 electrodes within a given hemisphere (left: 84.4%, right: 93.8%), and the solid black line indicates the accuracy using all 16 electrodes (96.9%), which also represents the accuracy achieved during the online experiments. Chance accuracy for the task is 2.8%.
4. Discussion
Multiple investigators have used BCI-based methods with scalp EEG and ECoG in humans to control movements through a prosthetic device (Hochberg, Serruya et al. 2006) or make cursors move on a computer monitor (Leuthardt, Schalk et al. 2004; Schalk, McFarland et al. 2004; McFarland, Sarnacki et al. 2005; Santhanam, Ryu et al. 2006; Felton, Wilson et al. 2007; Schalk, Miller et al. 2008; Blakely, Miller et al. 2009; Wilson, Schalk et al. 2009). Our previous study shows that electrical recordings from human cortex can be translated by P300-based BCI systems to produce accurate and reliable language output at least equal to and possibly superior to recordings obtained from scalp EEG (Krusienski and Shih 2011). We have subsequently shown that stereotactic depth electrodes placed in the temporal lobes can record signals to control a BCI-based language communication system (Krusienski and Shih 2011). These findings open a new avenue for research on improving communication devices for patients with ALS, spinal cord injuries, stroke, and severe inflammatory polyradiculopathies. As the risks associated with implantation of chronic intracranial electrodes continue to decrease with advances in electrode design and surgical techniques, an intracranial electrode-based P300 Speller may become a viable option for severely disabled individuals with no reliable means of communication.
The present case demonstrates as proof of concept that recording electrodes in the lateral ventricle adjacent to hippocampus can be used to control a brain-computer interface. Since the classifiers were trained and tested using data from successive days, the favorable classification performance indicates that the ERPs are consistent across multiple days. Although maximum classification accuracy (96.9%) was achieved using all electrodes, the 84.4% accuracy achieved by the electrodes within the ventricle is sufficient for effective communication. Additionally, the examination of adjacent electrode pairs indicates that it may be possible to achieve comparable performance by using only a few strategically position electrodes. It remains to be shown whether the signals from left or right hippocampal formation are superior for this application, or if this is subject-dependent. An additional patient performing the identical task also produced superior signals from the right hippocampal formation (Krusienski and Shih 2011), but it is not clear whether this is circumstantial, pervasive, or due to the disease. In the case that such a hemispherical bias exists, it is possible that electrodes positioned in the right ventricle may have produced superior performance.
The ability to utilize a SDE-based P300 Speller for communication improves the risk/benefit ratio for chronic intracranial implantation compared to ECoG with grid electrodes. SDE are often implanted through occipital burr holes with stereotactic guidance. In contrast, a craniotomy procedure is most commonly used to place grid or strip electrodes. Postoperative steroids to reduce brain swelling is used after grid/strip implantation, but not after SDE inserted through the occipital approach (personal communication, Robert Wharen, MD, Chief of Neurosurgery, Mayo Clinic Florida). Surgical case series (Behrens, Schramm et al. 1997; Burneo, Steven et al. 2006; Lee, Hwang et al. 2008; Wong, Birkett et al. 2009) suggest epilepsy patients undergoing SDE as opposed to subdural grids/extended strips have less morbidity. Intraventricular depth electrodes potentially may have even less long-term morbidity as they reside in the ventricles and are less likely to provoke the foreign body reactions (Winslow, Christensen et al. 2010) seen with electrodes residing within brain tissue.
P300 ERPs can be recorded from SDE in the human hippocampus (Halgren, Baudena et al. 1995; Clarke, Halgren et al. 1999). Ludowig et al. (Ludowig, Bien et al. 2010) studied the topography of the medial temporal P300 and found the highest signal amplitude in the anterior subiculum and posterior hippocampal body. All of these previous studies have obtained the P300 from intraparenchymal depth electrodes. However, previous work in epilepsy patients have shown that hippocampal electrical activity can be recorded from electrodes positioned in the inferior horn of the lateral ventricles adjacent to the hippocampal body (Song, Abou-Khalil et al. 2003). Our findings are consistent with these results. We were able to use the intraventricular electrodes to record seizure activity onset and acquire the necessary clinical data to proceed to successful resection of the epileptic focus. Although the amplitude of signals recorded from the intraventricular electrodes is lower than that seen with intraparenchymal recordings, our data demonstrate that these signals can still be accurately classified for BCI purposes. Further studies will be needed to compare the overall feasibility of intraventricular electrodes compared to scalp EEG, ECoG, or hippocampal depth electrodes in controlling a P300 Speller.
Acknowledgments
This work was funded in part by the National Science Foundation (1064912) and the National Institutes of Health (NIBIB/NINDS EB00856).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akhtari M, Bryant HC, et al. Conductivities of three-layer human skull. Brain Topogr. 2000;13(1):29–42. doi: 10.1023/a:1007882102297. [DOI] [PubMed] [Google Scholar]
- Behrens E, Schramm J, et al. Surgical and neurological complications in a series of 708 epilepsy surgery procedures. Neurosurgery. 1997;41(1):1–9. doi: 10.1097/00006123-199707000-00004. discussion 9–10. [DOI] [PubMed] [Google Scholar]
- Blakely T, Miller KJ, et al. Robust, long-term control of an electrocorticographic brain-computer interface with fixed parameters. Neurosurg Focus. 2009;27(1):E13. doi: 10.3171/2009.4.FOCUS0977. [DOI] [PubMed] [Google Scholar]
- Burneo JG, Steven DA, et al. Morbidity associated with the use of intracranial electrodes for epilepsy surgery. Can J Neurol Sci. 2006;33(2):223–227. doi: 10.1017/s0317167100005023. [DOI] [PubMed] [Google Scholar]
- Clarke JM, Halgren E, et al. Intracranial ERPs in humans during a lateralized visual oddball task: II. Temporal, parietal, and frontal recordings. Clin Neurophysiol. 1999;110(7):1226–1244. doi: 10.1016/s1388-2457(99)00064-4. [DOI] [PubMed] [Google Scholar]
- Farwell LA, Donchin E. Talking off the top of your head: toward a mental prosthesis utilizing event-related brain potentials. Electroencephalogr Clin Neurophysiol. 1988;70(6):510–523. doi: 10.1016/0013-4694(88)90149-6. [DOI] [PubMed] [Google Scholar]
- Felton EA, Wilson JA, et al. Electrocorticographically controlled brain-computer interfaces using motor and sensory imagery in patients with temporary subdural electrode implants. Report of four cases. J Neurosurg. 2007;106(3):495–500. doi: 10.3171/jns.2007.106.3.495. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, et al. Intracerebral potentials to rare target and distractor auditory and visual stimuli II. Medial, lateral and posterior temporal lobe. Electroencephalogr Clin Neurophysiol. 1995;94(4):229–250. doi: 10.1016/0013-4694(95)98475-n. [DOI] [PubMed] [Google Scholar]
- Hochberg LR, Serruya MD, et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442(7099):164–171. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- Krusienski DJ, Sellers EW, et al. Toward enhanced P300 speller performance. J Neurosci Methods. 2008;167(1):15–21. doi: 10.1016/j.jneumeth.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusienski DJ, Shih JJ. Control of a brain-computer interface using stereotactic depth electrodes in and adjacent to the hippocampus. J Neural Eng. 2011;8(2):025006. doi: 10.1088/1741-2560/8/2/025006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusienski DJ, Shih JJ. Control of a visual keyboard using an electrocorticographic brain-computer interface. Neurorehabil Neural Repair. 2011;25(4):323–331. doi: 10.1177/1545968310382425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Hwang YS, et al. Surgical complications of epilepsy surgery procedures : experience of 179 procedures in a single institute. J Korean Neurosurg Soc. 2008;44(4):234–239. doi: 10.3340/jkns.2008.44.4.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhardt A, Kaper M, et al. An adaptive P300-based online brain-computer interface. IEEE Trans Neural Syst Rehabil Eng. 2008;16(2):121–130. doi: 10.1109/TNSRE.2007.912816. [DOI] [PubMed] [Google Scholar]
- Leuthardt EC, Gaona C, et al. Using the electrocorticographic speech network to control a brain-computer interface in humans. Journal of neural engineering. 2011;8(3):036004. doi: 10.1088/1741-2560/8/3/036004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuthardt EC, Schalk G, et al. A brain-computer interface using electrocorticographic signals in humans. J Neural Eng. 2004;1(2):63–71. doi: 10.1088/1741-2560/1/2/001. [DOI] [PubMed] [Google Scholar]
- Ludowig E, Bien CG, et al. Two P300 generators in the hippocampal formation. Hippocampus. 2010;20(1):186–195. doi: 10.1002/hipo.20603. [DOI] [PubMed] [Google Scholar]
- McFarland DJ, Sarnacki WA, et al. Brain-computer interface (BCI) operation: signal and noise during early training sessions. Clin Neurophysiol. 2005;116(1):56–62. doi: 10.1016/j.clinph.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Nijboer F, Sellers EW, et al. A P300-based brain-computer interface for people with amyotrophic lateral sclerosis. Clin Neurophysiol. 2008;119(8):1909–1916. doi: 10.1016/j.clinph.2008.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam G, Ryu SI, et al. A high-performance brain-computer interface. Nature. 2006;442(7099):195–198. doi: 10.1038/nature04968. [DOI] [PubMed] [Google Scholar]
- Schalk G, McFarland DJ, et al. BCI2000: a general-purpose brain-computer interface (BCI) system. IEEE Trans Biomed Eng. 2004;51(6):1034–1043. doi: 10.1109/TBME.2004.827072. [DOI] [PubMed] [Google Scholar]
- Schalk G, Miller KJ, et al. Two-dimensional movement control using electrocorticographic signals in humans. J Neural Eng. 2008;5(1):75–84. doi: 10.1088/1741-2560/5/1/008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers EW, Vaughan TM, et al. A brain-computer interface for long-term independent home use. Amyotroph Lateral Scler. 2010;11(5):449–455. doi: 10.3109/17482961003777470. [DOI] [PubMed] [Google Scholar]
- Serby H, Yom-Tov E, et al. An improved P300-based brain-computer interface. IEEE Trans Neural Syst Rehabil Eng. 2005;13(1):89–98. doi: 10.1109/TNSRE.2004.841878. [DOI] [PubMed] [Google Scholar]
- Song JK, Abou-Khalil B, et al. Intraventricular monitoring for temporal lobe epilepsy: report on technique and initial results in eight patients. J Neurol Neurosurg Psychiatry. 2003;74(5):561–565. doi: 10.1136/jnnp.74.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansteensel MJ, Hermes D, et al. Brain-computer interfacing based on cognitive control. Annals of neurology. 2010;67(6):809–816. doi: 10.1002/ana.21985. [DOI] [PubMed] [Google Scholar]
- Vaughan TM, McFarland DJ, et al. The Wadsworth BCI Research and Development Program: at home with BCI. IEEE Trans Neural Syst Rehabil Eng. 2006;14(2):229–233. doi: 10.1109/TNSRE.2006.875577. [DOI] [PubMed] [Google Scholar]
- Wilson JA, Schalk G, et al. Using an EEG-based brain-computer interface for virtual cursor movement with BCI2000. J Vis Exp. 2009;(29) doi: 10.3791/1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow BD, Christensen MB, et al. A comparison of the tissue response to chronically implanted Parylene-C-coated and uncoated planar silicon microelectrode arrays in rat cortex. Biomaterials. 2010;31(35):9163–9172. doi: 10.1016/j.biomaterials.2010.05.050. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Birbaumer N, et al. Brain-computer interfaces for communication and control. Clin Neurophysiol. 2002;113(6):767–791. doi: 10.1016/s1388-2457(02)00057-3. [DOI] [PubMed] [Google Scholar]
- Wong CH, Birkett J, et al. Risk factors for complications during intracranial electrode recording in presurgical evaluation of drug resistant partial epilepsy. Acta Neurochir (Wien) 2009;151(1):37–50. doi: 10.1007/s00701-008-0171-7. [DOI] [PubMed] [Google Scholar]