Abstract
Objective
The goal of the present study was to investigate the clinical utility of measurements of ear-canal reflectance (ECR) in a population of patients with conductive hearing loss in the presence of an intact, healthy tympanic membrane (TM) and an aerated middle ear. We also sought to compare the diagnostic accuracy of umbo velocity (VU) measurements and measurements of ECR in the same group of patients.
Design
This prospective study comprised 31 adult patients with conductive hearing loss, of which 14 had surgically-confirmed stapes fixation due to otosclerosis, 6 had surgically-confirmed ossicular discontinuity, and 11 had CT- and VEMP-confirmed superior semicircular canal dehiscence (SCD). Measurements on all 31 ears included pure-tone audiometry for 0.25 – 8 kHz, ECR for 0.2 – 6 kHz using the Mimosa Acoustics HearID system, and VU for 0.3 – 6 kHz using the HLV-1000 laser Doppler vibrometer (Polytec Inc). We analyzed power reflectance |ECR|2 as well as the absorbance level = 10×log10(1−|ECR|2). All measurements were made prior to any surgical intervention. The VU and ECR data were plotted against normative data obtained in a companion study of 58 strictly defined normal ears (Rosowski et al. 2011).
Results
Small increases in |ECR|2 at low-to-mid frequencies (400–1000 Hz) were observed in cases with stapes fixation, while narrow-band decreases were seen for both SCD and ossicular discontinuity. The SCD and ossicular discontinuity differed in that the SCD had smaller decreases at mid-frequency (~1000 Hz), while ossicular discontinuity had larger decreases at lower frequencies (500–800 Hz). SCD tended to have less air-bone gap at high frequencies (1–4 kHz) compared to stapes fixation and ossicular discontinuity. The |ECR|2 measurements, in conjunction with audiometry, could successfully separate 28 of the 31 cases into the three pathologies. By comparison, VU measurements, in conjunction with audiometry, could successfully separate various pathologies in 29 of 31 cases.
Conclusions
The combination of |ECR|2 with audiometry showed clinical utility in the differential diagnosis of conductive hearing loss in the presence of an intact TM and an aerated middle ear, and appears to be of similar sensitivity and specificity to measurements of VU plus audiometry. Additional research is needed to expand upon these promising preliminary results.
INTRODUCTION
It is common to see patients in an otologic practice who have conductive hearing loss (defined as an air-bone gap on audiometry) in the presence of an intact, healthy tympanic membrane (TM) and an aerated middle ear. The vast majority of such cases result from one of three pathologic conditions: ossicular fixation, ossicular discontinuity, or a third window lesion of the inner ear (Merchant and Rosowski 2010). In the general clinic most cases of ossicular fixation result from a fixed stapes due to otosclerosis, while the majority of third window lesions are patients with superior semicircular canal dehiscence (SCD). The most common site of ossicular discontinuity is between the incus and stapes; the discontinuity may be complete, with no contact between the disconnected ends, or partial, wherein normal bony continuity is replaced by a band of fibrous tissue.
In contemporary clinical practice, there are no diagnostic tests that can reliably differentiate between the pathologic conditions (described above) responsible for conductive hearing loss. The size and frequency-dependence of the air-bone gap on standard audiometry shows large variations within and among these disorders, so that the audiogram alone cannot provide a differential diagnosis (Rappaport and Provencal 2002). Stapes fixation, partial discontinuity and SCD cannot be reliably distinguished by tympanometry (Harford 1980, Fowler and Shanks 2002). A computed tomographic (CT) scan is of diagnostic value for third window lesions such as SCD, but its resolution at present is not sufficient to permit reliable diagnosis of ossicular pathology (Chakers and Augustyn 2003). High-resolution CT is also relatively expensive and involves exposure of patients to radiation; hence it is not practical to subject every patient with conductive hearing loss to a CT scan. Presence or absence of acoustic reflex is useful to distinguish ossicular disorders from third-window lesions (Minor 2005), but it does not help differentiate between ossicular fixation and discontinuity. Some patients have absent acoustic reflex despite normal middle-ear function (Mukerji et al. 2010), thus a third-window lesion can be missed. Vestibular evoked myogenic potential (VEMP) testing is helpful in diagnosing SCD (Minor 2005), but cannot differentiate or diagnose ossicular pathologies; moreover, VEMP testing is not widely available.
For patients with air-bone gaps, normal tympanic membrane and aerated middle ear, a diagnostic test that differentiates among these disorders and that can be used in an office setting would be of clinical value. Patients with a third window lesion would be spared unnecessary surgical exploration of the middle ear. Furthermore, pre-surgical knowledge of the type of ossicular pathology likely to be encountered at surgery (e.g., stapes fixation versus ossicular discontinuity) would help an otologist in preoperative counseling of patients regarding surgical risks and in preoperative planning.
In 1999, Polytec Inc. (Waldbronn, Germany) introduced its HLV-1000 laser Doppler vibrometer (LDV) as a new diagnostic tool for measurement of sound-induced umbo velocity (VU). The umbo is the most inferior part of the mannubrium of the malleus that is attached near the center of the pars tensa of the tympanic membrane in humans. Our group and others have demonstrated that VU measurement, coupled with audiometry, enables reliable pre-surgical differentiation among ossicular fixations, ossicular discontinuity and third window disorders (Goode et al. 1996; Jorge-Rodriguez et al. 1997; Huber et al. 2001; Rosowski et al. 2003, 2008). Otologists from our institution and others in the Boston area often refer patients with conductive hearing loss to our laboratory for diagnostic testing using VU measurements and pre-surgical assessment. However, there are practical limitations with VU measurements. The LDV systems that are commercially available are not FDA approved for clinical application and are therefore restricted to research laboratories, such as ours. Measuring VU requires a clinician with comfort and experience in oto-microscopy, i.e. skill is required to successfully and consistently aim the laser on the umbo. Furthermore, a second professional is typically needed to make VU measurements: a trained computer operator to gather and evaluate the data. The relatively high cost (~ $100,000) of the Polytec device is an additional limiting factor.
Ear-canal reflectance (ECR) is a variation of clinical acoustic immittance testing that has been described by many investigators (Keefe et al. 1993; Voss and Allen 1994; Hunter and Margolis 1997; Puria and Allen, 1998; Feeney and Keefe, 1999, 2001; Feeney et al. 2003; Feeney and Sanford, 2004; Allen et al. 2005). Power reflectance, the square of the magnitude of ECR (|ECR|2) is the fraction of the incident acoustic power that is reflected by the tympanic membrane back into the ear canal (see the companion paper for a detailed description, Rosowski et al. 2011). ECR measurements can be performed with a commercially-available, relatively inexpensive device (~ $10,000 for the Mimosa device) that received FDA approval in 2006 for clinical use; the device has been used by several groups (Allen et al. 2005; Shahnaz and Bork, 2006; Van der Werff et al. 2007; Withnel et al. 2009; Hunter et al. 2010; Voss et al. 2008). The Mimosa device requires minimal training and ECR measurements can be performed by a single individual. We have used this device to make ECR measurements in normal and pathological ears over the course of the past 18 months. As described in a companion paper (Rosowski et al. 2011), a practical difficulty we faced with the Mimosa device was calibrating the foam inserts. However, once calibration was successful, the measurements were straightforward and simple to perform.
Power reflectance has shown promise in the differential diagnosis of some middle ear disorders. For example, Feeney et al. (2003) presented |ECR|2 data from two patients with stapes fixation due to otosclerosis and one patient with ossicular discontinuity showing distinct diagnostic patterns of |ECR|2 in the two conditions. Allen et al. (2005) presented data from a patient with bilateral stapes fixation due to otosclerosis. Shahnaz et al. (2009) presented data from 28 patients with stapes fixation due to otosclerosis, and described how |ECR|2 was of diagnostic value in differentiating otosclerotic ears from normal ears. However, it has not been determined whether |ECR|2 can perform well in differentiating among various pathologies, as would be desired in a clinical setting.
The goal of the present study was to investigate the clinical utility of |ECR|2 measurements in a population of patients with conductive hearing loss (due to different etiologies) in the presence of an intact, healthy TM and an aerated middle ear. We also sought to compare the diagnostic accuracy of VU measurements (which we have been using for many years) and measurements of |ECR|2 in the same patients.
MATERIAL AND METHODS
This prospective study was approved by the institutional review board of the Massachusetts Eye and Ear Infirmary (MEEI). The material consisted of 104 patients referred to our laboratory for VU and ECR measurements from the Otology Clinic at MEEI between November 2009 and February 2011. To be included in the present analysis, patients had to meet the following criteria: (1) an air bone gap of more than 10 dB on pure-tone audiometry; (2) an intact and healthy tympanic membrane consistent with an aerated middle ear on otoscopic examination; (3) a diagnosis of stapes fixation, ossicular discontinuity or SCD made subsequent to our measurements, as described below. For middle-ear disease, the air-bone gap was averaged over 500, 1000 and 2000 Hz. For SCD, air-bone gap was calculated from 250, 500 and 1000 Hz because SCD mainly affects low frequencies (Mikulec et al. 2004, Merchant et al. 2007, Merchant & Rosowski 2008).
Ears with stapes fixation were included if the diagnosis was confirmed at subsequent surgery, and the post-stapedectomy air-bone gap was less than 10 dB averaged over the frequencies 500, 1000, and 2000 Hz (thus ensuring that other potentially confounding pathologies such as malleus fixation or a third window lesion were not responsible for some of the hearing loss). Ears with ossicular discontinuity were included if the diagnosis was confirmed at subsequent surgery. Ears with SCD were included if a high-resolution CT scan confirmed a dehiscence and if VEMP thresholds were abnormally sensitive (Minor 2005). Fourteen ears with stapes fixation due to otosclerosis, 6 ears with ossicular discontinuity (4 with complete, and 2 with partial discontinuity), and 11 ears with SCD met the criteria for inclusion and were analyzed in detail in the present study (a total of 31 ears). Among the 31 ears, the age range was 22 to 72 years; 15 were males and 16 were females. There were 11 right and 20 left ears.
Of the 73 patients that were not included in this study, 19 patients did not have conductive hearing loss. History of previous surgery excluded 16 patients (8 tympanoplasty, 7 stapedectomy, 1 mastoidectomy). Diagnosis was not confirmed in 22 patients (surgery had not been performed in 12 patients, post-op air-bone-gap did not close or follow-up did not occur in 10 patients). Nine patients had tympanic membrane pathologies evident on otoscopy, 3 had mixed hearing loss with a large sensorineural component and 4 were pediatric patients.
Measurements of ECR were made for 0.2 – 6 kHz at 60 dB SPL using the Mimosa Acoustics HearID system, and measurements of VU were made for 0.3 – 6 kHz at 70 – 90 dB SPL using the HLV-1000 laser Doppler vibrometer (Polytec Inc) in our laboratory. Generally, before patients were referred to our laboratory, patients underwent pure-tone audiometry for 0.25 – 8 kHz (Interacoustic Equinox audiometers) and standard 226 Hz tympanometry (GSI Tympstar Middle Ear Analyzers) performed in a sound booth by various audiologists. Exceptions included 3 ears with stapes fixation that did not undergo tympanometry because tympanometry had not been ordered by the patients’ otologist as part of their diagnostic work-up. The details of our measurement techniques for VU and ECR are described in the companion paper (Rosowski et al. 2011). We analyzed |ECR|2 as well as the absorbance level = 10 × log10(1 − |ECR|2). |ECR|2 describes the fraction of the incident power that is reflected at the TM. The absorbance level is the dB descriptor of the fraction of incident power that is absorbed. The VU and |ECR|2 data were plotted against normative data obtained in the companion study of 58 strictly defined normal ears (Rosowski et al. 2011).
RESULTS
Stapes fixation
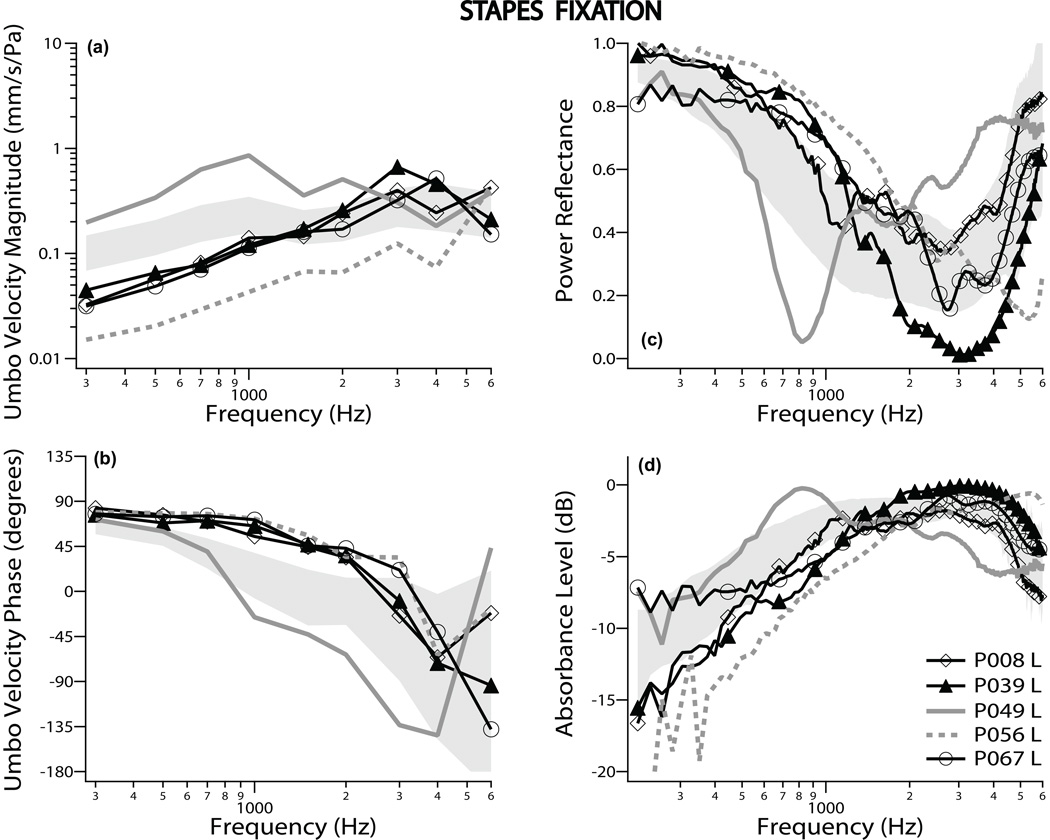
Figures 1a and b show VU measurements from 5 representative ears that include the largest [P049L] and smallest [P056L] VU magnitudes (gray lines) among all 14 ears found to have stapes fixation. The gray shaded regions in Fig. 1 show normative data of ± one standard deviation (SD) around the mean. Most VU magnitudes in ears with fixed stapes were lower than the normal-hearing average at low frequencies, and the VU phase tended to stay closer to 90 degrees at frequencies below 3 kHz as compared to normal. These results, indicative of a stiffer system, are similar to those previously published by us (Rosowski et al. 2008), and are also similar to VU data after experimental stapes fixation in cadaveric temporal bones (Nakajima et al. 2004, 2005).
Figure 1.
Umbo velocity and ear-canal reflectance data for stapes fixation: Umbo velocity magnitude and phase referenced to ear-canal pressure are shown in panels (a) and (b), respectively. Power reflectance data are shown in panel (c), while absorbance level is plotted in panel (d). All four panels display results from 5 representative ears, out of 14 measured. The 5 cases include the two ears (gray lines) that showed the most extreme measurements. The shaded region represents normative data of ± one standard deviation around the normal mean.
|ECR|2 data for the same 5 representative patients are plotted in Fig. 1c. Generally, the |ECR|2 was higher than normal at low frequencies (400–1000 Hz), indicative of a stiffer system. We also observed an inverse relationship between VU and power reflectance. The ear with largest VU at frequencies less than 3 kHz (P049L) had the lowest |ECR|2 between 0.4 and 1.5 kHz. The ear with the lowest VU magnitude (P056L) had the highest |ECR|2 over the same frequency range. The absorbance level is plotted in Fig. 1d for the same 5 ears, and had a tendency to be lower than the normal-hearing mean at low frequencies. Generally, the VU, |ECR|2 and absorbance level showed results that were consistent with a stiffer system, except for ear P049L which had a more compliant system than the normal mean. The two ears, [P049L, the most compliant] and [P056L, the most stiff] were consistently the two extreme outliers for all three measurements, VU, |ECR|2 and absorbance level. The most compliant ear [P049 L] had a tympanogram that was normal, and the stiffest ear [P056L] had a round-shaped tympanogram, consistent with a stiffer-than-normal system.
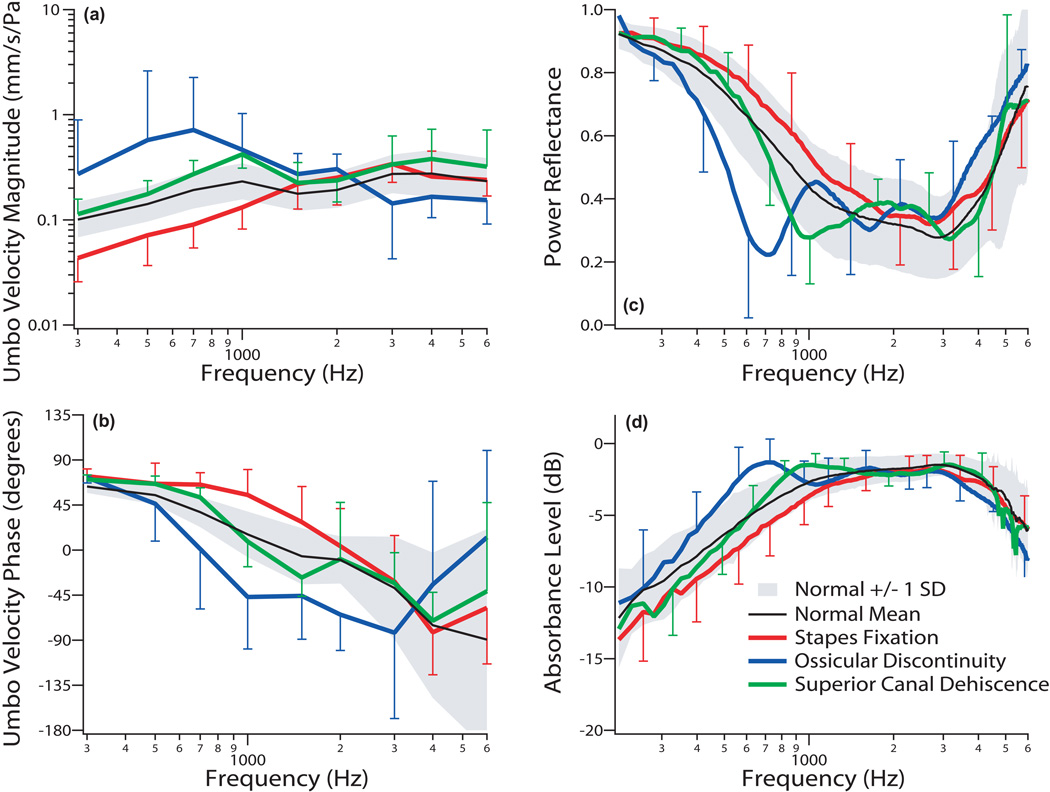
The mean ± one SD of the VU measurements for all 14 patients with stapes fixation are plotted in Fig. 4a & b with red lines, revealing an average reduction in VU magnitude of approximately 2 standard deviations from normal mean for frequencies below 1 kHz. The average phase was near 90 degrees at higher frequencies compared to normal. Both magnitude and phase data are consistent with a stiffer system. Mean |ECR|2 showed a small increase and mean absorbance level showed a small decrease for frequencies below 1 kHz compared to the normal-hearing mean, as shown in Figs. 4 c & d (red lines). The |ECR|2 results are similar to Shahnaz et al. (2009). There are large regions of overlap between the mean ±1 SD ranges of the normal and fixed-stapes measurements in all four panels of Figure 4.
Figure 4.
The mean ±1 standard deviation (SD) of umbo velocity and power reflectance data for all 31 ears in the study are shown, displayed separately by diagnosis. There were 14 ears with stapes fixation (red), 6 ears with ossicular discontinuity (blue), and 11 ears with superior canal dehiscence (green). Umbo velocity magnitude and phase referenced to ear-canal pressure are shown in panels (a) and (b), respectively. Power reflectance data are shown in panel (c), while absorbance level is plotted in panel (d). The shaded region represents normative data of ± 1 SD around the normal mean (black line).
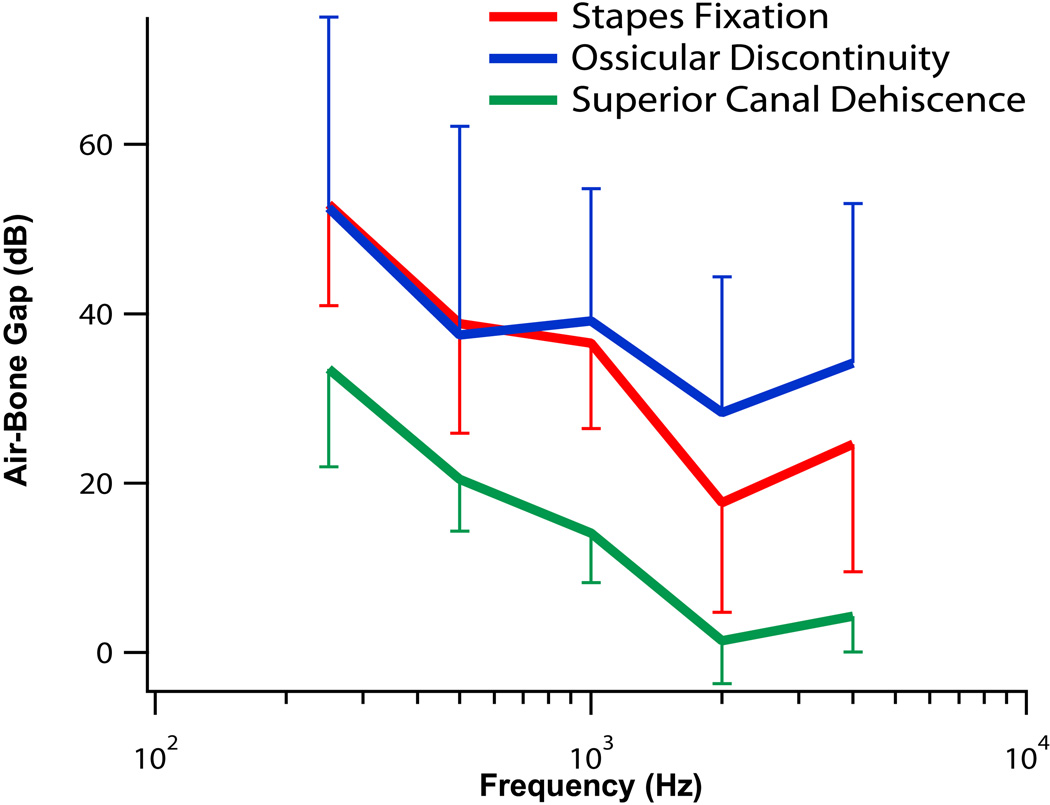
The mean of the air-bone gaps are plotted in Fig. 5. Stapes fixation (red line) showed large conductive losses (40 – 60 dB) at low frequencies, with smaller air-bone gaps at high frequencies (~20 dB). Of the 11 patients who underwent tympanometry, 10 had normal tympanograms; one ear [P056L] had a round-shaped tympanogram, consistent with a tympanic membrane and/or ossicular chain stiffer than normal.
Figure 5.
The mean ±1 standard deviations of the air-bone gaps versus frequency for the 3 pathologic conditions are displayed. There were 14 ears with stapes fixation (red), 6 ears with ossicular discontinuity (blue), and 11 ears with superior canal dehiscence (green).
Ossicular Discontinuity
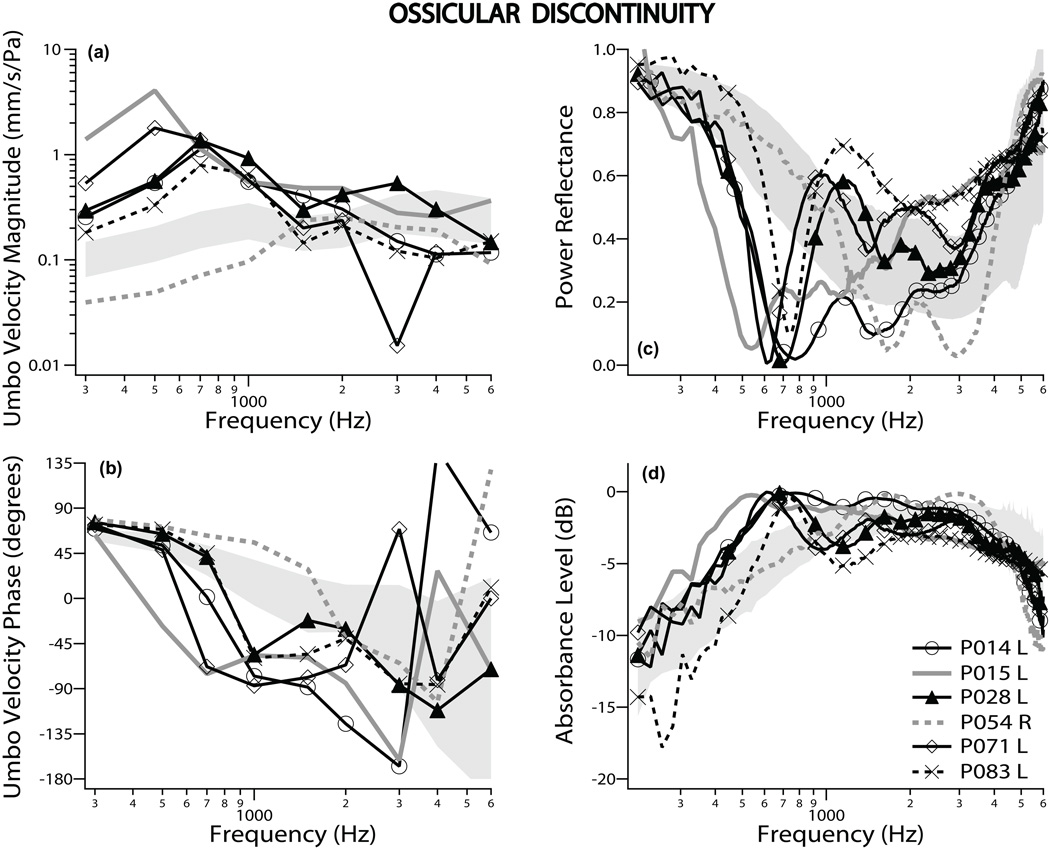
There were 6 ears with discontinuity of the ossicular chain. Five of the 6 had normal tympanograms; one ear [P054R] had an abnormally round-shaped tympanogram, consistent with a middle-ear system stiffer than normal. Figures 2a and b show plots of the VU magnitudes for all 6 ears with ossicular discontinuity, with gray lines to indicate the most extreme outliers. Five out of the 6 individuals showed an increase of VU magnitude of more than two standard deviations from normal mean at frequencies below 1 kHz, and VU phase that decreased abruptly around 400–800 Hz, similar to previous results (Rosowski et al. 2008). One ear, (P054R, with a round-shaped tympanogram) had a low VU magnitude without an abrupt decrease in phase, consistent with a stiffened system. Four ears had complete (solid lines) and two had partial (dashed lines) ossicular discontinuity. There was a tendency for VU magnitude to be higher and phase to change abruptly at lower frequencies for complete discontinuity as compared to partial discontinuity.
Figure 2.
Umbo velocity and ear-canal reflectance data for ossicular discontinuity: Umbo velocity magnitude and phase referenced to ear-canal pressure are shown in panels (a) and (b), respectively. Power reflectance data are shown in panel (c), while absorbance level is plotted in panel (d). All four panels display results from all 6 ears measured. The two ears that showed the most extreme measurements are plotted as gray lines. Solid lines signify complete discontinuity, while the dashed lines partial discontinuity. The shaded region represents normative data of ± one standard deviation around the normal mean.
ECR measurements showed a notch in |ECR|2 and a peak in absorbance level between 500 Hz and 800 Hz in 5 of the 6 ears with discontinuity (Figs. 2c and d). These results are similar to that published for one case by Feeney et al. (2003) and temporal bone studies by Feeney et al. (2009). The large notch in |ECR|2 and peak in absorbance level are consistent with a compliant system. The one ear with the round-shaped (stiffer) tympanogram (P054R, gray dotted line) did not have this notch or peak in the |ECR|2 or absorbance level, respectively. This was the same ear in which the VU was of low magnitude and phase that stayed near 90 degrees for higher frequencies, consistent with a stiffer system. |ECR|2 for complete discontinuity (solid lines) was lower than for partial discontinuity (dashed lines) around 500–600 Hz. Measurements of VU, |ECR|2 and absorbance level were consistent in ear P054R (with the rounded tympanogram peak): All three measurements showed a stiffer than normal characteristic.
Figures 4a and b show the mean VU for the 6 ears with ossicular discontinuity (blue lines); the mean magnitude was more than 2 standard deviations above the normal mean below 1 kHz, while phase decreased abruptly at low frequencies. The mean |ECR|2 (Figs. 4c and d) of these 6 ears in the area of the notch (500–800 Hz) was more than two SD below that of normal subjects. Thus, both VU and |ECR|2 measurements by themselves can generally separate ears with discontinuity of the ossicular chain from normal ears. The larger standard deviation around 600–800 Hz is due to the variation in frequency of the notch; this variation also widens the shape of the average notch as compared to the individual recordings.
The mean air-bone gap for ossicular discontinuity is plotted in Fig. 5 with a blue line. Similar to stapes fixation, ossicular discontinuity resulted in large air-bone gaps (40–60 dB) at low frequencies. However, at higher frequencies the air-bone gap for ossicular discontinuity was larger than for stapes fixation. For the 2 patients with partial discontinuity, the average air-bone gap across the frequency range of 250 – 4000 Hz was 20 dB and 17 dB. For the 4 patients with complete discontinuity, the average air-bone gap across the frequency range of 250 – 4000 Hz was higher, ranging from 40 to 59 dB. Although the number of ears is small, this data suggests that the air-bone gap is dependent on the degree of discontinuity.
Superior Semicircular Canal Dehiscence (SCD)
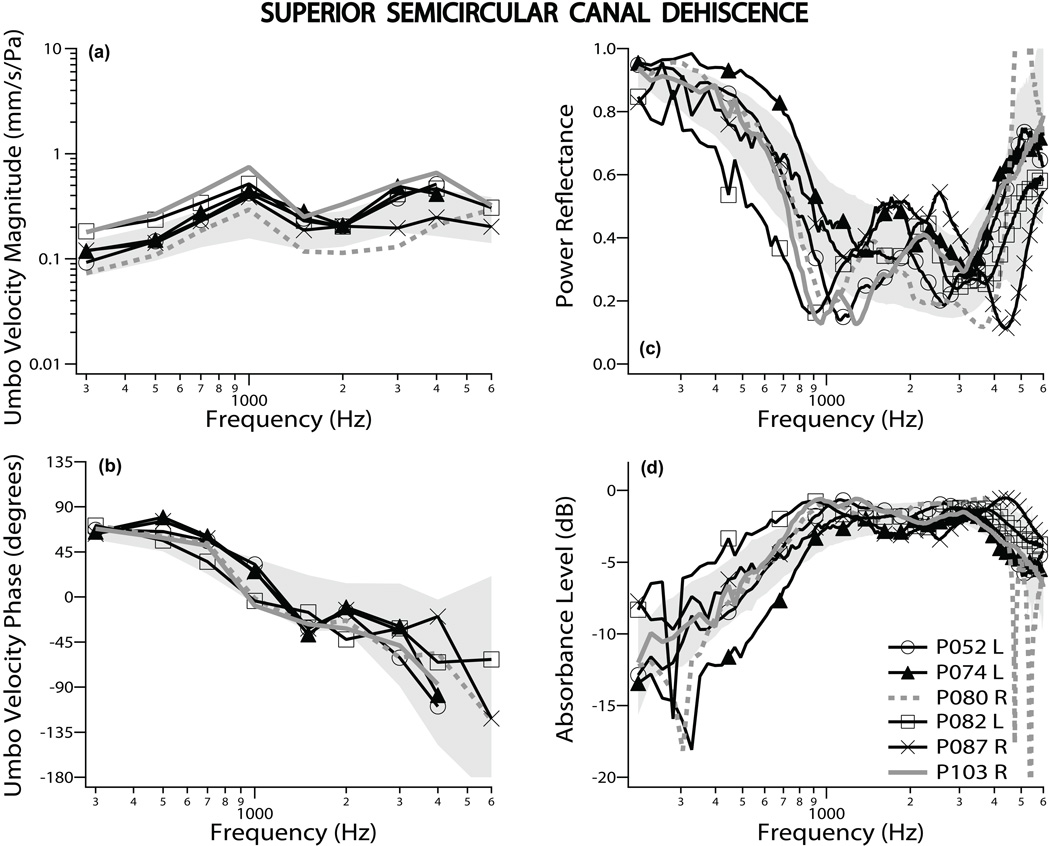
All 11 ears with SCD had normal tympanograms. Figure 3 shows 6 representative ears with SCD, including the most extreme magnitudes of VU [P103R, P080R plotted with gray lines] and |ECR|2 [P074L, P082L]. Note that unlike stapes-fixation or ossicular interruption, the ears with extreme measurement values in VU were not extreme in power reflectance. Most measurements of VU had a magnitude larger than the mean and a phase somewhat smaller than the mean at frequencies near and below 1 kHz, similar to previous data (Rosowski et al. 2008). The |ECR|2 measurements showed a notch with a value about two standard deviations below the normal mean at frequencies near 1000 Hz.
Figure 3.
Umbo velocity and ear-canal reflectance data for superior semicircular canal dehiscence: Umbo velocity magnitude and phase referenced to ear-canal pressure are shown in panels (a) and (b), respectively. Power reflectance data are shown in panel (c), while absorbance level is plotted in panel (d). All four panels display results from 6 representative ears, out of 11 measured. The gray lines depict the two ears that showed the most extreme umbo velocity magnitudes. The shaded region represents normative data of ± one standard deviation around the normal mean.
The mean SCD data for all 11 ears are plotted in Fig. 4, showing that SCD generally caused an increase in VU magnitude and a more abrupt decrease in VU phase around 1 kHz compared to the normal mean. SCD also caused a notch/decrease in |ECR|2 or peak/increase in absorbance level at around 1 kHz. Because of the overlap between normal and pathologic results, VU measurements in ears with SCD were only slightly different from those in normal ears. |ECR|2 measurements near 750–1000 Hz in SCD were more distinguishable from normal on average, but there was also significant overlap between normal and SCD ears. In general, the differences in VU and power reflectance that we observe between normal and SCD ears are reduced versions of the differences observed between normals and ears with ossicular interruption, with smaller changes in VU magnitude, VU angle, |ECR|2 and absorbance level extending to higher frequencies for SCD compared to ossicular discontinuity.
The air-bone gaps experienced by SCD patients were more pronounced at low frequencies than at high frequencies (Fig. 5), and were smaller (by about 20 dB) when compared to the gaps produced by fixation or interruption at the same frequency.
DISCUSSION
We made pre-diagnostic measurements comprising audiometry, tympanometry, VU and |ECR|2 in 31 patients with conductive hearing loss. These 31 patients were ultimately diagnosed as having stapes fixation, ossicular discontinuity or superior canal dehiscence as the cause of their air-bone gap. The three pathologies investigated in the study are representative of the majority of cases of conductive hearing losses seen in patients who have a healthy tympanic membrane and an aerated middle ear on otoscopic exam.
The data showed large overlaps in the size and frequency dependence of air-bone gaps within and between the three pathologies, so that audiometry by itself did not permit accurate preoperative diagnosis of the etiology for the conductive loss. Similarly, tympanometry was not sensitive in diagnosing the ears studied: 10 of 11 ears with fixed stapes, 5 of 6 with ossicular discontinuity and all 11 ears with SCD had normal tympanograms. Furthermore, the one ear with an abnormal tympanogram and interrupted ossicular chain actually had a round-shaped tympanogram, consistent with a stiffer middle ear, which would suggest fixation rather than the true diagnosis. As can be seen in Fig. 4, although VU and |ECR|2 showed a clearly distinctive pattern for ossicular discontinuity, there was sufficient overlap between the fixed stapes and SCD cases such that neither VU nor |ECR|2 alone could serve as a highly sensitive and specifc stand-alone diagnostic tool to separate out the three pathologic conditions.
In the results section and Figs. 1–4, we compared our measurements on ears with conductive hearing loss to the “normal” data from the companion paper. These comparisons are important to understand how the frequency responses of mechanical measurements differ. It will also be important to compare such data with future temporal bone experiments and computational modeling studies to understand details of the mechanisms contributing to various pathologies. Such comparison of data from pathological ears to data from normal ears is useful to answer basic science questions.
However, in the clinical setting, patients can be readily separated into those with normal hearing and those with conductive hearing loss by standard audiometry. The task that is difficult for otologists is to differentiate among etiologies responsible for conductive hearing loss that have a normal-appearing TM and an aerated middle ear (it is not necessary clinically to have a new test to differentiate pathological ears from normal-hearing ears because standard audiometry already works). Hence, we have focused on developing diagnostic algorithms using tools such as VU and ECR to differentiate between various pathologies responsible for conductive hearing loss.
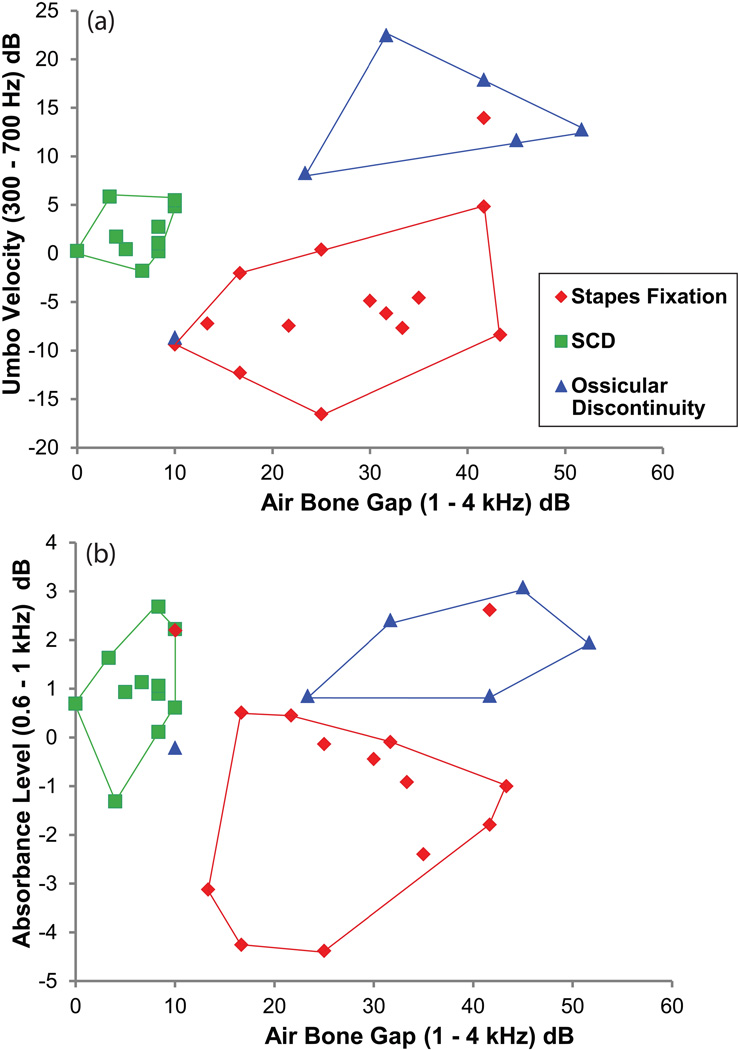
We have shown in the past that VU measurements in conjunction with audiometric data can differentiate various pathologies that cause conductive hearing loss (Rosowski et al. 2008). We performed a similar analysis for the dataset in the present study. In Figure 6, we show plots of umbo velocity (in 6a) or absorbance level (in 6b) versus the air-bone gap in all 31 ears. In Fig. 6a, for each ear, VU magnitudes measured for the ears with conductive hearing loss normalized to the VU magnitudes of normal-hearing mean (averaged over 300 – 700 Hz) were plotted against the air-bone gap (averaged over 1 – 4 kHz). For example, looking at one of the curves for SCD in Fig. 3a, the curve was first referenced (subtracted in dB) to the mean VU magnitudes measured on normal-hearing ears (described in the companion paper). Then the referenced VU magnitudes were averaged over 300–700 Hz (in dB). This value was plotted against the air-bone gap averaged over 1–4 kHz, resulting in the SCD square data points in Fig. 6a. The different symbols represent each of the three pathologies. It can be appreciated from the boundaries drawn that 29 of the 31 ears were easily separated into the three pathological groups. Exceptions included one ear with a fixed stapes that fell into the ossicular discontinuity area, and one ossicular discontinuity in the stapes fixation area. The choice of the 1 – 4 kHz range for the average air-bone gap was dictated by the range where we saw large differences between the three pathologies. Similarly, the VU magnitudes had the most separation between the three pathologies between 300 – 700 Hz.
Figure 6.
In top panel (a), Umbo velocity magnitudes referenced to the normal-hearing mean (averaged over 300–700 Hz) are plotted against the air bone gap (averaged over 1–4 kHz). The different symbols and colors represent each of the three pathologies. All three disorders were separable from each other for 29/31 ears. Exceptions included one ear with a fixed stapes in the ossicular discontinuity area, and one ossicular discontinuity in the stapes fixation area.
Bottom panel (b) shows absorbance level measurements where each ear was referenced to the normal-hearing mean (averaged over 0.6–1 kHz), and plotted against the air-bone gap (averaged over 1–4 kHz). Using absorbance level in combination with the air-bone gap also allowed separation of the 3 pathologies for 28/31 ears. There were 3 exceptions: 1 stapes fixation in the area bounded by ossicular discontinuity, 1 ossicular discontinuity not bounded by a particular pathology but in-between the SCD and stapes fixation area, and 1 stapes fixation that was at the border of the SCD area.
A similar scatter plot (Fig. 6b) was made for absorbance level measurements where each ear was referenced to the mean of the absorbance level measured in normal-hearing subjects (obtained from the companion paper), which was averaged over 0.6 – 1 kHz, and plotted against the air-bone gap (averaged over 1 – 4 kHz). Figure 4d demonstrates that absorbance level data for the three pathologies were most different from each other in the 0.6 – 1 kHz range. Using absorbance level in combination with the air-bone gap also separated 28 of the 31 ears into the three pathological groups. There were 3 exceptions: 1 stapes fixation fell into the area bounded by ossicular discontinuity, 1 ossicular discontinuity fell between the SCD and stapes fixation area, and 1 stapes fixation fell on the border of the SCD area. The fixed stapes ear that was in the ossicular discontinuity area for both the VU and absorbance level scatter plots was ear [P049L], which had VU and absorbance level measurements consistent with a compliant middle ear (Fig. 1, discussed in the results section). The ossicular discontinuity case, found at the lower-left corner of the stapes fixation area in the VU scatter plot (Fig. 6a) and between the SCD and stapes fixation area in the absorbance level scatter plot (Fib. 6b), was ear [P054R], which had the tympanogram, VU and absorbance level measurements consistent with a stiffer system (Fig. 2, discussed in the results section).
The simple analysis shown in Fig. 6 illustrates that either VU or absorbance level, in conjunction with audiometric data, was able to correctly identify nearly all of the ears into the three different pathologies. The computed sensitivity and specificity based on the two-dimensional projections of Figure 6 are listed in Table 1. In the definition of stapes fixation, umbo velocity had slightly higher sensitivity but lower specificity than absorbance level. For ossicular discontinuity, umbo velocity and absorbance level had the same sensitivity and specificity. For SCD, umbo velocity had the same sensitivity, but slightly higher specificity than absorbance level. Overall, for both types of diagnostic measurements, the range of sensitivity was 83–100% and specificity 94–100%.
Table 1.
Sensitivity and Specificity of Umbo Velocity and Absorbance Level Measurements
| Umbo Velocity with Air-Bone Gap Measurements | ||
|---|---|---|
| Pathology | Sensitivity | Specificity |
| Stapes Fixation | 93% | 94% |
| Ossicular Discontinuity | 83% | 96% |
| SCD | 100% | 100% |
| Absorbance Level with Air-Bone Gap Measurements | ||
| Pathology | Sensitivity | Specificity |
| Stapes Fixation | 86% | 100% |
| Ossicular Discontinuity | 83% | 96% |
| SCD | 100% | 95% |
The diagnostic scheme shown in Figure 6 used a two-dimensional approach where the separation of the 3 pathologies was based on the combination of air-bone gap and absorbance level or umbo velocity. Close inspection of Figure 6 should make it obvious that a large fraction of the sensitivity and specificity of the two-dimensional groupings came from our choice of the average air-bone gap for the 1–4 kHz range as the x-coordinate. This choice separated many of the SCD ears from the others. Without this separation along the x-coordinate, there is significantly more overlap between the different groups, especially in the absorbance level scores. Such dependence on the mid- and high-frequency air-bone gap would create difficulty in accurate diagnosis of a hypothetical patient with early stapes fixation and a small air-bone gap, whose audiogram would be more like the mean of our SCD population than the mean of our mature pre-operative fixed-stapes population.
Because of the larger overlap in the absorbance level values of the three groups plotted in Figure 6b, use of the air bone gap is more critical for absorbance level than for umbo velocity in these simple diagnostic schemes. However, the simple schemes we illustrate may not be the most sensitive or specific. An alternative scheme that needs evaluation depends on the differences in the frequency dependence of the reflectance scores for the three pathologies. Reflectance in SCD shows a notch (a narrow-band decrease) around 1000 Hz, ossicular discontinuity shows a deeper notch at lower frequencies, while stapes fixation shows a slight increase at mid-to-low frequencies (see Figure 4c). Thus, more refined analytical methods, such as determining the frequency of the notch and integrating to calculate the notch depth, as well as quantifying the low-frequency increase in power reflectance, may yield more sensitive and specific separations. Future work in this direction may increase diagnostic accuracy, especially for a larger patient population with possibly a larger variety of responses for each pathological condition.
This preliminary study demonstrates that ECR measurements can aid in the differential diagnosis of pathologies causing conductive hearing loss in the setting of an intact and healthy TM and an aerated middle ear by otoscopy. Additional measurements are needed using a greater number of patients, so as to further test and refine these preliminary results. Future research is also needed to determine the diagnostic value of ECR measurements in other types of middle ear disorders such as malleus fixation, perforations and other abnormalities of the tympanic membrane, and effusions of the middle ear.
CONCLUSIONS
There is clinical value in being able to pre-surgically diagnose the cause of a conductive hearing loss in the setting of an intact tympanic membrane and an aerated middle ear. The frequency dependence of the power reflectance measurements was different for the three pathologies: stapes fixation resulted in small increase from normal at low-to-mid frequencies, while large narrow-band decreases from normal were seen for ossicular discontinuity (between 500–800 Hz) and SCD (around 1 kHz). SCD tended to produce a smaller air-bone gap at high frequencies compared to stapes fixation and ossicular discontinuity. Power reflectance measurements with audiometry showed clinical utility in the differential diagnosis of these disorders, exhibiting high sensitivity and specificity, similar to the combination of umbo velocity and audiometry. Additional research is needed to further investigate these promising preliminary results.
ACKNOWLEDGMENTS
We thank our otology colleagues – Drs. Michael McKenna, Dan Lee, Jennifer Smullen and Ron DeVencia – who referred and helped to recruit their patients for this work. Our audiologic colleagues performed the auditory testing and contributed to the analysis of the audiometric data. We are also thankful to Susan Voss who provided MATLAB software to aid in the analysis of ear-canal reflectance measurements. Supported by NIH grant R01 DC004798 and a donation from Mr. Lakshmi Mittal.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Allen J, Jeng P, Levitt H. Evaluation of human middle ear function via an acoustic power assessment. J Rehabil Res Dev. 2005;42:63–78. doi: 10.1682/jrrd.2005.04.0064. [DOI] [PubMed] [Google Scholar]
- Chakers DW, Augustyn MA. Temporal bone imaging. In: Som PM, Curtin HD, editors. Head and Neck Imaging. 4th Edition. St. Louis, MO: Mosby; 2003. pp. 1093–1108. [Google Scholar]
- Feeney M, Grant I, Marryott L. Wideband energy reflectance in adults with middle-ear disorders. J Speech Lang Hear Res. 2003;46:901–911. doi: 10.1044/1092-4388(2003/070). [DOI] [PubMed] [Google Scholar]
- Feeney MP, Grant IL, Mills DM. Wideband energy reflectance measurements of ossicular chain discontinuity and repair in human temporal bone. Ear Hear. 2009;30:391–400. doi: 10.1097/AUD.0b013e3181a283ed. [DOI] [PubMed] [Google Scholar]
- Feeney MP, Keefe DH. Acoustic reflex detection using wide-band acoustic reflectance, admittance, and power measurements. J Speech Lang Hear Res. 1999;42:1029–1041. doi: 10.1044/jslhr.4205.1029. [DOI] [PubMed] [Google Scholar]
- Feeney MP, Keefe DH. Estimating the acoustic reflex threshold from wideband measures of reflectance, admittance, and power. Ear Hear. 2001;22:316–332. doi: 10.1097/00003446-200108000-00006. [DOI] [PubMed] [Google Scholar]
- Feeney M, Sanford C. Age effects in the human middle ear: Wideband acoustical measures. J Acoust Soc Am. 2004;116:3546–3558. doi: 10.1121/1.1808221. [DOI] [PubMed] [Google Scholar]
- Fowler CG, Shanks JE. Tympanometry. In: Katz J, Burkard RF, Medwetsky L, editors. Handbook of Clinical Audiology. 5th Edition. Philadelphia, PA: Lippincott, Williams and Wilkins; 2002. pp. 175–204. [Google Scholar]
- Goode RL, Ball G, Nishihara S, et al. Laser Doppler vibrometer (LDV) a new clinical tool for the otologist. Am J Otol. 1996;17:813–822. [PubMed] [Google Scholar]
- Harford E. Tympanometry. In: Jerger J, Northern J, editors. Clinical Impedance Audiometry. 2nd Edition. Acton, MA: American Electromedics Corp; 1980. pp. 40–64. [Google Scholar]
- Huber AM, Schwab C, Linder T, et al. Evaluation of eardrum laser doppler interferometry as a diagnostic tool. The Laryngoscope. 2001;111:501–507. doi: 10.1097/00005537-200103000-00022. [DOI] [PubMed] [Google Scholar]
- Hunter LL, Feeney MP, Lapsley Miller JA, et al. Wideband Reflectance in Newborns: Normative Regions and Relationship to Hearing-Screening Results. Ear Hear. 2010;31:599–610. doi: 10.1097/AUD.0b013e3181e40ca7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter LL, Margolis RH. Effects of tympanic membrane abnormalities on auditory function. J Am Acad Audiol. 1997;8:431–446. [PubMed] [Google Scholar]
- Jorge-Rodriguez J, Zenner HP, Hemmert W, et al. Laser vibrometry. A middle ear and cochlear analyzer for noninvasive studies of middle and inner ear function disorders. HNO. 1997;45:997–1007. doi: 10.1007/s001060050185. [DOI] [PubMed] [Google Scholar]
- Keefe DH, Bulen JC, Arehart KH, et al. Ear-canal impedance and reflection coefficient in human infants and adults. J Acoust Soc Am. 1993;94:2617–2638. doi: 10.1121/1.407347. [DOI] [PubMed] [Google Scholar]
- Merchant SN, Rosowski JJ, McKenna MJ. Superior semicircular canal dehiscence mimicking otosclerotic hearing loss. Adv Otorhinolaryngol. 2007;65:137–145. doi: 10.1159/000098790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant SN, Rosowski JJ. Conductive hearing loss caused by third-window lesions of the inner ear. Otol Neurotol. 2008;29(3):282–289. doi: 10.1097/mao.0b013e318161ab24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant SN, Rosowski JJ. Acoustics and Mechanics of the Middle Ear. In: Gulya AJ, Minor LB, Poe DS, editors. Glasscock-Shambaugh Surgery of the Ear. 6th Edition. Shelton, CT: People's Medical Publishing House-USA; 2010. pp. 49–72. [Google Scholar]
- Mikulec AA, McKenna MJ, Ramsey MJ, et al. Superior semicircular canal dehiscence presenting as conductive hearing loss without vertigo. Otol Neurotol. 2004;25(2):121–129. doi: 10.1097/00129492-200403000-00007. [DOI] [PubMed] [Google Scholar]
- Minor LB. Clinical manifestations of superior semicircular canal dehiscence. The Laryngoscope. 2005;115:1717–1727. doi: 10.1097/01.mlg.0000178324.55729.b7. [DOI] [PubMed] [Google Scholar]
- Mukerji S, Windsor AM, Lee DJ. Auditory brainstem circuits that mediate the middle ear muscle reflex. Trends in Amplification. 14:170–191. doi: 10.1177/1084713810381771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima HH, Ravicz ME, Merchant SN, et al. Experimental ossicular fixations and the middle ear’s response to sound: evidence for a flexible ossicular chain. Hearing Research. 2005;204:60–77. doi: 10.1016/j.heares.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Nakajima HH, Ravicz ME, Rosowski JJ, et al. Experimental and clinical studies of malleus fixation. Laryngoscope. 2004;115:147–154. doi: 10.1097/01.mlg.0000150692.23506.b7. [DOI] [PubMed] [Google Scholar]
- Puria S, Allen JB. Measurements and model of the cat middle ear: evidence of tympanic membrane acoustic delay. J Acoust Soc Am. 1998;104:3463–3481. doi: 10.1121/1.423930. [DOI] [PubMed] [Google Scholar]
- Rappaport JM, Provencal C. Neurotology for audiologists. In: Katz J, Burkard RF, Medwetsky L, editors. Handbook of Clinical Audiology. 5th Edition. Philadelphia, PA: Lippincott, Williams and Wilkins; 2002. pp. 9–32. [Google Scholar]
- Rosowski JJ, Mehta RP, Merchant SN. Diagnostic utility of laser doppler vibrometry in conductive hearing loss with normal tympanic membrane. Otol Neurotol. 2003;24:165–175. doi: 10.1097/00129492-200303000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosowski JJ, Nakajima HH, Hamade MA, et al. Energy reflectance, umbo velocity and tympanometry in normal hearing adults. Ear Hear. 2011 doi: 10.1097/AUD.0b013e31822ccb76. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosowski JJ, Nakajima HH, Merchant SN. Clinical utility of laser-doppler vibrometer measurements in live normal and pathologic human ears. Ear Hear. 2008;29:3–19. doi: 10.1097/AUD.0b013e31815d63a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahnaz N, Bork K. Wideband reflectance norms for Caucasian and Chinese young adults. Ear Hear. 2006;27:774–788. doi: 10.1097/01.aud.0000240568.00816.4a. [DOI] [PubMed] [Google Scholar]
- Shahnaz N, Bork K, Polka L, et al. Energy reflectance and tympanometry in normal and otosclerotic ears. Ear Hear. 2009;30:219–233. doi: 10.1097/AUD.0b013e3181976a14. [DOI] [PubMed] [Google Scholar]
- Van der Werff KR, Prieve BA, Georgantas LM. Test-retest reliability of wideband reflectance measures in infants under screening and diagnostic test conditions. Ear Hear. 2007;28:669–681. doi: 10.1097/AUD.0b013e31812f71b1. [DOI] [PubMed] [Google Scholar]
- Voss SE, Allen J. Measurement of acoustic impedance and reflectance in the human ear canal. J Acoust Soc Am. 1994;95:372–384. doi: 10.1121/1.408329. [DOI] [PubMed] [Google Scholar]
- Voss SE, Horton NJ, Woodbury RR, et al. Sources of variability in reflectance measurements on normal cadaver ears. Ear Hear. 2008;29:651–655. doi: 10.1097/AUD.0b013e318174f07c. [DOI] [PubMed] [Google Scholar]
- Withnell RH, Jeng PS, Waldvogel K, et al. An in situ calibration for hearing thresholds. J Acoust Soc Am. 2009;125:1605–1611. doi: 10.1121/1.3075551. [DOI] [PubMed] [Google Scholar]