Abstract
Pituitary metastasis is an infrequent clinical problem occurring in 1%–5% of various autopsy series. Differentiated thyroid carcinoma as the primary malignancy was reported in only 2.1% of the cases. A 53-year-old Filipina presented with 7 months history of progressive loss of vision and headaches. She underwent thyroidectomy 2 years prior to admission for an enlarging neck mass. After then, she was lost to follow-up. Physical examination revealed visual field loss, galactorrhea and a 3×4 cm firm suprasternal mass. Imaging showed a 4.5×5×5 cm mass in the sphenoid and ethmoid sinuses with extension into the sella and suprasellar regions. Biopsy of the mass was consistent with papillary thyroid carcinoma, metastatic. For that, she underwent completion thyroidectomy, followed by surgical debulking of the sellar mass. Postoperatively, there was minimal improvement in vision and 13 months after, she is still on constant follow-up in our clinic, and is due for radioiodine therapy.
Background
Pituitary metastases (PM) are a rare clinical entity occurring in only 1%–5% of various autopsy series, with mostly the breast and lung carcinoma being the primary malignancy. Thyroid carcinoma being relatively rare with very low incidence of sellar-pituitary region metastases is reported to occur in only 2.1%. In the literature, there are only 13 published cases of differentiated thyroid carcinoma (DTC) with PM. It is important that we know the various presentations of such cases especially with the rising incidence of PM due to improvement in cancer survival rates in the last decade and the more sensitive imaging techniques used nowadays.
In this article, we will discuss the difference in clinical presentation, radiologic findings, the treatment and the outcome of PM from a carcinoma other than the thyroid, from that coming from a primary DTC. In contrast to the five previously reported cases of papillary thyroid carcinoma (PTC) with PM, in which all were diagnosed cases of PTC for several years prior to the discovery of PM, our patient presented first with the compressive symptoms pertinent to an enlarging pituitary gland due to the metastases prior to the diagnosis of a PTC. A discussion differentiating a pituitary adenoma from PM other than from a DTC, and PM from a DTC will also be presented.
Case presentation
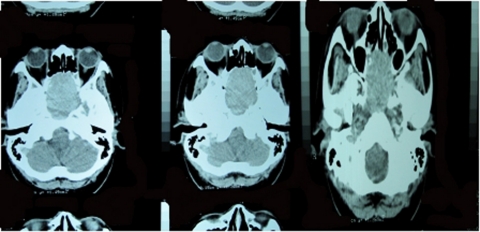
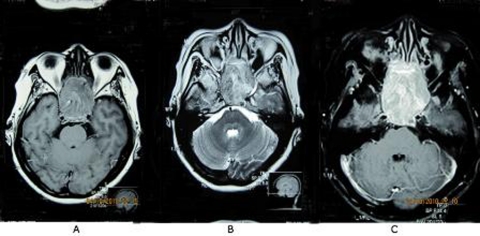
Our case is a 53-year-old Filipino female who came in for blurring of vision of her left eye that started 7 months prior to consultation. This was associated with headache, initially frontal radiating to the occipital area, relieved by bouts of vomiting. There was no history of antecedent trauma, neither weakness nor numbness of extremities, and there was no changes in sensorium. After seeking consultation with an ophthalmologist, CT scan of the orbits was requested showing a slightly enhancing soft tissue mass within both the sphenoid and ethmoid sinuses extending anteriorly to the posterior nasal chambers, with noted erosion and remodelling along the lateral walls of the sphenoid sinuses as well as the inferior wall of the dorsum sella (figure 1). A follow-up MRI of the brain showed a large well-defined intensely enhancing 4.5×5×5 cm tumour mass in the inferior frontal lobe with tumour extension into the sphenoid and the posterior ethmoid sinus regions, with secondary compression of the underlying sellar floor (figure 2). With time, bouts of headaches and vomiting were still persistent, blurring of vision was progressive eventually involving her right eye, and patient also complained of galactorrhea. There were no complains of neither polyuria nor polydypsia. Surgical intervention was advised, hence referred to our department for endocrine co management and clearance.
Figure 1.
CT scan of the orbits showing a slightly enhancing soft tissue mass within the ethmoid and sphenoid sinuses extending anteriorly to the posterior nasal chambers, with erosion and remodelling of the lateral walls of the sphenoid sinuses and the inferior wall of the dorsum sella.
Figure 2.
MRI images of the brain showing a large well-defined isointense tumour mass in the inferior frontal lobe measuring approximately 4.5×5×5 cm that is isointense on T1W1 (A), hyperintense on T2 (B) and intensely enhancing after gadolinium contrast injection (C).
Our patient is not previously known hypertensive, nor is she diabetic. She had a history of thyroidectomy in a local medical mission in their area 2 years prior to consultation for a gradually enlarging anterior neck mass that started 22 years prior, with no associated palpitations, no tremors, no heat intolerance and no weight loss. The extent of thyroidectomy was unknown. No pathology specimen was submitted, and the patient was eventually lost to follow-up.
She had her menarche at age 16 with regular monthly intervals thereafter. She is G7P5 (4-0-2-4), with one of her children dying from lymphoma at the age of 14. Aside from a family history of goitre and hypertension, she had no other significant heredofamilial diseases.
On physical examination, her vital signs were normal with a blood pressure of 120/80, and a body mass index of 27 kg/m2. A horizontal anterior neck scar was noted, with a 3×4 cm palpable suprasternal mass that was firm and does not move with deglutition. Galactorrhea was present. On confrontation, she had a central visual field loss on the right, and an inferior nasal quadrant visual field loss on the left. She was normoreflexive, with no noted tremors, and no exophthalmos. The rest of the physical examination was unremarkable. There was no moon facies, no dorsocervical fat pad and no purplish abdominal striae.
Investigations
Hormonal evaluation showed a decreased free thyroxine (FT4) hormone at 9.2 pmol/l, with an inappropriately normal thyroid stimulating hormone (TSH) at 1.7 mIU/l. Serum prolactin was at 1 862.7 mIU/l (67.2 nmol/l) consistent with pituitary stalk compression. She had a normal 8 A.M serum cortisol, normal serum sodium at 137 meq/l and urine specific gravity of 1.010.
Ultrasound of the thyroid showed a normal right thyroid lobe, but with a recurrent left thyroid solid mass measuring 6.64×6.20×3.67 cm with extension to the upper thoracic region, with its most superior portion in the inferior thyroid bed. This seems to imply that the patient underwent left thyroid lobectomy 2 years prior to consultation. Fine needle aspiration was done revealing only bloody aspirate.
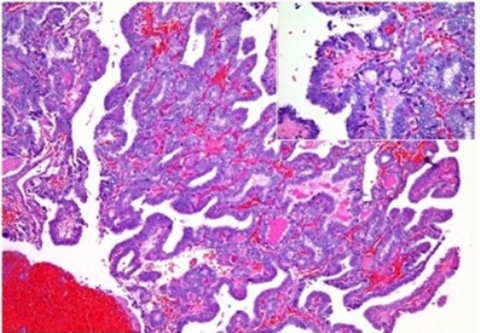
Because of ethmoid/sphenoid mass extending into the posterior nasal chamber, a biopsy of the said mass was easily done revealing papillary carcinoma, metastatic (figure 3).
Figure 3.
Right intranasal mass. Multiple papillary fronds are lined by cuboidal to low columnar cells forming colloid follicles. (Inset) Nuclear overlapping with occasional nuclear clearing. The papillary structures are lined by endothelial cells.
Treatment
Thyroid hormone replacement was initiated with levothyroxine at a dose of 1.6 mcg/kg body weight in preparation for surgery. Subsequent FT4 determinations were normal at 18.02 pmol/l, 15.1 pmol/l and 23.17 pmol/l, all taken 2 months apart.
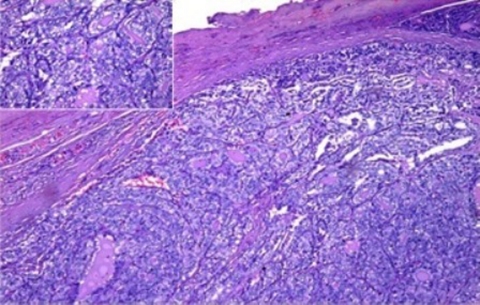
The patient underwent completion thyroidectomy with debulking of the sternal mass, however not much of the intrathoracic extension was removed due to significant bleeding. Pathology of the right thyroid was negative for tumour, suprasternal mass however was signed out as papillary carcinoma metastatic to the lymph node (figure 4).
Figure 4.
‘Suprasternal mass’. Neoplastic thyrocytes forming colloid follicles are haphazardly arranged within a fibrous capsule. (Inset) The neoplastic cells are cuboidal to low columnar showing nuclear overlapping, clearing and papillary formation.
Eventually, surgical debulking of the sphenoid/ethmoid mass was done via transnasal debulking endoscopic approach using a microdebrider. Intraoperative findings showed that the mass was smooth and erythematous medial to the middle turbinate. Due to sellar and suprasellar extension of the mass, she was covered with hydrocortisone 50 mg intravenous q8 perioperatively and eventually had unremarkable postoperative course.
Outcome and follow-up
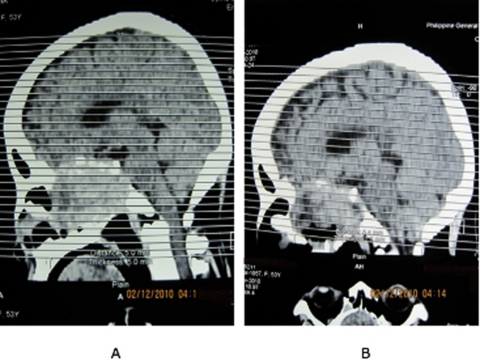
Our patient is still alive at 13 months after her initial complaint of blurring of vision. Postoperatively, her vision had minimal improvement but with much resolution of the headache and vomiting. Repeat cranial CT scan revealed a significant residual mass, mostly in the sellar-suprasellar region (figure 5). Due to the high probability of a blunted TSH rise after levothyroxine withdrawal, the use of recombinant human TSH (rhTSH) was contemplated; but due to financial constraints, this may not be possible. Our team’s plan is to withdraw levothyroxine for 3 weeks to give time for the endogenous TSH to rise before giving iodine-131 therapy. She would also be given steroids prior to and during the radioiodine therapy to prevent possible cerebral oedema given the significant residual mass. It was decided not to give radiotherapy prior to iodine-131 therapy because of the possible subsequent decrease in radioiodine uptake following tumour fibrosis that the prior can cause.
Figure 5.
Cranial CT scans, sagittal view, preoperatively (A) and postoperatively (B) showing significant residual mass.
Discussion
PM are infrequent clinical problem reported in 0.14%–28.1% of all brain metastases.1 In various autopsy series, this was reported to be as low as 1% to as high 5%.2–4 As reported by Kominos, neoplasms from almost every tissue have been reported to metastasise to the pituitary, with the breast cancer for females, and lung cancer for males accounting for approximately two thirds of the cases. In this series, PM from a primary thyroid malignancy accounted for only 2.1%.
PM occurs through the following routes of spread: (1) Direct haematogenous spread to the pituitary parenchyma or diaphragma sellae; (2) Spread from the hypothalmo-hypophysial or infundibulum metastases through the portal vessels; (3) Direct extension from the juxtasellar or skull base metastases; (4) Meningeal spread through the suprasellar cistern.1 Our patient most likely had direct extension from the primary ethmoid/sphenoid metastases to the sella, and then to the suprasellar area explaining her progressive loss of vision.
The posterior lobe, either alone or in combination with the anterior lobe was most commonly involved in an autopsy series of 201 cases done by McCormick et al,5 occurring in 84.6% of the cases, while the anterior lobe in 15.4%. Such predilection of metastases to the posterior lobe was attributed to the lack of direct arterial supply to the anterior lobe, plus the fact that the posterior lobe was in larger area of contact with the adjacent dura. The anterior lobe, on the other hand, is affected usually by direct contiguous spread from the posterior lobe, making it also susceptible to ischemic infarcts when associated with large metastatic lesions of the posterior lobe.1 Given this, the most common symptom then would be related to a posterior pituitary hormone deficiency. In one autopsy series studying seven cases of symptomatic PM, five patients presented with diabetes insipidus (DI), one with panhypopituitarism, and the other with neurologic deficit and hypoadrenalism.6 A similar series on eight patients with symptomatic PM, seven (70%) presented with DI, which was even the initial manifestation of the systemic cancer in two of the subjects. Other symptoms include headache, visual field defects which include oculomotor and trigeminal nerve dysfunction.7
One of the most common causes of a sellar-suprasellar mass however is a non-functioning pituitary adenoma. A pituitary adenoma however usually presents with visual field defects and an early anterior pituitary dysfunction, with DI being reported in only 2% of the cases. In contrast, PM usually presents with DI with the symptoms of anterior pituitary dysfunction developing only at a later onset. The presence of following therefore would suggest PM: (1) A rapidly growing tumour especially in a patient treated with dopamine agonists; (2) A sudden onset of DI; (3) Ophthalmoplegia; (4) Headaches; (5) Age >50 years old. All these criteria being irrespective of malignancy.1
Radiologic evaluation in differentiating an adenoma from PM has not been helpful. Both can present with sellar enlargement and deformity with various erosions and invasions. PM usually shows isointensity on T1W1, moderate hyperintensity on T2W1 and would usually show enhancement after giving gadolinium diethylenetriaminepentaacetic acid. One of the more specific MRI findings of a PM, however, is a dumbbell shaped intrasellar and suprasellar tumour, with a clear indentation at the level of the diaphragma sella (figure 6), a rare finding in an adenoma the reason being their difference in growth patterns. An adenoma usually grows slowly and can expand or destroy the diaphragm, whereas a PM has a rapid growth and they respect the diaphragm early in their course.7
Figure 6.
Dumbell shape morphology of pituitary metastases on brain MRI.7
Our patient however did not have signs and symptoms of DI, nor did her mass present with the characteristic dumbbell shaped morphology of PM on MRI. In contrast to PM from other primary carcinomas, PM from a DTC usually presents with signs and symptoms of mass effect such as blurring of vision, ptosis, oculomotor and abducens nerve palsies and headache.
These symptoms suggest parasellar lesion with relatively fast growth rather than an intrasellar lesion that destroys pituitary tissue or disconnects the pituitary stalk. This behaviour is also characteristic of thyroid carcinoma in loco, which grows outwardly rather than invasively.4 This explains the absence of DI, and the dumbbell shaped morphology on MRI in our patient. On MRI, PM from DTC is usually hypointense on T1W1, hyperintense on T2 weighted images destroying the enclosing bony structures.
As of this writing, there are only 13 reported cases of DTC metastasising to the pituitary, two from a medullary thyroid carcinoma, six from follicular and five from a PTC.4 Centering on PTC with PM, all cases were diagnosed cases of PTC ranging from 7 to 25 years with several regional sites of metastases prior to the diagnosis of PM. One presented with symptoms of panhypopituitarism, three with visual field defects and one with a blunted TSH response on levothyroxine withdrawal in preparation for an radioactive iodine (RAI) therapy. Four of these patients underwent debulking by transphenoidal resection, eventually followed by RAI therapy. Three patients had survived more than 1 year from diagnosis of PM, one died within 2 months after refusing further intervention, and another died due to massive intrathoracic haemorrhage from metastases.8
Currently there are no established therapeutic guidelines on how to manage PM. On review of the literature, PM is usually treated palliatively depending on the symptoms and extent of the systemic disease.9 With reports of surgery usually indicating the lesions to be firm, diffuse, invasive, vascular and haemorrhagic, complete surgical resection is unlikely. Hence, surgical exploration and decompression, alone or combined with radiation, is essential if clarification of diagnosis is likely to affect therapy or if suprasellar extension causes progressive deterioration of vision or pain.1 10 In view of the limitations of resectability, it appears logical to recommend radiation as the initial course of treatment.7 Concurrently, there are no guidelines on how to manage DTC with PM. With the reported cases, their mode of treatment mostly includes resection, postoperative ablation with iodine-131 and radiotherapy. One patient underwent stereotactic radiosurgery. As with PM from other carcinomas, surgical debulking and decompression are necessary to relieve pressure symptoms, especially if with progressive loss of vision. As stated in American Thyroid Associciation (ATA) guidelines in 2009, it was recommended that for DTC with central nervous system metastases, and not specifically on those with sellar metastases, that are not amenable to surgery should be considered for external beam irradiation. Also, if RAI therapy is being considered, prior external beam radiotherapy and concomitant glucocorticoid therapy is strongly recommended to minimise the effects of a potential TSH-induced increase in tumour size and the subsequent inflammatory effects of the RAI therapy (recommendation C).
Due to a high probability of a blunted TSH response after levothyroxine withdrawal postoperatively, and knowing that the uptake of radioiodine into thyroidal tissue is TSH dependent, the use of recombinant human TSH (rhTSH) prior to the RAI therapy may therefore deem necessary. The use of rhTSH is a safe and effective tool to stimulate radioiodine uptake.11 It is recommended by the ATA in 2009 to use rhTSH in patients with pituitary disease who are unable to raise their endogenous TSH.12
Prognosis of patients with PM is generally poor not because of the location but due to aggressiveness of the primary neoplasia.1 Mean survival length is 6–7 months, with 10% of patients dying with 1 year after diagnosis, and the longest survival being 3 years.13 14 Surgery however can control or limit the growth of the tumour and may increase the period of survival to more than a year in some patients.15 Prognosis of PM specifically from a DTC is less established with most of the reported cases were on constant follow-up at the time of their reporting. Two patients died, one from a primary medullary thyroid carcinoma and one from PTC, within 2 months after surgery due to intercurrent infection and after refusing further interventions, respectively. Another patient died 13 months after surgery due to massive intrathoracic haemorrhage. Our patient is still on constant follow-up 13 months after her initial complaint of blurring of vision and headache.
Learning points.
-
▶
Pituitary metastases should always be considered in patients with primary malignancy presenting with DI.
-
▶
Consider pituitary metastases in patients with differentiated thyroid carcinoma having a blunted TSH rise after levothyroxine withdrawal, symptoms of mass effect and ophthalmoplegia.
-
▶
DI is the most important criterion in differentiating a pituitary adenoma from PM.
-
▶
No guidelines in the management of PM, specifically that from DTC, but mostly includes surgical debulking, iodine-131 ablation and radiotherapy. More research in this field is needed.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Kominos J, Vlassopoulou V, Protopapa D, et al. Tumors metastatic to the pituitary gland: a case report and literature review. JCEM 2004;89:574. [DOI] [PubMed] [Google Scholar]
- 2.Nelson PB, Robinson AG, Martinez AJ. Metastatic tumor of the pituitary gland. Neurosurgery 1987;21:941–4 [DOI] [PubMed] [Google Scholar]
- 3.Yilmazlar S, Kocaeli H, Cordan T. Sella turcica metastases from follicular carcinoma of the thyroid. Neurological Research 2004;26:74–8 [DOI] [PubMed] [Google Scholar]
- 4.Prodam F, Pagano L, Belcastro S, et al. Pituitary metastases from follicular thyroid carcinoma. Thyroid 2010;20:823–30 [DOI] [PubMed] [Google Scholar]
- 5.McCormick PC, Post KD, Kandji AD, et al. Metastatic carcinoma to the pituitary gland. Br J Neurosurg 1989;3:71–9 [DOI] [PubMed] [Google Scholar]
- 6.Schubiger O, Haller D. Metastases to the pituitary-hypothalamic axis. Neuroradiology 1992;34:131–4 [DOI] [PubMed] [Google Scholar]
- 7.Sioutos P, Yen V, Arbit E. Pituitary gland metastases. Ann Surg Oncol 1996;3:94–9 [DOI] [PubMed] [Google Scholar]
- 8.Sziklas JJ, Mathews J, Spencer RP, et al. Thyroid carcinoma metastatic to the pituitary. J Nucl Med 1985;26:1097. [PubMed] [Google Scholar]
- 9.Jia-Lin X, Yong-Sheng W. Papillary thyroid carcinoma metastatic to the pituitary gland: a case report and literature review. J Chin Clin Med 2010;5:116–19 [Google Scholar]
- 10.Bell CD, Kovacs K, Horvath E, et al. Papillary carcinoma of thyroid metastatic to the pituitary gland. Arch Pathol Lab Med 2001;125:935–8 [DOI] [PubMed] [Google Scholar]
- 11.Masiukiewicz US, Nakchbandi IA, Stewart AF, et al. Papillary thyroid carcinoma metastatic to the pituitary gland. Thyroid 1999;9:1023–7 [DOI] [PubMed] [Google Scholar]
- 12.Cooper DS, Doherty GM, Haugen BR, et al. Revised American thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167–214 [DOI] [PubMed] [Google Scholar]
- 13.Ntyonga-Pono MP, Thomopoulos P, Luton JP. [Pituitary metastases. 3 cases]. Presse Med 1999;28:1567–71 [PubMed] [Google Scholar]
- 14.Nelson PB, Robinson AG, Martinez AJ. Metastatic tumor of the pituitary gland. Neurosurgery 1987;21:941–4 [DOI] [PubMed] [Google Scholar]
- 15.Scully R. Case records of the Massachusetts General Hospital. N Engl J Med 2001;345:1483–8 [DOI] [PubMed] [Google Scholar]