Abstract
A man in his 60s presents with chronic dyspnoea and cough for 3 years. EKG and nuclear stress test were not diagnostic. An echocardiogram revealed moderate pericardial effusion. His symptoms improved with ibuprofen temporarily and a repeat echocardiogram showed resolution of the effusion. However, when his symptoms recurred, re-imaging showed a large intracardiac tumour causing right ventricular outflow obstruction. Subsequent histological examination revealed metastatic paraganglioma. He was found to carry a germline mutation in the SDHB gene which is associated with higher malignant risk. Knowledge of his underlying mutation allowed the patient and his family to receive appropriate gene-specific counselling and surveillance.
Background
Paragangliomas (PGL), while uncommon, often present late as a result of space-occupying complications. This case highlights the importance of early recognition and diagnosis of paraganliomas as they arise adjacent to important vascular structures such as the heart, aorta and carotid vessels in the neck. We wish to highlight the role that inherited genetic mutations have on the malignant potential of PGL. A germline SDHB mutation, such as in this case, is associated with extra-adrenal location and with malignant potential. This information also allows the patient’s family to receive appropriate genetic counselling, predictive testing and clinical surveillance as PGL grow insidiously, but can often be resected curatively when malignant transformation is detected early. It is important for clinicians to note that PGL can mimic common symptoms, are not all indolent and may prove deadly if detected late.
Case presentation
In April 2010, a 62-year-old man, with a medical history of prostate cancer, diabetes, hyperlipidaemia and occasional tobacco use, presented with chronic dyspnea and cough. He first consulted an cardiologist in May 2007 for exertional fatigue. Clinical examination was unremarkable. Biochemical and haematological investigations were normal. An EKG was non-diagnostic with predominantly upsloping ST-segment depression; nuclear stress test was normal. An April 2008-echocardiogram performed for persistent dyspnea and cough revealed a moderate pericardial effusion, which persisted through September. He was treated with ibuprofen. His cardiologist was considering a chest CT if symptoms continued; however, repeat echocardiogram in November 2008 revealed near resolution of the pericardial effusion, and his symptoms also disappeared.
He returned to his cardiologist in April 2010 with progressive exertional dyspnoea and cough. Echocardiogram was reportedly normal apart from mildly elevated pulmonary artery pressure at 30.9 mm Hg (normal range 17–28). The patient sought a second opinion at this point. Chest x-ray was suspicious for collapse of the right middle lobe; chest CT performed for further evaluation demonstrated a 6.8×3.8 cm soft tissue density arising from the anterior heart (figure 1). 18F-fluorodeoxyglucose positron emission tomography showed intense activity in the mass related to the right ventricular outflow tract (figure 2A). Thoracoscopy with biopsy of an enlarged aortopulmonary-window lymph node was positive for metastatic PGL. Subsequent plasma catecholamine and metanephrine levels were obtained with dopamine significantly elevated at 898 pg/ml (0–20). The patient denied classic symptoms of secretory PGL including hypertension, headache, palpitations, or diaphoresis.
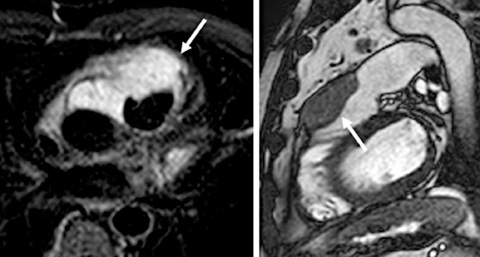
Figure 1.
Cardiac MRI. (A) Axial T2-weighted short τ inversion recovery cardiac MRI image shows a mass with high signal intensity (arrow) anterior to the aorta and the pulmonary artery/right ventricular (RV) outflow tract, extending to the space in between them. (B) Sagittal steady state free precession MRI image shows the mass anterior to the RV outflow tract and pulmonary artery and invading the anterior RV wall and proximal pulmonary artery.
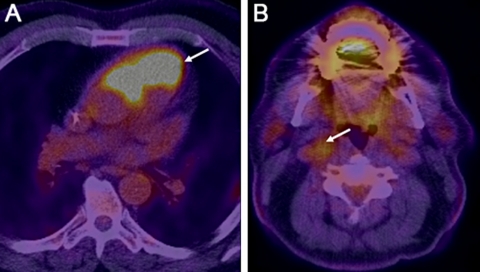
Figure 2.
Paragangliomas revealed by 18F-fluorodeoxyglucose positron emission tomography. (A) Fused positron emission tomography (PET)-CT image of the chest shows a 6.8×3.8 cm hypermetabolic soft tissue mass with max standardised uptake value (SUV) of 39.6 arising anterior to and extending in between aorta and the pulmonary artery/right ventricular outflow tract. (B) Fused PET-CT image of the neck shows a 1.7×1.3 cm hypermetabolic mass with max SUV of 4.2 s playing the right carotid bifurcation. This mass also showed intense contrast enhancement on CT (not shown here).
Investigations
(Included in above vignette).
Differential diagnosis
Cardiac tumours are uncommon and it is important that clinicians do not overlook them, because the symptomatology mimics common heart and lung conditions. The persistent and progressive nature of the patient’s symptoms would suggest a space-occupying lesion or an underlying cardiac cause. His echocardiogram did not show any valvular pathology and was not suggestive of any cardiomyopathy. Together with the presence of a moderate-sized pericardial effusion, and lack of other signs to suggest inflammation, drug-related or infection as a cause for the pericardial effusion, one should suspect a tumour. It is possible that symptomatic treatment with non-steroidal anti-inflammatory drugs such as ibuprofen could have temporarily led to symptom improvement and resolution of the effusion. Moderate-sized pericardial effusions should be investigated thoroughly prior to symptomatic treatment. Common malignant causes of moderate-sized pericardial effusions include metastatic disease, non-Hodgkin’s lymphomas, Hodgkin disease and other less common cardiac tumours such as PGL as illustrated by this case.
Treatment
Given the location of the tumour, resection was not favoured and the patient underwent alcohol ablation and coiling of the conus branch of the right coronary artery supplying the PGL. He is currently receiving palliative chemotherapy.
Discussion
Phaeochromocytomas (PHEO) and PGL are rare neuroendocrine tumours with an estimated prevalence of 1:4500 and 1:1700 and with an annual incidence of 3–8 cases per 1 million a year in the general population.1 PHEO/PGLs arise from three anatomically and functionally distinct parts of the neural crest-derived symphatho-adrenal medulla, sympathetic and parasympathetic paraganglia. PGLs arise from parasympathetic- (most commonly along the cranial nerves, eg, carotid body tumour) and from extra-adrenal sympathetic associated chromaffin tissues. The latter are diffuse scattered throughout the human body which is why these tumours may be found practically in any location for example, cardiac as in this case. According to autopsy findings, only 1–2% of all PGLs have a thoracic origin,2 with cardiac lesions occurring even less uncommonly. Cardiac PGLs can arise within the pericardium or can be intracardiac.3–6
From the autopsy findings, there is a 0.05% prevalence of PHEO/PGLs in an unselected population, suggesting that many such tumours may be overlooked in clinical practice. It is noteworthy that while we emphasise the classical symptom triad of headache, sweating and palpitation combined with persistent or paraxysmal hypertension in the medical curriculum, a recent study of 201 patients with PHEOs reveal that only 10% of patient present with the typical triad of symptoms.7 A further 10% of patients were asymptomatic and 6% were normotensive. Clinical suspicion for PHEO/PGLs should be maintained even in the absence of classical symptoms.
Between a quarter and a third of PHEO/ PGLs have a familial aetiology.8 9 This group is heterogeneous and includes the following syndromes: von Hiddel-Lindau disease, multiple endocrine neoplasia type 2, neurofibromatosis type 1 and succinate dehydrogenase (SDHx) mutation-related PGL syndromes. Despite the fact that different germline SDHx mutations occur in one subunit of a multi-unit enzyme, they have phenotypic heterogeneity (table 1).
Table 1.
Characteristics of succinate dehydrogenase-related PGL
| Gene | SDHA | SDHB | SDHC | SDHD | SDHAF2 |
| Chromosome location | 5p15 | 1p35-p36.1 | 1q21 | 11q23 | 11q13.1 |
| Clinical features | Necrotising encephalopathy with homozygous mutations, PGL with heterozygous mutation | PHEO/PGL, papillary thyroid cancer, renal cell carcinoma | PHEO/PGL | PHEO/PGL | PHEO/PGL |
| Syndrome | Leigh | PGL4 | PGL3 | PGL1 | PGL2 |
| Inheritance | Autosomal recessive in Leigh syndrome, Autosomal dominant in PGL | Autosomal dominant | Autosomal dominant | Autosomal dominant with maternal imprinting | Autosomal dominant with maternal imprinting |
| Malignant risk1–3 | Unknown | 14–34% | Unknown, likely low | 0–8% | Unknown, likely low |
| Multifocal disease | Unknown | Frequent | Unknown | Frequent | Frequent |
| Most common primary site | Unknown | Extra-adrenal abdominal, thoracic | Head and neck | Head and neck, adrenal | Head and neck |
PGL, paraganglioma; PHEO, phaeochromocytomas.
SDH is a mitochondrial enzyme, carrying dual function in the process of mitochondrial energy production. It consists of four functionally different subunits: A, B, C and D. All four subunits of the SDH complex are implicated in PHEO/PGL development, in addition to SDHAF2 (SDH assembly factor 2), a cofactor of SDHA. Genes for SDHA, SDHB, SDHC and SDHD are nuclear-encoded genes and the mode of inheritance is autosomal dominant with maternal imprinting for SDHD and SDHAF2. Germline mutations result in loss of function of SDH.
Numerous studies have explored the clinical features and malignant potential associated with SDH-related PHEO/PGLs.10 11 Germline SDHB mutations as seen in our patient are related to high malignant potential, with risk for metastases. The majority of patients with SDHB mutations have extra-adrenal abdominal or thoracic disease. About 10% of SDHB-related tumours are biochemically silent or produce only dopamine12 13 which may account for a subset of patients who are normotensive and have few classical symptoms.
Biochemical diagnosis of SDH-related PHEO/PGL follows expert recommendations from the International Symposium on Pheochromocytoma for initial biochemical testing that includes measurements of fractionated metanephrines in urine or plasma or both.14 15 Both CT scan and MRI techniques are sensitive anatomical imaging modalities but newer functional imaging techniques have improved specificity.16 The mainstay of treatment for any PHEO/PGL is surgical resection with perioperative attention at preventing catecholamine induced complications like hypertensive crisis or cardiac arrythmias. There are unfortunately no effective treatments for malignant tumours and the usefulness of surgical debulking is not clear. The 5-year survival rate for metastatic PGLs is variable but is estimated to be about 50%.17 For patients who have vital organs such as the heart affected, PGLs can be rapidly lethal when left undiagnosed.
Learning points.
-
▶
SDH-related PGL are rare, with potentially lethal conditions.
-
▶
Biochemically, these tumours can be secretory or silent.
-
▶
A subset of these tumours have a malignant potential, especially in individuals who carry SDHB mutations. Treatment in these patients may require early surgical intervention especially if within or in proximity to vital structures.
-
▶
Up to a third of phaeochromocytomas or PGLs are associated with an underlying germline mutation in SDH-related genes.
-
▶
Identifying the underlying gene and mutation will allow for gene-specific tailored surveillance and management for the patient, and predictive testing and gene-specific management for the family.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Pacak KC, Chrousos GP, Koch CA, et al. Pheochromocytoma:Progress in Diagnosis, Therapy and Genetics. First Edition New Jersey: Humana Press; 2001 [Google Scholar]
- 2.McNeil AR, Blok BH, Koelmeyer TD, et al. Phaeochromocytomas discovered during coronial autopsies in Sydney, Melbourne and Auckland. Aust N Z J Med 2000;30:648–52 [DOI] [PubMed] [Google Scholar]
- 3.Besterman E, Bromley LL, Peart WS. An intrapericardial phaeochromocytoma. Br Heart J 1974;36:318–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gopalakrishnan R, Ticzon AR, Cruz PA, et al. Cardiac paraganglioma (chemodectoma): a case report and review of the literature. J Thorac Cardiovasc Surg 1978;76:183–9 [PubMed] [Google Scholar]
- 5.Karabinos I, Rouska E, Charokopos N. A primary cardiac paraganglioma. Eur Heart J. 2011 (In Press). doi: 10.1093/eurheartj/ehr294. [DOI] [PubMed] [Google Scholar]
- 6.Sharma SK, Sharma S, Mukhopadhyay S. Mediastinal paraganglioma presenting as an intracardiac mass with superior vena caval obstruction. Thorax 1993;48:1181–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopetschke R, Slisko M, Kilisli A, et al. Frequent incidental discovery of phaeochromocytoma: data from a German cohort of 201 phaeochromocytoma. Eur J Endocrinol 2009;161:355–61 [DOI] [PubMed] [Google Scholar]
- 8.Neumann HP, Bausch B, McWhinney SR, et al. Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med 2002;346:1459–66 [DOI] [PubMed] [Google Scholar]
- 9.Erlic Z, Rybicki L, Peczkowska M, et al. Clinical predictors and algorithm for the genetic diagnosis of pheochromocytoma patients. Clin Cancer Res 2009;15:6378–85 [DOI] [PubMed] [Google Scholar]
- 10.Neumann HP, Pawlu C, Peczkowska M, et al. Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA 2004;292:943–51 [DOI] [PubMed] [Google Scholar]
- 11.Burnichon N, Rohmer V, Amar L, et al. The succinate dehydrogenase genetic testing in a large prospective series of patients with paragangliomas. J Clin Endocrinol Metab 2009;94:2817–27 [DOI] [PubMed] [Google Scholar]
- 12.Timmers HJ, Kozupa A, Eisenhofer G, et al. Clinical presentations, biochemical phenotypes, and genotype-phenotype correlations in patients with succinate dehydrogenase subunit B-associated pheochromocytomas and paragangliomas. J Clin Endocrinol Metab 2007;92:779–86 [DOI] [PubMed] [Google Scholar]
- 13.Timmers HJ, Gimenez-Roqueplo AP, Mannelli M, et al. Clinical aspects of SDHx-related pheochromocytoma and paraganglioma. Endocr Relat Cancer 2009;16:391–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pacak K, Eisenhofer G, Ahlman H, et al. Pheochromocytoma: recommendations for clinical practice from the First International Symposium. October 2005. Nat Clin Pract Endocrinol Metab 2007;3:92–102 [DOI] [PubMed] [Google Scholar]
- 15.Grossman A, Pacak K, Sawka A, et al. Biochemical diagnosis and localization of pheochromocytoma: can we reach a consensus? Ann N Y Acad Sci 2006;1073:332–47 [DOI] [PubMed] [Google Scholar]
- 16.Timmers HJ, Chen CC, Carrasquillo JA, et al. Comparison of 18F-fluoro-L-DOPA, 18F-fluoro-deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab 2009;94:4757–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenhofer G, Bornstein SR, Brouwers FM, et al. Malignant pheochromocytoma: current status and initiatives for future progress. Endocr Relat Cancer 2004;11:423–36 [DOI] [PubMed] [Google Scholar]