Abstract
Primary mediastinal desmoplastic small round cell cancer is an uncommon tumour usually located in the abdomen and pelvis. Here the authors report an extremely rare case of a young male with a primary desmoplastic small round cell tumour in the anterior and middle mediastinum. The patient had non-specific complaints but an abnormal shadow was seen in a routine chest x-ray. He was diagnosed as having mediastinal mass with few lung parenchymal deposits on CT. Mediastinoscopy and guided biopsy revealed desmoplastic small round cell tumour. Desmoplastic small round cell tumour is a rare and aggressive tumour which rarely involves the mediastinum as a primary site. The nature of the lesion and its prognosis were explained to the patient. He was offered chemotherapy and radiotherapy for the tumour management. He refused treatment and left against medical advice.
Background
Desmoplastic small round cell tumour is a very rare but aggressive tumour having an extremely poor prognosis with a life span of less than 2 years. Here, we report an extremely rare case of a young male with primary desmoplastic small round cell tumour in the anterior and middle mediastinum. Although this case is rare, desmoplastic tumours should be included among possible differential diagnoses of an anterior or middle mediastinal mass.
Case presentation
The patient, a young 22-year-old male, presented with cough with minimal expectoration of 1 month’s duration, followed by intermittent episodes of fever for 2–3 weeks. The fever was low grade. It was associated with loss of appetite and weight loss of 4 kilograms.
Examination revealed a thin young man, afebrile, with normal pulse, blood pressure and respiratory rate. Pallor was present.
Investigations
His investigations showed a haemoglobin of 9.4 g/dl, and red blood cells were normocytic and normochromic. Total leucocyte count and platelet count were within normal limits. The erythrocyte sedimentation rate was 35 mm. Serum lactate dehydrogenase was 649 U/l. Rest of the biochemical parameters were within normal limits.
The chest radiograph revealed a large non-homogenous opacity in the left para-mediastinal region with a few pulmonary deposits (figure 1). Contrast-enhanced CT (CECT) of chest showed a large, lobulated hetergenously enhancing lesion involving the mediastinum and para-mediastinal areas on the left side of chest, with necrotic and calcific foci within (figures 2 and 3). There was also evidence of nodular pleural deposits. The abdomen and pelvis appeared normal on CECT.
Figure 1.
X-ray chest (posteroanterior view)-the chest radiograph revealed a large non-homogenous opacity in the left para-mediastinal region with a few pulmonary deposits.
Figure 2.
(a, b) The biopsy revealed a cellular tumour comprising of nests and groups of polygonal to spindle shaped pleomorphic cells, separated by fibrous septa, having high nuclear to cytoplasmic ratio, high mitosis and some areas of necrosis (a-photomicrograph 40x, b-photomicrograph 200x).
Figure 3.
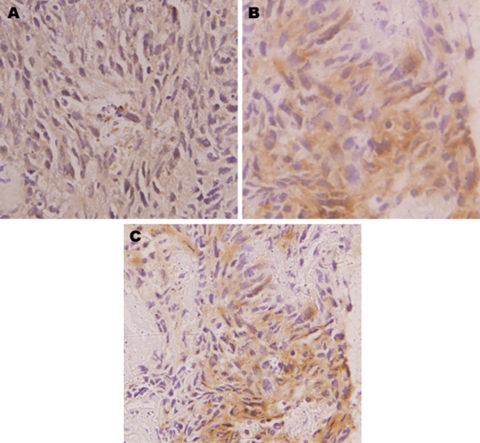
(a–c) On immunohistochemistry, the tumour cells were negative for leucocyte common antigen (a), strongly expressed cytokeratin (b), vimentin (c).
The patient was negative for HIV by ELISA. Also, his sputum was negative for acid fast bacilli. An initial impression of primary germ cell tumour was made.
Mediastinoscopy and biopsy of the mass were performed. The biopsy revealed a cellular tumour comprising of nests and groups of polygonal to spindle shaped pleomorphic cells, separated by fibrous septa, having high nuclear to cytoplasmic ratio, high mitosis and some areas of necrosis. On immunohistochemistry, the tumour cells were negative for LCA (leucocyte common antigen), strongly expressed cytokeratin, vimentin, desmin, neuron specific enolase and epithelial membrane antigen suggestive of a desmoplastic small round cell tumour (figure 4b).
Figure 4.
(A–C) Contrast-enhanced CT of chest-showed a large, lobulated heterogeneously enhancing lesion involving the mediastinum and para-mediastinal areas on the left side of chest, with necrotic and calcific foci within. There was also evidence of nodular pleural deposits. No evidence of any abdominal and pelvic involvement.
The nature of the lesion and its prognosis were explained to the patient. He was offered chemotherapy and radiotherapy for his malignancy. He refused treatment and left against medical advice.
Differential diagnosis
Non-seminomatous germ cell tumour
Hodgkins lymphoma
Non-hodgkins lymphoma
Thymoma.
Outcome and follow-up
The patient was offered chemotherapy and radiotherapy for his malignancy. He refused the treatment and left against medical advice.
Discussion
Desmoplastic small round cell tumour (DSRCT) was first described in 1989 by Gerald and Rosai, who described a distinct type of small blue round cell tumour. It had a predilection for serosal surfaces such as the peritoneum and the tunica vaginalis, and affected mostly caucasian males in the second or third decade of life. DSRCT is generally associated with aggressive features and a poor prognosis. Tumour cells co-express epithelial, mesenchymal and neuronal markers and are thought to originate from a mesothelial or submesothelial progenitor cells with the potential to undergo multi-lineage differentiation.1
Pathologically, desmoplastic small round cell tumour consists of round, blue cells embedded in a desmoplastic stroma. To distinguish it from other small cell tumours, immuno-histochemical markers are used. In a study by Ordonez et al, a variety of epithelial, mesenchymal and neuronal markers were detected in 39 tumours studied; cytokeratin in 37/39, epithelial membrane antigen in 24/25, desmin in 39/39, vimentin in 22/27, neuron-specific enolase in 18/25, synaptophysin in 3/19, chromogranin in 1/22, WT1 protein in 8/9, muscle-specific actin in 3/19 and α-smooth-muscle actin in 3/16.2 Lee and Hsiao observed that all the tumour cells were reactive to cytokeratin, desmin, vimentin and WT-1. EWS-WT1 fusion gene was identified in three patients.3 So, although initially thought to be having mesodermal origin due to its site of origin, this tumour is now hypothesised to arise from a progenitor cell with multi-phenotypic differentiation.4
In most cases, DSRCT presents as an abdominal mass with peritoneal and omental implants. The most common presentation is bulky abdominal disease present in a young adult, often male. Abdominal pain, palpable abdominal mass, abdominal distention due to mass or ascites, hydronephrosis due to obstruction of the urinary tract by the tumour, hepatomegaly, bowel obstruction due to pressure effect of the tumour, intraabdominal lymphadenopathy and liver metastasis are the common modes of presentation of this tumour.5 Other reported sites of disease include pleura, ethmoid sinuses, scalp, hand, posterior cranial fossa, pancreas, ovary, para-testicular region and kidney.5–8 However, the mediastinum as the primary site is unusual and never reported in literature.
DSRCT is a very aggressive neoplasm with a 5-year survival of less than 15%. Treatment options include surgery, radiotherapy, chemotherapy (with or without stem cell transplantation) and recently introduced molecular-targeted therapies. Unfortunately, there is no standard therapeutic regimen described since no modality is clearly superior to any other. Surgery is usually extensive and often includes excision of the omentum, splenectomy and lymph node resection in case of abdominal involvement as the primary site. Due to the invasive nature of this tumour, complete resection with negative margins is usually not possible. Debulking surgery has been described as an attempt to eliminate 90% of the tumour bulk.9
In addition to surgery and radiation therapy, local control options for DSRCT (particularly metastatic disease) include radiofrequency ablation, γ-knife, cryo-ablation, embolisation and chemo-embolisation. These are usually performed in academic centres after careful consideration of individual cases.
Chemotherapeutic agents used are cisplatin, carboplatin, topotecan, temozolamide, vinorelbine and irinotecan. High-dose chemotherapy with autologous stem-cell rescue has also been attempted, however no significant impact in long-term survival has been achieved after transplant.10
Although DSRCTs are generally sensitive to chemotherapy, the response is not enough to achieve cure since patients almost invariably relapse. This could potentially be a reflection of the heterogeneity of the cells within the tumour; where a distinct population of cells (cancer stem cells) that are less sensitive to chemotherapy and radiotherapy possess the ability to self-renew and retain the capacity to regenerate the tumour bulk after it has been eradicated. This represents a highly attractive hypothesis since it could explain tumour behaviour and lead to the identification of new targets for more effective therapies. Unlike other small round blue cell tumours like Ewing’s sarcoma,11 such a stem cell has not been yet identified in DSRCTs.12,13
In a series of 66 patients reported by Memorial Sloan-Kettering Cancer Center, New York,9 individuals who received multimodal treatment consisting of debulking surgery, whole abdomino-pelvic radiation14 and chemotherapy regimen had a 3-year survival of 55% (29/66), compared to 27% in those patients who did not receive all three therapies. In this series, patients who underwent tumour resection had a 58% 3-year survival rate whereas no survivors were found in the non-surgical group. This may represent a difference in tumour extension at the time of diagnosis; however a therapeutic advantage of surgical resection has been suggested.
Biswas G et al have recommended multimodality approach comprising of chemotherapy, radiotherapy and surgery for treatment of desmoplastic small round cell tumour. They found that surgical excision improves survival but is not possible in all the cases. They also observed that desmoplastic small round cell tumours are chemosensitive but overall results of multimodality approach were unsatisfactory with overall median survival in their study being only 6.5 months.15 In another study by Lal et al, multimodality approach resulted in significant improvement in survival and also observed that surgical resection correlated with improved outcome.9 Recently, Temsirolimus has been used for the treatment of metastatic desmoplastic small round cell tumour.16
Overall, the average survival of desmoplastic small round cell tumour is less than 2 years.17 The differential diagnosis of such tumours are:
Small cell mesothelioma- This tumour is extremely rare in children and young adults. Negative staining for cytokeratin 5/6 and calretinin and dot like staining for desmin can distinguish desmoplastic small round cell tumour from mesothelioma.
Primitive neuroectodermal tumour (PNET)/Ewing’s sarcoma family of tumours- The age of presentation is similar. Histologically, they are composed of blue undifferentiated cells with scant cytoplasm. Immunohistologically, PNET/Ewing’s sarcomas usually differentiate towards neural elements. The abnormal translocation in PNET/Ewing’s sarcoma is t(11;22)(q24;q12).
Malignant non-Hodgkins lymphoma- It is excluded by the negative immunostaining for LCA of the tumour cells.
Small cell carcinoma- There is lack of desmoplastic stroma and tumour cells stain negative for desmin and S100.
Diagnosis of desmoplastic small round cell tumour is clinched by specific pathological and tumour specific immunohistochemical staining pattern not seen in other tumours.
Learning points.
-
▶
DSRCT is a rare but aggressive malignancy with poor outcome.
-
▶
It is usually an abdomino-pelvic malignancy that demonstrates distinct histological appearances and a unique cytogenetic profile.
-
▶
The mediastinum as the primary site of involvement of this tumour was never reported previously in literature.
-
▶
Although the mediastinum is a very rare site, it could still be a differential diagnosis of a rapidly developing mediastinal mass in young adult males.
-
▶
An aggressive approach to the treatment using multiple modalities can offer some temporary benefit in survival.
Acknowledgments
To my family and my patients, my sweet and inspiring friend Dr Neha Chopra.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Gerald WL, Miller HK, Battifora H, et al. Intra-abdominal desmoplastic small round-cell tumor. Report of 19 cases of a distinctive type of high-grade polyphenotypic malignancy affecting young individuals. Am J Surg Pathol 1991;15:499–513 [PubMed] [Google Scholar]
- 2.Ordóñez NG. Desmoplastic small round cell tumor: I: a histopathologic study of 39 cases with emphasis on unusual histological patterns. Am J Surg Pathol 1998;22:1303–13 [DOI] [PubMed] [Google Scholar]
- 3.Lee YS, Hsiao CH. Desmoplastic small round cell tumor: a clinicopathologic, immunohistochemical and molecular study of four patients. J Formos Med Assoc 2007;106:854–60 [DOI] [PubMed] [Google Scholar]
- 4.Granja NM, Begnami MD, Bortolan J, et al. Desmoplastic small round cell tumour: Cytological and immunocytochemical features. Cytojournal 2005;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang F. Desmoplastic small round cell tumors: cytologic, histologic, and immunohistochemical features. Arch Pathol Lab Med 2006;130:728–32 [DOI] [PubMed] [Google Scholar]
- 6.Su MC, Jeng YM, Chu YC. Desmoplastic small round cell tumor of the kidney. Am J Surg Pathol 2004;28:1379–83 [DOI] [PubMed] [Google Scholar]
- 7.Bismar TA, Basturk O, Gerald WL, et al. Desmoplastic small cell tumor in the pancreas. Am J Surg Pathol 2004;28:808–12 [DOI] [PubMed] [Google Scholar]
- 8.Finke NM, Lae ME, Lloyd RV, et al. Sinonasal desmoplastic small round cell tumor: a case report. Am J Surg Pathol 2002;26:799–803 [DOI] [PubMed] [Google Scholar]
- 9.Lal DR, Su WT, Wolden SL, et al. Results of multimodal treatment for desmoplastic small round cell tumors. J Pediatr Surg 2005;40:251–5 [DOI] [PubMed] [Google Scholar]
- 10.Bisogno G, Ferrari A, Rosolen A, et al. Sequential intensified chemotherapy with stem cell rescue for children and adolescents with desmoplastic small round-cell tumor. Bone Marrow Transplant 2010;45:907–11 [DOI] [PubMed] [Google Scholar]
- 11.Suvà ML, Riggi N, Stehle JC, et al. Identification of cancer stem cells in Ewing’s sarcoma. Cancer Res 2009;69:1776–81 [DOI] [PubMed] [Google Scholar]
- 12.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 2008;8:755–68 [DOI] [PubMed] [Google Scholar]
- 13.Rosen JM, Jordan CT. The increasing complexity of the cancer stem cell paradigm. Science 2009;324:1670–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodman KA, Wolden SL, La Quaglia MP, et al. Whole abdominopelvic radiotherapy for desmoplastic small round-cell tumor. Int J Radiat Oncol Biol Phys 2002;54:170–6 [DOI] [PubMed] [Google Scholar]
- 15.Biswas G, Laskar S, Banavali SD, et al. Desmoplastic small round cell tumor: extra abdominal and abdominal presentations and the results of treatment. Indian J Cancer 2005;42:78–84 [DOI] [PubMed] [Google Scholar]
- 16.Thijs AM, van der Graaf WT, van Herpen CM. Temsirolimus for metastatic desmoplastic small round cell tumor. Pediatr Blood Cancer 2010;55:1431–2 [DOI] [PubMed] [Google Scholar]
- 17.Kempson RL, Fletcher CDM, Evans HL, et al. Tumours of the Soft tissue. Atlas of Tumour Pathology. Third Series, Fascicle 30 Washington, DC: Armed Forces Institute of Pathology; 2001:452–8 [Google Scholar]