Abstract
Mitochondria are involved in cellular functions that go beyond the traditional role of these organelles as the power plants of the cell. Mitochondria have been implicated in several human diseases, including cardiac dysfunction, and play a role in the aging process. Many aspects of our knowledge of mitochondria stem from studies performed on the isolated organelle. Their relative inaccessibility imposes experimental difficulties to study mitochondria in their natural environment – the cytosol of intact cells – and has hampered a comprehensive understanding of the plethora of mitochondrial functions. Here we review currently available methods to study mitochondrial function in intact cardiomyocytes. These methods primarily use different flavors of fluorescent dyes and genetically encoded fluorescent proteins in conjunction with high-resolution imaging techniques. We review methods to study mitochondrial morphology, mitochondrial membrane potential, Ca2+ and Na+ signaling, mitochondrial pH regulation, redox state and ROS production, NO signaling, oxygen consumption, ATP generation and the activity of the mitochondrial permeability transition pore. Where appropriate we complement this review on intact myocytes with seminal studies that were performed on isolated mitochondria, permeabilized cells, and in whole hearts.
Keywords: Cardiomyocytes, Mitochondrial function, Methods
Introduction: Mitochondrial structure and function
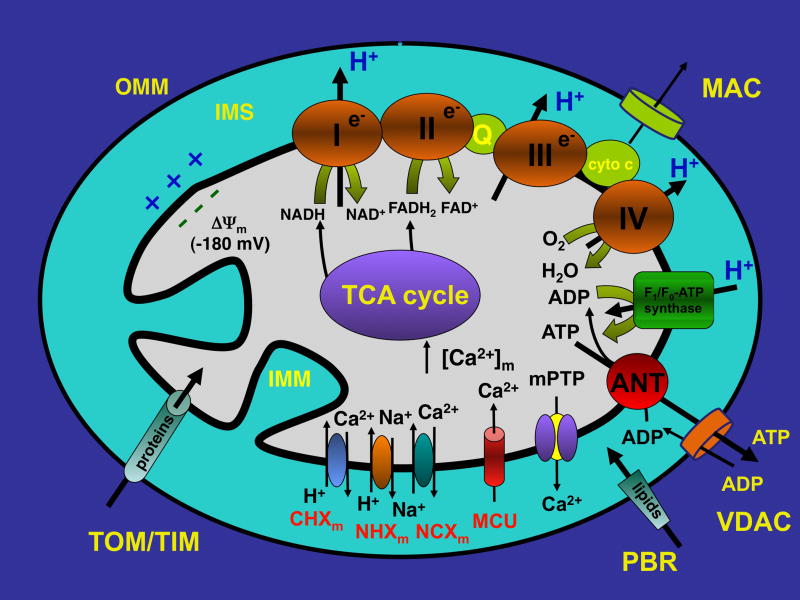
Mitochondria are cytosolic double-membrane organelles, which for their ability to generate ATP for most of the cellular energy demands, are often referred to as the ‘power plants’ of the cell [1, 2]. However, mitochondria are also involved in a range of other processes, such as signaling, cellular ion homeostasis, oxidative stress, apoptotic and necrotic cell death, as well as the control of cell cycle and cell growth [3]. The cellular number of mitochondria varies widely by species, cell and tissue type. An adult ventricular myocyte contains ~7000 mitochondria, which occupy ~35% of the cell volume [4, 5] to match the high energy demands of these cells. Mitochondria dynamically change their morphology through the processes of mitochondrial fusion and fission to form an extensive interconnected mitochondrial network or a fragmented discrete phenotype [6–9]. Indeed, the name “mitochondrion” originating from the Greek words “mitos” (thread), and “chondrion” (grain or granule) reflects the heterogeneity of mitochondrial morphology. In adult cardiomyocytes, the size, shape and metabolic activity of mitochondria also depend on intracellular location. Three subpopulations of mitochondria in the adult heart have been identified as interfibrillar, subsarcolemmal and perinuclear mitochondria [7, 8, 10]. Interfibrillar mitochondria are aligned in longitudinal rows between myofibrils [4, 8, 10] in close proximity to sarcoplasmic reticulum (SR) Ca2+ release sites [10]. They often span a single sarcomere from Z-band to Z-band and are relatively uniform in size and shape (rod-shaped organelles 0.5–1 μm in width and 1–2 μm in length) [8, 10]. Subsarcolemmal and perinuclear mitochondria appear less organized and more variable in shape and size [8, 10], possibly as a result of less restraint fission and fusion compared to interfibrillar mitochondria [7, 8]. In contrast to adult myocytes, mitochondria of neonatal cardiomyocytes are organized in extensive cytoplasmic membrane networks undergoing continuous fission, fusion, and movement rather than individual rod-shaped organelles [8]. Mitochondria composed of compartments that carry out specialized functions: the outer mitochondrial membrane (OMM), the intermembrane space (IMS), the inner mitochondrial membrane (IMM) with the cristae and the matrix (Figure 1). Mitochondria contain their own genome that is distinct from the genome of the cell. The OMM encloses the entire organelle but is freely permeable to molecules of up to 5000 daltons due to the presence of pores (about 2–3 nm) formed by the Voltage-Dependent Anion Channel (VDAC). VDAC is the most abundant protein of the OMM and is present in 3 distinct isoforms in eukaryotic cells (VDAC1, VDAC2 and VDAC3) [11, 12]. VDAC is involved in transporting metabolites, including ADP and ATP, between mitochondria and cytosol, and in its ‘closed confirmation’ it maintains a pore of ~1.8 angstroms diameter, that permits passage of protons and other ions [13], making the concentration of small molecules such as ions and sugars in the IMS similar to the cytosol. Although all three VDAC isoforms are equivalent in allowing mitochondrial Ca2+ loading upon IP3-releasing agonist stimulation in HeLa cells, silencing of VDAC1 selectively impairs the transfer of a low-amplitude apoptotic (e.g., oxidative stress in form of 1 mM H2O2) Ca2+ signal to mitochondria [14]. Larger molecules like proteins, however, can only cross the OMM by active transport through mitochondrial membrane transport proteins making the IMS a compartment that contains a distinct set of proteins including cytochrome c. The vast majority of proteins destined for the mitochondrial matrix are encoded in the nucleus and synthesized outside mitochondria. Mitochondrial protein import involves the TIM/TOM complex (TIM: Transporter Inner Membrane; TOM: Transporter Outer Membrane) [15, 16]. Besides their protein transport role, members of this translocation machinery also participate in processes leading to apoptosis. For example, the Peripheral Benzodiazepine Receptor (PBR, also known as translocator protein of the outer membrane or TSPO) of the OMM serves the cholesterol transport and steroid synthesis [17], but is also involved in OMM permeabilization in apoptosis in conjunction with the pro-apoptotic Bcl family of proteins [18]. Members of the Bcl-2 protein family regulate apoptosis by controlling the formation of the Mitochondrial Apoptosis-Induced Channel (MAC, see Figure 1) in the OMM in response to certain apoptotic stimuli [19] where the pro-apoptotic members Bax and/or Bak form MAC [19, 20], and the anti-apoptotic members Bcl-2 or Bcl-xL prevent MAC formation. MAC formation is an early marker of the onset of apoptosis [21, 22] and MAC activation mediates the release of cytochrome c to the cytosol, triggering the commitment step of the mitochondrial apoptotic cascade. Pharmacological inhibition (e.g. with Bax Channel Inhibitors [23] and MAC inhibitors [24]) or genetically knocking down main components of MAC result in prevention of cytochrome c release and apoptosis.
Figure 1. Schematic overview of mitochondrial structure and transport mechanisms.
For details and abbreviations see text.
In striking contrast to the OMM, the IMM is highly impermeant. Ions, metabolites and proteins require specialized membrane transporters and exchangers to access the matrix compartment. The TIM translocase complex is responsible for protein import and metabolite passage. The IMM forms internal compartments called cristae. The IMM contains the phopholipid Cardiolipin which significantly reduces the IMM permeability to protons and therefore allows to establish a proton-motive force across the IMM. The proton-motive force is generated by the complexes of the IMM respiratory chain (or electron transport chain, ETC) which uses free energy released during electron transport to translocate protons (H+) from the mitochondrial matrix into the IMS. The main components of the ETC are complexes I to IV, coenzyme Q and cytochrome c. The proton-motive force is composed of the mitochondrial membrane potential (ΔΨm) which arises from the net movement of positive charges across the IMM, and the pH gradient. Influx of protons back into the matrix is mediated by the F1F0-ATP synthase. This multi-subunit protein complex includes a H+ channel coupled to an ATP synthase which generates ATP. ΔΨm across the IMM is highly negative (~−180 mV), due to the chemiosmotic gradient for protons across the IMM. The proton gradient provides the energy for ATP synthesis by the respiratory chain. ΔΨm also provides the driving force for Ca2+ uptake into mitochondria by the Ca2+-uniporter (MCU) [25–29] which has been identified recently as a 40-kDa protein of the IMM [28, 29]. Increases in matrix Ca2+ concentration ([Ca2+]m) enhance the activities of ATP synthase and enzymes of the tricarboxylic acid (TCA) cycle, thus stimulating ATP production rates [30]. However cytosolic Ca2+ overload and excessive Ca2+ uptake by the MCU can depolarize mitochondria and limit Ca2+ entry, but may also abolish the driving force for H+ entry and ATP synthase function [31]. Matrix acidification by the H+ flow through the ATP synthase required for ATP synthesis also inhibits the MCU and thereby allows to maintain [Ca2+]m at optimal levels [32, 33]. In order to maintain mitochondrial Ca2+ homeostasis, Ca2+ uptake is balanced by Ca2+ extrusion primarily via mitochondrial Na+/Ca2+ exchanger (NCXm), with a small contribution of Ca2+/H+ exchanger and Na+/H+ exchanger which serves as the pathway for Na+ extrusion from the matrix [25, 26]. Elevated levels of matrix Ca2+ and reactive oxygen species (ROS) activate the mitochondrial permeability transition pore (mPTP) leading to necrotic or apoptotic cell death [25, 34]. The irreversible mPTP opening is associated with a collapse of ΔΨm, release of cytochrome c and perhaps more ROS generation, resulting in a vicious cycle of further amplification of ROS production, mitochondrial Ca2+ overload, and increasingly irreversible cell damage [35]. While it has been postulated that the mPTP mediates the lethal permeability changes of the mitochondrial membranes leading to mitochondria-mediated cell death, recent studies indicate that brief transient openings of the mPTP (cf. Figure 2 and [36, 37]) can provide protection against cellular injury by serving as a physiological Ca2+ release mechanism during mitochondrial Ca2+ overload [38–41]. A rise of intramitochondrial Ca2+ can also activate a putative mitochondrial nitric oxide synthase (mtNOS) which can generate NO locally but also can become as source of ROS under certain pathological conditions [26, 42]. Even though the existence of the mitochondrial nitric oxide synthase (mtNOS) in cardiac mitochondria is still controversial and the enzyme has not been identified at the protein level (see [26, 43–46] for reviews), several functional studies demonstrated that an increase in mitochondrial Ca2+ uptake could generate local NO [42, 46–53] which can serve as a protective mechanism by inhibiting mitochondrial Ca2+ accumulation and respiration.
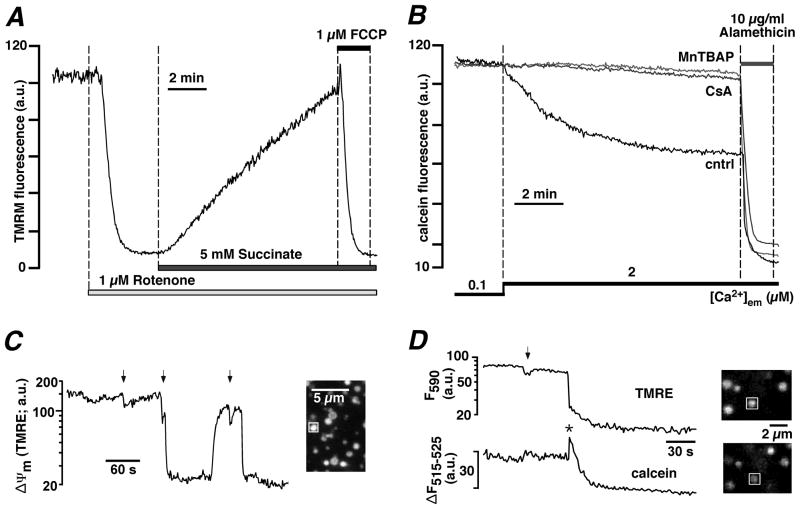
Figure 2. Measurements ofΔΨm and mPTP activity in permeabilized ventricular myocytes and isolated mitochondria.
A, ΔΨm monitored with the membrane potential sensitive dye TMRM in permeabilized rabbit ventricular myocytes upon inhibition of ETC complex I with rotenone and subsequent exposure to ETC complex II substrate succinate. The mitochondrial uncoupler FCCP completely depolarized Ψm. B, mPTP activity monitored with mitochondria entrapped calcein (620 Da) in permeabilized cat ventricular myocytes. Opening of mPTP was induced by enhanced ROS formation (prevented by superoxide and peroxynitrite scavenger MnTBAP, 50 μM) resulting from Ca2+-induced stimulation of mtNOS in the absence of L-arginine. Opening of mPTP resulted in the loss of calcein and a decrease of fluorescence. The pore forming antibiotic alamethicin induced maximum calcein release. Cyclosporine A (CsA, 5 μM) blocked mPTP opening. C, mPTP openings recorded from isolated mitochondria with TMRE. PTP opening can occur as brief incomplete (arrows) and prolonged maximal depolarizations. D, simultaneous monitoring of mPTP activity as changes in ΔΨm (TMRE) and loss of calcein in isolated mitochondria. Brief incomplete depolarizations (arrow) did not cause loss of calcein, however prolonged and complete depolarizations were accompanied by loss of calcein. The decline of calcein fluorescence was preceded by a transient increase (asterisk) caused by calcein dequenching. (Panel B: from Dedkova and Blatter, The Journal of Physiology, 2009, with permission; panels C and D: from Hüser, Rechenmacher and Blatter, Biophysical Journal, 1998, with permission).
Methods to measure mitochondrial function
In the following paragraphs we will review methods to study mitochondrial morphology, mitochondrial membrane potential, Ca2+ and Na+ signaling, mitochondrial pH regulation, redox state and ROS production, NO signaling, oxygen consumption, ATP generation and the activity of the mitochondrial permeability transition pore. This review focuses on studies performed on intact cardiac myocytes. Considering the fact that single cell studies have their own inherent limitations (e.g. elimination of putative interactions with cells of the surrounding tissue, differences in oxygen and metabolite supply) we complement, where appropriate, this review with seminal studies that were performed on isolated mitochondria, permeabilized cells, and in whole hearts.
Measurement of mitochondrial morphology
Given the relatively rigid and well-defined arrangement of mitochondria in the adult heart (see above), it may be difficult to appreciate the relevance of changes in mitochondrial morphology to this organ. This characteristic arrangement of mitochondria develops during cardiac maturation, since in neonatal cardiomyocytes [8] mitochondria are organized in filamentous networks similar to many other non-cardiac cell types [54]. Emerging evidence suggests that changes in mitochondrial morphology may be relevant to various aspects of cardiovascular biology, including cardiac development [55], the response to ischemia-reperfusion injury [56], heart failure [57], diabetes mellitus [55, 58] and apoptosis [59]. Traditionally the vast majority of our knowledge about mitochondrial morphology stems from electron-microscopic studies (see [8, 10] for detailed reviews). However, this method only provides a snapshot in space and time, and cannot visualize mitochondrial dynamics directly. However, several novel techniques have been developed recently to monitor dynamic changes in mitochondrial morphology, fission and fusion. For example, mitochondrial fusion has been studied dynamically with an assay based on mitochondrial expression of red and green fluorescent proteins and monitoring of matrix content mixing after fusion [60]. More recently, a quantitative assay for mitochondrial fusion based on the Renilla luciferase complementation assay has been described [61]. The process of mitochondrial fusion can bee tracked also using a photoactivatable mitochondrial green fluorescent protein (PA-GFPmt) [62]. PA-GFPmt is nonfluorescent but can be activated in a highly localized manner by ultraviolet illumination. The photoactivated GFP diffuses rapidly within the entire mitochondrial matrix and can be therefore be used to tag individual mitochondria. In adult murine myocytes infected with adenovirus to deliver photoactivatable mitochondrial-targeted PA-GFP [56] elongated interfibrillar mitochondria could be identified. The length of these elongated mitochondria ranged from 2 to 6 μm (corresponding to 1 to 3 sarcomeres). Cell treatment with mitochondrial division inhibitor-1, a pharmacological inhibitor of the mitochondrial fission protein dynamin-related protein 1 (Drp1), increased the percentage of the elongated mitochondria approximately fourfold [56]. Moreover, inhibition of mitochondrial division reduced cell death and inhibited mPTP opening after simulated ischemia-reperfusion, and in vivo treatment with this inhibitor reduced myocardial infarct size in mice after coronary artery occlusion and reperfusion [56] suggesting that inhibition of the mitochondrial fission could provide a novel pharmacological approach for cardioprotection.
Measurement of mitochondrial membrane potential
In intact cells the mitochondrial matrix compartment is not accessible with standard electrophysiological techniques using sharp or patch-clamp microelectrodes. Therefore, ΔΨm is typically estimated with fluorescent voltage-sensitive dyes which are membrane-permeant fluorescent lipophilic cationic compounds that distribute across the IMM [63–67] following the Nernst equation. The most commonly used compounds are rhodamine 123 (Rho123), its derivatives tetramethylrhodamine methyl and ethyl esters (TMRM and TMRE) (Figure 2), and the carbocyanine compound JC-1 [68, 69]. While in principle the use of these voltage-sensitive indicators is straight forward, there are several caveats to consider when interpreting results obtained in intact cells. First, the dye must be kept in all superfusion solution thoughout the experiment in order to maintain its equilibrium distribution. Otherwise, with each solution change, dye will leak out of the cell, resulting in changes in fluorescence that are independent of in ΔΨm [70]. For cardiomyocytes, it is necessary to load cells with dyes for 30–60 min at 37°C in order to assure appropriate mitochondrial localization [70, 71]. Dye concentration should be kept in the low nanomolar range (5–50 nM for TMRE and TMRM, for example). Even when cells are loaded with low (nanomolar) concentrations of the dye, its concentration in mitochondria can reach several hundred micromolar [72]. TMRM, for example, can concentrate about 10 fold across the plasma membrane and 400–600 fold across the mitochondria membrane (with ΔΨm ~ −180 mV) [70, 71]. Moreover, the concentration of these dyes in mitochondria not only depends on ΔΨm but may also be affected by changes in the volume of these organelles, e.g. when mitochondrial swelling occurs. Mitochondrial movement in and out of the focal plane (e.g. due to fusion and fission, see above) can pose another problem for correct ΔΨm monitoring. It is possible to correct for movement artifacts by applying a ratiometric approach. For example, the simultaneous loading of mitochondria with targeted PA-GFPm (a matrix-targeted protein used in mitochondrial morphology studies, see above) and TMRE [62] has been used to measure ΔΨm ratiometrically and to circumvent movement-dependent changes in TMRE fluorescence [62]. The simultaneous presence of two fluorophores, however can potentially be an additional source of error resulting from interactions between the two dyes (see below). Additionally, many of these dyes exhibit significant binding to mitochondrial structures and enhanced mitochondrial accumulation which causes deviations from Nernstian behavior [67, 73]. Dyes can leak out of mitochondria when mPTP opening occurs, due to simple diffusion rather than partition in accordance with membrane potential. All these factors should be considered when ΔΨm calibration is made (see [62, 67, 72] for details). In intact cells, ΔΨm measurements are complicated by the role that the plasma membrane plays for the accumulation and distribution of the probe. First, it has been shown that cells expressing plasma membrane glycoproteins responsible for multidrug resistance (MDR) have a limited ability to accumulate TMRM and JC-1 [74] (an issue to keep in mind when comparing different cell types, cardiac cell lines and species). All potentiometric dyes are good substrates for MDR carriers, which will export the dye from the cells leading to a decreased dye accumulation [70]. Typically, in cells expressing MDR, dye loading will be extremely slow and can be greatly enhanced by treatment with MDR inhibitors (progesterone, verapamin, and cyclosprorin A) [70, 74]. Second, the cytosolic dye concentration is also affected by the plasma membrane potential which drives, together with ΔΨm, the distribution of the dye between mitochondrial matrix, cytosol and extracellular space [71]. Typically, upon mitochondrial depolarization, TMRM is released from mitochondria to the cytosol, causing a decrease of mitochondrial fluorescence and an increase of cytosolic fluorescence without any significant changes of the signal averaged over the whole cell. Increased cytosolic TMRM will redistribute into the extracellular solution if the depolarization persists for a sufficiently long period of time [70] leading to a decrease of whole cell fluorescence, or back into mitochondria if depolarization is transient [40] without any significant changes in whole cell fluorescence. Along the same line changes in plasma membrane potential will also cause a redistribution of TMRM from cytosol to the extracellular buffer and may cause a change in the mitochondrial fluorescence signal, even though ΔΨm remains unchanged.
Voltage-sensitive dyes have also been used at higher concentration to monitor ΔΨm (e.g. 1–20 μM TMRM loaded for 10–15 min with subsequent washout) non-confocally from whole cells [70]. In this case mitochondrial fluorophore accumulation leads to formation of non-fluorescent dye aggregates [75], called J-aggregates [63, 67], that causes fluorescence quenching. J-aggregate formation is not unique to TMRM and has also been observed with Rho123 and other fluorophores. Depolarization of ΔΨm and subsequent redistribution of TMRM out of the matrix compartment results in TMRM ‘de-quench’ and a fluorescence increase reflecting the average change in ΔΨm [70, 71, 76]. In this quench/de-quench mode small depolarization in ΔΨm induced much larger (2-order of magnitude) changes in TMRM fluorescence compared to that obtained in redistribution mode (low concentration approach) [77]. However, caution should be used when applying the de-quench approach in combination with confocal imaging. Laser illumination of TMRM loaded in mitochondria at high concentrations generates ROS from the fluorescent probe that provokes mPTP opening [37, 76]. (This ‘side effect’ has actually been used as experimental model for simulation of oxidative stress and to induce mPTP opening observed during ischemia-reperfusion [76, 78] rather than for ΔΨm monitoring).
Monitoring ΔΨm has also been achieved successfully in intact heart. This may be of particular importance for studying mitochondrial function in the diseased heart. Despite a number of technical difficulties related to the visualization of mitochondrial function in intact heart, optical mapping in ex vivo perfused guinea pig [79, 80] and rat [81–83] hearts has been performed. Using real time 2-photon [79–81] or high-resolution CCD camera [82, 83] imaging, complex spatiotemporal heterogeneities in ΔΨm, together with complex spatial patterns (e.g. ΔΨm waves) and dynamic fluctuations in ΔΨm could be observed during global ischemia.
Overall, the different available voltage-sensitive dyes have been used in cardiac tissue with variable success. Rho123 has turned out to be problematic since mitochondrial uncouplers failed to induce any changes in ΔΨm in cardiomyocytes [70, 84]. Moreover, Rho123 was reported to inhibit ADP-stimulated respiration and mitochondrial ATP synthase at high matrix concentrations [85]. JC-1 was first reported in adult cardiac myocytes to measure ΔΨm during anoxia and reoxygenation [86]. JC-1 emits light at red and green wavelengths depending on concentration. At high concentrations J-aggregates form and emit red light, whereas at low concentration the monomer form emits green light [86]. While, in principle JC-1 offers the advantage to measure ΔΨm ratiometrically by monitoring the ratio of green/red (530/590 nm) fluorescence [86], data obtained with JC-1 should also be interpreted with caution [70, 86]. J-aggregates are formed across the whole cell only when higher concentrations (0.5–1 μM) and longer loading times (30 min to 2 hours) were used. Otherwise J-aggregates tend to accumulate in the periphery only due to slow diffusion and redistribution rates of the dye [70, 74] which may preclude the detection of rapid changes in ΔΨm. Furthermore, the equilibrium between monomer and J-aggregates is also influenced by pH, osmolarity, and oxidative environment of the cells [87, 88]. Moreover, high concentrations of JC-1 can be toxic to mitochondria detected as formation of crystalline-like structures [70, 74]. As a rule, the use of high (more than 1 μM) concentrations of the potentiometric dyes should be avoided since they can cause metabolic inhibition [63]. Of commonly used fluorophores, TMRM exhibits the least mitochondrial toxicity [67]. To minimize phototoxicity and ensure a linear response, low concentrations of TMRM should be used in the dye re-distribution mode described above.
TMRM is often used in combination with other fluorescent dyes to monitor simultaneously additional mitochondrial parameters such as [Ca2+]m, mitochondrial ROS generation (with dichlorofluorescein diacetate, DCF) or the opening of the mPTP (with calcein). The simultaneous presence of two fluorophores can result in FRET phenomena (Förster resonance energy transfer or fluorescence resonance energy transfer) that can lead to erroneous interpretations of the results. Both DCF [80, 89] and calcein [37, 90] have been described as FRET partners of TMRM.
The plethora of limitations and drawbacks associated with the use of voltage-sensitive dyes also renders these indicators difficult to calibrate quantitatively (for discussion of signal calibration see e.g. (see [62, 67, 72]). Often experimental data with potentiometric probes are expressed as a percentage changes from basal levels. As a minimum recommendation it is suggested to expose cells to the protonophore FCCP (1–10 μM) at the end of an experiment to equilibrate H+ concentration in all compartments and therefore abolish the ΔΨm. This provides a minimum ΔΨm level that is required for signal normalization between different cells. An interesting suggestion for signal calibration takes into consideration the standard deviation of the measured fluorescence signal. Polarized mitochondria concentrate the dye into the organelle causing a bright punctate signal with a high standard deviation (SD) across the cell (i.e., much brighter signal over mitochondria compared to cytosolic signal) while depolarization causes a decrease in SD as the dye is evenly distributed throughout the cell (see [70] for more details). The ratio of mean fluorescence to SD (mean/SD) gives a quantitative measure of dye localization and can be used to calibrate ΔΨm.
Measurement of [Ca2+]m and mitochondrial Ca2+ transport
Different methodologies have been used to measure [Ca2+]m in intact living cardiomyocytes with fluorescent Ca2+ indicators. Typical strategies employed entrapping membrane-permeant forms of fluorescent Ca2+ indicators (typically acetoxymethyl (AM) esters) in the matrix compartment and subsequently eliminating the cytosolic contribution to the fluorescence signal. De-esterification by intracellular (cytosolic and intramitochondrial) esterases entraps the indicator in the respective compartments. However, the potential presence of cytosolic dye may confound or even preclude [Ca2+]m measurements. Cytosolic dye can be eliminated by accelerating extrusion at higher temperature [84, 91], perfusion of the cytosol or by quenching with Mn2+ [92–94] or Co2+ [71]. De-esterification at 37°C for several hours may preferentially eliminate cytosolic loading [95, 96], however it can also reduce the Ca2+ responsiveness of the dye [70, 71]. Cytosolic perfusion with dye-free pipette solution can be achieved using the whole cell patch clamp method [93, 97]. The cytosolic dye quench technique has been used successfully [92–94, 98, 99], however is potentially complicated by the fact that the MCU can transport Mn2+ (and thus quench matrix dye) and Mn2+ may interfere with mitochondrial Ca2+ transport [92, 100]. Among the available fluorescent Ca indicators, indo-1 [92–94, 98], fura-2 [99], fluo-3 [101], dihydrorhod-2 [102], rhod-2 [103] and X-rhod-1 [71] have been used for [Ca2+]m measurements. Rhodamine compounds (Rhod-2, Rhod-2FF, dihydrorhod-2 and X-rhod-1) were originally developed with the goal to measure [Ca2+]m selectively in mitochondria. This was based on the fact that esterification with AM results in a cationic molecule that will accumulate preferentially (but not exclusively!) in mitochondria due to the large negative ΔΨm and remain in the mitochondrial matrix after cleavage by intramitochondrial esterases. The Kd of rhod-2 for Ca2+ is relatively low (~ 570 nM), which can lead to dye saturation during mitochondrial Ca2+ overload, and is affected by changes in pH as they might occur during ischemia [104]. The related probe X-rhod-1 may be more suitable for measuring higher [Ca2+]m levels because of its higher Kd (~ 700 nM).
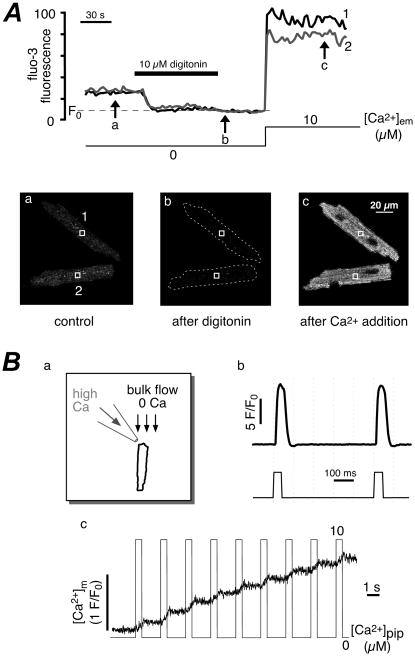
Moreover, it has been reported that rhod-2 and rhod-FF induced Ca2+-dependent inhibition of mitochondrial Ca2+ uptake since increase in [Ca2+]m was blunted during repetitive stimulation [105]. Also, changes in mitochondrial morphology and membrane potential, which were amplified by cell exposure to the excitation light, were obsereved after cell loading with these dyes at low concentrations (1–2 μM) [105]. At higher dye concentrations (>5 μM) cell damage occured, that partially could be explained by the generation of toxic byproduct induced by dye illumination. A common potential disadvantage of all rhodamin-derived indicators is their non-ratiometric nature, which make [Ca2+]m measurement susceptible to errors stemming from changes in dye concentration and motion artifacts. Classical ratiometric indicators like fura-2 [99] and indo-1 [92–94, 98] have been used to measure [Ca2+]m, however their relatively low Kd and the potential presence in the cytosol imposes serious limitations to the use of these indicators for [Ca2+]m measurements in intact cells. Despite their limitations and shortcomings conventional fluorescent Ca2+ indicators have been used to study the role of mitochondria for Ca2+ homeostasis in cardiac myocytes. For example, Sedova et al. [101] addressed the controversial question (for reviews see [26, 106]) whether [Ca2+]m reflects the cytosolic beat-to-beat Ca2+ transients, using a novel approach which imitates conditions of an intact cytosolic environment in membrane-permeabilized cells. Permeabilized cells have the unique advantage that the cytosolic environment can be controlled precisely while the arrangements and interactions between intracellular membranes and organelles (SR, mitochondria) remain structurally and functionally intact [107, 108]. [Ca2+]m was measured with fluo-3 trapped inside mitochondria and cytosolic indicator was removed by plasma membrane permeabilization with digitonin (Figure 3). Cells were exposed to rapid ejections of Ca2+ from a micropipette mimicking cytosolic Ca2+ transients. With this technique the lack of beat-to-beat mitochondrial Ca2+ spikes could be demonstrated.
Figure 3. Measurements of [Ca2+]m with compartmentalized fluo-3 in single permeabilized ventricular myocytes.
A, top: changes in fluo-3 fluorescence following membrane permeabilization with digitonin and increase in extramitochondrial [Ca2+] ([Ca2+]em). Images a to c (bottom) were taken at times indicated by arrows. B, changes of [Ca2+]m in response to rapid cytosolic Ca2+ transients. a and b, technique used to generate rapid changes in [Ca2+]em to simulate cytosolic Ca2+ transients. Cells were placed in the laminar flow of zero-Ca2+ solution (1 mM EGTA) and computer controlled pressure ejections of 10 μM Ca2+-containing solution from a glass micropipette. c, changes of [Ca2+]m in a permeabilized myocyte in response to stimulation with Ca2+ pulses applied at 0.5 Hz (pulse duration = 0.5 s; [Na+]em = 40 mM; [Ca2+]pip = 10 μM). (From Sedova, Dedkova & Blatter, Am. J. Physiol. Cell Physiol., 2006; Am. Physiol. Soc., used with permission)
Alternatively, [Ca2+]m can be measured using a fusion protein consisting of aequorin and a mitochondrial targeting sequence. Advantages of this chemiluminescence approach are its selectivity for measuring [Ca2+]m and the steadily improved spatial resolution of the technique [109, 110], however its applicability has been restricted to use with cultured cells (e.g. cultured neonatal rat ventricular myocytes [111]). In addition, the signal generated by the photoprotein is typically too weak to be monitored with single cell imaging [109–112]. Ca2+-dependent consumption of the photoprotein and the requirement of the exogenous cofactor co-elenterazine could cause problem during continuous monitoring of [Ca2+]m [109]. A similar technique (adenoviral infection with aqueorin targeted to cytosol and mitochondria) was employed in adult rat ventricular myocytes [112]. Using this technique it has been demonstrated that electrical stimulation at 2 Hz, [Ca2+]m increased simultaneously with [Ca2+]i, but the decay of [Ca2+]m was much slower. Adenoviral infection requires myocytes to be put into short-term culture, which might negatively affect the excitation-contraction coupling process due to structural and electrical remodeling.
Recent years have seen the development of novel mitochondrial Ca2+ probes that are specifically targeted to the mitochondrial matrix compartment by taking advantage of molecular biology techniques. Mt-pericam, a mitochondrial matrix targeted, circularly permuted green fluorescent protein fused to calmodulin and its target peptide M13 [113] was developed with the goal to monitor [Ca2+]m ratiometrically. At 405 nm excitation wavelength pericam emission is sensitive to changes in [Ca2+]m, however emission in response to excitation at 488 nm is sensitive to changes in pH [114]. Taking advantage of these properties it has been proposed that this ratiometric pericam can be used for simultaneous Ca2+ and H+ detection, since its Kd and pKa values are suitable for measuring mitochondrial [Ca2+] and pH [113]. Packaging the pericam into adenoviral vectors allowed for in vivo cardiac infection [115] and subsequent [Ca2+]m monitoring in mouse myocytes. Using this technique Belke et al.[115] clearly demonstrated that basal [Ca2+]m increased slowly from resting levels during electrical pacing at 1 Hz, however no distinct [Ca2+]m transients were observed.
The Ca2+-sensitive inverse pericam Mitycam [113] was targeted to mitochondria with a standard mitochondrial-targeting sequence (subunit VIII of human cytochrome c oxidase) and adenovirally expressed in cardiomyocytes [116]. Mitochondrial location of Mitycam was confirmed by the characteristic fluorescence pattern that overlapped with the signal form the mitochondrial marker MitoTracker Red, and by the sensitivity of the fluorescence signal to mitochondrial inhibitors. Mitycam was capable of reporting beat-to-beat mitochondrial Ca2+ transients.
Most recently, a novel fluorescent indicator for [Ca2+]m, GCaMP2-mt, was developed by adding a mitochondrial targeting sequence to a high signal-to-noise Ca2+ sensor protein GCaMP2 [117] to measure oxidant-induced responses of [Ca2+]m in cultured neonatal myocytes.
Several tools are routinely used to study mitochondrial Ca2+ transport. Mitochondrial Ca2+ uptake via MCU can be inhibited pharmacologically with ruthenium red [118–121] or Ru360 (a more MCU-specific ruthenium red derivative) [122–125], or by collapsing ΔΨm with the protonophore FCCP [126–128] to eliminate the driving force for uptake. Oligomycin [129–131] should be used in combination with FCCP to prevent ATP depletion by mitochondrial ATP synthase operating in the reverse mode. In addition, mitochondrial Ca2+ extrusion via NCXm can be blocked efficiently by clonazepam [132, 133] or by the more NCXm-specific benzothiazepine compound CGP-37157 [133–135].
Measurements of mitochondrial Na+ concentration
Through the action of mitochondrial and plasma membrane NCX mitochondrial and cytosolic Ca2+ and Na+ regulation is tightly interconnected. For example, [Na+]i raises significantly in heart failure [136] which stimulates NCXm and leads to reduced steady-state [Ca2+]m [97, 137] and presumably increased [Na+]m, decreased activity of Ca2+-activated dehydrogenases, hampered regeneration of NADH and NAD(P)H levels [137], to ROS generation [138] and energy starvation [97, 139] during excitation-contraction coupling. The majority of [Na+]m measurements was performed in isolated mitochondria [140] or permeabilized cells loaded with fluorescent Na+ indicators such as SBFI [141], Sodium Green [142] and CoroNa Green [143]. In rat ventricular myocytes loaded with SBFI [Na+]m was significantly lower than [Na+]i but increased after metabolic inhibition [141]. [Na+]m is ~5 mM which was about 2 times lower than [Na+]i reported in these cells [141], and similar to [Na+]m and [Na+]i estimates obtained with electron microprobe analysis [144, 145].
The CoroNa Red sodium indicator is a cationic dye that can be loaded into the cell without an acetoxymethyl ester groups [146, 147]. Because of its positive charge CoroNa Red is taken up preferentially by polarized mitochondria, and has been successfully utilized in intact renal epithelial cells [146] and astrocytes [147, 148].
Measurement of intramitochondrial pH
Mitochondrial matrix pH (pHm) has been difficult to study in intact cells and attempts have been made using the fluorescent pH indicators BCECF [149] or carboxy-SNARF [150], however measurements are complicated by cytosolic contamination of the fluorescence signal. Improvements come from Green Fluorescent Protein (GFP) mutants that emit light in a pH-dependent fashion [151] and can be directed to various organellar compartments by expression as fusion proteins linked to targeted peptides. One such mutant (pH-GFP) with a near-linear increase fluorescence response in the pH range 7.5–8.5, was directed to the mitochondrial matrix using an N-terminal COX-IV leader sequence and successfully used to measure matrix pH in apoptosis conditions [151, 152].
The recently reported ratiometric pericam used to monitor mitochondrial Ca2+ (as described above), also measures changes in mitochondrial pH independently of Ca2+ [114] thus allowing recording of mitochondrial Ca2+ and pH.
Measurement of mitochondrial redox state
In mitochondria, the major net energy conversion is based on transport of electrons released in the TCA cycle from reduced nicotinamide adenine dinucleotide (NADH) to molecular O2 via a series of catabolic reactions in the respiratory chain, whereby NADH is oxidized to NAD+. The respiratory chain converts the potential energy of the electrons into a proton gradient across the IMM, which drives ATP production. Mitochondrial NADH content is determined by NADH generation and NADH turnover. NADH is generated in the TCA cycle and by β-oxidation of fatty acid carnitine esters, and its turnover is determined by the rate of oxidative phosphorylation, which in turn is governed by ATP demand, NADH redox state, O2 supply [153], and the functional state of the respiratory chain complexes. Unlike its conjugate electron acceptor NAD+ (which is non-fluorescent) NADH is fluorescent (with emission maximum at 460 nm) upon excitation with near-ultraviolet light of 365 nm. Thus, NADH-fluorometry can be used to estimate mitochondrial NADH/NAD+ ratio. NADH is also present in cytosol, however in cardiac tissue the NADH fluorescence signal is primarily mitochondrial [154, 155], and contributions from NADPH (nicotinamide adenine dinucleotide phosphate, a cellular source for reducing equivalents used in protective redox reactions against ROS toxicity and in biosynthesis) are negligible, due to the fact that it is present in much smaller amounts [156], and because its fluorescence quantum yield is 3–4 times lower than that of NADH [157, 158]. When excited at 488 nm, the autofluorescence (520 nm) of cardiac cells arises predominantly from mitochondrial dehydrogenases containing flavin adenine dinucleotide (FAD), whose redox state is linked to that of mitochondrial NADH [159]. Thus, changes in flavoprotein fluorescence serve as endogenous local reporter of mitochondrial matrix redox potential (Figure 4). In contrast to NADH, it is the oxidized FAD that is fluorescent, such that the total fluorescence is inversely proportional to the ratio of reduced to oxidized flavoprotein. The flavoprotein signal decreases to a minimum in the presence of the cytochrome oxidase inhibitor cyanide (CN−), and increases to a maximum in the presence of the mitochondrial uncoupler FCCP, allowing an in-situ calibration of the redox signal. NADH/FAD autofluorescence measurements have been conducted successfully in intact hearts to monitor redox state at the organ level [160–163]. In the intact heart ischemia initially caused an increase in NADH autofluorescence and a decrease in FAD autofluorescence indicating a more reduced redox state during the onset of ischemia [160]. During late ischemia mitochondria became less reduced as revealed by the decline in NADH fluorescence without a significant change in FAD fluorescence. Both signals become highly oxidized, however, during reperfusion [160].
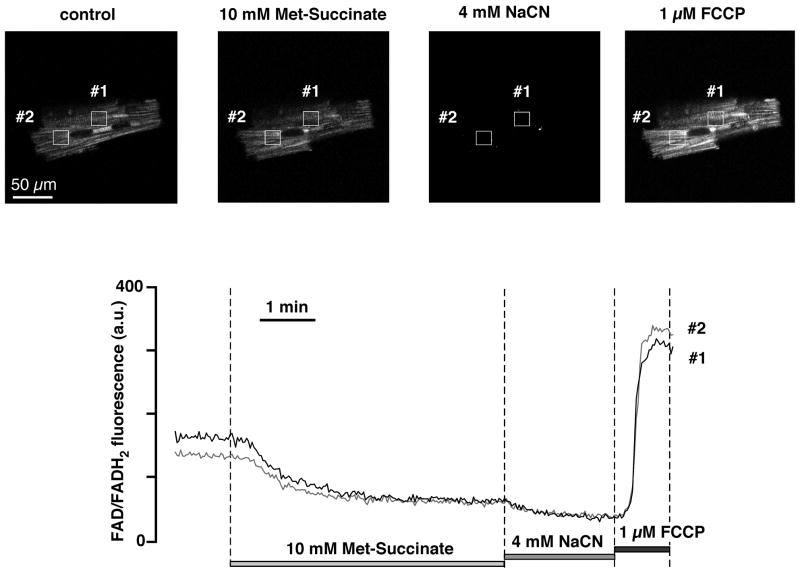
Figure 4. Monitoring mitochondrial redox potential in intact ventricular myocytes by measuring FAD autofluorescence.
Top, from left to right: confocal images of ventricular myocyte FAD autofluorescence (excitation 488 nm, emission 510–525 nm) before (cntrl) and after exposure to ETC complex II substrate methyl succinate (Met-Succinate), ETC complex IV inhibitor sodium cyanide (NaCN), and mitochondrial uncoupler FCCP. Bottom, time course of changes in FAD/FADH2 fluorescence recorded from the regions of interest shown on top. Exposure to NaCN represents maximal reduction of FAD (i.e., 0% fluorescence from FAD with maximal formation of FADH2), while exposure to FCCP reflects maximal oxidation of FAD.
Recently developed redox-sensitive green fluorescent proteins (roGFPs) [164, 165] opened the way to monitor the ambient redox potential changes in intact cells ratiometrically (excitation maxima at ~ 400 and 490 nm). This ratiometric approach provides a great advantage of avoiding the differences in protein expression between individual cells and the absolute optical sensitivity of the protein. This indicator is genetically encoded and therefore can be targeted. For example, roGFP1 (GFP with mutations C48S, S147C, and Q204C) is specifically expressed in mitochondrial matrix by fusing the plasmid with the pyruvate dehydrogenase E1α subunit leader sequence [164]. This probe was used successfully in HeLa cells [164], however its use in larger cells such as cardiomyocytes will require an adenoviral construct.
Measurement of mitochondrial oxygen consumption
Measurements of mitochondrial oxygen (O2) consumption together with estimation of the rates of oxidative phosphorylation (OXPHOS) are good indicators of the preservation or deterioration of mitochondrial integrity. Traditionally, studies of mitochondrial O2 consumption and oxidative phosphorylation are performed polarographically on isolated mitochondria with Clark-type oxygen electrodes [166–169] or with an optical approach that is based on fluorescence quenching of a ruthenium compound by O2 [170, 171]. Growing evidence that structural and functional interactions between mitochondria and cytoskeleton [172], other organelles [173] and cytosol play a significant role in OXPHOS reactions, has led to the development of new methods that can probe these reactions and monitor O2 consumption in intact [174, 175] and permeabilized [107, 176] cells, and together with the use of specific substrates and inhibitors help determine the activities of various elements of the respiratory chain [107, 176–178].
Measurement of mitochondrial ROS generation
Myocytes have multiple cytosolic and organellar sources for ROS production including leakage from the mitochondrial electron transport chain, cytosolic/caveolar, sarcoplasmic reticulum and mitochondrial NOS, NADPH oxidase and xanthine oxidase. For example, superoxide (O2•−) is generated in the cytosol via NADPH oxidases and xanthine oxidase, however mitochondria consume >90% of oxygen and therefore can become a major site of O2•− generation in response to a variety of cellular stresses. The IMM is impermeable to O2•−, making the mitochondria a separate cellular compartment for O2•−. The multiple ROS sources make ROS measurements challenging, particularly the identification of the subcellular origin. The most suitable fluorescent dye for mitochondrial O2•− detection in intact cardiomyocytes is MitoSox Red, a hydroethidium (HE) derivative which has been covalently modified with a hexyl linker to a triphenylphosphonium cation to form Mito-HE [179–181]. A positive charge and the lipophilic nature of the triphenylphosphonium (TPP) cation allow Mito-HE to accumulate preferentially in mitochondria [179, 180]. During cellular oxidative stress Mito-HE is oxidized by O2•− to form a hydroxylated product (HO-Mito-Ethidium+) that becomes fluorescent upon irreversible binding to the mitochondrial DNA (excitation at 510 nm and emission at 580 nm)[179]. Ethidium becomes highly fluorescent when intercalated into DNA, double stranded RNA or DNA/RNA complexes [180]. It is recommended to load myocytes with lower concentrations (0.3–0.5 μM) of MitoSox Red for 30 min at 37°C. Higher concentrations of Mito-HE are toxic to mitochondria and can lead to rupture of the IMM and redistribution of fluorescence to the nucleus [179, 180]. Duration of light exposure should be minimized in order to prevent photo-oxidation of Mito-HE. Endogenous ROS species can oxidize HE resulting in multiple fluorescent compounds that complicate the measurement of O2•− with HE, however only O2•−- dependent oxidation generates a hydroxylated product (2-OH-E) that has a unique excitation at 396 nm in addition to the typical HE excitation at 510 nm [179]. Results obtained exclusively with HE excitation at 510 nm must be interpreted with caution as they likely overestimate the contributions of O2•− while underestimating contributions from other ROS [182].
The fluorescent membrane-permeable indicators 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA, also known as dichlorofluorescein diacetate (DCF)) and its chloromethyl (CM-H2DCFDA) or carboxyl (carboxy-H2DCFDA) derivatives that exhibit much better retention in live cells, are the most commonly used reagents for detecting intracellular ROS. Oxidation of the dye by ROS results in formation of a highly fluorescent DCF product which can be detected at 530 nm upon excitation at 488 nm [183]. While DCFs are mostly used for hydrogen peroxide (H2O2) detection (Figure 5), they also react with a wide variety of ROS species including, but not limited to peroxyl (ROO•) and hydroxyl (HO•) radicals and the peroxynitrite anion (ONOO−). In addition to the lack of specificity, DCF and his derivatives have limitations and disadvantages that include photooxidation and generation of ROS upon light exposure (which results in artificial signal amplification) and the irreversible nature of the binding reaction that generates the fluorescent molecule (which precludes the measurement of decreases of ROS levels).
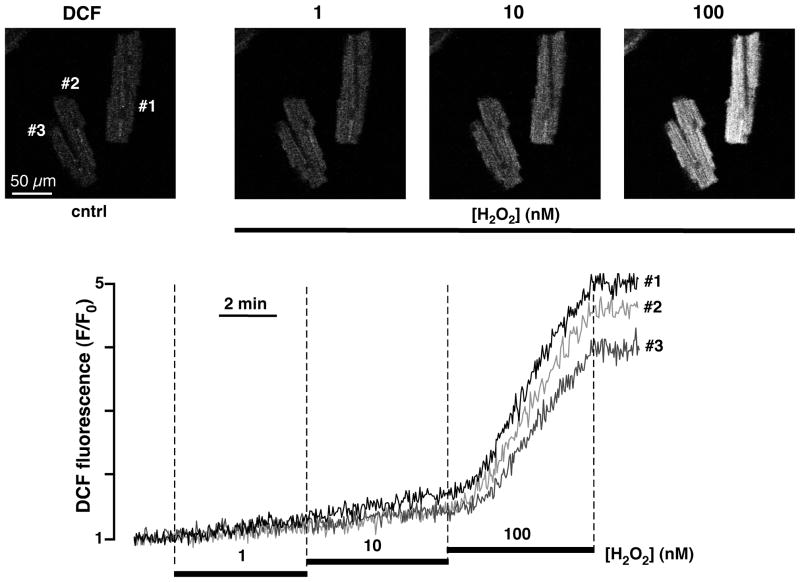
Figure 5. Measurements of the mitochondrial reactive oxygen species (ROS) generation with DCF in intact ventricular myocytes.
Top, from left to right: confocal images of ventricular myocytes loaded with ROS-sensitive dye DCF (excitation 488 nm, emission 510–525 nm) before (cntrl) and after exposure to hydrogene peroxide (H2O2; 1 – 100 nM). Bottom, time course of DCF fluorescence in response to 1, 10 and 100 nM H2O2.
To avoid some of these disadvantages, specific H2O2 sensitive fluorescent probes that do not depend on probe oxidation were created [184], including Peroxy Green 1 (PG1) and Peroxy Crimson 1 (PC1) which have been successfully used to measure cellular hydrogen peroxide (H2O2) levels. Shortcomings of these probes are the relative slow and irreversible nature of their response [183, 185].
Recent developments generated genetically encoded fluorescent sensors that can be targeted to specific subcellular locations and compartments [186]. The probe pHyPer-dMito is based on the yellow fluorescent protein (YFP) inserted into the regulatory domain of E. coli protein OxyR (OxyR-RD) [187] and is capable of detection of mitochondrial H2O2 [188]. pHyPer-dMito has submicromolar affinity to H2O2, is insensitive to other oxidants tested such as O2•−, oxidized glutathione, nitric oxide and peroxynitrite [188], and other than MitoSox Red and DCF, the fluorescent signal is reversible which allows dynamic real-time measurements of ROS changes in living cells. Indeed, using a similar circularly permuted YFP (cpYFP) bursts of O2•− generation, termed ‘superoxide flashes’, could be demonstrated in individual mitochondria of adult myocytes [189]. In contrast to pHyrPer-dMito, cpYFP emission was unchanged by H2O2 (0.1–10 mM) and peroxynitrite, and was decreased by hydroxyl radical and nitric oxide. Physiological levels of Ca2+, ATP, ADP, NAD(P)+, and NAD(P)H, and (unlike GFP-based redox biosensors [165, 190]) variations in redox potential had no significant effects on cpYFP fluorescence [189].
SNAP-tag (Covalys, Bioscience, Switzerland) is a genetically encoded fluorescent probe specific for H2O2 [191] that can be targeted to the nucleus, to the sarcoplasmic reticulum and to mitochondria by fusing SNAP-tag to the carboxyl terminus of cytochrome c oxidase subunit 8 (SNAP-Cox8A) [183]. SNAP-tag allows to monitor H2O2 changes with subcellular resolution, however shares the disadvantages of all irreversible probes.
Most recently, a ratiometric mass spectrometry probe has been developed [192] to assess mitochondrial matrix H2O2 levels in vivo. The probe MitoB comprises a TPP cation (driving mitochondrial accumulation) conjugated to arylboronic acid that reacts with H2O2 to form the phenol MitoP. Quantifying the MitoP/MitoB ratio by liquid chromatography-tandem mass spectrometry enables the measurement of average mitochondrial H2O2.
Measurement of the mitochondrial nitric oxide (NO) production
Despite growing evidence that mitochondrial Ca2+ uptake stimulates NO production inside mitochondria (see [26, 42, 48–50, 193] for more details), the direct measurement of mitochondrial NO production ([NO]m) in intact cells has remained difficult due to the coexistence of multiple sources of NO (mitochondria, caveolae, SR) and the high diffusibility of NO. The most direct evidence for mitochondrial NO generation in cardiomyocytes stems from experiments in membrane-permeabilized (digitonin) cardiomyocytes using the NO-sensitive fluorescent dyes DAF-2 DA (4,5-diaminofluorescein diacetate) or DAF-FM DA (4-Amino-5-methylamino-2′,7′-difluorofluorescein) [42]. Activation of mitochondrial Ca2+ uptake in the presence of the NOS substrate L-arginine caused an increase in DAF-2 fluorescence (Figure 6), presumably due to activation mtNOS. Caveolar and SR NOS could be excluded as the source of NO in these experiments and NO production was abolished after inhibition of mitochondrial Ca2+ uptake pinpointing mtNOS as the source of NO [42]. The peak level of NO produced by a single cardiac mitochondrion upon stimulation of mitochondrial Ca2+ uptake was estimated to be ~15–28 nM [42, 48]. Although the question of existence and nature of mtNOS is highly controversial (see [26, 43–46] for reviews) and the molecular identity of mtNOS remains to be determined, genetic[48] and pharmacological [42] approaches identified mtNOS as a Ca2+/calmodulin-dependent nNOS isoform. When mtNOS was deprived from L-arginine and the cofactor tetrahydrobiopterin (BH4) mtNOS became a source of ROS production that significantly increased the propensity for opening of the mPTP (Figure 2B). The DAF-type NO indicators are currently the best choice for fluorescent NO measurements at the cellular and organellar level, despite the disadvantage of the irreversibility of the reaction inducing fluorescence and concerns that DAF dyes not only react with NO but also with ROS species [194].
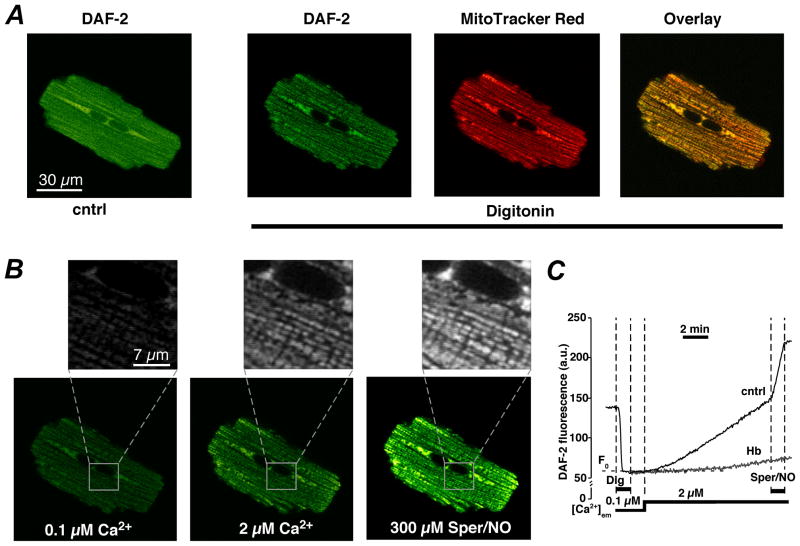
Figure 6. Measurements of mitochondrial NO production with DAF-2.
A, from left to right: confocal images of a ventricular myocyte loaded simultaneously with DAF-2 (green, emission 510–525 nm) before (cntrl) and after cell permeabilization with digitonin, and MitoTracker Red (red, emission >590 nm). The right panel shows colocalization of DAF-2 and MitoTracker Red represented by shades of yellow. B, confocal images of a permeabilized DAF-2 loaded cell under unstimulated conditions ([Ca2+]em = 0.1 μM), after an increase in [Ca2+]em to 2 μM, and after subsequent addition of the exogenous NO donor Sper/NO. Insets revel NO signals from individual mitochondria. C, time course of DAF-2 fluorescence recorded from a subcellular region of interest (≤40 μm2) under conditions shown in A and B. Treatment with the NO scavenger hemoglobin (Hb, 10 μM) prevented the Ca2+- and Sper/NO-induced increase in DAF-2 fluorescence. (From: Dedkova and Blatter, The Journal of Physiology 2009; with permission).
Detection of the mitochondrial permeability transition pore opening
The mitochondrial permeability transition defines a sudden increase in the permeability of the IMM to ions and solutes with molecular weights up to 1500 Da [41]. This process is attributed to the opening of a voltage- and Ca2+-dependent, cyclosporin A (CsA)-sensitive, high-conductance channel that is termed mitochondrial permeability transition pore (mPTP) [34, 195]. The molecular identity of mPTP is largely unknown (see [34, 195, 196] for recent reviews) and the original model which proposed a complex that includes the adenine nucleotide translocase (ANT; cf. Fig. 1) in the IMM, the VDAC of the OMM, and the regulatory matrix protein cyclophilin D (CypD), has been challenged by recent genetic studies [197, 198]. In the heart [199, 200] the mPTP plays a crucial role in myocyte injury following oxidative stress. In isolated mitochondria, opening of the mPTP causes matrix swelling with subsequent distension and local disruption of the OMM (whose surface is smaller than that of the IMM because of crista formation), release of soluble products from the IMS, dissipation of ΔΨm, and release of small molecules from the matrix [201, 202]. Therefore, in isolated mitochondria, measurements of matrix swelling [203], Ca2+ fluxes [203], ΔΨm or fluorescent dye loading [37] (cf. Figure 2) have successfully been employed to monitor mPTP activity. In IMM preparations (mitoblasts) ion currents through the pore could be measured with the patch clamp technique [202, 204]. In intact cells, however, these methods are either unsuitable or complicated by concomitant cytosolic and surface membrane processes. Nonetheless, mitochondrial swelling in conjunction with mPTP activity has also been assessed in intact cardiomyocytes [205]. Since mPTP opening is typically accompanied with IMM depolarization, ΔΨm monitoring (as described above) in intact cells can provide an indirect estimate of the mPTP activity as evidenced by the sensitivity to the mPTP inhibitor cyclosporin A and facilitation by high Ca2+ and decreased pH [90]. A more direct approach probes the release of mitochondria-entrapped fluorescent dyes calcein (MW~620 Da) [42, 206] (Figure 2) or calcein red-orange (MW~790) [207] and can be employed in intact myocytes, however the approach may require elimination of cytosolic dye by cytosolic perfusion through a patch pipette, plasma membrane permeabilization [42, 47] or Co2+ quenching [36]. This method has been validated in isolated mitochondria [37] and the decline in mitochondrial calcein fluorescence should be tested for CsA-sensitivity to ensure the involvement of the mPTP. In perfused hearts, 2-deoxy[3H]glucose entrapment technique [208–210] has been successfully used to monitor mPTP activity, based on the fact that 2-deoxy[3H]glucose will only enter mitochondria when the mPTP is open.
Methods for ATP measurement in single living cells
Adenosine-5′-trisphosphate (ATP) fuels numerous energy-requiring cellular processes and the failure of maintaining adequate cytosolic ATP levels ([ATP]i) are at the heart of the changes that take place during metabolic distress. [ATP]i levels can be measured in whole hearts by noninvasive P31 NMR, however the technique allows to measure only slow changes of total ATP [211, 212]. To follow the fast changes in intracellular ATP ([ATP]i), an indirect [ATP]i detection technique based on measurements of free magnesium ([Mg2+ ]i) with fluorescent indicators was proposed [213, 214]. This method is based on the fact that a large portion of the intracellular Mg2+ pool is bound to ATP and present as MgATP. Since free [Mg2+]i is kept constant within a rather narrow range, ATP hydrolysis leads to a concomitant increase in free [Mg2+]i as measured with fluorescent Mg2+ indicators such as mag-indo [213], magnesium Green [214], magFura-2 [215], and mag-Fluo-4 [216].
To distinguish between cytosolic and mitochondrial compartmentalized ATP, a luciferase-based adenoviral transfection method was developed [112, 217] which allows to measure ATP in pH- and Ca2+-independent manner [218, 219] and with the possibility of targeting the enzyme to individual organelles. This can be achieved by fusing of the luciferase gene with a cDNA encoding a localization sequence such as the mitochondrial import sequence of cytochrome c oxidase subunit VIII. Using either untargeted (cytosolic) or mitochondrially targeted luciferase, it has been demonstrated that no changes in ATP occured during the contractile cycle with or without beta-adrenergic stimulation, indicating that even if Ca2+ enters the mitochondria on a beat-to-beat basis [111, 112, 116], this is not translated into ATP changes on this timescale. Only large workloads led to an initial drop in ATP concentration followed by a recovery with an overshoot increase in ATP above basal level [112, 217]. Furthermore, mitochondrial NADH or NAD(P)H measurements [220–222] can be utilized as indirect indicators of the energetic state of mitochondria, and therefore reflect changes in ATP concentration.
Conclusions
Mitochondria are essential for normal cardiac function as the dominant source of ATP but also because of the crucial role in Ca2+ homeostasis, programmed cell death, and ROS signaling. An understanding of the mitochondrial function in normal and pathophysiological states is critical for the development of new therapeutical tools for treatment of mitochondria-related diseases. Here we briefly summarized the current state of experimental approaches to study mitochondria of intact cardiac cells under normal and pathological conditions.
Highlights.
Mitochondria are organelles referred to as the ‘power plants’ for cellular energy demands.
Mitochondria participate in a wide range of cellular processes.
Mitochondria have been implicated in human diseases including cardiac dysfunction.
Understanding mitochondrial functions leads to new therapies for cardiac diseases.
This review describes methods to study mitochondrial function in intact cardiomyocytes.
Acknowledgments
This work was supported by the National Institutes of Health Grants HL62231, HL80101 and HL101235, and the Leducq Foundation (to LAB) and the American Heart Association (AHA) National Scientist Development Grant AHA 0735071N and Rush University Medical Center New Investigator Grant-in-Aid 31196 (to END).
Footnotes
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005 Jul;85(3):1093–129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 2.Saks V, Favier R, Guzun R, Schlattner U, Wallimann T. Molecular system bioenergetics: regulation of substrate supply in response to heart energy demands. J Physiol. 2006 Dec 15;577(Pt 3):769–77. doi: 10.1113/jphysiol.2006.120584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006 Jul 25;16(14):R551–60. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 4.Barth E, Stammler G, Speiser B, Schaper J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J Mol Cell Cardiol. 1992 Jul;24(7):669–81. doi: 10.1016/0022-2828(92)93381-s. [DOI] [PubMed] [Google Scholar]
- 5.Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. 2. Dordrecht, Netherlands: Kluwer; 2001. [Google Scholar]
- 6.Liesa M, Palacin M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009 Jul;89(3):799–845. doi: 10.1152/physrev.00030.2008. [DOI] [PubMed] [Google Scholar]
- 7.Ong SB, Hausenloy DJ. Mitochondrial morphology and cardiovascular disease. Cardiovasc Res. 2010 Oct 1;88(1):16–29. doi: 10.1093/cvr/cvq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hom J, Sheu SS. Morphological dynamics of mitochondria--a special emphasis on cardiac muscle cells. J Mol Cell Cardiol. 2009 Jun;46(6):811–20. doi: 10.1016/j.yjmcc.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iglewski M, Hill JA, Lavandero S, Rothermel BA. Mitochondrial fission and autophagy in the normal and diseased heart. Curr Hypertens Rep. 2010 Dec;12(6):418–25. doi: 10.1007/s11906-010-0147-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lukyanenko V, Chikando A, Lederer WJ. Mitochondria in cardiomyocyte Ca2+ signaling. Int J Biochem Cell Biol. 2009 Oct;41(10):1957–71. doi: 10.1016/j.biocel.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blachly-Dyson E, Forte M. VDAC channels. IUBMB Life. 2001 Sep–Nov;52(3–5):113–8. doi: 10.1080/15216540152845902. [DOI] [PubMed] [Google Scholar]
- 12.Colombini M. VDAC: the channel at the interface between mitochondria and the cytosol. Mol Cell Biochem. 2004 Jan-Feb;256–257(1–2):107–15. doi: 10.1023/b:mcbi.0000009862.17396.8d. [DOI] [PubMed] [Google Scholar]
- 13.Matsuyama S, Reed JC. Mitochondria-dependent apoptosis and cellular pH regulation. Cell Death Differ. 2000 Dec;7(12):1155–65. doi: 10.1038/sj.cdd.4400779. [DOI] [PubMed] [Google Scholar]
- 14.De Stefani D, Bononi A, Romagnoli A, Messina A, De Pinto V, Pinton P, et al. VDAC1 selectively transfers apoptotic Ca(2+) signals to mitochondria. Cell Death Differ. 2011 Jul 1; doi: 10.1038/cdd.2011.92. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfanner N, Meijer M. The Tom and Tim machine. Curr Biol. 1997 Feb 1;7(2):R100–3. doi: 10.1016/s0960-9822(06)00048-0. [DOI] [PubMed] [Google Scholar]
- 16.Muro C, Grigoriev SM, Pietkiewicz D, Kinnally KW, Campo ML. Comparison of the TIM and TOM channel activities of the mitochondrial protein import complexes. Biophys J. 2003 May;84(5):2981–9. doi: 10.1016/S0006-3495(03)70024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casellas P, Galiegue S, Basile AS. Peripheral benzodiazepine receptors and mitochondrial function. Neurochem Int. 2002 May;40(6):475–86. doi: 10.1016/s0197-0186(01)00118-8. [DOI] [PubMed] [Google Scholar]
- 18.Obame FN, Zini R, Souktani R, Berdeaux A, Morin D. Peripheral benzodiazepine receptor-induced myocardial protection is mediated by inhibition of mitochondrial membrane permeabilization. J Pharmacol Exp Ther. 2007 Oct;323(1):336–45. doi: 10.1124/jpet.107.124255. [DOI] [PubMed] [Google Scholar]
- 19.Dejean LM, Martinez-Caballero S, Manon S, Kinnally KW. Regulation of the mitochondrial apoptosis-induced channel, MAC, by BCL-2 family proteins. Biochim Biophys Acta. 2006 Feb;1762(2):191–201. doi: 10.1016/j.bbadis.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Dejean LM, Martinez-Caballero S, Guo L, Hughes C, Teijido O, Ducret T, et al. Oligomeric Bax is a component of the putative cytochrome c release channel MAC, mitochondrial apoptosis-induced channel. Mol Biol Cell. 2005 May;16(5):2424–32. doi: 10.1091/mbc.E04-12-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pavlov EV, Priault M, Pietkiewicz D, Cheng EH, Antonsson B, Manon S, et al. A novel, high conductance channel of mitochondria linked to apoptosis in mammalian cells and Bax expression in yeast. J Cell Biol. 2001 Nov 26;155(5):725–31. doi: 10.1083/jcb.200107057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo L, Pietkiewicz D, Pavlov EV, Grigoriev SM, Kasianowicz JJ, Dejean LM, et al. Effects of cytochrome c on the mitochondrial apoptosis-induced channel MAC. Am J Physiol Cell Physiol. 2004 May;286(5):C1109–17. doi: 10.1152/ajpcell.00183.2003. [DOI] [PubMed] [Google Scholar]
- 23.Hetz C, Vitte PA, Bombrun A, Rostovtseva TK, Montessuit S, Hiver A, et al. Bax channel inhibitors prevent mitochondrion-mediated apoptosis and protect neurons in a model of global brain ischemia. J Biol Chem. 2005 Dec 30;280(52):42960–70. doi: 10.1074/jbc.M505843200. [DOI] [PubMed] [Google Scholar]
- 24.Peixoto PM, Ryu SY, Bombrun A, Antonsson B, Kinnally KW. MAC inhibitors suppress mitochondrial apoptosis. Biochem J. 2009 Nov 1;423(3):381–7. doi: 10.1042/BJ20090664. [DOI] [PubMed] [Google Scholar]
- 25.Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999 Oct;79(4):1127–55. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- 26.Dedkova EN, Blatter LA. Mitochondrial Ca(2+) and the heart. Cell Calcium. 2008 Jul;44(1):77–91. doi: 10.1016/j.ceca.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, et al. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature. 2010 Sep 16;467(7313):291–6. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011 Jun 19; doi: 10.1038/nature10234. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011 Jun 19; doi: 10.1038/nature10230. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szabadkai G, Duchen MR. Mitochondria: the hub of cellular Ca2+ signaling. Physiology (Bethesda) 2008 Apr;23:84–94. doi: 10.1152/physiol.00046.2007. [DOI] [PubMed] [Google Scholar]
- 31.Kauffman RF, Lardy HA. Biphasic uptake of Ca2+ by rat liver mitochondria. J Biol Chem. 1980 May 10;255(9):4228–35. [PubMed] [Google Scholar]
- 32.Moreau B, Nelson C, Parekh AB. Biphasic regulation of mitochondrial Ca2+ uptake by cytosolic Ca2+ concentration. Curr Biol. 2006 Aug 22;16(16):1672–7. doi: 10.1016/j.cub.2006.06.059. [DOI] [PubMed] [Google Scholar]
- 33.Moreau B, Parekh AB. Ca2+-dependent inactivation of the mitochondrial Ca2+ uniporter involves proton flux through the ATP synthase. Curr Biol. 2008 Jun 3;18(11):855–9. doi: 10.1016/j.cub.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 34.Di Lisa F, Bernardi P. A CaPful of mechanisms regulating the mitochondrial permeability transition. J Mol Cell Cardiol. 2009 Jun;46(6):775–80. doi: 10.1016/j.yjmcc.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004 Oct;287(4):C817–33. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 36.Petronilli V, Miotto G, Canton M, Brini M, Colonna R, Bernardi P, et al. Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys J. 1999 Feb;76(2):725–34. doi: 10.1016/S0006-3495(99)77239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huser J, Rechenmacher CE, Blatter LA. Imaging the permeability pore transition in single mitochondria. Biophys J. 1998 Apr;74(4):2129–37. doi: 10.1016/S0006-3495(98)77920-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elrod JW, Wong R, Mishra S, Vagnozzi RJ, Sakthievel B, Goonasekera SA, et al. Cyclophilin D controls mitochondrial pore-dependent Ca(2+) exchange, metabolic flexibility, and propensity for heart failure in mice. J Clin Invest. 2010 Oct 1;120(10):3680–7. doi: 10.1172/JCI43171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Broekemeier KM, Klocek CK, Pfeiffer DR. Proton selective substate of the mitochondrial permeability transition pore: regulation by the redox state of the electron transport chain. Biochemistry. 1998 Sep 22;37(38):13059–65. doi: 10.1021/bi980820c. [DOI] [PubMed] [Google Scholar]
- 40.Huser J, Blatter LA. Fluctuations in mitochondrial membrane potential caused by repetitive gating of the permeability transition pore. Biochem J. 1999 Oct 15;343(Pt 2):311–7. [PMC free article] [PubMed] [Google Scholar]
- 41.Bernardi P, Broekemeier KM, Pfeiffer DR. Recent progress on regulation of the mitochondrial permeability transition pore; a cyclosporin-sensitive pore in the inner mitochondrial membrane. J Bioenerg Biomembr. 1994 Oct;26(5):509–17. doi: 10.1007/BF00762735. [DOI] [PubMed] [Google Scholar]
- 42.Dedkova EN, Blatter LA. Characteristics and function of cardiac mitochondrial nitric oxide synthase. J Physiol. 2009 Feb 15;587(Pt 4):851–72. doi: 10.1113/jphysiol.2008.165423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valdez LB, Boveris A. Mitochondrial nitric oxide synthase, a voltage-dependent enzyme, is responsible for nitric oxide diffusion to cytosol. Front Biosci. 2007;12:1210–9. doi: 10.2741/2139. [DOI] [PubMed] [Google Scholar]
- 44.Kato K, Giulivi C. Critical overview of mitochondrial nitric-oxide synthase. Front Biosci. 2006;11:2725–38. doi: 10.2741/2002. [DOI] [PubMed] [Google Scholar]
- 45.Brookes PS. Mitochondrial nitric oxide synthase. Mitochondrion. 2004 Mar;3(4):187–204. doi: 10.1016/j.mito.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Valdez LB, Zaobornyj T, Alvarez S, Bustamante J, Costa LE, Boveris A. Heart mitochondrial nitric oxide synthase. Effects of hypoxia and aging. Mol Aspects Med. 2004 Feb-Apr;25(1–2):49–59. doi: 10.1016/j.mam.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 47.Dedkova EN, Blatter LA. Modulation of mitochondrial Ca2+ by nitric oxide in cultured bovine vascular endothelial cells. Am J Physiol Cell Physiol. 2005 Oct;289(4):C836–45. doi: 10.1152/ajpcell.00011.2005. [DOI] [PubMed] [Google Scholar]
- 48.Kanai AJ, Pearce LL, Clemens PR, Birder LA, VanBibber MM, Choi SY, et al. Identification of a neuronal nitric oxide synthase in isolated cardiac mitochondria using electrochemical detection. Proc Natl Acad Sci U S A. 2001 Nov 20;98(24):14126–31. doi: 10.1073/pnas.241380298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghafourifar P, Richter C. Nitric oxide synthase activity in mitochondria. FEBS Lett. 1997 Dec 1;418(3):291–6. doi: 10.1016/s0014-5793(97)01397-5. [DOI] [PubMed] [Google Scholar]
- 50.Giulivi C. Functional implications of nitric oxide produced by mitochondria in mitochondrial metabolism. Biochem J. 1998 Jun 15;332( Pt 3):673–9. doi: 10.1042/bj3320673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanai A, Epperly M, Pearce L, Birder L, Zeidel M, Meyers S, et al. Differing roles of mitochondrial nitric oxide synthase in cardiomyocytes and urothelial cells. Am J Physiol Heart Circ Physiol. 2004 Jan;286(1):H13–21. doi: 10.1152/ajpheart.00737.2003. [DOI] [PubMed] [Google Scholar]
- 52.Zenebe WJ, Nazarewicz RR, Parihar MS, Ghafourifar P. Hypoxia/Reoxygenation of isolated rat heart mitochondria causes cytochrome c release and oxidative stress; evidence for involvement of mitochondrial nitric oxide synthase. J Mol Cell Cardiol. 2007 Oct;43(4):411–9. doi: 10.1016/j.yjmcc.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nazarewicz RR, Zenebe WJ, Parihar A, Larson SK, Alidema E, Choi J, et al. Tamoxifen induces oxidative stress and mitochondrial apoptosis via stimulating mitochondrial nitric oxide synthase. Cancer Res. 2007 Feb 1;67(3):1282–90. doi: 10.1158/0008-5472.CAN-06-3099. [DOI] [PubMed] [Google Scholar]
- 54.Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, et al. HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci U S A. 1998 Mar 17;95(6):2979–84. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Makino A, Suarez J, Gawlowski T, Han W, Wang H, Scott BT, et al. Regulation of mitochondrial morphology and function by O-GlcNAcylation in neonatal cardiac myocytes. Am J Physiol Regul Integr Comp Physiol. 2011 Jun;300(6):R1296–302. doi: 10.1152/ajpregu.00437.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010 May 11;121(18):2012–22. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 57.Gupta A, Gupta S, Young D, Das B, McMahon J, Sen S. Impairment of ultrastructure and cytoskeleton during progression of cardiac hypertrophy to heart failure. Lab Invest. 2010 Apr;90(4):520–30. doi: 10.1038/labinvest.2010.43. [DOI] [PubMed] [Google Scholar]
- 58.Yu T, Sheu SS, Robotham JL, Yoon Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res. 2008 Jul 15;79(2):341–51. doi: 10.1093/cvr/cvn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001 Oct;1(4):515–25. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- 60.Legros F, Lombes A, Frachon P, Rojo M. Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Mol Biol Cell. 2002 Dec;13(12):4343–54. doi: 10.1091/mbc.E02-06-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang H, Choi SY, Frohman MA. A quantitative assay for mitochondrial fusion using Renilla luciferase complementation. Mitochondrion. 2010 Aug;10(5):559–66. doi: 10.1016/j.mito.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Twig G, Graf SA, Wikstrom JD, Mohamed H, Haigh SE, Elorza A, et al. Tagging and tracking individual networks within a complex mitochondrial web with photoactivatable GFP. Am J Physiol Cell Physiol. 2006 Jul;291(1):C176–84. doi: 10.1152/ajpcell.00348.2005. [DOI] [PubMed] [Google Scholar]
- 63.Lemasters JJ, Ramshesh VK. Imaging of mitochondrial polarization and depolarization with cationic fluorophores. Methods Cell Biol. 2007;80:283–95. doi: 10.1016/S0091-679X(06)80014-2. [DOI] [PubMed] [Google Scholar]
- 64.Ehrenberg B, Montana V, Wei MD, Wuskell JP, Loew LM. Membrane potential can be determined in individual cells from the nernstian distribution of cationic dyes. Biophys J. 1988 May;53(5):785–94. doi: 10.1016/S0006-3495(88)83158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Farkas DL, Wei MD, Febbroriello P, Carson JH, Loew LM. Simultaneous imaging of cell and mitochondrial membrane potentials. Biophys J. 1989 Dec;56(6):1053–69. doi: 10.1016/S0006-3495(89)82754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johnson LV, Walsh ML, Bockus BJ, Chen LB. Monitoring of relative mitochondrial membrane potential in living cells by fluorescence microscopy. J Cell Biol. 1981 Mar;88(3):526–35. doi: 10.1083/jcb.88.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scaduto RC, Jr, Grotyohann LW. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J. 1999 Jan;76(1 Pt 1):469–77. doi: 10.1016/S0006-3495(99)77214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Griffiths EJ. Mitochondria--potential role in cell life and death. Cardiovasc Res. 2000 Apr;46(1):24–7. doi: 10.1016/s0008-6363(00)00020-1. [DOI] [PubMed] [Google Scholar]
- 69.Mathur A, Hong Y, Kemp BK, Barrientos AA, Erusalimsky JD. Evaluation of fluorescent dyes for the detection of mitochondrial membrane potential changes in cultured cardiomyocytes. Cardiovasc Res. 2000 Apr;46(1):126–38. doi: 10.1016/s0008-6363(00)00002-x. [DOI] [PubMed] [Google Scholar]
- 70.Duchen MR, Surin A, Jacobson J. Imaging mitochondrial function in intact cells. Methods Enzymol. 2003;361:353–89. doi: 10.1016/s0076-6879(03)61019-0. [DOI] [PubMed] [Google Scholar]
- 71.Davidson SM, Yellon D, Duchen MR. Assessing mitochondrial potential, calcium, and redox state in isolated mammalian cells using confocal microscopy. Methods Mol Biol. 2007;372:421–30. doi: 10.1007/978-1-59745-365-3_30. [DOI] [PubMed] [Google Scholar]
- 72.O’Reilly CM, Fogarty KE, Drummond RM, Tuft RA, Walsh JV., Jr Quantitative analysis of spontaneous mitochondrial depolarizations. Biophys J. 2003 Nov;85(5):3350–7. doi: 10.1016/S0006-3495(03)74754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rottenberg H. Membrane potential and surface potential in mitochondria: uptake and binding of lipophilic cations. J Membr Biol. 1984;81(2):127–38. doi: 10.1007/BF01868977. [DOI] [PubMed] [Google Scholar]
- 74.Diaz G, Diana A, Falchi AM, Gremo F, Pani A, Batetta B, et al. Intra- and intercellular distribution of mitochondrial probes and changes after treatment with MDR modulators. IUBMB Life. 2001 Feb;51(2):121–6. doi: 10.1080/15216540119470. [DOI] [PubMed] [Google Scholar]
- 75.Bunting JR, Phan TV, Kamali E, Dowben RM. Fluorescent cationic probes of mitochondria. Metrics and mechanism of interaction. Biophys J. 1989 Nov;56(5):979–93. doi: 10.1016/S0006-3495(89)82743-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hausenloy DJ, Yellon DM, Mani-Babu S, Duchen MR. Preconditioning protects by inhibiting the mitochondrial permeability transition. Am J Physiol Heart Circ Physiol. 2004 Aug;287(2):H841–9. doi: 10.1152/ajpheart.00678.2003. [DOI] [PubMed] [Google Scholar]
- 77.Voronina SG, Barrow SL, Gerasimenko OV, Petersen OH, Tepikin AV. Effects of secretagogues and bile acids on mitochondrial membrane potential of pancreatic acinar cells: comparison of different modes of evaluating DeltaPsim. J Biol Chem. 2004 Jun 25;279(26):27327–38. doi: 10.1074/jbc.M311698200. [DOI] [PubMed] [Google Scholar]
- 78.Bhamra GS, Hausenloy DJ, Davidson SM, Carr RD, Paiva M, Wynne AM, et al. Metformin protects the ischemic heart by the Akt-mediated inhibition of mitochondrial permeability transition pore opening. Basic Res Cardiol. 2008 May;103(3):274–84. doi: 10.1007/s00395-007-0691-y. [DOI] [PubMed] [Google Scholar]
- 79.Akar FG, Aon MA, Tomaselli GF, O’Rourke B. The mitochondrial origin of postischemic arrhythmias. J Clin Invest. 2005 Dec;115(12):3527–35. doi: 10.1172/JCI25371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Slodzinski MK, Aon MA, O’Rourke B. Glutathione oxidation as a trigger of mitochondrial depolarization and oscillation in intact hearts. J Mol Cell Cardiol. 2008 Nov;45(5):650–60. doi: 10.1016/j.yjmcc.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Matsumoto-Ida M, Akao M, Takeda T, Kato M, Kita T. Real-time 2-photon imaging of mitochondrial function in perfused rat hearts subjected to ischemia/reperfusion. Circulation. 2006 Oct 3;114(14):1497–503. doi: 10.1161/CIRCULATIONAHA.106.628834. [DOI] [PubMed] [Google Scholar]
- 82.Jin H, Nass RD, Joudrey PJ, Lyon AR, Chemaly ER, Rapti K, et al. Altered spatiotemporal dynamics of the mitochondrial membrane potential in the hypertrophied heart. Biophys J. 2010 May 19;98(10):2063–71. doi: 10.1016/j.bpj.2010.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lyon AR, Joudrey PJ, Jin D, Nass RD, Aon MA, O’Rourke B, et al. Optical imaging of mitochondrial function uncovers actively propagating waves of mitochondrial membrane potential collapse across intact heart. J Mol Cell Cardiol. 2010 Oct;49(4):565–75. doi: 10.1016/j.yjmcc.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Griffiths EJ, Stern MD, Silverman HS. Measurement of mitochondrial calcium in single living cardiomyocytes by selective removal of cytosolic indo 1. Am J Physiol. 1997 Jul;273(1 Pt 1):C37–44. doi: 10.1152/ajpcell.1997.273.1.C37. [DOI] [PubMed] [Google Scholar]
- 85.Emaus RK, Grunwald R, Lemasters JJ. Rhodamine 123 as a probe of transmembrane potential in isolated rat-liver mitochondria: spectral and metabolic properties. Biochim Biophys Acta. 1986 Jul 23;850(3):436–48. doi: 10.1016/0005-2728(86)90112-x. [DOI] [PubMed] [Google Scholar]
- 86.Di Lisa F, Blank PS, Colonna R, Gambassi G, Silverman HS, Stern MD, et al. Mitochondrial membrane potential in single living adult rat cardiac myocytes exposed to anoxia or metabolic inhibition. J Physiol. 1995 Jul 1;486( Pt 1):1–13. doi: 10.1113/jphysiol.1995.sp020786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reers M, Smith TW, Chen LB. J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry. 1991 May 7;30(18):4480–6. doi: 10.1021/bi00232a015. [DOI] [PubMed] [Google Scholar]
- 88.Chinopoulos C, Tretter L, Adam-Vizi V. Depolarization of in situ mitochondria due to hydrogen peroxide-induced oxidative stress in nerve terminals: inhibition of alpha-ketoglutarate dehydrogenase. J Neurochem. 1999 Jul;73(1):220–8. doi: 10.1046/j.1471-4159.1999.0730220.x. [DOI] [PubMed] [Google Scholar]
- 89.Aon MA, Cortassa S, Maack C, O’Rourke B. Sequential opening of mitochondrial ion channels as a function of glutathione redox thiol status. J Biol Chem. 2007 Jul 27;282(30):21889–900. doi: 10.1074/jbc.M702841200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Honda HM, Korge P, Weiss JN. Mitochondria and ischemia/reperfusion injury. Ann N Y Acad Sci. 2005 Jun;1047:248–58. doi: 10.1196/annals.1341.022. [DOI] [PubMed] [Google Scholar]
- 91.Griffiths EJ. Species dependence of mitochondrial calcium transients during excitation-contraction coupling in isolated cardiomyocytes. Biochem Biophys Res Commun. 1999 Sep 24;263(2):554–9. doi: 10.1006/bbrc.1999.1311. [DOI] [PubMed] [Google Scholar]
- 92.Miyata H, Silverman HS, Sollott SJ, Lakatta EG, Stern MD, Hansford RG. Measurement of mitochondrial free Ca2+ concentration in living single rat cardiac myocytes. Am J Physiol. 1991 Oct;261(4 Pt 2):H1123–34. doi: 10.1152/ajpheart.1991.261.4.H1123. [DOI] [PubMed] [Google Scholar]
- 93.Zhou Z, Matlib MA, Bers DM. Cytosolic and mitochondrial Ca2+ signals in patch clamped mammalian ventricular myocytes. J Physiol. 1998 Mar 1;507( Pt 2):379–403. doi: 10.1111/j.1469-7793.1998.379bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schreur JH, Figueredo VM, Miyamae M, Shames DM, Baker AJ, Camacho SA. Cytosolic and mitochondrial [Ca2+] in whole hearts using indo-1 acetoxymethyl ester: effects of high extracellular Ca2+ Biophys J. 1996 Jun;70(6):2571–80. doi: 10.1016/S0006-3495(96)79828-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Trollinger DR, Cascio WE, Lemasters JJ. Selective loading of Rhod 2 into mitochondria shows mitochondrial Ca2+ transients during the contractile cycle in adult rabbit cardiac myocytes. Biochem Biophys Res Commun. 1997 Jul 30;236(3):738–42. doi: 10.1006/bbrc.1997.7042. [DOI] [PubMed] [Google Scholar]
- 96.Kim JS, Jin Y, Lemasters JJ. Reactive oxygen species, but not Ca2+ overloading, trigger pH- and mitochondrial permeability transition-dependent death of adult rat myocytes after ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2006 May;290(5):H2024–34. doi: 10.1152/ajpheart.00683.2005. [DOI] [PubMed] [Google Scholar]
- 97.Maack C, Cortassa S, Aon MA, Ganesan AN, Liu T, O’Rourke B. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ Res. 2006 Jul 21;99(2):172–82. doi: 10.1161/01.RES.0000232546.92777.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Di Lisa F, Gambassi G, Sprurgeon H, Hansford RG. Intramitochondrial free calcium in cardiac myocytes in relation to dehydrogenase activation. Cardiovascular Research. 1993;27:1840–4. doi: 10.1093/cvr/27.10.1840. [DOI] [PubMed] [Google Scholar]
- 99.Abdallah Y, Iraqi W, Said M, Kasseckert SA, Shahzad T, Erdogan A, et al. Interplay between Ca(2+) cycling and mitochondrial permeability transition pores promotes reperfusion-induced injury of cardiac myocytes. J Cell Mol Med. 2010 Dec 28; doi: 10.1111/j.1582-4934.2010.01249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gavin CE, Gunter KK, Gunter TE. Manganese and calcium efflux kinetics in brain mitochondria. Relevance to manganese toxicity. Biochem J. 1990 Mar 1;266(2):329–34. doi: 10.1042/bj2660329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sedova M, Dedkova EN, Blatter LA. Integration of rapid cytosolic Ca2+ signals by mitochondria in cat ventricular myocytes. Am J Physiol Cell Physiol. 2006 Nov;291(5):C840–50. doi: 10.1152/ajpcell.00619.2005. [DOI] [PubMed] [Google Scholar]
- 102.Hajnoczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995 Aug 11;82(3):415–24. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 103.Jou MJ, Peng TI, Sheu SS. Histamine induces oscillations of mitochondrial free Ca2+ concentration in single cultured rat brain astrocytes. J Physiol. 1996 Dec 1;497( Pt 2):299–308. doi: 10.1113/jphysiol.1996.sp021769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stamm C, Friehs I, Choi YH, Zurakowski D, McGowan FX, del Nido PJ. Cytosolic calcium in the ischemic rabbit heart: assessment by pH- and temperature-adjusted rhod-2 spectrofluorometry. Cardiovasc Res. 2003 Sep 1;59(3):695–704. doi: 10.1016/s0008-6363(03)00467-x. [DOI] [PubMed] [Google Scholar]
- 105.Fonteriz RI, de la Fuente S, Moreno A, Lobaton CD, Montero M, Alvarez J. Monitoring mitochondrial [Ca(2+)] dynamics with rhod-2, ratiometric pericam and aequorin. Cell Calcium. 2010 Jul;48(1):61–9. doi: 10.1016/j.ceca.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 106.O’Rourke B, Blatter LA. Mitochondrial Ca2+ uptake: tortoise or hare? J Mol Cell Cardiol. 2009 Jun;46(6):767–74. doi: 10.1016/j.yjmcc.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saks VA, Veksler VI, Kuznetsov AV, Kay L, Sikk P, Tiivel T, et al. Permeabilized cell and skinned fiber techniques in studies of mitochondrial function in vivo. Mol Cell Biochem. 1998 Jul;184(1–2):81–100. [PubMed] [Google Scholar]
- 108.Fiskum G, Kowaltowksi AJ, Andreyev AY, Kushnareva YE, Starkov AA. Apoptosis-related activities measured with isolated mitochondria and digitonin-permeabilized cells. Methods Enzymol. 2000;322:222–34. doi: 10.1016/s0076-6879(00)22023-5. [DOI] [PubMed] [Google Scholar]
- 109.Rutter GA, Burnett P, Rizzuto R, Brini M, Murgia M, Pozzan T, et al. Subcellular imaging of intramitochondrial Ca2+ with recombinant targeted aequorin: significance for the regulation of pyruvate dehydrogenase activity. Proc Natl Acad Sci U S A. 1996 May 28;93(11):5489–94. doi: 10.1073/pnas.93.11.5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Robert V, Pinton P, Tosello V, Rizzuto R, Pozzan T. Recombinant aequorin as tool for monitoring calcium concentration in subcellular compartments. Methods Enzymol. 2000;327:440–56. doi: 10.1016/s0076-6879(00)27295-9. [DOI] [PubMed] [Google Scholar]
- 111.Robert V, Gurlini P, Tosello V, Nagai T, Miyawaki A, Di Lisa F, et al. Beat-to-beat oscillations of mitochondrial [Ca2+] in cardiac cells. Embo J. 2001 Sep 3;20(17):4998–5007. doi: 10.1093/emboj/20.17.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bell CJ, Bright NA, Rutter GA, Griffiths EJ. ATP regulation in adult rat cardiomyocytes: time-resolved decoding of rapid mitochondrial calcium spiking imaged with targeted photoproteins. J Biol Chem. 2006 Sep 22;281(38):28058–67. doi: 10.1074/jbc.M604540200. [DOI] [PubMed] [Google Scholar]
- 113.Nagai T, Sawano A, Park ES, Miyawaki A. Circularly permuted green fluorescent proteins engineered to sense Ca2+ Proc Natl Acad Sci U S A. 2001 Mar 13;98(6):3197–202. doi: 10.1073/pnas.051636098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jiang D, Zhao L, Clapham DE. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science. 2009 Oct 2;326(5949):144–7. doi: 10.1126/science.1175145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Belke DD, Swanson E, Suarez J, Scott BT, Stenbit AE, Dillmann WH. Increased expression of SERCA in the hearts of transgenic mice results in increased oxidation of glucose. Am J Physiol Heart Circ Physiol. 2007 Apr;292(4):H1755–63. doi: 10.1152/ajpheart.00884.2006. [DOI] [PubMed] [Google Scholar]
- 116.Kettlewell S, Cabrero P, Nicklin SA, Dow JA, Davies S, Smith GL. Changes of intra-mitochondrial Ca2+ in adult ventricular cardiomyocytes examined using a novel fluorescent Ca2+ indicator targeted to mitochondria. J Mol Cell Cardiol. 2009 Jun;46(6):891–901. doi: 10.1016/j.yjmcc.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 117.Iguchi M, Kato M, Nakai J, Takeda T, Matsumoto-Ida M, Kita T, et al. Direct monitoring of mitochondrial calcium levels in cultured cardiac myocytes using a novel fluorescent indicator protein, GCaMP2-mt. Int J Cardiol. 2011 Feb 3; doi: 10.1016/j.ijcard.2011.01.034. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 118.Reed KC, Bygrave FL. A low molecular weight ruthenium complex inhibitory to mitochondrial Ca2+ transport. FEBS Lett. 1974 Sep 15;46(1):109–14. doi: 10.1016/0014-5793(74)80346-7. [DOI] [PubMed] [Google Scholar]
- 119.Reed KC, Bygrave FL. The inhibition of mitochondrial calcium transport by lanthanides and ruthenium red. Biochem J. 1974 May;140(2):143–55. doi: 10.1042/bj1400143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ferrari R, di Lisa F, Raddino R, Visioli O. The effects of ruthenium red on mitochondrial function during post-ischaemic reperfusion. J Mol Cell Cardiol. 1982 Dec;14(12):737–40. doi: 10.1016/0022-2828(82)90186-9. [DOI] [PubMed] [Google Scholar]
- 121.Carry MM, Mrak RE, Murphy ML, Peng CF, Straub KD, Fody EP. Reperfusion injury in ischemic myocardium: protective effects of ruthenium red and of nitroprusside. Am J Cardiovasc Pathol. 1989;2(4):335–44. [PubMed] [Google Scholar]
- 122.Matlib MA, Zhou Z, Knight S, Ahmed S, Choi KM, Krause-Bauer J, et al. Oxygen-bridged dinuclear ruthenium amine complex specifically inhibits Ca2+ uptake into mitochondria in vitro and in situ in single cardiac myocytes. J Biol Chem. 1998 Apr 24;273(17):10223–31. doi: 10.1074/jbc.273.17.10223. [DOI] [PubMed] [Google Scholar]
- 123.Ying WL, Emerson J, Clarke MJ, Sanadi DR. Inhibition of mitochondrial calcium ion transport by an oxo-bridged dinuclear ruthenium ammine complex. Biochemistry. 1991 May 21;30(20):4949–52. doi: 10.1021/bi00234a016. [DOI] [PubMed] [Google Scholar]
- 124.Zazueta C, Sosa-Torres ME, Correa F, Garza-Ortiz A. Inhibitory properties of ruthenium amine complexes on mitochondrial calcium uptake. J Bioenerg Biomembr. 1999 Dec;31(6):551–7. doi: 10.1023/a:1005464927366. [DOI] [PubMed] [Google Scholar]
- 125.Garcia-Rivas Gde J, Carvajal K, Correa F, Zazueta C. Ru360, a specific mitochondrial calcium uptake inhibitor, improves cardiac post-ischaemic functional recovery in rats in vivo. Br J Pharmacol. 2006 Dec;149(7):829–37. doi: 10.1038/sj.bjp.0706932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Terada H. The interaction of highly active uncouplers with mitochondria. Biochim Biophys Acta. 1981 Dec 30;639(3–4):225–42. doi: 10.1016/0304-4173(81)90011-2. [DOI] [PubMed] [Google Scholar]
- 127.Heytler PG. Uncouplers of oxidative phosphorylation. Methods Enzymol. 1979;55:462–42. doi: 10.1016/0076-6879(79)55060-5. [DOI] [PubMed] [Google Scholar]
- 128.Seren S, Caporin G, Galiazzo F, Lippe G, Ferguson SJ, Sorgato MC. Current-voltage relationships for proton flow through the F0 sector of the ATP-synthase, carbonylcyanide-p-trifluoromethoxyphenylhydrazone or leak pathways in submitochondrial particles. Eur J Biochem. 1985 Oct 15;152(2):373–9. doi: 10.1111/j.1432-1033.1985.tb09207.x. [DOI] [PubMed] [Google Scholar]
- 129.Penefsky HS. Mechanism of inhibition of mitochondrial adenosine triphosphatase by dicyclohexylcarbodiimide and oligomycin: relationship to ATP synthesis. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1589–93. doi: 10.1073/pnas.82.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Masini A, Ceccarelli-Stanzani D, Muscatello U. The effect of oligomycin on rat liver mitochondria respiring in state 4. FEBS Lett. 1983 Aug 22;160(1–2):137–40. doi: 10.1016/0014-5793(83)80953-3. [DOI] [PubMed] [Google Scholar]
- 131.Rouslin W, Erickson JL, Solaro RJ. Effects of oligomycin and acidosis on rates of ATP depletion in ischemic heart muscle. Am J Physiol. 1986 Mar;250(3 Pt 2):H503–8. doi: 10.1152/ajpheart.1986.250.3.H503. [DOI] [PubMed] [Google Scholar]
- 132.Griffiths EJ, Wei SK, Haigney MC, Ocampo CJ, Stern MD, Silverman HS. Inhibition of mitochondrial calcium efflux by clonazepam in intact single rat cardiomyocytes and effects on NADH production. Cell Calcium. 1997 Apr;21(4):321–9. doi: 10.1016/s0143-4160(97)90120-2. [DOI] [PubMed] [Google Scholar]
- 133.Chiesi M, Schwaller R, Eichenberger K. Structural dependency of the inhibitory action of benzodiazepines and related compounds on the mitochondrial Na+-Ca2+ exchanger. Biochem Pharmacol. 1988 Nov 15;37(22):4399–403. doi: 10.1016/0006-2952(88)90623-5. [DOI] [PubMed] [Google Scholar]
- 134.Cox DA, Conforti L, Sperelakis N, Matlib MA. Selectivity of inhibition of Na(+)-Ca2+ exchange of heart mitochondria by benzothiazepine CGP-37157. J Cardiovasc Pharmacol. 1993 Apr;21(4):595–9. doi: 10.1097/00005344-199304000-00013. [DOI] [PubMed] [Google Scholar]
- 135.Cox DA, Matlib MA. Modulation of intramitochondrial free Ca2+ concentration by antagonists of Na(+)-Ca2+ exchange. Trends Pharmacol Sci. 1993 Nov;14(11):408–13. doi: 10.1016/0165-6147(93)90063-P. [DOI] [PubMed] [Google Scholar]
- 136.Despa S, Islam MA, Weber CR, Pogwizd SM, Bers DM. Intracellular Na(+) concentration is elevated in heart failure but Na/K pump function is unchanged. Circulation. 2002 May 28;105(21):2543–8. doi: 10.1161/01.cir.0000016701.85760.97. [DOI] [PubMed] [Google Scholar]
- 137.Liu T, O’Rourke B. Enhancing mitochondrial Ca2+ uptake in myocytes from failing hearts restores energy supply and demand matching. Circ Res. 2008 Aug 1;103(3):279–88. doi: 10.1161/CIRCRESAHA.108.175919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kohlhaas M, Liu T, Knopp A, Zeller T, Ong MF, Bohm M, et al. Elevated cytosolic Na+ increases mitochondrial formation of reactive oxygen species in failing cardiac myocytes. Circulation. 2010 Apr 13;121(14):1606–13. doi: 10.1161/CIRCULATIONAHA.109.914911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kohlhaas M, Maack C. Adverse bioenergetic consequences of na+-Ca2+ exchanger-mediated Ca2+ influx in cardiac myocytes. Circulation. 2010 Nov 30;122(22):2273–80. doi: 10.1161/CIRCULATIONAHA.110.968057. [DOI] [PubMed] [Google Scholar]
- 140.Jung DW, Apel LM, Brierley GP. Transmembrane gradients of free Na+ in isolated heart mitochondria estimated using a fluorescent probe. Am J Physiol. 1992 Apr;262(4 Pt 1):C1047–55. doi: 10.1152/ajpcell.1992.262.4.C1047. [DOI] [PubMed] [Google Scholar]
- 141.Donoso P, Mill JG, O’Neill SC, Eisner DA. Fluorescence measurements of cytoplasmic and mitochondrial sodium concentration in rat ventricular myocytes. J Physiol. 1992 Mar;448:493–509. doi: 10.1113/jphysiol.1992.sp019053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gandhi S, Wood-Kaczmar A, Yao Z, Plun-Favreau H, Deas E, Klupsch K, et al. PINK1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell. 2009 Mar 13;33(5):627–38. doi: 10.1016/j.molcel.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Meier SD, Kovalchuk Y, Rose CR. Properties of the new fluorescent Na+ indicator CoroNa Green: comparison with SBFI and confocal Na+ imaging. J Neurosci Methods. 2006 Sep 15;155(2):251–9. doi: 10.1016/j.jneumeth.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 144.Wendt-Gallitelli MF, Isenberg G. X-ray microanalysis of single cardiac myocytes frozen under voltage-clamp conditions. Am J Physiol. 1989 Feb;256(2 Pt 2):H574–83. doi: 10.1152/ajpheart.1989.256.2.H574. [DOI] [PubMed] [Google Scholar]
- 145.Wendt-Gallitelli MF, Isenberg G. Total and free myoplasmic calcium during a contraction cycle: x-ray microanalysis in guinea-pig ventricular myocytes. J Physiol. 1991 Apr;435:349–72. doi: 10.1113/jphysiol.1991.sp018514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Baron S, Caplanusi A, van de Ven M, Radu M, Despa S, Lambrichts I, et al. Role of mitochondrial Na+ concentration, measured by CoroNa red, in the protection of metabolically inhibited MDCK cells. J Am Soc Nephrol. 2005 Dec;16(12):3490–7. doi: 10.1681/ASN.2005010075. [DOI] [PubMed] [Google Scholar]
- 147.Azarias G, Van de Ville D, Unser M, Chatton JY. Spontaneous NA+ transients in individual mitochondria of intact astrocytes. Glia. 2008 Feb;56(3):342–53. doi: 10.1002/glia.20619. [DOI] [PubMed] [Google Scholar]
- 148.Bernardinelli Y, Azarias G, Chatton JY. In situ fluorescence imaging of glutamate-evoked mitochondrial Na+ responses in astrocytes. Glia. 2006 Oct;54(5):460–70. doi: 10.1002/glia.20387. [DOI] [PubMed] [Google Scholar]
- 149.Chacon E, Reece JM, Nieminen AL, Zahrebelski G, Herman B, Lemasters JJ. Distribution of electrical potential, pH, free Ca2+, and volume inside cultured adult rabbit cardiac myocytes during chemical hypoxia: a multiparameter digitized confocal microscopic study. Biophys J. 1994 Apr;66(4):942–52. doi: 10.1016/S0006-3495(94)80904-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Takahashi A, Zhang Y, Centonze E, Herman B. Measurement of mitochondrial pH in situ. Biotechniques. 2001 Apr 30;4:804–8. 10, 12. doi: 10.2144/01304rv01. passim. [DOI] [PubMed] [Google Scholar]
- 151.Matsuyama S, Llopis J, Deveraux QL, Tsien RY, Reed JC. Changes in intramitochondrial and cytosolic pH: early events that modulate caspase activation during apoptosis. Nat Cell Biol. 2000 Jun;2(6):318–25. doi: 10.1038/35014006. [DOI] [PubMed] [Google Scholar]
- 152.Llopis J, McCaffery JM, Miyawaki A, Farquhar MG, Tsien RY. Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proc Natl Acad Sci U S A. 1998 Jun 9;95(12):6803–8. doi: 10.1073/pnas.95.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Balaban RS. Regulation of oxidative phosphorylation in the mammalian cell. Am J Physiol. 1990 Mar;258(3 Pt 1):C377–89. doi: 10.1152/ajpcell.1990.258.3.C377. [DOI] [PubMed] [Google Scholar]
- 154.Kanaide H, Yoshimura R, Makino N, Nakamura M. Regional myocardial function and metabolism during acute coronary artery occlusion. Am J Physiol. 1982 Jun;242(6):H980–9. doi: 10.1152/ajpheart.1982.242.6.H980. [DOI] [PubMed] [Google Scholar]
- 155.Eng J, Lynch RM, Balaban RS. Nicotinamide adenine dinucleotide fluorescence spectroscopy and imaging of isolated cardiac myocytes. Biophys J. 1989 Apr;55(4):621–30. doi: 10.1016/S0006-3495(89)82859-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Klingenberg M, Slenczka W. Pyridine nucleotide in liver mitochondria. An analysis of their redox relationships. Biochem Z. 1959;331:486–517. [PubMed] [Google Scholar]
- 157.Estabrook RW. Fluorometric measurement of reduced pyridine nucleotide in cellular and subcellular particles. Anal Biochem. 1962 Sep;4:231–45. doi: 10.1016/0003-2697(62)90006-4. [DOI] [PubMed] [Google Scholar]
- 158.Jobsis FF, Duffield JC. Oxidative and glycolytic recovery metabolism in muscle. J Gen Physiol. 1967 Mar;50(4):1009–47. doi: 10.1085/jgp.50.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Romashko DN, Marban E, O’Rourke B. Subcellular metabolic transients and mitochondrial redox waves in heart cells. Proc Natl Acad Sci U S A. 1998 Feb 17;95(4):1618–23. doi: 10.1073/pnas.95.4.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Aldakkak M, Stowe DF, Lesnefsky EJ, Heisner JS, Chen Q, Camara AK. Modulation of mitochondrial bioenergetics in the isolated Guinea pig beating heart by potassium and lidocaine cardioplegia: implications for cardioprotection. J Cardiovasc Pharmacol. 2009 Oct;54(4):298–309. doi: 10.1097/FJC.0b013e3181b2b842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Aldakkak M, Stowe DF, Heisner JS, Spence M, Camara AK. Enhanced Na+/H+ exchange during ischemia and reperfusion impairs mitochondrial bioenergetics and myocardial function. J Cardiovasc Pharmacol. 2008 Sep;52(3):236–44. doi: 10.1097/FJC.0b013e3181831337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Aldakkak M, Stowe DF, Chen Q, Lesnefsky EJ, Camara AK. Inhibited mitochondrial respiration by amobarbital during cardiac ischaemia improves redox state and reduces matrix Ca2+ overload and ROS release. Cardiovasc Res. 2008 Jan 15;77(2):406–15. doi: 10.1016/j.cardiores.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 163.Aldakkak M, Camara AK, Heisner JS, Yang M, Stowe DF. Ranolazine reduces Ca(2+) overload and oxidative stress and improves mitochondrial integrity to protect against ischemia reperfusion injury in isolated hearts. Pharmacol Res. 2011 Jun 29; doi: 10.1016/j.phrs.2011.06.018. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hanson GT, Aggeler R, Oglesbee D, Cannon M, Capaldi RA, Tsien RY, et al. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J Biol Chem. 2004 Mar 26;279(13):13044–53. doi: 10.1074/jbc.M312846200. [DOI] [PubMed] [Google Scholar]
- 165.Dooley CT, Dore TM, Hanson GT, Jackson WC, Remington SJ, Tsien RY. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J Biol Chem. 2004 May 21;279(21):22284–93. doi: 10.1074/jbc.M312847200. [DOI] [PubMed] [Google Scholar]
- 166.Chance B, Williams GR. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- 167.Gnaiger E. Bioenergetics at low oxygen: dependence of respiration and phosphorylation on oxygen and adenosine diphosphate supply. Respir Physiol. 2001 Nov 15;128(3):277–97. doi: 10.1016/s0034-5687(01)00307-3. [DOI] [PubMed] [Google Scholar]
- 168.Gnaiger E, Mendez G, Hand SC. High phosphorylation efficiency and depression of uncoupled respiration in mitochondria under hypoxia. Proc Natl Acad Sci U S A. 2000 Sep 26;97(20):11080–5. doi: 10.1073/pnas.97.20.11080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lanza IR, Nair KS. Functional assessment of isolated mitochondria in vitro. Methods Enzymol. 2009;457:349–72. doi: 10.1016/S0076-6879(09)05020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Aon MA, Cortassa S, O’Rourke B. Redox-optimized ROS balance: a unifying hypothesis. Biochim Biophys Acta. 2010 Jun-Jul;1797(6–7):865–77. doi: 10.1016/j.bbabio.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Aon MA, Cortassa S, Wei AC, Grunnet M, O’Rourke B. Energetic performance is improved by specific activation of K+ fluxes through K(Ca) channels in heart mitochondria. Biochim Biophys Acta. 2010 Jan;1797(1):71–80. doi: 10.1016/j.bbabio.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lin A, Krockmalnic G, Penman S. Imaging cytoskeleton--mitochondrial membrane attachments by embedment-free electron microscopy of saponin-extracted cells. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8565–9. doi: 10.1073/pnas.87.21.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Csordas G, Varnai P, Golenar T, Roy S, Purkins G, Schneider TG, et al. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol Cell. 2010 Jul 9;39(1):121–32. doi: 10.1016/j.molcel.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mamchaoui K, Saumon G. A method for measuring the oxygen consumption of intact cell monolayers. Am J Physiol Lung Cell Mol Physiol. 2000 Apr;278(4):L858–63. doi: 10.1152/ajplung.2000.278.4.L858. [DOI] [PubMed] [Google Scholar]
- 175.Renner K, Amberger A, Konwalinka G, Kofler R, Gnaiger E. Changes of mitochondrial respiration, mitochondrial content and cell size after induction of apoptosis in leukemia cells. Biochim Biophys Acta. 2003 Sep 23;1642(1–2):115–23. doi: 10.1016/s0167-4889(03)00105-8. [DOI] [PubMed] [Google Scholar]
- 176.Veksler VI, Kuznetsov AV, Sharov VG, Kapelko VI, Saks VA. Mitochondrial respiratory parameters in cardiac tissue: a novel method of assessment by using saponin-skinned fibers. Biochim Biophys Acta. 1987 Jun 29;892(2):191–6. doi: 10.1016/0005-2728(87)90174-5. [DOI] [PubMed] [Google Scholar]
- 177.Kunz WS, Kuznetsov AV, Gellerich FN. Mitochondrial oxidative phosphorylation in saponin-skinned human muscle fibers is stimulated by caffeine. FEBS Lett. 1993 May 24;323(1–2):188–90. doi: 10.1016/0014-5793(93)81477-h. [DOI] [PubMed] [Google Scholar]
- 178.Kunz WS, Kuznetsov AV, Schulze W, Eichhorn K, Schild L, Striggow F, et al. Functional characterization of mitochondrial oxidative phosphorylation in saponin-skinned human muscle fibers. Biochim Biophys Acta. 1993 Aug 16;1144(1):46–53. doi: 10.1016/0005-2728(93)90029-f. [DOI] [PubMed] [Google Scholar]
- 179.Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, et al. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci U S A. 2006 Oct 10;103(41):15038–43. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Robinson KM, Janes MS, Beckman JS. The selective detection of mitochondrial superoxide by live cell imaging. Nat Protoc. 2008;3(6):941–7. doi: 10.1038/nprot.2008.56. [DOI] [PubMed] [Google Scholar]
- 181.Dickinson BC, Srikun D, Chang CJ. Mitochondrial-targeted fluorescent probes for reactive oxygen species. Curr Opin Chem Biol. 2010 Feb;14(1):50–6. doi: 10.1016/j.cbpa.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zielonka J, Kalyanaraman B. Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: another inconvenient truth. Free Radic Biol Med. 2010 Apr 15;48(8):983–1001. doi: 10.1016/j.freeradbiomed.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rhee SG, Chang TS, Jeong W, Kang D. Methods for detection and measurement of hydrogen peroxide inside and outside of cells. Mol Cells. 2010 Jun;29(6):539–49. doi: 10.1007/s10059-010-0082-3. [DOI] [PubMed] [Google Scholar]
- 184.Maeda H, Yamamoto K, Nomura Y, Kohno I, Hafsi L, Ueda N, et al. A design of fluorescent probes for superoxide based on a nonredox mechanism. J Am Chem Soc. 2005 Jan 12;127(1):68–9. doi: 10.1021/ja047018k. [DOI] [PubMed] [Google Scholar]
- 185.Miller EW, Tulyathan O, Isacoff EY, Chang CJ. Molecular imaging of hydrogen peroxide produced for cell signaling. Nat Chem Biol. 2007 May;3(5):263–7. doi: 10.1038/nchembio871. [DOI] [PubMed] [Google Scholar]
- 186.Malinouski M, Zhou Y, Belousov VV, Hatfield DL, Gladyshev VN. Hydrogen peroxide probes directed to different cellular compartments. PLoS One. 2011;6(1):e14564. doi: 10.1371/journal.pone.0014564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Choi H, Kim S, Mukhopadhyay P, Cho S, Woo J, Storz G, et al. Structural basis of the redox switch in the OxyR transcription factor. Cell. 2001 Apr 6;105(1):103–13. doi: 10.1016/s0092-8674(01)00300-2. [DOI] [PubMed] [Google Scholar]
- 188.Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, et al. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods. 2006 Apr;3(4):281–6. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- 189.Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, et al. Superoxide flashes in single mitochondria. Cell. 2008 Jul 25;134(2):279–90. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ostergaard H, Henriksen A, Hansen FG, Winther JR. Shedding light on disulfide bond formation: engineering a redox switch in green fluorescent protein. EMBO J. 2001 Nov 1;20(21):5853–62. doi: 10.1093/emboj/20.21.5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gautier A, Juillerat A, Heinis C, Correa IR, Jr, Kindermann M, Beaufils F, et al. An engineered protein tag for multiprotein labeling in living cells. Chem Biol. 2008 Feb;15(2):128–36. doi: 10.1016/j.chembiol.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 192.Cocheme HM, Quin C, McQuaker SJ, Cabreiro F, Logan A, Prime TA, et al. Measurement of H2O2 within living Drosophila during aging using a ratiometric mass spectrometry probe targeted to the mitochondrial matrix. Cell Metab. 2011 Mar 2;13(3):340–50. doi: 10.1016/j.cmet.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dedkova EN, Ji X, Lipsius SL, Blatter LA. Mitochondrial calcium uptake stimulates nitric oxide production in mitochondria of bovine vascular endothelial cells. Am J Physiol Cell Physiol. 2004 Feb;286(2):C406–15. doi: 10.1152/ajpcell.00155.2003. [DOI] [PubMed] [Google Scholar]
- 194.Roychowdhury S, Luthe A, Keilhoff G, Wolf G, Horn TF. Oxidative stress in glial cultures: detection by DAF-2 fluorescence used as a tool to measure peroxynitrite rather than nitric oxide. Glia. 2002 Apr 15;38(2):103–14. doi: 10.1002/glia.10024. [DOI] [PubMed] [Google Scholar]
- 195.Zorov DB, Juhaszova M, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Regulation and pharmacology of the mitochondrial permeability transition pore. Cardiovasc Res. 2009 Jul 15;83(2):213–25. doi: 10.1093/cvr/cvp151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol. 2009 Jun;46(6):821–31. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 197.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005 Mar 31;434(7033):658–62. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 198.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007 May;9(5):550–5. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J. 1988 Oct 1;255(1):357–60. [PMC free article] [PubMed] [Google Scholar]
- 200.Crompton M, Costi A, Hayat L. Evidence for the presence of a reversible Ca2+-dependent pore activated by oxidative stress in heart mitochondria. Biochem J. 1987 Aug 1;245(3):915–8. doi: 10.1042/bj2450915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, et al. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 2006 May;273(10):2077–99. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- 202.Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim Biophys Acta. 1995 Jul 17;1241(2):139–76. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 203.Ruiz-Meana M, Garcia-Dorado D, Miro-Casas E, Abellan A, Soler-Soler J. Mitochondrial Ca2+ uptake during simulated ischemia does not affect permeability transition pore opening upon simulated reperfusion. Cardiovasc Res. 2006 Sep 1;71(4):715–24. doi: 10.1016/j.cardiores.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 204.Petronilli V, Szabo I, Zoratti M. The inner mitochondrial membrane contains ion-conducting channels similar to those found in bacteria. FEBS Lett. 1989 Dec 18;259(1):137–43. doi: 10.1016/0014-5793(89)81513-3. [DOI] [PubMed] [Google Scholar]
- 205.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, et al. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004 Jun;113(11):1535–49. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Dedkova EN, Blatter LA. Mitochondrial calcium uptake stimulates nitric oxide and ROS production by mitochondria-specific nitric oxide synthase (mtNOS) in cat ventricular myocytes. Biophysical Journal. 2006;90:521a. [Google Scholar]
- 207.Seidlmayer LK, Blatter LA, Pavlov E, Dedkova EN. Decreased levels of inorganic polyphosphates prevent opening of the mitochondrial permeability transition pore in ischemia-reperfusion injury. Circulation. 2010;122:A17373. [Google Scholar]
- 208.Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J. 1995 Apr 1;307( Pt 1):93–8. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Halestrap AP, Connern CP, Griffiths EJ, Kerr PM. Cyclosporin A binding to mitochondrial cyclophilin inhibits the permeability transition pore and protects hearts from ischaemia/reperfusion injury. Mol Cell Biochem. 1997 Sep;174(1–2):167–72. [PubMed] [Google Scholar]
- 210.Javadov SA, Clarke S, Das M, Griffiths EJ, Lim KH, Halestrap AP. Ischaemic preconditioning inhibits opening of mitochondrial permeability transition pores in the reperfused rat heart. J Physiol. 2003 Jun 1;549(Pt 2):513–24. doi: 10.1113/jphysiol.2003.034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chmelik M, Schmid AI, Gruber S, Szendroedi J, Krssak M, Trattnig S, et al. Three-dimensional high-resolution magnetic resonance spectroscopic imaging for absolute quantification of 31P metabolites in human liver. Magn Reson Med. 2008 Oct;60(4):796–802. doi: 10.1002/mrm.21762. [DOI] [PubMed] [Google Scholar]
- 212.Kingsley-Hickman PB, Sako EY, Ugurbil K, From AH, Foker JE. 31P NMR measurement of mitochondrial uncoupling in isolated rat hearts. J Biol Chem. 1990 Jan 25;265(3):1545–50. [PubMed] [Google Scholar]
- 213.Silverman HS, Di Lisa F, Hui RC, Miyata H, Sollott SJ, Hanford RG, et al. Regulation of intracellular free Mg2+ and contraction in single adult mammalian cardiac myocytes. Am J Physiol. 1994 Jan;266(1 Pt 1):C222–33. doi: 10.1152/ajpcell.1994.266.1.C222. [DOI] [PubMed] [Google Scholar]
- 214.Leyssens A, Nowicky AV, Patterson L, Crompton M, Duchen MR. The relationship between mitochondrial state, ATP hydrolysis, [Mg2+]i and [Ca2+]i studied in isolated rat cardiomyocytes. J Physiol. 1996 Oct 1;496( Pt 1):111–28. doi: 10.1113/jphysiol.1996.sp021669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Collins TJ, Lipp P, Berridge MJ, Li W, Bootman MD. Inositol 1,4,5-trisphosphate-induced Ca2+ release is inhibited by mitochondrial depolarization. Biochem J. 2000 Apr 15;347(Pt 2):593–600. doi: 10.1042/0264-6021:3470593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Aromolaran AS, Zima AV, Blatter LA. Role of glycolytically generated ATP for CaMKII-mediated regulation of intracellular Ca2+ signaling in bovine vascular endothelial cells. Am J Physiol Cell Physiol. 2007 Jul;293(1):C106–18. doi: 10.1152/ajpcell.00543.2006. [DOI] [PubMed] [Google Scholar]
- 217.Bell CJ, Manfredi G, Griffiths EJ, Rutter GA. Luciferase expression for ATP imaging: application to cardiac myocytes. Methods Cell Biol. 2007;80:341–52. doi: 10.1016/S0091-679X(06)80017-8. [DOI] [PubMed] [Google Scholar]
- 218.Jovanovic S, Du Q, Sukhodub A, Jovanovic A. A dual mechanism of cytoprotection afforded by M-LDH in embryonic heart H9C2 cells. Biochim Biophys Acta. 2009 Aug;1793(8):1379–86. doi: 10.1016/j.bbamcr.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Du Q, Jovanovic S, Sukhodub A, Jovanovic A. Infection with AV-SUR2A protects H9C2 cells against metabolic stress: a mechanism of SUR2A-mediated cytoprotection independent from the K(ATP) channel activity. Biochim Biophys Acta. 2010 Mar;1803(3):405–15. doi: 10.1016/j.bbamcr.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Brandes R, Bers DM. Increased work in cardiac trabeculae causes decreased mitochondrial NADH fluorescence followed by slow recovery. Biophys J. 1996 Aug;71(2):1024–35. doi: 10.1016/S0006-3495(96)79303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Brandes R, Bers DM. Intracellular Ca2+ increases the mitochondrial NADH concentration during elevated work in intact cardiac muscle. Circ Res. 1997 Jan;80(1):82–7. doi: 10.1161/01.res.80.1.82. [DOI] [PubMed] [Google Scholar]
- 222.Heineman FW, Balaban RS. Effects of afterload and heart rate on NAD(P)H redox state in the isolated rabbit heart. Am J Physiol. 1993 Feb;264(2 Pt 2):H433–40. doi: 10.1152/ajpheart.1993.264.2.H433. [DOI] [PubMed] [Google Scholar]