Abstract
The stomach is a sensitive digestive organ that is susceptible and exposed to exogenous pathogens from the diet. In response to such pathogens, the stomach induces oxidative stress, which might be related to the development of gastric organic disorders such as gastritis, gastric ulcers, and gastric cancer, as well as functional disorders such as functional dyspepsia. In particular, the bacterium Helicobacter pylori plays a major role in eliciting and confronting oxidative stress in the stomach. The present paper summarizes the pathogenesis of oxidative stress in the stomach during the development of various stomach diseases.
Keywords: gastric mucosa, oxidative stress, Helicobacter pylori
Oxidative Stress in the Process of Gastric Mucosal Injury
Physiological responses to stressors include increased activity of the hypothalamic-pituitary-adrenal axis as well as changes in gastrointestinal tissue. According to Selye’s formulation of the general adaptation syndrome, an increase in adrenocortical activity is related to an increase in the incidence of gastric ulceration. The main candidate for the cause of stress ulcers is oxidative stress. There is some evidence that psychological stress, in addition to physical stress such as surgical intervention and microbial infection including Helicobacter pylori (H. pylori),(1) leads to oxidative stress in the stomach. Oxidative stress, which is a state of elevated levels of reactive oxygen species (ROS), causes a variety of conditions that stimulate either additional ROS production or a decline in antioxidant defenses. Oxidative stress is not only involved in the pathogenesis of gastric inflammation, ulcerogenesis, and carcinogenesis in H. pylori infection, but also in that of lifestyle-related diseases including atherosclerosis, hypertension, diabetes mellitus, ischemic heart diseases, and malignancies.(2) Several phenotypes of gastrointestinal diseases, such as peptic ulcer disease and gastroparesis, are known to be related to antioxidant property dysfunction.
Ethanol
The effects of ethanol on gastric mucosa are complicated and multifaceted. They may be associated with a disturbance in the balance between gastric mucosal protective and aggressive factors. Gastric mucosa is exposed to gastric acid, pepsin, and stimulants among others, while gastroprotective factors maintain the integrity of the gastric mucous layer, microcirculatory system, HCO3−, prostaglandins (PGs), epidermal growth factor synthesis, and epithelial cell restitution. Ethanol injures the vascular endothelial cells of the gastric mucosa and induces microcirculatory disturbance and hypoxia, linking to the overproduction of oxygen radicals.
Pan et al.(3) report the role of mitochondrial energy charge in the pathogenesis of ethanol-induced gastric mucosal injury. The gastric mucosal lesion index is correlated with the thiobarbituric acid (TBA)-reactive substance (TBARS) content in gastric mucosa. As the concentration of ethanol increases and the exposure time to ethanol is extended, the TBARS content in gastric mucosa and the extent of gastric mucosal damage increase. The ultrastructural pathological changes in mitochondria are positively related to ethanol concentration and exposure time. The expressions of mitochondrial DNA ATPase subunits 6 and 8 mRNA decline with increasing TBARS content in gastric mucosa produced as a result of ethanol gavage. As mentioned above, ethanol-induced gastric mucosal injury is related to oxidative stress, which disturbs the energy metabolism of mitochondria and plays a critical role in the pathogenesis of ethanol-induced gastric mucosal injury.
Ischemia/reperfusion injury
Ischemia/reperfusion damages the gastric mucosa by inducing oxidative stress. Specifically, ROS such as superoxide (O2−) and hydrogen peroxide (H2O2) induce inflammatory responses and tissue damage by fragmenting cellular DNA. In the gut, ROS can also be generated by non-steroidal anti-inflammatory drugs (NSAIDs), cold stress, ethanol, and H. pylori infection. NADPH oxidase found in phagocytic cells, vascular smooth muscle cells, endothelial cells, fibroblasts, and adipocytes convert oxygen into superoxide anions. Nakagiri et al.(4) recently reported that NADPH oxidase activity is elevated in ischemia and ischemia/reperfusion and is involved in the resultant gastric mucosal damage. This increased NADPH oxidase activity may also upregulate cyclooxygenase-2.
Peskar et al.(5) report that during ischemia/reperfusion, inhibitors of the cyclooxygenase and lipoxygenase pathways increase gastric mucosal damage in a dose-dependent manner. The synergism observed as a result of the combination of cyclooxygenase and lipoxygenase inhibitors suggests that both pathways are important in gastric mucosal defense during ischemia/reperfusion. PGE2 theoretically antagonizes the effects of cyclooxygenase and lipoxygenase inhibitors. Similarly, lipoxin A4, a lipoxygenase-derived product of arachidonate metabolism, also antagonizes the effects of cyclooxygenase and lipoxygenase inhibitors; moreover, it could replace PGE2 for the prevention of gastric mucosal damage caused by cyclooxygenase inhibitors during ischemia/reperfusion.
Portal hypertensive gastropathy
Portal hypertensive gastropathy (PHG) is a common complication of liver cirrhosis and is associated with impaired gastric mucosal healing. PHG may be related to increased ROS and lipid peroxide (LPO) production. Kinjo et al.(4) report increased levels of LPO and nitrotyrosine, an indicator of nitration of tyrosine residues due to peroxynitrite, in the gastric mucosa of portal hypertensive rats; in addition, they report impaired ERK1/2 phosphorylation related to increased nitration by peroxynitrite. The gastroprotective, anti-inflammatory agent rebamipide prevents free radical production by scavenging hydroxyl radicals.(5,6) Rebamipide decreases LPO and nitrotyrosine levels, normalizes ERK1/2 phosphorylation, and improves the ulcer index; this suggests that defects in the mitogen-activated protein kinase (MAPK) pathway are involved in the increased susceptibility to gastric mucosal injury observed in portal hypertensive gastropathy, indicating a potential role of rebamipide for treatment.
NSAIDs and aspirin
In addition to inhibiting cyclooxygenase and decreasing prostaglandin production, NSAIDs induce mucosal damage via ROS produced by recruited leukocytes. ROS-mediated mitochondrial damage as well as lipid, protein, and DNA oxidation lead to apoptosis and mucosal injury. Proton pump inhibitor (PPI) therapy is thought to primarily protect gastric mucosa by inhibiting gastric acid secretion. Nevertheless, Maity et al.(7) recently demonstrated that a PPI, lansoprazole, also inhibits NSAID-induced gastropathy by inhibiting mitochondrial and Fas-mediated apoptosis pathways. Lansoprazole’s anti-apoptotic activity appears to be mediated by preventing NSAID-induced reductions in anti-apoptotic genes (e.g., Bcl and Bcl-2) while inhibiting increases in Fas and Fas ligand as well as pro-apoptotic genes (e.g., Bax and Bak).
On the other hand, aspirin increases the permeability of cultured gastric epithelial cell monolayers. The disruption in barrier integrity is mediated by p38 MAPK and involves the downregulation of claudin-7, a protein component of tight junctions.(8) This differs from the effects of other NSAIDs (i.e., non-aspirin NSAIDs), which increase epithelial permeability coupled to cyclooxygenase-1 inhibition; this increase can be restored by PGE2 administration.
Heat shock proteins
Heat shock proteins, especially HSP70, provide cellular protection against stressor-induced tissue damage by refolding or degrading denatured proteins produced as a result of these stressors. Otaka et al.(9) recently used affinity chromatography to identify cytoskeletal myosin and actin as the first molecules bound by HSP70 after gastric mucosal injury in rats. Transcriptional upregulation of HSPs occurs via the binding of the transcription factor, heat shock factor 1 (HSF1), to heat shock element, which is located upstream of the HSP genes. In HSF1 null and HSP70-expressing transgenic mice, HSPs protect against irritant (e.g., ethanol or NSAIDs)-induced gastric lesions; moreover, geranylgeranylacetone (GGA), a gastroprotective agent, induces HSPs. Furthermore, HSP70 protects the gastric mucosa by inhibiting apoptosis, proinflammatory cytokines, and cell adhesion molecules involved in leukocyte infiltration. After induction by GGA, HSPs exhibit protective effects in mouse models of inflammatory bowel disease as well as in NSAID-induced lesions of the small intestine. Therefore, HSP inducers such as GGA may have therapeutic benefits in numerous diseases.
Enlarged Fold Gastritis and Oxidative Stress
H. pylori eradication therapy increases Runt domain transcription factor 3 (RUNX3) expression in glandular epithelial cells in enlarged-fold gastritis. Recently, we reported that RUNX3 is expressed in gastric epithelial cells and that H. pylori eradication significantly increases RUNX3 expression in the glandular epithelium of the corpus; however, no changes were observed in the antrum.(10) The mucosal chemiluminescence value, a marker of oxidative stress, is 4-fold higher in the corpus than in the antrum. H. pylori eradication significantly decreases the mucosal chemiluminescence values in both portions of the stomach to nearly undetectable levels. We conclude that the glandular epithelium is exposed to high levels of carcinogenic oxidative stress and expresses low levels of the tumor-suppressing molecule, RUNX3; however, RUNX3 expression was restored after eradication, suggesting a high risk of carcinogenesis associated with H. pylori-induced enlarged-fold gastritis of the corpus.(10)
Oxidative Stress during H. pylori Infection
Antioxidant ability of H. pylori to establish chronic infection
H. pylori infection induces a strong inflammatory host response, leading to the generation of a number of ROS and reactive nitrogen species (RNS), which are mediated by neutrophils and macrophages.(11) The generation of ROS and/or RNS is an important host immune response against persistent pathogens. Therefore, H. pylori must combat oxidative stress generated by the host immune response using an antioxidant protein in order to establish long-term colonization.(12,13) The mechanisms for ROS detoxification are of particular interest in understanding the H. pylori-associated pathogenesis. It is well known that H. pylori has a variety of enzymes acting as antioxidant systems to combat the toxic effects of ROS including catalase (KatA), iron-cofactored superoxide dismutase (SodB), and alkyl hydroperoxide reductase (AhpC).(13,14)
Superoxide dismutase (SOD) catalyzes the conversion of superoxide anions to hydrogen peroxide, which is degraded to oxygen and water by catalase.(13,15) SOD is a metalloenzyme; 3 structurally different forms have been identified depending on the metal cofactor. In general, organisms encode different sets of SOD enzymes. For example, Escherichia coli has 3 SODs: Fe-SOD (SodB) and Mn-SOD (SodA) in the cytoplasm and Cu/Zn-SOD in the periplasm.(16) On the other hand, H. pylori produces only a single SodB encoded by the sodB gene.(17,18) It was recently reported that sodB deletion in H. pylori causes the bacterium to lose its capacity for gastric mucosal colonization in mice.(19) This indicates that SodB is an important determinant of the host colonization capability of H. pylori. The regulation of sodB mRNA expression is also important for ROS detoxification. The mRNA expression of sodB in H. pylori is directly regulated by ferric uptake regulator (Fur) protein.(20) Fur functions as a global transcriptional regulator in H. pylori.(21–24) It is reported that Fur binds to ferrous iron (Fe2+) and that the genes for iron uptake are suppressed by the iron-binding form of Fur.(25,26) On the other hand, sodB expression is suppressed by the iron-free form of Fur (apo-Fur).(20) Apo-Fur binds to a specific consensus sequence called Fur-Box located on the sodB promoter, blocking the binding of RNA polymerase.(20,27,28) It was recently reported that nucleic-acid mutations in Fur-Box and/or amino-acid mutations in Fur decrease the affinity of apo-Fur for Fur-Box in H. pylori, halting the suppression of sodB mRNA expression.(29–31) In particular, stopping the suppression of sodB mRNA expression in H. pylori by amino-acid mutations in Fur (i.e., C78Y and P114S) determines the development of metronidazole (Mtz) resistance.(31) This is because, when Mtz enters cells, its antimicrobial toxicity is dependent on the reduction of its nitro group to nitro anion radicals and the generation of superoxide.(32,33)
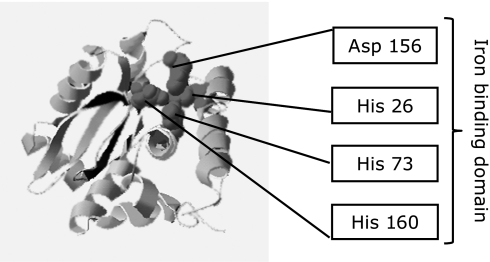
The SodB of H. pylori shares 53% sequence identity with the corresponding protein from E. coli. Interestingly, H. pylori SodB is significantly different from other Fe-SODs; its most distinguishing characteristic is its extended C-terminal tail,(34) although the role of this tail remains unclear. The structure of SodB has been clarified; it is now known to exist as a dimer composed of 2 identical subunits.(34) Furthermore, it is identified as having 4 ferrous ion (Fe2+)-coordinating residues (i.e., an iron-binding domain; His 26, His 73, His 160, and Asp 156) (Fig. 1). In fact, SodB needs to recruit ferrous ion (Fe2+) to express its activity.(15,34) It is expected that SodB activity might be suppressed by preventing the uptake of iron ion (Fe2+ and/or Fe3+).
Fig. 1.
Homology modeling of Helicobacter pylori SodB. Four amino acids (His 26, His 73, His 160, and Asp 156) of SodB are ferrous ion (Fe2+)-coordinating residues (i.e., an iron-binding domain).
Generation of oxidative stress as a virulence factor in H. pylori-infected hosts
ROS released from activated neutrophils are also potential virulence factors involved in H. pylori-infected host cells. In H. pylori-infected host cells, hypochlorous anions (OCl−) are generated from H2O2 in the presence of Cl−. The hypochlorous anions subsequently react with ammonia (NH3), which is derived from urea by urease produced by H. pylori, ultimately yielding monochloramine (NH2Cl). NH2Cl induces mucosal cytotoxicity due to its lipophilic properties and freely penetrates biological membranes to oxidize intracellular components.(35–37)
In addition, it is well known that H. pylori produces a γ-glutamyltranspeptidase (EC 2.3.2.2; GGT) in the periplasm.(38) H. pylori GGT catalyzes the transpeptidation and hydrolysis of the γ-glutamyl groups of glutamine and glutathione. Interestingly, these findings indicate that H. pylori GGT performs 2 functions. First, GGT functions in the physiological functioning of H. pylori. H. pylori is unable to take up extracellular glutamine and glutathione directly; GGT hydrolyzes these substances to glutamate. The glutamate is then transported into H. pylori cells via a Na+-dependent reaction and is mainly incorporated into the TCA cycle.(39) Second, GGT acts as a virulence factor by disrupting the antioxidant ability of host cells. Although glutathione has antioxidant potential in host cells, H. pylori GGT reduces extracellular glutathione levels. In fact, H. pylori GGT reduces the ROS resistance of the host cells and induces apoptosis or necrosis.(38,40)
Excess ROS are produced in H. pylori-colonized human stomachs; this induces oxidative stress to both the gastric mucosa and H. pylori. Because H. pylori has a deft capability of detoxifying ROS using a variety of enzymes to establish long-term colonization,(12,13) excess ROS leads solely to host cell damage.
Oxidative Stress in the Progression of Gastric Motility Disorders
Gastric motility disorders can occur in many clinical settings with a wide variety in the severity of symptoms with or without gastric mucosal injuries. Gastric motility disorders are attributable to either damage within the smooth muscle itself or dysfunctions within the neuromuscular components including the enteric nerves and interstitial cells of Cajal (ICC), which regulate smooth muscle function. How oxidative stress is involved in these dysfunctions is discussed in the following situations.
Gastrointestinal Complications in Sepsis
Oxygen radicals are implicated as relevant mediators in sepsis and septic shock in animals including humans.(41,42) Sepsis is a systemic response caused by bacterial endotoxins such as lipopolysaccharide, which induce the release of ROS and the generation of numerous pro-inflammatory factors and nitric oxide. During sepsis, the most frequent complications within the gastrointestinal tract are gastrointestinal motility disturbances and mucosal barrier dysfunction. Experimental administration of LPS delays gastric emptying and upregulates inducible nitric oxide synthase in order to downregulate neuronal nitric oxide synthase (nNOS) and synthesize PGs.(43) It is also reported that SOD reverses the endotoxin-induced delay in gastric emptying and diminishes the presence of nitrotyrosine, 4-hydroxy-2-nonenal in gastric mucosa, and inducible nitric oxide synthase-positive residential macrophages in the external musculature; these suggest the involvement of oxidative and nitrosative stresses in the pathogenesis of lipopolysaccharide-induced gastrointestinal dysmotility.(44)
Gastrointestinal Complications in Ischemia/Reperfusion Injury
The gastrointestinal tract is one of the most susceptible organ systems to ischemia. Previous investigations demonstrate that ischemia/reperfusion is a major contributor to gastric mucosal injury caused by stresses such as burn stress or hemorrhagic shock, NSAIDs, and H. pylori infection. In addition to mucosal injuries, delayed gastric emptying is also reported after gastric ischemia/reperfusion associated with disruption of the ICC network and nNOS-positive neurons.(45) ICC play critical roles in gastrointestinal motility in that they are the source of the electrical slow waves underlying the phasic contractions of the gastric musculature and mediate excitatory and inhibitory inputs to the musculature from the enteric motor neurons. Neuronal NOS generates neuronally derived NO, which is the major inhibitory neurotransmitter in the gastrointestinal tract. ICC and nNOS-positive neurons are both important factors for gastric emptying. Oxidative stress produced by the xanthine–xanthine oxidase system after ischemia/reperfusion may play a major role in these events, although the precise mechanism is unclear.
Diabetes Mellitus
Oxidative stress is a strong pathogenic co-factor involved in the development of complications of diabetes. Increased glucose levels in diabetes react non-enzymatically with proteins and become advanced glycation end products (AGEs); AGEs activate endothelial NADPH oxidase and increase endothelial ROS,(46) which occurs in animal models of diabetes(47) and diabetes patients.(48) Gastric neuromuscular dysfunction occurs in up to 30–50% of patients after 10 years of type 1 or 2 diabetes associated with histological changes including the loss of nNOS and ICC in both humans and animal models. It is reported that increased oxidative stress is attributable to the loss of upregulation of heme oxygenase-1; this results in the loss of ICC, decreased nNOS expression, and delayed gastric emptying in non-obese diabetic mice. These changes can be reversed by heme oxygenase-1 induction, demonstrating an important role of oxidative stress in the development of diabetic gastroparesis.(49)
Aging and Oxidative Stress
Gastrointestinal function declines with aging, including delayed gastric emptying, decreased peristalsis, and slowed colonic transit; these impair quality of life and increase morbidity and mortality. Notable changes in gut neuromuscular function that accompany advanced age are reported in human and animal models.(50,51) Cowen et al.(52) report a 50% reduction in ileal myenteric neurons in 24-month-old Sprague-Dawley rats fed ad libitum; this was prevented by caloric restriction, which reduces oxidative stress.(53) In the study using progeric mice deficient in the anti-aging peptide Klotho, progeric mice exhibited a gastric phenotype resembling that of human aging involving profound ICC loss with reduced slow wave amplitude and nitrergic inhibitory junction potentials. Klotho protects ICC by preserving their precursors, limiting oxidative stress, and maintaining nutritional status and normal levels of trophic factors important for ICC differentiation.(54) Increased oxidative stress in combination with a decrease in circulating and tissue factors that regulate ICC differentiation and survival contribute to the profound depletion of mature ICC and impair gastric function.
Conclusion
In conclusion, oxidative stress is one of the major contributors to the development of stomach diseases. Recent therapeutic options such as gastroprotective agents including antioxidant properties (e.g., rebamipide) can modulate the level of oxidative stress to enhance anti-inflammatory or antioxidant capacity. The stomach is an organ in direct contact with external pathogens; by presenting a strong acid environment, it has a special biological defense mechanism that eliminates such pathogens. However, H. pylori manages to live in the stomach by breaking through this defensive line. In response to the colonization of this bacterium, gastric mucosa can be exposed to severe oxidative stress with considerable levels of inflammatory cell accumulation, which might be related to the development of gastric mucosal as well as neuromuscular disorders.
Acknowledgments
The present study was supported by a Grant-in-Aid for Scientific Research (B) from the Japan Society for the promotion of Science (No. 22300169, to H.S.), a grant of the Adaptable and Seamless Technology transfer Program through target-driven R&D (A-STEP) (AS231Z00132G to H.S.) from the Japan Science and Technology Agency (JST), a grant from the Smoking Research Foundation (to H.S.), the Keio Gijuku Academic Development Fund (to H.S.), and a Nateglinide Memorial Toyoshima Research and Education Fund (to H.S.).
Abbreviations
- AGEs
advanced glycation end products
- GGA
geranylgeranylacetone
- GGT
γ-glutamyltranspeptidase
- HSF
heat shock factor
- ICC
interstitial cells of Cajal
- LPO
lipid peroxide
- MAPK
mitogen-activated protein kinase
- Mtz
metronidazole
- nNOS
neuronal nitric oxide synthase
- NSAIDs
non-steroidal anti-inflammatory drugs
- PGs
prostaglandins
- PHG
portal hypertensive gastropathy
- PPI
proton pump inhibitor
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RUNX3
Runt domain transcription factor 3
- SOD
superoxide dismutase
- TBARS
thiobarbituric acid-reactive substance
References
- 1.Suzuki H, Iwasaki E, Hibi T. Helicobacter pylori and gastric cancer. Gastric Cancer. 2009;12:79–87. doi: 10.1007/s10120-009-0507-x. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki H, Matsuzaki J, Hibi T. Ghrelin and oxidative stress in gastrointestinal tract. J Clin Biochem Nutr. 2011;48:122–125. doi: 10.3164/jcbn.10-16GFR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan JS, He SZ, Xu HZ, et al. Oxidative stress disturbs energy metabolism of mitochondria in ethanol-induced gastric mucosa injury. World J Gastroenterol. 2008;14:5857–5867. doi: 10.3748/wjg.14.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinjo N, Kawanaka H, Akahoshi T, et al. Significance of ERK nitration in portal hypertensive gastropathy and its therapeutic implications. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1016–G1024. doi: 10.1152/ajpgi.90329.2008. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki M, Miura S, Mori M, et al. Rebamipide, a novel antiulcer agent, attenuates Helicobacter pylori induced gastric mucosal cell injury associated with neutrophil derived oxidants. Gut. 1994;35:1375–1378. doi: 10.1136/gut.35.10.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishizawa T, Suzuki H, Nakagawa I, et al. Rebamipide-promoted restoration of gastric mucosal sonic hedgehog expression after early Helicobacter pylori eradication. Digestion. 2009;79:259–262. doi: 10.1159/000213241. [DOI] [PubMed] [Google Scholar]
- 7.Maity P, Bindu S, Choubey V, et al. Lansoprazole protects and heals gastric mucosa from non-steroidal anti-inflammatory drug (NSAID)-induced gastropathy by inhibiting mitochondrial as well as Fas-mediated death pathways with concurrent induction of mucosal cell renewal. J Biol Chem. 2008;283:14391–14401. doi: 10.1074/jbc.M800414200. [DOI] [PubMed] [Google Scholar]
- 8.Oshima T, Miwa H, Joh T. Aspirin induces gastric epithelial barrier dysfunction by activating p38 MAPK via claudin-7. Am J Physiol Cell Physiol. 2008;295:C800–C806. doi: 10.1152/ajpcell.00157.2008. [DOI] [PubMed] [Google Scholar]
- 9.Otaka M, Odashima M, Izumi Y, et al. Target molecules of molecular chaperone (HSP70 family) in injured gastric mucosa in vivo. Life Sci. 2009;84:664–667. doi: 10.1016/j.lfs.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki M, Suzuki H, Minegishi Y, Ito K, Nishizawa T, Hibi T. H. pylori-eradication therapy increases RUNX3 expression in the glandular epithelial cells in enlarged-fold gastritis. J Clin Biochem Nutr. 2010;46:259–264. doi: 10.3164/jcbn.09-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bagchi D, Bhattacharya G, Stohs SJ. Production of reactive oxygen species by gastric cells in association with Helicobacter pylori. Free Radic Res. 1996;24:439–450. doi: 10.3109/10715769609088043. [DOI] [PubMed] [Google Scholar]
- 12.Allen LA. Phagocytosis and persistence of Helicobacter pylori. Cell Microbiol. 2007;9:817–828. doi: 10.1111/j.1462-5822.2007.00906.x. [DOI] [PubMed] [Google Scholar]
- 13.Wang G, Alamuri P, Maier RJ. The diverse antioxidant systems of Helicobacter pylori. Mol Microbiol. 2006;61:847–860. doi: 10.1111/j.1365-2958.2006.05302.x. [DOI] [PubMed] [Google Scholar]
- 14.Olczak AA, Olson JW, Maier RJ. Oxidative-stress resistance mutants of Helicobacter pylori. J Bacteriol. 2002;184:3186–3193. doi: 10.1128/JB.184.12.3186-3193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bereswill S, Neuner O, Strobel S, Kist M. Identification and molecular analysis of superoxide dismutase isoforms in Helicobacter pylori. FEMS Microbiol Lett. 2000;183:241–245. doi: 10.1111/j.1574-6968.2000.tb08965.x. [DOI] [PubMed] [Google Scholar]
- 16.Benov LT, Fridovich I. Escherichia coli expresses a copper- and zinc-containing superoxide dismutase. J Biol Chem. 1994;269:25310–25314. [PubMed] [Google Scholar]
- 17.Spiegelhalder C, Gerstenecker B, Kersten A, Schiltz E, Kist M. Purification of Helicobacter pylori superoxide dismutase and cloning and sequencing of the gene. Infect Immun. 1993;61:5315–5325. doi: 10.1128/iai.61.12.5315-5325.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pesci EC, Pickett CL. Genetic organization and enzymatic activity of a superoxide dismutase from the microaerophilic human pathogen, Helicobacter pylori. Gene. 1994;143:111–116. doi: 10.1016/0378-1119(94)90614-9. [DOI] [PubMed] [Google Scholar]
- 19.Seyler RW, Jr., Olson JW, Maier RJ. Superoxide dismutase-deficient mutants of Helicobacter pylori are hypersensitive to oxidative stress and defective in host colonization. Infect Immun. 2001;69:4034–4040. doi: 10.1128/IAI.69.6.4034-4040.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ernst FD, Homuth G, Stoof J, et al. Iron-responsive regulation of the Helicobacter pylori iron-cofactored superoxide dismutase SodB is mediated by Fur. J Bacteriol. 2005;187:3687–3692. doi: 10.1128/JB.187.11.3687-3692.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ernst FD, Bereswill S, Waidner B, et al. Transcriptional profiling of Helicobacter pylori Fur- and iron-regulated gene expression. Microbiology. 2005;151:533–546. doi: 10.1099/mic.0.27404-0. [DOI] [PubMed] [Google Scholar]
- 22.Lee HW, Choe YH, Kim DK, Jung SY, Lee NG. Proteomic analysis of a ferric uptake regulator mutant of Helicobacter pylori: regulation of Helicobacter pylori gene expression by ferric uptake regulator and iron. Proteomics. 2004;4:2014–2027. doi: 10.1002/pmic.200300740. [DOI] [PubMed] [Google Scholar]
- 23.Bijlsma JJ, Waidner B, Vliet AH, et al. The Helicobacter pylori homologue of the ferric uptake regulator is involved in acid resistance. Infect Immun. 2002;70:606–611. doi: 10.1128/iai.70.2.606-611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi YW, Park SA, Lee HW, Lee NG. Alteration of growth-phase-dependent protein regulation by a Fur mutation in Helicobacter pylori. FEMS Microbiol Lett. 2009;294:102–110. doi: 10.1111/j.1574-6968.2009.01557.x. [DOI] [PubMed] [Google Scholar]
- 25.Delany I, Pacheco AB, Spohn G, Rappuoli R, Scarlato V. Iron-dependent transcription of the frpB gene of Helicobacter pylori is controlled by the Fur repressor protein. J Bacteriol. 2001;183:4932–4937. doi: 10.1128/JB.183.16.4932-4937.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Vliet AH, Stoof J, Vlasblom R, et al. The role of the ferric uptake regulator (Fur) in regulation of Helicobacter pylori iron uptake. Helicobacter. 2002;7:237–244. doi: 10.1046/j.1523-5378.2002.00088.x. [DOI] [PubMed] [Google Scholar]
- 27.Tiss A, Barre O, Michaud-Soret I, Forest E. Characterization of the DNA-binding site in the ferric uptake regulator protein from Escherichia coli by UV crosslinking and mass spectrometry. FEBS Lett. 2005;579:5454–5460. doi: 10.1016/j.febslet.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 28.Baichoo N, Helmann JD. Recognition of DNA by Fur: a reinterpretation of the Fur box consensus sequence. J Bacteriol. 2002;184:5826–5832. doi: 10.1128/JB.184.21.5826-5832.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carpenter BM, Gancz H, Gonzalez-Nieves RP, et al. A single nucleotide change affects fur-dependent regulation of sodB in H. pylori. PLoS One. 2009;4:e5369. doi: 10.1371/journal.pone.0005369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi SS, Chivers PT, Berg DE. Point mutations in Helicobacter pylori’s fur regulatory gene that alter resistance to metronidazole, a prodrug activated by chemical reduction. PLoS One. 2011;6:e18236. doi: 10.1371/journal.pone.0018236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsugawa H, Suzuki H, Satoh K, et al. Two amino acids mutation of ferric uptake regulator determines Helicobacter pylori resistance to metronidazole. Antioxid Redox Signal. 2011;14:15–23. doi: 10.1089/ars.2010.3146. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Reyes E, Kalyanaraman B, Mason RP. The reductive metabolism of metronidazole and ronidazole by aerobic liver microsomes. Mol Pharmacol. 1980;17:239–244. [PubMed] [Google Scholar]
- 33.Rao DN, Mason RP. Generation of nitro radical anions of some 5-nitrofurans, 2- and 5-nitroimidazoles by norepinephrine, dopamine, and serotonin. A possible mechanism for neurotoxicity caused by nitroheterocyclic drugs. J Biol Chem. 1987;262:11731–11736. [PubMed] [Google Scholar]
- 34.Esposito L, Seydel A, Aiello R, et al. The crystal structure of the superoxide dismutase from Helicobacter pylori reveals a structured C-terminal extension. Biochim Biophys Acta. 2008;1784:1601–1606. doi: 10.1016/j.bbapap.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki M, Miura S, Suematsu M, et al. Helicobacter pylori-associated ammonia production enhances neutrophil-dependent gastric mucosal cell injury. Am J Physiol. 1992;263:G719–G725. doi: 10.1152/ajpgi.1992.263.5.G719. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki H, Mori M, Suzuki M, Sakurai K, Miura S, Ishii H. Extensive DNA damage induced by monochloramine in gastric cells. Cancer Lett. 1997;115:243–248. doi: 10.1016/s0304-3835(97)04745-9. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki H, Seto K, Mori M, Suzuki M, Miura S, Ishii H. Monochloramine induced DNA fragmentation in gastric cell line MKN45. Am J Physiol. 1998;275:G712–G716. doi: 10.1152/ajpgi.1998.275.4.G712. [DOI] [PubMed] [Google Scholar]
- 38.Shibayama K, Kamachi K, Nagata N, et al. A novel apoptosis-inducing protein from Helicobacter pylori. Mol Microbiol. 2003;47:443–451. doi: 10.1046/j.1365-2958.2003.03305.x. [DOI] [PubMed] [Google Scholar]
- 39.Shibayama K, Wachino J, Arakawa Y, Saidijam M, Rutherford NG, Henderson PJ. Metabolism of glutamine and glutathione via gamma-glutamyltranspeptidase and glutamate transport in Helicobacter pylori: possible significance in the pathophysiology of the organism. Mol Microbiol. 2007;64:396–406. doi: 10.1111/j.1365-2958.2007.05661.x. [DOI] [PubMed] [Google Scholar]
- 40.Flahou B, Haesebrouck F, Chiers K, et al. Gastric epithelial cell death caused by Helicobacter suis and Helicobacter pylori γ-glutamyl transpeptidase is mainly glutathione degradation-dependent. Cell Microbiol. 2011;13:1933–1955. doi: 10.1111/j.1462-5822.2011.01682.x. [DOI] [PubMed] [Google Scholar]
- 41.Goode HF, Webster NR. Free radicals and antioxidants in sepsis. Crit Care Med. 1993;21:1770–1776. doi: 10.1097/00003246-199311000-00029. [DOI] [PubMed] [Google Scholar]
- 42.Albuszies G, Brückner UB. Antioxidant therapy in sepsis. Intensive Care Med. 2003;29:1632–1636. doi: 10.1007/s00134-003-1861-5. [DOI] [PubMed] [Google Scholar]
- 43.Calatayud S, García-Zaragozá E, Hernández C, et al. Downregulation of nNOS and synthesis of PGs associated with endotoxin-induced delay in gastric emptying. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1360–G1367. doi: 10.1152/ajpgi.00168.2002. [DOI] [PubMed] [Google Scholar]
- 44.de Winter BY, van Nassauw L, de Man JG. Role of oxidative stress in the pathogenesis of septic ileus in mice. Neurogastroenterol Motil. 2005;17:251–261. doi: 10.1111/j.1365-2982.2004.00618.x. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki S, Suzuki H, Horiguchi K, et al. Delayed gastric emptying and disruption of the interstitial cells of Cajal network after gastric ischaemia and reperfusion. Neurogastroenterol Motil. 2010;22:585–593. doi: 10.1111/j.1365-2982.2009.01444.x. e126. [DOI] [PubMed] [Google Scholar]
- 46.Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001;280:E685–E694. doi: 10.1152/ajpendo.2001.280.5.E685. [DOI] [PubMed] [Google Scholar]
- 47.Hink U, Li H, Mollnau H, Oelze M, et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:E14–E22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- 48.Guzik TJ, West NE, Black E, et al. Vascular superoxide production by NAD(P)H oxidase: association with endothelial dysfunction and clinical risk factors. Circ Res. 2000;86:E85–E90. doi: 10.1161/01.res.86.9.e85. [DOI] [PubMed] [Google Scholar]
- 49.Choi KM, Gibbons SJ, Nguyen TV, et al. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology. 2008;135:2055–2064. doi: 10.1053/j.gastro.2008.09.003. 2064 e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Horowitz M, Maddern GJ, Chatterton BE, Collins PJ, Harding PE, Shearman DJ. Changes in gastric emptying rates with age. Clin Sci (Lond) 1984;67:213–218. doi: 10.1042/cs0670213. [DOI] [PubMed] [Google Scholar]
- 51.Phillips RJ, Powley TL. As the gut ages: timetables for aging of innervation vary by organ in the Fischer 344 rat. J Comp Neurol. 2001;434:358–377. doi: 10.1002/cne.1182. [DOI] [PubMed] [Google Scholar]
- 52.Cowen T, Johnson RJ, Soubeyre V, Santer RM. Restricted diet rescues rat enteric motor neurons from age related cell death. Gut. 2000;47:653–660. doi: 10.1136/gut.47.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 54.Izbeki F, Asuzu DT, Lorincz A, et al. Loss of Kitlow progenitors, reduced stem cell factor and high oxidative stress underlie gastric dysfunction in progeric mice. J Physiol. 2010;588:3101–3117. doi: 10.1113/jphysiol.2010.191023. [DOI] [PMC free article] [PubMed] [Google Scholar]