Abstract
On March 11, 2011, an earthquake led to major problems at the Fukushima Daiichi Nuclear Power Plant. A 14-m high tsunami triggered by the earthquake disabled all AC power to Units 1, 2, and 3 of the Power Plant, and carried off fuel tanks for emergency diesel generators. Despite many efforts, cooling systems did not work and hydrogen explosions damaged the facilities, releasing a large amount of radioactive material into the environment. In this review, we describe the environmental impact of the nuclear accident, and the fundamental biological effects, acute and late, of the radiation. Possible medical countermeasures to radiation exposure are also discussed.
Keywords: Fukushima nuclear power plant, nuclear accident, environmental contamination, reactive oxygen species, low dose effect, radiation mitigator
Introduction
On March 11, 2011, an earthquake and tsunami of unprecedented scale led to major problems with the stabilization of nuclear power plants (NPP) in northeastern Japan. Operating reactors shut down automatically, with control rods inserting into the reactor cores. However, the 14-meter tsunami triggered by the earthquake disabled all AC power to Units 1, 2, and 3 of the Fukushima Daiichi Power Plant, carrying away fuel tanks for emergency diesel generators. Water injection failed in the emergency core cooling system of Units 1, 2, and 3. Since the normal cooling system was inoperable, a pressure valve was opened manually to reduce the pressure in the reactor container. In spite of such efforts, hydrogen explosions damaged the facilities. Eventually, a large amount of radioactive material was released into the environment.
The Environmental Impact of the Nuclear Accident at Tokyo Electric Power Company (TEPCO) Fukushima Daiichi Nuclear Power Plant
This chapter provides an overview of the accident and the activities of Hirosaki University in Fukushima.
Sequence of events at Fukushima Daiichi
Table 1 demonstrates the Fukushima Daiichi event sequence from March 11 to 15.(1) It was derived from information collected by Japan’s national nuclear regulator, the Nuclear and Industrial Safety Agency.
Table 1.
Fukushima Daiichi event sequence (March 11 through 15)
| Date | Time | Events |
|---|---|---|
| March 11 | 2:46 p.m. | A 9.0 magnitude earthquake strikes. Ground acceleration triggers automatic shutdown of all three reactors in operation. |
| 3:42 p.m. | A 14-meter tsunami triggered by the earthquake disables all AC power to Units 1, 2 and 3. | |
| 3:45 p.m. | Fuel tanks for emergency diesel generators are carried off by the tsunami. | |
| 4:46 p.m. | Water injection fails in the emergency core cooling systems of Units 1 and 2. | |
| March 12 | 9:07 p.m. | A pressure relief valve is opened on the Unit 1 pressure vessel. |
| 3:36 p.m. | A hydrogen explosion damages the structure of the Unit 1 reactor building. | |
| 8:20 p.m. | Seawater injection to the Unit 1 pressure vessel begins. | |
| 5:58 a.m. | Water injection fails in the emergency core cooling system of Unit 3. | |
| March 13 | 9:20 a.m. | A pressure relief valve is opened on the Unit 3 pressure vessel. |
| 4:46 p.m. | Water injection fails in the emergency core cooling systems of Unit 1 and 2. | |
| 11:01 a.m. | A hydrogen explosion damages the external structure of the Unit 3 reactor building. | |
| March 14 | 1:25 p.m. | The water level in the Unit 2 pressure vessel is found to be low, leading operators to conclude that the reactor cooling system is no longer functional. |
| 4:34 p.m. | Seawater injection into the Unit 2 pressure vessel begins. | |
| 6:20 a.m. | An explosion sound is heard at Unit 2 and it concluded to indicate an abnormality in the pressure suppression pool. At the same time, part of a wall in the operation area of Unit 4 is damaged. | |
| 9:38 a.m. | A fire breaks out in the Unit 4 reactor building. | |
| March 15 | 12:29 p.m. | The unit 4 fire is extinguished. |
Activities of Hirosaki University
On March 13, members of the Radiation Safety Council at Hirosaki University convened a meeting to discuss responses to this accident.(2) At the request of the Japanese government, the council decided to dispatch university staff members to Fukushima to support the people living there. The first team was sent on March 15.
The teams had two major functions. The first was to perform screening tests for radioactive contamination among the general public in Fukushima Prefecture. The second was to assist with temporary visits by evacuees to the homes in a 20-km zone. Radiation measurements and sampling were also conducted.
Screening tests for radioactive contamination
Twenty teams consisting of a radiation expert, a nurse and a clerk were dispatched to Fukushima by the end of July. Some 82 people were involved from the university. More than 5,000 people were examined. A GM survey meter (e.g. TGS-146B, Aloka, Co., Japan) was used for screening. Fig. 1 illustrates a screening test carried out by a university staff member. A count exceeding 100,000 cpm was regarded as indicative of radioactive contamination, requiring decontamination. A decontamination room was prepared by the Self-Defence Forces. For a count of between 13,000 and 100,000 cpm, decontamination was advised. If the count was less than 13,000 cpm, no decontamination was needed.
Fig. 1.
Screening test carried out by Hirosaki University staff.
Temporary visits by evacuees to homes in a 20-km zone
To support temporary visits, 11 teams consisting of a medical doctor, a nurse, a radiation expert and a clerk, were dispatched to Fukushima from late May through early August. Some 51 people were involved from the university.
Radiation measurements in the field
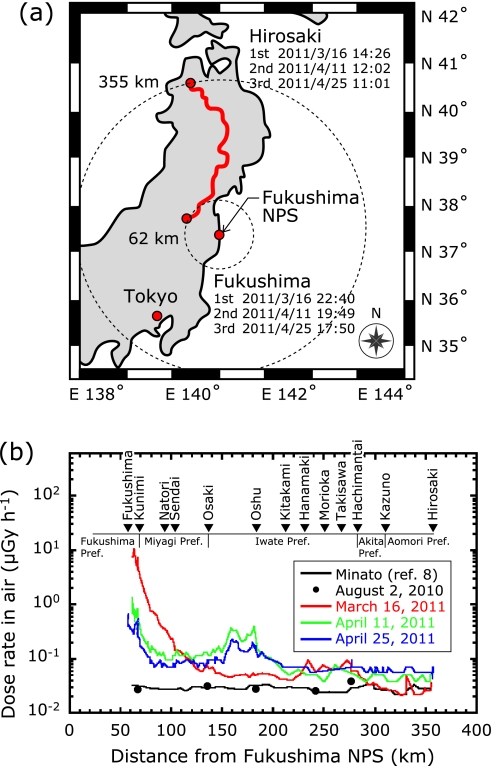
Dose rates in air along an expressway passing northwest of the Fukushima Daiichi NPP were measured in a car-borne survey.(3) Car-borne surveys are commonly used for the rapid assessment of dose rates in emergency situations. The measurements were conducted 3 times: on March 16, April 11 and April 25, 2011. The distance between the Fukushima NPP and measurement points ranged from 60 to 355 km, and the typical distance between measurements points was approximately 3 km. Total distance on the expressway was about 1,256 km. A 1'' × 1'' NaI (Tl) scintillation survey meter (TCS-171, ALOKA Co., Japan) was used, and measurements were carried out every minute. The meter was calibrated with a 2'' × 2'' NaI (Tl) scintillation spectrometer (SPA-3, Eberline Co., New Mexico). Latitude and longitude at each measurement point were measured using a global positioning system (WPL-2000, Wintec Co., Ltd., Taiwan). More than 100 measurements were obtained in each survey. Shielding by the car body was estimated by making measurements inside and outside of the car at 56 points. A shielding factor of 1.9 ± 0.04 was used.
Fig. 2 shows the temporal variation of dose rates in air before and after the start of the Fukushima NPP crisis. In the first survey, dose rates along the expressway between Fukushima City and Osaki City were found to have increased markedly, from about 0.08 to up to 11 µGy h-1. In the remaining part of the transect, however, the dose rates increased little, by a factor of about 1.5 to 2.5. In the second and third surveys, the dose rate profile along the transect looked quite different. The effect was particularly striking around Oshu City, where the dose rate increased by a factor of up to 10 between the first and the second surveys.
Fig. 2.
Expressway survey route for measuring dose rates in air from Hirosaki City to Fukushima City. Temporal variation of dose rates in air before and after the start of the Fukushima NPS crisis.
Reprinted by permission from Macmillan Publishers Ltd. Scientific Reports 1, Article number 87, Figure 1, 7 September 2011, online only, copyright 2011.
Early Stages of Biological Effects of Radiation and Radiation Damage
On exposure to ionizing radiation, water, which is makes up more than 60% of the body, is ionized generating reactive oxygen species (ROS), such as hydrated electron (eaq−) and hydrogen radical (•H).(4) These mayors can react withother substances in the body to initiate damage. This type of effect on living organisms is indirect. On the other hand, radiation can also directly inactivate substances in the body. Actually, both the direct and indirect effects of radiation cause biological damage. However, the difference is due to the type of radiation and physical and/or chemical conditions. Modulatory action can be explained by indirect effects.
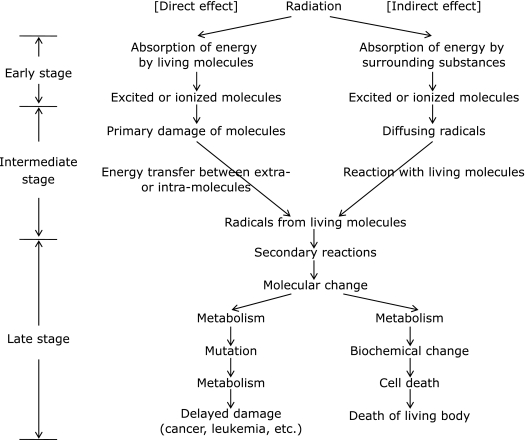
As for the biological effects of radiation, damage such as cancer is mainly considered. When living organisms are exposed to radiation, the main effects are divided into early, intermediate and late as shown in Fig. 3.(5) Although the three cannot be completely distinguished, the generation of ROS occurs between the early and intermediate stages and the influence of ROS should appear in the late stage.
Fig. 3.
Time-dependent process of biological effects after exposure of radiation.
| H2O → H2O+ + eaq− | (1) |
The cation (H2O+) thus formed may give the hydroxyl radical (•OH) as follows:
| H2O+ → H+ + •OH | (2) |
The •H is generated from the reaction of hydrated ion with a water molecule as follows:
| eaq− + H2O → OH− + •H | (3) |
Except for these simple reactions, the direct dissociation of an excited (about 7 eV) water molecule may generate •H and •OH as follows:
| H2O → H2O* → •H + •OH (* shows an excited state) | (4) |
Table 2 shows G values of radiation with low ionized density at pH 3–10.(6)
Table 2.
Yield of primary products generated from radiolysis of water
| Product | G value |
|---|---|
| eaq− | 2.7 |
| •H | 0.55 |
| •OH | 2.7 |
| H2 | 0.45 |
| H2O2 | 0.7 |
| H2O+ | 2.7 |
Further, molecular oxygen usually exists in living cells, and then, eaq− or •H generated from the radiolysis of water reacts with molecular oxygen (O2) to yield superoxide ion (O2•−) or its conjugated acid, the hydroperoxyl radical (HO2•) as follows:
| eaq− + O2 → O2•− | (5) |
| •H + O2 → HO2• | (6) |
| HO2• ⇄ H+ + O2•− | (7) |
| (pKa = 4.5–4.9)(7) |
Thus, the damage to living organisms from the indirect effects of radiation may be caused by the reactive species mentioned above. The effect of radiation is strengthened by the formation of many kinds of ROS and free radicals due to the existence of water in the living body. This ”oxygen effect” is 2.5–3 times that in the absence.
As for radiation damage, those reactive species mentioned above can react with DNA either directly or after metal-catalyzed transformation in the cell. As many as 100 different DNA modifications have been identified after exposure to ionizing radiation.(4) These include single- and double-strand breaks, base modifications, abasic sites and cross-links. These lesions are primary sites for radiation-induced cell lethality, mutations and malignant transformation. The double-strand breaks in DNA are particularly important to the lethal effect of ionizing radiation. However, the biological importance of base modifications has not been clearly established.
There are two types of radiation injuries, acute radiation injury and delayed radiation injury. Both types are observed in many radiation accidents.
Acute radiation injury
When whole-body is exposed to high-dose radiation, many types of damage occur depending on the radiation-exposed dose. Such damage is known as acute radiation injury. Table 3 summarizes the effect on living organisms of exposure to ionizing radiation.
Table 3.
Doses and effects on exposure to ionizing radiation
| Dose (mSv) | Effect |
|---|---|
| 0.1–0.3 | chest X-ray |
| 3–4 | world average dose per year of exposure to radiation |
| 0.6–2.7 | stomach radiography |
| 7–20 | CT scan |
| 50 | dose limit per year among radiation workers |
| 200 | lifetime exposure to natural radiation |
| 500 | decrease in lymphocytes, cataracts |
| 1000 | acute radiation damage, nausea, vomiting |
| 2000 | 5% of those exposed die within several weeks |
| 3000–5000 | 50% of those exposed die within several weeks |
| 7000–10000 | 95% of those exposed die within several weeks |
| 20000–60000 | cerebral edema, respiratory distress, diarrhea, fever, circulatory failure within 1–2 weeks |
| 100000 | instant coma, death within hours |
Late radiation injury
When whole-body is exposed to low-dose radiation or repeatedly exposed to low dose-rate radiation, some effects appear over a period of years or decades. Such damage is known as delayed radiation injury. Among them, radiation carcinogenesis, such as leukemia and other malignancies, is thought to have no threshold dose and is classified as a stochastic effect. Cataracts are also a form of late radiation injury, but have a threshold dose for onset and are classified as deterministic effects.
Health Effects of Radiation at Low Doses
DNA damage and health effects
DNA is deemed the most important target of radiation leading to effects on health. Ionizing radiation causes several types of DNA damage directly or indirectly. While the repair machinery in the cell processes the damage, some areas could remain unrepaired or be misrepaired.
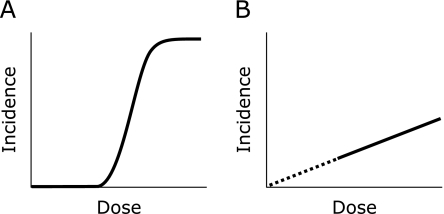
When unrepaired/misrepaired damage unduly accumulates in a cell, or when the damage results in a lethal mutation, the cell deteriorates and dies. This can manifest as impaired organ function and a destruction of tissue structure. This kind of effect is called an adverse tissue reaction or deterministic effect. If the fraction of deteriorated/dead cells in the tissue remains small, no symptoms will become clinically evident since the defects are compensated for by the large majority of normal cells. Hence, adverse tissue reactions appear at doses above a certain threshold, and follow the dose response illustrated in Fig. 4A. The threshold value differs among tissues. The most sensitive organ is the testis with a threshold dose of 100 mGy for temporary infertility. An embryo is also sensitive, and malformation could be induced at doses above 100 mGy during the period of major organogenesis.(8) In the Fukushima accident, no one seems to have been exposed to doses above any threshold for tissue reactions, and this type of effect is not an issue.
Fig. 4.
Dose response for the health effects of radiation. A: Adverse tissue reactions (deterministic effects). B: Cancer and heritable effects (stochastic effects).
Misrepaired DNA damage is not always lethal to cells. The resultant mutation is often compatible with cell viability. Although most viable mutated cells are thought to have little or no influence on health, some could contribute to malignant transformation. Germ cells could also carry critical mutations that result in heritable diseases in later generations. Given that cancers and heritable effects arise from a single mutated cell, their incidence is thought to increase with dose with no threshold as shown in Fig. 4B. The cancerous and heritable effects of radiation are collectively called stochastic effects.
Epidemiological evidence
A dose-dependent increase of cancer in rates has been demonstrated by epidemiological studies. Among them, the study of atomic-bomb survivors of Hiroshima and Nagasaki is regarded as the most powerful and reliable. A recent report indicated that the excess relative risk (ERR) of solid cancer increases linearly with dose up to 2 Gy, and the gender-averaged estimate of ERR per Gy is 0.47 for a person aged 70 exposed to the bombings at age 30.(9) It should be noted the increase is not statistically significant at doses below ~150 mGy. Some case-control studies, particularly what is known as the Oxford survey, suggest excess risk of childhood cancer associated with lower levels of exposure, a few tens of mGy.(10) The interpretation of these studies is controversial, however, since comparable excess has not been identified in any cohort study.
In the case of internal exposure, there is considerable uncertainty in estimating dose and risk. Nevertheless epidemiological studies for internal emitters suggest that the risk per unit dose is not remarkably high in comparison with external exposure, based on the current internal dose assessment system.(11) There is an argument that precancerous lesions of the urinary bladder were induced in the Ukrainian population by very low levels of internal exposure to 137Cs.(12) However, the claim is quite unlikely since the bladder doses of those subjects from 137Cs are estimated to be much lower than the doses from natural 40K. The proposed association between precancerous lesions and internal 137Cs can be attributed to selection bias and confounding factors.
Unlike for cancer, there is no epidemiological evidence for the induction of heritable effects.(8) Offspring of a-bomb survivors, radiologists, and those living in areas of high background radiation have been studied, but no indication of trans-generational effects of radiation has been found. This may be because radiation-induced mutations are often deletions of a large DNA segment that are incompatible with live birth. Although the possibility of heritable effects can not be ruled out, the epidemiological data suggest the risk is small in comparison with the risk of cancer.
Biological features of low dose tumorigenesis
The biological mechanism of radiation tumorigenesis has been studied intensively because of major concern about low dose effects. Among various types of radiation-induced DNA damage, double-strand breaks (DSBs) are believed to be a source of chromosomal aberrations and somatic cell mutations that are responsible for tumorigenesis. There are two major pathways for DSB repair, nonhomologous end joining (NHEJ) and homologous recombination (HR).(13) While HR is a high fidelity process, a perfectly matching sequence required for repair is available only in the late S to G2 phase of the cell cycle. Consequently, most DSBs are processed by NHEJ. A significant proportion of the DSBs caused by radiation is associated with other closely spaced lesions, creating clustered damage.(14) For this type of damage, the broken ends will require additional processing prior to ligation, which may result in a loss of base pairs. Moreover a complex form of clustered damage that involves two or more DSBs can compromise the fidelity of NHEJ, increasing the chances of misrejoining.(15) Considering that even a single electron track traversing a cell nucleus can induce clustered DNA damage, chromosome/gene alterations would be inevitable however small the dose may be. In fact a large-scale analysis of chromosomal aberrations in human lymphocytes demonstrated a linear dose response down to 20 mGy.(16)
While the nature of radiation-induced DNA damage as well as imperfect repair forms a strong basis for the linear non-threshold (LNT) dose response for cancer induction, things may change when higher order protective functions are taken into account. Misrepaired, mutated cells could be eliminated by apoptosis, cell competition, and immunological surveillance. If such protective functions work more efficiently at lower doses, the dose-response would be non-linear, and there might be a threshold. Furthermore, some phenomena mainly found in cultured cells could possibly complicate the situation, including adaptive responses in cells pre-exposed to a low dose of radiation, bystander effects in cells that are not directly irradiated, and genomic instability that manifests in the progeny of irradiated cells many generations after exposure. Currently it is unclear to what extent these three phenomena are active in vivo, and how they are inter-related.(17)
Risk of radiation-induced cancer
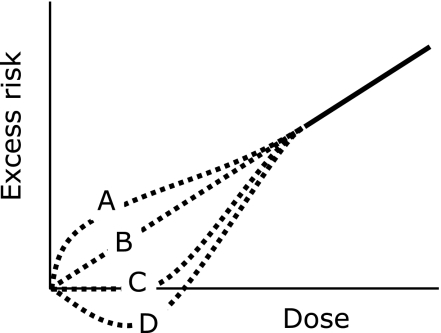
Summing up the epidemiological data and biological findings, we are still unsure about the shape of the dose response curve in the low dose range. While several dose response curves are possible as illustrated in Fig. 5, the LNT model is usually adopted as a best estimate for low dose risk. Based on this model, the lifetime cancer risk per unit dose is estimated to average ~5%/Sv over the whole population.(8) This means cancer mortality will be 20.5% if all members are exposed to 100 mSv of radiation, on the assumption of a background cancer mortality rate of 20%. The value should not be interpreted as a certain prediction, but an estimate with uncertainty.
Fig. 5.
Possible dose response curves for cancer induction at low doses. A: supralinear, B: LNT (linear non-threshold), C: threshold, D: hormetic.
In theory there is a possibility of cancer among people exposed in the accident at the Fukushima Daiichi NPP. Assuming the LNT model represents the reality of radiation-induced cancer at low doses, however, significant excess risk due to exposure is unlikely to be detected for the emergency workers and the public living around the site unless their doses have been seriously underestimated.(18) This notion would be supported by the absence of clear findings for the Chernobyl liquidators or residents in high background radiation areas.(19,20) In this context, accident-related epidemiological studies should place more focus on the effects of anxiety, distress, and resulting changes in lifestyle factors rather than exposure.
Possible Medical Countermeasures to Exposure
There are several ways to countermeasure exposure to radiation. One is to use medicines to prevent or reduce radiation-induced injuries. Such agents can be classified into two groups, absorbents and radiation modifiers. People can be exposed internally to radionuclides by ingesting or inhaling them, or through direct contact. Absorbents protect agaist internal exposure by preventing the accumulation or accelerating the exclusion of radionuclides. Iodine tablets (stable potassium iodide) can be used to prevent the absorption of radioactive iodine such as 131I in the thyroid gland (http://www.bt.cdc.gov/radiation/ki.asp). Prussian blue can be used to remove 137Cs from the human body by binding to 137Cs. (http://emergency.cdc.gov/radiation/prussianblue.asp). Diethylene-triamine-pentaacetic acid (DTPA) is expected to chelate plutonium, removing it through the kidneys (http://emergency.cdc.gov/radiation/dtpa.asp). Since such chemicals have been stocked for possible use after nuclear accidents and a certain amount of 131I was released by the Fukushima accident, iodine tablets were used by workers at the contaminated site as a preventive measure. The tablets were not delivered to people living around the power plant (http://ajw.asahi.com/article/0311disaster/fukushima/AJ201108298140).
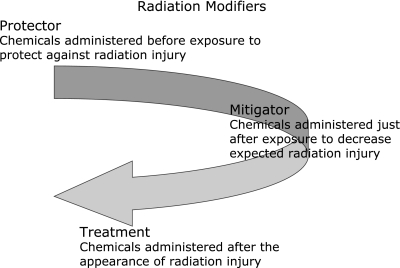
Radiation modifiers may be used to prevent or reduce injuries by modifying chemical/biological responses to radiation in the living body.(21) One can classify compounds that ameliorate radiation injuries into three categories with respect to the timing of administration: compounds for the prophylaxis, mitigation, and treatment of radiation injuries (Fig. 6). As shown above, since the first event caused by ionizing radiation is the formation of free radicals from water existing in the living body, major damage is caused by aqueous free radicals. Therefore, compounds that quench or scavenge free radicals should be effective at reducing radiation damage. Most of the radiation modifiers reported to date are protectors, used for prophylaxis, and should be present in the appropriate position in the living body before exposure. In contrast, to counteract accidental overexposure, it is necessary to use compounds that are effective when administered after exposure. These compounds are called mitigators and the relatively few agents reported so far include LC9018,(22) tocopherol-mono-glucoside (TMG),(23,24) mineral yeast,(25) and CBLB502.(26)
Fig. 6.
Classification of radiation modifiers with respect to timing of administration.
Only amifostine has been approved by the U.S. Food and Drug Administration (FDA) for use as a protector in limited cases of radiotherapy (http://www.drugs.com/pro/amifostine.html). However, the toxicity of amifostine is not negligible. Regardless of whether an agent is a protector or mitigator, it should have low toxicity even if the radiation modifying activity is not very high, if it is to be uses on large numbers of people. Therefore, chemicals existing in the body or natural products that have been used for a long time are possible candidates for radiation modifiers for civilian populations.
Melatonin (N-acetyl-5-methoxytryptamine) is one such compound. Melatonin is a naturally occurring compound found in animals, plants, and microbes. In animals, it is a pineal hormone and circulating levels of it vary in a daily cycle producing circadian rhythms. Melatonin has reportedly radioprotective features in both animal and human studies.(27–32) Because of its very low toxicity, high availability, and ease of self-administration, some leading researchers of melatonin claim that it may be useful in providing protection against ionizing radiation in disasters like the one at Fukushima.(33,34)
Another type of compound is natural antioxidants. Although many compounds, especially from plants, have been studied as radiation protectors in the laboratory, their effectiveness is relatively low. Based on the antioxidant activity of hydrogen,(35) water containing a high concentration of hydrogen was recently reported to be a radiation protector.(36–39) It may become a new type of radiation modifier with low toxicity.
In the Fukushima accident, no acute radiation injuries have been observed even among people associated with the operation of the plant or responding to the accident in contrast to the Chernobyl accident where a number of people suffered acute radiation injuries. The anxiety among most of the civilian population is the future increase in the possibility of tumorigenesis. For high dose exposure, some compounds have been reported to reduce specific tumorigenesis. For example, curcumin, a component of turmeric, reportedly reduced the rate of breast cancer in female rats irradiated while lactating.(40) However, compounds effective at reducing the probability of tumorigenesis caused by low-dose exposure have not been reported as far as we know. As stated in chapter III, in vivo low dose tumorigenesis is still a matter of controversy. Animal experiments are needed but numerous animals must be used to achieve significance because of the very low probability of an event and very long time needed to observe an end point. This must be overcome in the future.
Acknowledgments
The authors are grateful to Dr. Masahiro Hosoda for his kind assistance throughout this work.
Abbreviations
- DSBs
double-strand breaks
- DTPA
diethylene-triamine-pentaacetic acid
- ERR
excess relative risk
- FDA
U.S. Food and Drug Administration
- HR
homologous recombination
- LNT
linear non-threshold
- NHEJ
nonhomologous end joining
- NPP
nuclear power plants
- ROS
reactive oxygen species
- TEPCO
Tokyo Electric Power Company
- TMG
tocopherol-mono-glucoside
References
- 1.Cox M. Nuclear News. 2011. Special Report: Fukushima Daiichi after the Earthquake and Tsunami; pp. 17–18. [Google Scholar]
- 2.Tokonami S. Proc of the 3rd Int Symp on Radiat Emerg Med in Hirosaki Univ. Measures against Nuclear Accident at TEPCO Fukushima Daiichi Nuclear Power Plant: activities carried out by Hirosaki University. in press. [Google Scholar]
- 3.Hosoda M, Tokonami S, Sorimachi A, et al. The time variation of dose rate artificially increased by the Fukushima nuclear crisis. Sci Rep. 2011;1:87. doi: 10.1038/srep00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boiteux S. Repair of ionizing radiation induced base damage: role of DNA glycosylases. In: Ozawa T, Tatsumi K, Hori T, editors. Biodefence Mechanisms Against Environmental Stress. Tokyo: Kodansha-Springer; 1998. pp. 107–114. [Google Scholar]
- 5.Ozawa T. Generation of active oxygen by environmental factors; radiation. Tanpakushitsu Kakusan Koso. 1988;33:2811–2817. [PubMed] [Google Scholar]
- 6.Bielski BHJ, Gebski JM. In: Free Radicals in Biology. Pryor WA, editor. Vol III. New York: Academic Press; 1977. pp. 1–51. [Google Scholar]
- 7.Michelson AM, McCord JM, Fridovich I. Superoxide and Superoxide Dismutase. New York: Academic Press; 1977. pp. 19–60. [Google Scholar]
- 8.International Commission on Radiological Protection The 2007 recommendations of the international commission on radiological protection. ICRP publication 103. Ann ICRP. 2007;37:1–332. doi: 10.1016/j.icrp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Preston DL, Ron E, Tokuoka S, et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res. 2007;168:1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 10.Doll R, Wakeford R. Risk of childhood cancer from fetal irradiation. Br J Radiol. 1997;70:130–139. doi: 10.1259/bjr.70.830.9135438. [DOI] [PubMed] [Google Scholar]
- 11.Committee Examining Radiation Risks of Internal Emitters report of the Committee Examining Radiation Risks of Internal Emitters (CERRIE), 2004. http://www.cerrie.org http://www.cerrie.org Retrieved from .
- 12.Romanenko A, Morimura K, Wanibuchi H, et al. Urinary bladder lesions induced by persistent chronic low-dose ionizing radiation. Cancer Sci. 2003;94:328–333. doi: 10.1111/j.1349-7006.2003.tb01441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 14.Nikjoo H, O’Neill P, Wilson WE, Goodhead DT. Computational approach for determining the spectrum of DNA damage induced by ionizing radiation. Radiat Res. 2001;156:577–583. doi: 10.1667/0033-7587(2001)156[0577:cafdts]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Rothkamm K, Kühne M, Jeggo PA, Löbrich M. Radiation-induced genomic rearrangements formed by nonhomologous end-joining of DNA double-strand breaks. Cancer Res. 2001;61:3886–3893. [PubMed] [Google Scholar]
- 16.Lloyd DC, Edwards AA, Leonard A, et al. Chromosomal aberrations in human lymphocytes induced in vitro by very low doses of X-rays. Int J Radiat Biol. 1992;61:335–343. doi: 10.1080/09553009214551021. [DOI] [PubMed] [Google Scholar]
- 17.Morgan WF, Sowa MB. Non-targeted effects of ionizing radiation: implications for risk assessment and the radiation dose response profile. Health Phys. 2009;97:426–432. doi: 10.1097/HP.0b013e3181ab98c7. [DOI] [PubMed] [Google Scholar]
- 18.Wakeford R. And now, Fukushima. J Radiol Prot. 2011;31:167–176. doi: 10.1088/0952-4746/31/2/E02. [DOI] [PubMed] [Google Scholar]
- 19.United Nations Scientific Committee on the Effects of Atomic Radiation . Sources and effects of ionizing radiation. UNSCEAR 2008 Report to the General Assembly with Scientific Annexes. Volume II, Scientific Annex D: health effects due to radiation from the chernobyl accident. New York: United Nations; 2011. [Google Scholar]
- 20.Nair RR, Rajan B, Akiba S, et al. Background radiation and cancer incidence in Kerala, India-Karanagappally cohort study. Health Phys. 2009;96:55–66. doi: 10.1097/01.HP.0000327646.54923.11. [DOI] [PubMed] [Google Scholar]
- 21.Brizel DM. Pharmacologic approaches to radiation protection. J Clin Oncol. 2007;25:4084–4089. doi: 10.1200/JCO.2007.11.5816. [DOI] [PubMed] [Google Scholar]
- 22.Nomoto K, Yokokura T, Tsuneoka K, Shikita M. Radioprotection of mice by a single subcutaneous injection of heat-killed Lactobacillus casei after irradiation. Radiat Res. 1991;125:293–297. [PubMed] [Google Scholar]
- 23.Satyamitra M, Uma Devi P, Murase H, Kagiya VT. In vivo postirradiation protection by a vitamin E analog, α-TMG. Radiat Res. 2003;160:655–661. doi: 10.1667/rr3077. [DOI] [PubMed] [Google Scholar]
- 24.Ueno M, Inano H, Onoda M, et al. Modification of mortality and tumorigenesis by tocopherol-mono-glucoside (TMG) administered after X irradiation in mice and rats. Radiat Res. 2009;172:519–524. doi: 10.1667/RR1695.1. [DOI] [PubMed] [Google Scholar]
- 25.Anzai K, Ikota N, Ueno M, Nyui M, Kagiya TV. Heat-treated mineral-yeast as a potent post-irradiation radioprotector. J Radiat Res (Tokyo) 2008;49:425–430. doi: 10.1269/jrr.07127. [DOI] [PubMed] [Google Scholar]
- 26.Burdelya LG, Krivokrysenko VI, Tallant TC, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320:226–230. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vijayalaxmi, Reiter RJ, Herman TS, Meltz ML. Melatonin and radioprotection from genetic damage: in vivo/in vitro studies with human volunteers. Mutat Res. 1996;371:221–228. doi: 10.1016/s0165-1218(96)90110-x. [DOI] [PubMed] [Google Scholar]
- 28.Vijayalaxmi, Meltz ML, Reiter RJ, Herman TS, Kumar KS. Melatonin and protection from whole-body irradiation: survival studies in mice. Mutat Res. 1999;425:21–27. doi: 10.1016/s0027-5107(98)00246-2. [DOI] [PubMed] [Google Scholar]
- 29.Koc M, Taipi S, Erin Buyukokurglu M, Bakan N. The effect of melatonin against oxdative damage during total-body irradiation in rats. Radiat Res. 2003;160:251–255. doi: 10.1667/3034. [DOI] [PubMed] [Google Scholar]
- 30.Vijayalaxmi, Reiter RJ, Tan DX, Herman TS, Thomas CR., Jr Melatonin as a radioprotective agent: a review. Int J Radiat Oncol Biol Phys. 2004;59:639–653. doi: 10.1016/j.ijrobp.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Manda K, Ueno M, Anzai K. AFMK, a melatonin metabolite, attenuates X-ray-induced oxidative damage to DNA, proteins and lipids in mice. J Pineal Res. 2007;42:386–393. doi: 10.1111/j.1600-079X.2007.00432.x. [DOI] [PubMed] [Google Scholar]
- 32.Shirazi A, Ghobadi G, Ghazi-Khansari M. A radiobiological review on melatonin: a novel radioprotector. J Radiat Res (Tokyo) 2007;48:263–272. doi: 10.1269/jrr.06070. [DOI] [PubMed] [Google Scholar]
- 33.Korkmaz A, Tan DX, Reiter RJ. Melatonin; an established radioprotective agent against Japan’s nuclear disaster. TAF Prev Med Bull. 2011;10:127–129. [Google Scholar]
- 34.Reiter RJ, Tan DX, Korkmaz A, Manchester LC. The disaster in Japan: utility of melatonin in providing protection against ionizing radiation. J Pineal Res. 2011;50:357–358. doi: 10.1111/j.1600-079X.2011.00881.x. [DOI] [PubMed] [Google Scholar]
- 35.Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 36.Qian L, Cao F, Cui J, et al. The potential cardioprotective effects of hydrogen in irradiated mice. J Radiat Res (Tokyo) 2010;51:741–747. doi: 10.1269/jrr.10093. [DOI] [PubMed] [Google Scholar]
- 37.Zhao L, Zhou C, Zhang J, et al. Hydrogen protects mice from radiation induced thymic lymphoma in BALB/c mice. Int J Biol Sci. 2011;7:297–300. doi: 10.7150/ijbs.7.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang KM, Kang YN, Choi IB, et al. Effects of drinking hydrogen-Rich water on the quality of life of patients treated with radiotherapy for liver tumors. Med Gas Res. 2011;1:11. doi: 10.1186/2045-9912-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Terasaki Y, Ohsawa I, Terasaki M, et al. Hydrogen therapy attenuates irradiation-induced lung damage by reducing oxidative stress. Am J Physiol Lung Cell Mol Physiol. 2011;301:L415–L426. doi: 10.1152/ajplung.00008.2011. [DOI] [PubMed] [Google Scholar]
- 40.Inano H, Onoda M, Inafuku N, et al. Potent preventive action of curcumin on radiation-induced initiation of mammary tumorigenesis in rats. Carcinogenesis. 2000;21:1835–1841. doi: 10.1093/carcin/21.10.1835. [DOI] [PubMed] [Google Scholar]