Abstract
Relapsing polychondritis and Sweet’s syndrome are rare systemic inflammatory conditions. The authors present a patient who developed Sweet’s syndrome 1 week after adalimumab therapy for refractory relapsing polychondritis. Coexistent relapsing polychondritis and Sweet’s syndrome is rare, however, is likely to represent a true disease association and signifies a high risk of myelodysplasia. Antitumour necrosis factor α (TNFα) therapy is a treatment option in both relapsing polychondritis and Sweet’s syndrome, and switching anti-TNFα agents may be feasible in the event of adverse reaction.
Background
This patient presented with the coexistence of two uncommon conditions; relapsing polychondritis (RP) and Sweet’s syndrome. Both RP and Sweet’s syndrome have been independently associated with myelodysplasia, however the incidence of malignancy appears to be much higher in patients with concurrent disease, and it is important to recognise this for appropriate screening.
This case also highlights diagnostic and treatment conundrums. The trigger for development of Sweet’s syndrome in this patient was not immediately clear and possibilities included infection, medication (adalimumab) and malignancy. A decision was made to treat the refractory RP complicated by Sweet’s syndrome with etanercept in view of his poor quality of life; it was felt that the risk of chronic high dose steroids outweighed the theoretical increased risk of malignant transformation associated with antitumour necrosis factor α (TNFα) agents.
To our knowledge, this is the first report that switching anti-TNFα agents may be effective in patients with RP.
Case presentation
A 66-year-old male presented to rheumatology in 2006 with a 5 month history of episodic fever, night sweats, weight loss and a limb rash comprised of tender erythematous plaques. Examination revealed an inflamed right pinna and nasal bridge, a hoarse voice and unilateral episcleritis. Inflammatory markers were elevated: C reactive protein (CRP) 84 mg/l (normal range (NR) 0–5), erythrocyte sedimentation rate (ESR) 44 mm/h (5–15). He was diagnosed with RP and initiated on prednisolone 40 mg daily with bone protection.
He responded well to treatment; however he failed to wean the prednisolone below 15 mg daily and developed complications of steroid therapy including Cushingoid facies and vertebral collapse. Oral methotrexate was commenced as a steroid-sparing agent, however doses above 15 mg weekly resulted in bone marrow suppression. He was switched to azathioprine 2.5 mg/kg with minimal response. He continued to flare frequently; these flares were treated effectively with short term escalation of prednisolone.
In view of persistent elevation of inflammatory markers and frequent disease relapses requiring chronic high dose steroid therapy, adalimumab 40 mg subcutaneously fortnightly was added to the azathioprine in April 2009. He responded dramatically within 1 week, both symptomatically and serologically with a reduction in CRP to 2 mg/l (NR 0–5).
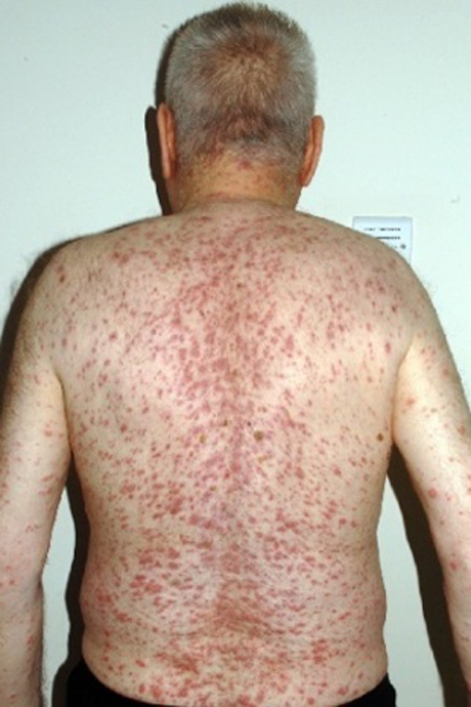
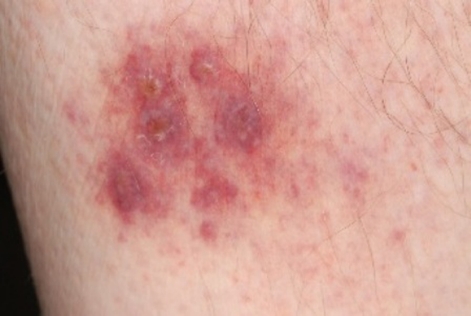
However, while attending for the second adalimumab injection, he reported a 4 day history of rigors, arthralgia and rash. The symptoms developed within hours of receiving root canal surgery, and failed to respond to an increase in prednisolone to 40 mg daily initiated by the patient. Examination revealed a fever of 39°C, an extensive pustular rash over the body and face, a purpuric rash on the legs and erythematous plaques on the dorsum of the left hand (figures 1 and 2). The immunosuppressive agents were withheld, a septic screen was performed, and he was treated with broad-spectrum antibiotics and intravenous acyclovir for presumed infection.
Figure 1.
Papular and pustular rash.
Figure 2.
Pustules with a purpuric base.
Investigations
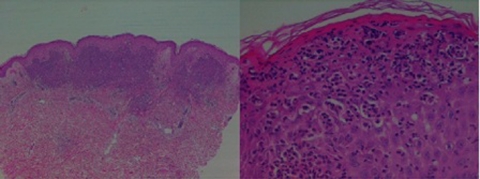
Investigations revealed a pancytopaenia and macrocytosis: haemoglobin 10.9 g/dl (NR 13–17.5), mean cell volume 108.5 fl (NR 80–100), white cell count 3.6×109/l (NR 3.7–11), platelets 125×109/l (NR 150–450). Inflammatory markers were elevated: ESR 97 mm/h, CRP 314 mg/l (NR 0–5). The septic screen was negative, including urinalysis, multiple sets of blood cultures, chest radiograph and transoesophageal echocardiogram. Antistreptolysin O titre and viral serology for varicella-zoster, Epstein–Barr virus, cytomegalovirus and parvovirus were also negative. A skin biopsy was consistent with acute neutrophilic dermatosis (Sweet’s syndrome) (figure 3A,B). A bone marrow biopsy was performed in view of the pancytopaenia and revealed abnormalities consistent with myelodysplastic syndrome of the refractory anaemia subtype.
Figure 3.
(A, B) Low and high power microscopy of skin biopsy consistent with Sweet’s syndrome: the epidermis shows focal parakeratosis, mild hyperkeratosis, mild acanthosis and focal dense neutrophilic infiltration forming microabscesses within. The superficial dermis is replaced by a dense acute inflammatory infiltrate, rich in polymorphs, admixed with nuclear debris and few lymphoid cells. The capillaries show leukocytoclasis, but no evidence of a vasculitic process.
Treatment
Intravenous methylprednisolone 1 g daily for 3 days was added to his therapy. His symptoms resolved and he was discharged home 9 days after admission on a reducing dose of prednisolone.
Outcome and follow-up
After discharge, he continued to suffer with recurrent flares of RP. Etanercept was commenced at 50 mg subcutaneously per week with excellent response. Inflammatory markers improved significantly (CRP 2 mg/l (NR 0–5), ESR 54 mm/h), and he felt well with a marked improvement in disease control.
Discussion
Relapsing polychondritis
RP is a rare systemic inflammatory disease characterised by episodic inflammation and destruction of cartilaginous tissues with a preponderance for auricular, nasal and laryngeal cartilage. Histological analysis of affected tissue characteristically shows neutrophilic or lymphocytic perichondritis. RP is associated with autoimmune or malignant conditions in approximately 30% of cases, most commonly rheumatoid arthritis (RA) and the myelodysplastic syndromes respectively.1 Treatment is largely empirical as there are no controlled therapeutic trials. Mild disease is often treated with non-steroidal anti-inflammatories or low dose steroids, while higher doses are reserved for more severe involvement. Steroid-sparing drugs are often prescribed, and there is anecdotal evidence supporting the use of most available disease-modifying agents. There are several case reports of successful treatment with antiTNFα agents in refractory RP.2 3
Sweet’s syndrome
Sweet’s syndrome is defined as an acute febrile neutrophilic dermatosis. Patients classically present with a sudden onset of fever, leucocytosis and tender erythematous skin lesions including plaques, papules and vesicles. Systemic involvement is rare. Biopsies typically show a neutrophil-predominant infiltration and leucocytoclasis within the upper dermis, often with marked dermal oedema. A rapid response to steroid therapy is characteristic.
Sweet’s syndrome commonly develops in association with other systemic disorders or identifiable triggers, including infection, medications and inflammatory disorders such as inflammatory bowel disease and RA. Sweet’s syndrome is associated with malignancy in 21% cases, most commonly haematological disorders such as acute myelogenous leukaemia.4 The temporal relationship between the onset of rash and the presentation of malignancy is variable; indeed, development of Sweet’s syndrome may signify relapse of previously treated neoplasia. Clinically, paraneoplastic Sweet’s syndrome may be indicated by the presence of anaemia, platelet count abnormalities, recurrent rash or the absence of neutrophilia.4 In general there are few differences between the appearance of the rash in idiopathic and malignancy-associated Sweet’s syndrome, however, vesicular, bullous or ulcerating skin lesions and oral involvement are more common in patients with underlying haematological malignancy.4 5 It is notable that our patient exhibited both a pancytopaenia and vesicular rash.
The aetiology of Sweet’s syndrome is unknown; it is considered to be a reactive dermatosis due to the wide variety of associated triggers and the prompt response to steroids. TNFα may be an important cytokine mediator.6 There are case reports of successful anti-TNFα therapy in patients with Sweet’s syndrome in association with another inflammatory disorder including Crohn’s disease, Sjogren’s syndrome and RA.6 7
Coexistent Sweet’s syndrome and relapsing polychondritis
To our knowledge, there are 19 cases in the published English literature of coexistent RP and Sweet’s syndrome.5 8–14 Several of these were identified in case series, with a variable temporal association between the two disorders.14 In a retrospective review examining dermatological involvement in 200 patients with RP, Sweet’s syndrome–like changes were detected on biopsy in seven cases (two with ‘pure’ RP, five with concurrent myelodysplasia).9 Of 48 patients with RP, three later developed Sweet’s syndrome.5 In a study of nine patients with Sweet’s syndrome and myelodysplasia, four subsequently developed RP.8
The coexistence of Sweet’s syndrome and RP is therefore likely to represent a true association, rather than the coincidental development of two uncommon conditions.15 A striking feature of these reported cases is the high incidence of associated myelodysplasia (10 of the 19 patients).8–10 It has been postulated that the development of Sweet’s syndrome in patients with RP and myelodysplasia may herald transformation of the haematological disease.10
In Vignon-Pennamen’s series of nine patients with Sweet’s syndrome and myelodysplasia, initial skin biopsy showed a mononuclear dermal infiltrate rather than the characteristic polymorphic infiltrate. However, biopsies from chronic disease showed the classical histological changes.8 As with our patient, the four individuals who later developed RP all presented with auricular and nasal chondritis in the absence of systemic involvement.8
There is one report of anti-TNFα therapy in a patient with active RP and Sweet’s syndrome. Although both the arthritis and rash responded quickly to infliximab, the patient died of septic shock.13
Learning points.
-
▶
We present a patient who developed Sweet’s syndrome 1 week after adalimumab therapy for refractory RP. He was later diagnosed with myelodysplastic syndrome.
-
▶
Both RP and Sweet’s syndrome have been independently associated with myelodysplasia, however the incidence of malignancy appears to be much higher in patients with coexistent disease. Vigilance is required for the development of neoplastic disorder in such patients.
-
▶
Anti-TNFα therapy presents a treatment option in both RP and Sweet’s syndrome. Switching anti-TNFα agents may be successful in the event of adverse reaction.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Diebold L, Rauh G, Jäger K, et al. Bone marrow pathology in relapsing polychondritis: high frequency of myelodysplastic syndromes. Br J Haematol 1995;89:820–30 [DOI] [PubMed] [Google Scholar]
- 2.Carter JD. Treatment of relapsing polychondritis with a TNF antagonist. J Rheumatol 2005;32:1413. [PubMed] [Google Scholar]
- 3.Seymour MW, Home DM, Williams RO, et al. Prolonged response to anti-tumour necrosis factor treatment with adalimumab (Humira) in relapsing polychondritis complicated by aortitis. Rheumatology (Oxford) 2007;46:1738–9 [DOI] [PubMed] [Google Scholar]
- 4.Cohen PR, Kurzrock R. Sweet’s syndrome and cancer. Clin Dermatol 1993;11:149–57 [DOI] [PubMed] [Google Scholar]
- 5.Fett DL, Gibson LE, Su WP. Sweet’s syndrome: systemic signs and symptoms and associated disorders. Mayo Clin Proc 1995;70:234–40 [DOI] [PubMed] [Google Scholar]
- 6.Foster EN, Nguyen KK, Sheikh RA, et al. Crohn’s disease associated with Sweet’s syndrome and Sjögren’s syndrome treated with infliximab. Clin Dev Immunol 2005;12:145–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamauchi PS, Turner L, Lowe NJ, et al. Treatment of recurrent Sweet’s syndrome with coexisting rheumatoid arthritis with the tumor necrosis factor antagonist etanercept. J Am Acad Dermatol 2006;54(3 Suppl 2):S122–6 [DOI] [PubMed] [Google Scholar]
- 8.Vignon-Pennamen MD, Juillard C, Rybojad M, et al. Chronic recurrent lymphocytic Sweet syndrome as a predictive marker of myelodysplasia: a report of 9 cases. Arch Dermatol 2006;142:1170–6 [DOI] [PubMed] [Google Scholar]
- 9.Francès C, el Rassi R, Laporte JL, et al. Dermatologic manifestations of relapsing polychondritis. A study of 200 cases at a single center. Medicine (Baltimore) 2001;80:173–9 [DOI] [PubMed] [Google Scholar]
- 10.Kawakami T, Kawase A, Takeuchi S, et al. Sweet syndrome subsequent to relapsing polychondritis and myelodysplastic syndrome in a Japanese patient. Acta Derm Venereol 2008;88:517–19 [DOI] [PubMed] [Google Scholar]
- 11.Astudillo L, Launay F, Lamant L, et al. Sweet’s syndrome revealing relapsing polychondritis. Int J Dermatol 2004;43:720–2 [DOI] [PubMed] [Google Scholar]
- 12.Fujimoto N, Tajima S, Ishibashi A, et al. Acute febrile neutrophilic dermatosis (Sweet’s syndrome) in a patient with relapsing polychondritis. Br J Dermatol 1998;139:930–1 [DOI] [PubMed] [Google Scholar]
- 13.Matzkies FG, Manger B, Schmitt-Haendle M, et al. Severe septicaemia in a patient with polychondritis and Sweet’s syndrome after initiation of treatment with infliximab. Ann Rheum Dis 2003;62:81–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vestergaard C, Soelvsten H, Ramsing M, et al. Concomitant Sweet’s syndrome and relapsing polychondritis. Acta Derm Venereol 2007;87:426–7 [DOI] [PubMed] [Google Scholar]
- 15.Cohen PR. Sweet’s syndrome and relapsing polychondritis: is their appearance in the same patient a coincidental occurrence or a bona fide association of these conditions? Int J Dermatol 2004;43:772–7 [DOI] [PubMed] [Google Scholar]