Abstract
The authors present a case of lupus mastitis which was initially diagnosed following an incisional biopsy of a breast lump, with similar pathology found 2 years later after an ultrasound guided biopsy of the same lump. The woman had been diagnosed 7 years before with systemic lupus erythematosus. The radiological and pathological features are presented in this report with discussion of similar cases in the literature.
Background
Lupus panniculitis (LP) is an uncommon entity, first described by Kaposi in1883.1 Lupus mastitis (LM) is a rare presentation of LP involving the deep subcutaneous adipose tissue of the breast. While affected patients are usually known to have systemic (SLE) or discoid lupus erythematosus (DLE), mastitis can also herald the onset of SLE or DLE. The pathological process that causes LM is not fully understood.2
SLE is an autoimmune disease in which cells undergo damage mediated by autoantibodies and immune complexes. On breast imaging, LM can mimic malignancy, however it has distinctive histologic features including a lobular lymphocytic panniculitis and hyaline fat necrosis, which enable specific diagnosis. Marked improvement of symptoms often occurs with immunosuppressive therapy and surgery should be avoided where possible, as this may trigger an additional flare of the disease.3
A case of LM is reported emphasising the radiological and pathological findings and briefly reviewing the literature.
Case presentation
A 34-year-old female presented to the emergency department with fever, right breast swelling and pain. She had known complications secondary to SLE including lupus-induced nephritis resulting in end stage renal failure, which was managed by haemodialysis. A combination of a right brachiocephalic fistula and superior vena cava stenosis led to right breast oedema that subsided following angioplasty and stenting.
Other medical history included mitral valve disease secondary to rheumatic fever with subsequent mechanical valve replacement, hypertension, staphylococcal endophthalmitis, recurrent pneumococcal sepsis and dyslipidaemia.
On examination of the right breast, swelling of the axillary tail of the breast and the tissues over the lateral thoracic wall was noted. The breast appeared inflamed, and was tender and warm to palpation. Other systems were normal.
Investigations
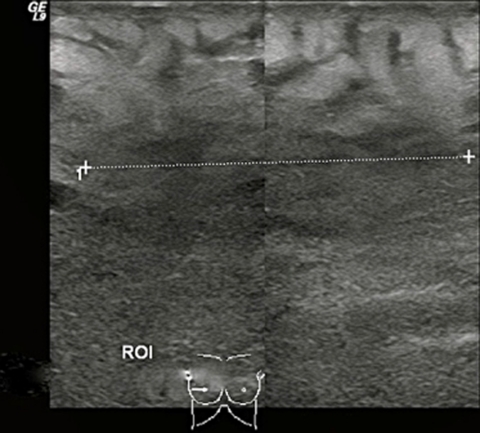
A breast ultrasound was performed to search for an abscess or collection. The breast appeared diffusely oedematous and an irregular hypoechoic area was noted laterally at the 9 o’clock position (figure 1).
Figure 1.
Right breast ultrasound: diffusely oedematous tissue throughout the breast with an irregular hypoechoic mass at the lateral aspect of the breast in the 9 o’clock position.
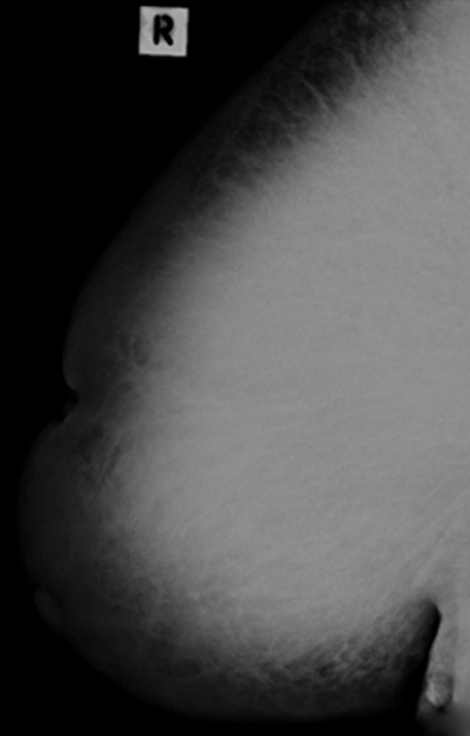
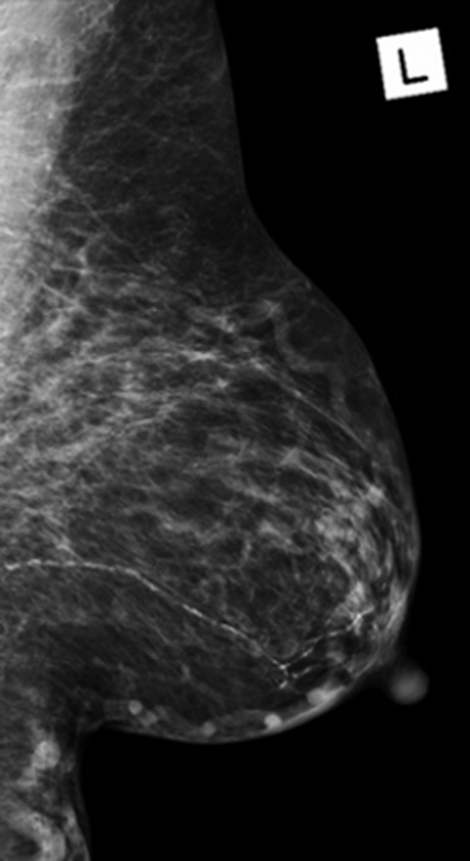
Mammography showed generalised increased density of the right breast compared with the left. No focal mass lesion or calcification was visible (figures 2 and 3).
Figure 2.
Right breast mammogram: gross asymmetric increase density involving the right breast when compared to the left. No focal mass lesion or calcification was noted.
Figure 3.
Left breast mammogram: gross asymmetric increase density involving the right breast when compared to the left. No focal mass lesion. Vascular calcification was noted.
The differential diagnosis for these appearances included mastitis with early abscess formation and malignancy with inflammatory carcinoma.
Surgical incision and drainage of the right breast was performed, however, there was no drainable collection and an excisional biopsy (30×25×15 mm) was performed.
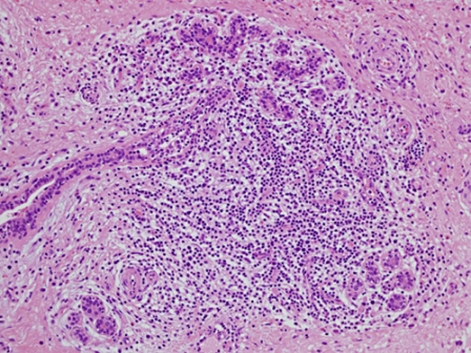
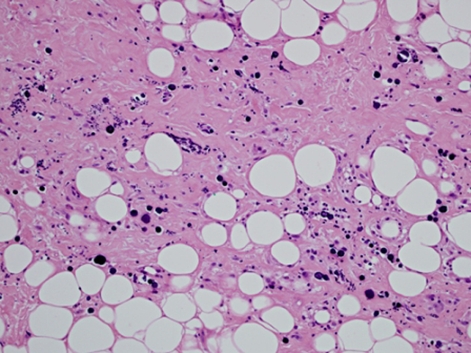
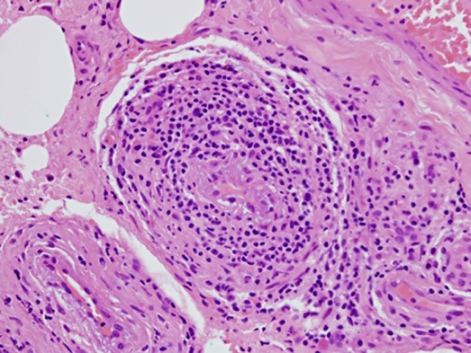
Histopathological analysis revealed hyaline fat necrosis with prominent calcification and sclerosis, periductal and perilobular lymphocytic infiltrates and vascular changes including lymphocytic vasculitis. No evidence of malignancy was seen and in the clinical setting of existing SLE, LM was diagnosed (figures 4–6).
Figure 4.
H&E stain: lymphocytic lobulitis with lobular atrophy, objective magnification x20.
Figure 6.
H&E stain: interstitial fibrosis and calcification within fat, objective magnification x20.
Figure 5.
H&E stain: perivascular lymphocytic infiltration, objective magnification x40.
Differential diagnosis
The most important differential diagnosis to exclude is inflammatory carcinoma of the breast. As the sonographic imaging features were suspicious, biopsy was performed to exclude malignancy.
Histopathological analysis demonstrated hyaline fat necrosis, sclerosis, calcification and lymphocytic vasculitis. These features are considered virtually pathognomonic for LM, with the presence of germinal centres excluding a subcutaneous panniculitis like T cell lymphoma.
Diabetic mastopathy can mimic LM histopathologically however, LM has a more extensive, less circumscribed lobular infiltrate.4
Primary medullary carcinoma also needs to be considered in the differential diagnosis, however in this case given the absence of both malignant epithelial cells on histology and the lack of a well-circumscribed lesion on mammogram and ultrasound, led this to be discounted.
Other differential diagnoses for a tender swollen breast included mastitis with abscess formation and breast oedema secondary to superior vena cava obstruction.
Treatment
While steroid therapy is the treatment of choice for LP, this was deemed inappropriate for our patient because Pseudomonas was isolated from a swab of the biopsy site placing her at risk of developing local infection or systemic complications, and in view of the history of recurrent sepsis. She continued on oral antibiotics and the changes in her right breast gradually improved.
Outcome and follow-up
Two years later, the patient presented with similar symptoms in the right breast with swelling, pain and fever. Clinical examination revealed peau d’orange and no discrete underlying palpable mass.
Breast ultrasound showed a 7 cm mass in the upper outer quadrant associated with generalised breast oedema and axillary lymphadenopathy. Ultrasound guided core biopsy again showed findings consistent with LM.
Discussion
Panniculitis is an inflammatory reaction in the subcutaneous adipose tissue that affects connective tissue septa of fat lobules.2
Panniculitis may be acute or chronic4 and when associated with SLE or DLE, is termed lupus erythematosus profundus or lupus panniculiltis (LP), a name first coined by Kaposi in 1883.1 LP is rare, occurring in only 2–3% of patients with SLE.5 6 LP is usually seen following the diagnosis of SLE/DLE but on rare occasions, may be the initial presentation.7 8
If breast tissue is involved, LP is called LM. Overlying skin can be normal erythematous, poikilodermic, hyperkeratotic or ulcerated.9 The aetiology of SLE is not well understood, but is thought to be an autoimmune disease in which organs, tissues and cells undergo damage mediated by tissue-binding autoantibodies and immune complexes.10
A literature search using PubMed revealed 30 previously described cases of LM6 9 11–14 in addition to ours, with the findings summarised in tables 1–3.
Table 1.
Demographic findings in LM
| Demographics | ||||
|---|---|---|---|---|
| Study | Age (y) | Sex | Race | Lupus history |
| Arsenovic and Terzic23 | 33 | F | NA | SLE 3 years |
| Bachmeyer et al18 | 30 | F | NA | DLE for 11 years |
| Bayar et al3 | 23 | F | NA | DLE for 2 years |
| Carducci et al7 | 62 | F | NA | DLE diagnosed after LM |
| Castro et al5 | 18 | F | Afro-Brazilian | SLE for 2 years |
| Cernea et al24 | 33 | F | White | DLE |
| Cerveira et al15 | 28 | F | NA | 5 Year history of SLE; 2 Year history of LP at other sites |
| Chen et al6 | 29 | F | African American | SLE for 5 years |
| Crevits et al16 | 50 | M | NA | SLE |
| De Bandt et al8 | 21 | F | African American | SLE diagnosed after LM presentation |
| Fernandez-Flores11 | 42 | M | NA | None identified |
| Fernandez-Torres11 | 57 | F | NA | None identified |
| Georgian-Smith et al17 | 44 | F | NA | SLE for 16 years |
| Guerre et al19 | 46 | F | NA | SLE for 10 years |
| Harris and Winkelmann28 | 36 | F | African American | DLE for 11 years |
| Harris and Winkelmann28 | 70 | F | White | DLE for 37 years |
| Holland et al20 | 49 | F | African American | DLE (length of disease not specified) |
| Holland et al20 | 26 | F | African American | SLE 6 years |
| Kinonen et al4 | 52 | F | African American | DLE |
| Kinonen et al4 | 58 | F | African American | SLE 15 years |
| Martella et al12 | 43 | M | NA | SLE and antiphospholipid antibody syndrome for 8 years |
| Nigar et al25 | 40 | F | NA | SLE for 1 year |
| Pons and Ortiz-Medina29 | 58 | F | Hispanic | None identified |
| Sabate et al26 | 33 | F | NA | SLE for 7 years with discoid lesions |
| Summers et al10 | 43 | F | African American | SLE (length of disease not specified) |
| Tuffanelli DL13 | 49 | F | White | SLE with discoid for 20 years |
| Vidal Pich et al14 | 13 | F | Hispanic | SLE (length of disease not specified) |
| Wang et al21 | 28 | F | Asian | SLE for 13 years |
| Wani et al22 | 26 | F | NA | SLE for 15 years |
| Current case | 36 | F | Australian Aboriginal | SLE for 8 years |
| Winkelmann9 | NA | M | NA | NA |
DLE, discoid lupus erythematosus; LM, lupus mastitis; LP, lupus panniculitis; SLE, systemic lupus erythematosus;
Table 3.
Histopathological findings in lupus mastitis
| Study | Location | Pathology |
|---|---|---|
| Arsenovic and Terzic23 | Right breast | Extensive hyaline fat necrosis associated with a lymphocytic infiltrate, both surrounding and in the lobular septa; microcalcifications present; lymphocytic vasculitis |
| Bachmeyer et al18 | Right breast | Voluminous calcifications with a fibrous reaction in breast parenchyma and rare ducts surrounded by lymphocytic infiltrate |
| Bayar et al3 | Right breast | Extensive stromal fibrosis, ductal and lobular atrophy and scattered stromal lymphocytes infiltrating some ducts |
| Carducci et al7 | Right breast | Lymphocytic lobular panniculitis with fat necrosis |
| Castro et al5 | Right breast | Lymphoplasmacytic infiltrate rich in xanthomatous histiocytes, fat necrosis and ductal hyperplasia |
| Cernea et al24 | Left breast | Hyalinisation of subcutaneous fat cells and collagen in the dermis with lymphocytic infiltrate |
| Cerveira et al15 | Left breast | Lobular and periseptal panniculitis with focal hyaline fat necrosis, lymphocytic infiltrate and coarse calcifications |
| Chen et al6 | Both breasts | Extensive mixed inflammatory cell infiltrate of lymphocytes, plasma cells around breast lobules and small vessel walls |
| Crevits et al16 | Right breast | No biopsy or excision performed |
| De Bandt et al8 | Both breasts | Hyaline fat coagulation, lymphocytic reaction and hyalinization of fat lobules with sclerosis/microcalcifications |
| Fernandez-Flores11 | Left breast | Lymphocytic panniculitis and vasculitis |
| Fernandez-Torres11 | Left breast | Hyaline fat necrosis/calcifications, lymphocytic vasculitis; lymphoplasmacytic infiltrate in the reticular dermis/subcutaneous fat, germinal centres present |
| Georgian-Smith et al17 | Left breast | Fat necrosis with microcalcification. Mastectomy confirmed the diagnosis of lupus mastitis |
| Guerre et al19 | Both breasts | Lymphocytic panniculitis with fat necrosis |
| Harris and Winkelmann28 | Left breast | Did not describe specific features – ‘consistent with lupus erythematosus panniculitis’ |
| Harris and Winkelmann28 | Both breasts | Biopsy never performed |
| Holland et al20 | Right breast | Fat necrosis and inflammation |
| Holland et al20 | Right breast | Chronic inflammation and fat necrosis compatible with panniculitis |
| Kinonen et al4 | Both breasts | Hyaline fat necrosis with lymphocytes in the subcutaneous fat, with germinal centre formation |
| Kinonen et al4 | Left breast | Prominent hyaline fat necrosis with widespread lymphocytic infiltration of the adipose tissue |
| Martella et al12 | Left breast | Panniculitis with areas of hyaline necrosis, perivascular inflammation and vasculitis |
| Nigar et al25 | Right breast | Inflammation and degenerative features consistent with lupus mastitis |
| Pons and Ortiz-Medina29 | Both breasts | Findings suggestive of lupus mastitis |
| Sabate et al26 | Left breast | Lobular panniculitis, including areas of hyaline and fat necrosis, perivascular lymphocytic inflammation and vasculitis |
| Summers et al10 | Right breast | Lobular lymphocytic panniculitis with lymphoplasmacytic infiltrates extending into fat and hyaline sclerosis of fat lobules |
| Tuffanelli DL13 | Right breast | Lymphocytic panniculitis |
| Vidal Pich et al14 | Right breast | Findings suggestive of lupus mastitis |
| Wang et al21 | Both breasts | Coarse dystrophic calcification, fatty necrosis and perivascular lymphocyte infiltration |
| Wani et al22 (BMJ case reports) | Left breast | Did not describe specific features – ‘consistent with lupus mastitis’ |
| Current case | Right breast | Fat necrosis with prominent calcifcation and sclerosis, lymphocytic infiltrates/vasculitis |
| Winkelmann9 | Breast | ductal calcification |
Table 2.
Imaging findings in lupus mastitis
| Study | Mammography | Ultrasound |
|---|---|---|
| Arsenovic and Terzic23 | Heterogeneous mass with multifocal coarse calcifications with poorly defined margins | Ill-defined heterogeneous mass with multifocal coarse calcifications |
| Bachmeyer et al18 | Coarse and curvilinear calcifications along the galactophoric ducts | Not suggested |
| Bayar et al3 | Dense tissue with coarse calcifications in the midline and lower half of the breast | Ill-defined, heterogeneous breast area with axillary lymphadenopathy. Parenchymal heterogeneity with skin thickening. No distinct mass |
| Carducci et al7 | An area of asymmetric density and hypodiaphania in the upper quadrant of the right breast | Subcutaneous tissue thickening. Ill-defined hyperechoic area in the subcutaneous fat with no associated vascularity |
| Castro et al5 | Dense breast tissue with an irregular, heterogeneous, ill-defined mass | Multiple nodular areas with inner echoes and ductal ectasia. Diffuse parenchyma texture and subcutaneous thickening |
| Cernea et al24 | Dense breast tissue with an irregular, heterogeneous, ill-defined mass | Ill defined, heterogeneous hyperechoic mass, extending into the subcutaneous fat |
| Cerveira et al15 | Bilateral coarse multiple calcifications | Coarse calcifications |
| Chen et al6 | Not suggested | Ill-defined isoechoic heterogeneous mass |
| Crevits et al16 | Coarse pleomorphic calcifications, diffusely spread all over the right breast | Not suggested |
| De Bandt et al8 | Homogenous round opacities | Not suggested |
| Fernandez-Flores11 | Not suggested | Not suggested |
| Fernandez-Torres11 | No significant abnormality | Not suggested |
| Georgian-Smith et al17 | Segmental heterogeneous calcifications. Diffuse increased density of fibroglandular tissue | Not suggested |
| Guerre et al19 | Bilateral non-specific breast calcification | Multiple cysts with generalised parenchymal oedema and skin thickening |
| Harris and Winkelmann28 | Mammograms were interpreted as carcinoma of the breast | Not suggested |
| Harris and Winkelmann28 | Tiny, benign-appearing nodule in the upper portion of the left breast | Not suggested |
| Holland et al20 | Scattered microcalcifications with a small amount of residual fibroglandular tissue | Not suggested |
| Holland et al20 | Right upper outer quadrant breast mass | Solid mass |
| Kinonen et al4 | Hazy, ill-defined, soft tissue density, but no discrete mass or calcifications | Ill-defined hyperechoic mass with mild to moderate vascularity |
| Kinonen et al4 | Focal irregular density in the upper central portion of the breast with microcalcifications | Heterogeneous area with increase vascularity |
| Martella et al12 | Not suggested | Echogenic subcutaneous lump, with irregular margins |
| Nigar et al25 | Irregular mass in the superior aspect of the right breast | Hypoechoic area with minimal vascularity |
| Pons and Ortiz-Medina29 | Not suggested | Not suggested |
| Sabate et al26 | Irregular mass with ill-defined margins involving the subcutaneous fat pad and skin thickening | Echogenic mass with ill-defined margins concerning the anterior fat pad involving the adjacent glandular parenchyma with skin thickening |
| Summers et al10 | Dense breast tissue with an irregular, heterogeneous, ill defined mass with calcifications | Not suggested |
| Tuffanelli DL13 | Not suggested | Not suggested |
| Vidal Pich et al14 | Not suggested | Not suggested |
| Wang et al21 | Multifocal, coarse calcifications with a linear pattern in both breasts | Several ill-defined hypoechoic areas in the breast parenchyma bilaterally. No associated vascularity |
| Wani et al22 | Diffuse calcifications bilaterally | Diffuse calcifications with acoustic shadowing |
| Current case | Widespread increased density compared to the left breast | Irregular nodular mass with multiple axillary lymph nodes showing thickened cortices |
| Winkelmann9 | Not suggested | Not suggested |
Of the 31 cases, 27 were female and 4 were male. The age of diagnosis ranged between 13 to 70 years in women (mean 39 years) and 42 to 50 years in men (mean 45 years).
In two of the female cases, SLE/DLE was diagnosed following the initial finding of LM.7 8
Mammogram
Frequency of mammographic findings in LM:
| Mammographic findings | Frequency | Percentage total (%) |
|---|---|---|
| Calcification of various types only | 93,15–22 | 30 |
| An irregular ill-defined mass associated with calcifications | 34,10,23 | 10 |
| An irregular non-calcified mass | 55,20,24–26 | 16 |
| An area of asymmetric density | 24,7 | 6 |
| No significant abnormality | 127 | 3 |
| Appearances suggesting breast carcinoma | 128 | 3 |
| Solitary benign nodule | 128 | 3 |
| Multiple round well-defined nodules | 18 | 3 |
| Widespread increased density | Current case | 3 |
| Not performed or findings not discussed | 76,9,11–14 | 23 |
Ultrasound
Frequency of ultrasound findings in LM:
| Ultrasound findings | Frequency | Percentage total (%) |
|---|---|---|
| Ill-defined mass with varying echogenicity | 103,4,6,7,12,21,23,24,26 | 33 |
| Coarse or diffuse calcification | 215,22 | 6 |
| Calcified mass | 123 | 3 |
| Solid mass | 120 | 3 |
| Nodular areas | 15 | 3 |
| Heterogeneous area with increased vascularity | 14 | 3 |
| Multiple cysts with generalised parenchymal oedema and skin thickening | 119 | 3 |
| Not performed or radiological report not discussed | 148–11,13,14,16–18,20,27–29 | 46 |
MRI
A single case of LM evaluated by MRI showed an ill-defined, lobulated heterogeneous rim enhancing mass involving the subcutaneous fat.26
Histopathology
Highly indicative (virtually pathognomonic) findings include lymphocytic lobular panniculitis with hyalinised fat necrosis.10
The lymphocytic infiltration is one of small, mature lymphocytes admixed with plasma cells mainly involving fat lobules however septal involvement may occur.
The presence of germinal centres may aid in differentiating LM from low-grade lymphoma.4
Treatment
LM is a medical disease with treatment consisting of antimalarial agents and corticosteroids for combination therapy. Surgical management can risk triggering an additional flare and should only be considered in patients who do not respond to medical treatment.3
Learning points.
-
▶
LM should be considered in the differential diagnosis of a suspicious breast mass on mammography or ultrasound particularly if the patient has a background of SLE/DLE.
-
▶
Histopathological findings of lymphocytic lobular panniculitis with hyalinised fat necrosis are virtually pathognomonic for LM.
-
▶
The diagnosis of LM is important to consider because the condition may be exacerbated by surgery.
-
▶
Antimalarial agents and corticosteroids are the first line treatment agents for LM.
-
▶
Careful patient follow-up is necessary to exclude malignancy.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Kaposi M. Pathologie und Therapie der Hautkrankheiten. Second Edition Vienna: Urban & Schwarzenberg; 1883:642 [Google Scholar]
- 2.Robbins S, Kumar V. Robbins and Cotran Pathologic Basis of Disease. Eight Edition Philadelphia, PA: Saunders and Elsevier; 2010 [Google Scholar]
- 3.Bayar S, Dusunceli E, Ceyhan K, et al. Lupus mastitis is not a surgical disease. Breast J 2007;13:187–8 [DOI] [PubMed] [Google Scholar]
- 4.Kinonen C, Gattuso P, Reddy VB. Lupus mastitis: an uncommon complication of systemic or discoid lupus. Am J Surg Pathol 2010;34:901–6 [DOI] [PubMed] [Google Scholar]
- 5.Castro GR, Appenzeller S, Soledade C, et al. Mastitis refractory to cyclophosphamide in systemic lupus erythematosus. Clin Exp Rheumatol 2004;22:786. [PubMed] [Google Scholar]
- 6.Chen X, Hoda SA, Delellis RA, et al. Lupus mastitis. Breast J 2005;11:283–4 [DOI] [PubMed] [Google Scholar]
- 7.Carducci M, Mussi A, Lisi S, et al. Lupus mastitis: a 2-year history of a single localization of lupus erythematosus mimicking breast carcinoma. J Eur Acad Dermatol Venereol 2005;19:260–2 [DOI] [PubMed] [Google Scholar]
- 8.De Bandt M, Meyer O, Grossin M, et al. Lupus mastitis heralding systemic lupus erythematosus with antiphospholipid syndrome. J Rheumatol 1993;20:1217–20 [PubMed] [Google Scholar]
- 9.Winkelmann RK. Panniculitis in connective tissue disease. Arch Dermatol 1983;119:336–44 [PubMed] [Google Scholar]
- 10.Summers TA, Jr, Lehman MB, Barner R, et al. Lupus mastitis: a clinicopathologic review and addition of a case. Adv Anat Pathol 2009;16:56–61 [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Flores A, Crespo LG, Alonso S, et al. Lupus mastitis in the male breast mimicking inflammatory carcinoma. Breast J 2006;12:272–3 [DOI] [PubMed] [Google Scholar]
- 12.Martella S, Matthes AG, Bassi F, et al. Lupus mastitis in male mimicking a breast lump. Int J Surg 2008;6:e67–9 [DOI] [PubMed] [Google Scholar]
- 13.Tuffanelli DL. Lupus erythematosus panniculitis (profundus). Arch Dermatol 1971;103:231–42 [PubMed] [Google Scholar]
- 14.Vidal Pich E, Bianchi C, Bianchi O, et al. Lupus eritematoso profundo y calcinosis cutis. Med Cut ILA 1972;6:259–64 [Google Scholar]
- 15.Cerveira I, Costa Matos L, Garrido A, et al. Lupus mastitis. Breast 2006;15:670–2 [DOI] [PubMed] [Google Scholar]
- 16.Crevits J, Van Steen A, Van Ongeval C, et al. Unilateral calcifying lupus mastitis in a male breast. Breast J 2009;15:307–8 [DOI] [PubMed] [Google Scholar]
- 17.Georgian-Smith D, Lawton TJ, Moe RE, et al. Lupus mastitis: radiologic and pathologic features. AJR Am J Roentgenol 2002;178:1233–5 [DOI] [PubMed] [Google Scholar]
- 18.Bachmeyer C, Goubin I, Berseneff H, et al. Coarse calcifications by mammography in lupus mastitis. Arch Dermatol 2006;142:398–9 [DOI] [PubMed] [Google Scholar]
- 19.Guerre AR, Pelletier F, Aubin F, et al. [Lupus mastitis associated with severe systemic erythematosus lupus]. Rev Med Interne 2009;30:540–2 [DOI] [PubMed] [Google Scholar]
- 20.Holland NW, McKnight K, Challa VR, et al. Lupus panniculitis (profundus) involving the breast: report of 2 cases and review of the literature. J Rheumatol 1995;22:344–6 [PubMed] [Google Scholar]
- 21.Wang YC, Chou CP, Levenson RB, et al. Imaging features of bilateral lupus mastitis. Breast J 2010;16:203–4 [DOI] [PubMed] [Google Scholar]
- 22.Arsenovic N, Terzic M. Lupus mastitis mimicking a breast tumor. J Obstet Gynaecol Res 2008;34:919–21 [DOI] [PubMed] [Google Scholar]
- 23.Arsenovic N, Terzic M. Lupus mastitis mimicking a breast tumor. J Obstet Gynaecol Res 2008;34:919–21 [DOI] [PubMed] [Google Scholar]
- 24.Cernea SS, Kihara SM, Sotto MN, et al. Lupus mastitis. J Am Acad Dermatol 1993;29(2 Pt 2):343–6 [DOI] [PubMed] [Google Scholar]
- 25.Nigar E, Contractor K, Singhal H, et al. Lupus mastitis - a cause of recurrent breast lumps. Histopathology 2007;51:847–9 [DOI] [PubMed] [Google Scholar]
- 26.Sabaté JM, Gómez A, Torrubia S, et al. Lupus panniculitis involving the breast. Eur Radiol 2006;16:53–6 [DOI] [PubMed] [Google Scholar]
- 27.Fernández-Torres R, Sacristán F, Del Pozo J, et al. Lupus mastitis, a mimicker of erysipelatoides breast carcinoma. J Am Acad Dermatol 2009;60:1074–6 [DOI] [PubMed] [Google Scholar]
- 28.Harris RB, Winkelmann RK. Lupus mastitis. Arch Dermatol 1978;114:410–2 [PubMed] [Google Scholar]
- 29.Pons S, Ortiz-Medina A. Lupus profundo mamario carcinomatoideo (lupus mastitis). Arch Argent Dermatol 1978;28:103–12 [Google Scholar]