Abstract
Temperature-dependent free radical reactions were investigated using nitroxyl radicals as redox probes. Reactions of two types of nitroxyl radicals, TEMPOL (4-hydroxyl-2,2,6,6-tetramethylpiperidine-N-oxyl) and carbamoyl-PROXYL (3-carbamoyl-2,2,5,5-tetramethylpyrrolidine-N-oxyl), were tested in this paper. Heating a solution containing a nitroxyl radical and a reduced form of glutathione (GSH) caused temperature-dependent decay of electron paramagnetic resonance (EPR) signal of the nitroxyl radical. Heating a solution of the corresponding hydroxylamine form of the nitroxyl radical showed EPR signal recovery. The GSH-dependent reduction of nitroxyl radicals at 70°C was suppressed by antioxidants, spin trapping agents, and/or bubbling N2 gas, although heating carbamoyl-PROXYL with GSH showed temporarily enhanced signal decay by bubbling N2 gas. Since SOD could restrict the GSH-dependent EPR signal decay of TEMPOL, O2•− is related with this reaction. O2•− was probably generated from dissolved oxygen in the reaction mixture. Oxidation of the hydroxylamines at 70°C was also suppressed by bubbling N2 gas. Heating a solution of spin trapping agent, DMPO (5,5-dimethyl-1-pyrroline-N-oxide) showed a temperature-dependent increase of the EPR signal of the hydroxyl radical adduct of DMPO. Synthesis of hydroxyl radical adduct of DMPO at 70°C was suppressed by antioxidants and/or bubbling N2 gas. The results suggested that heating an aqueous solution containing oxygen can generate O2•−.
Keywords: reactive oxygen species, electron paramagnetic resonance, redox probe, nitroxyl radical, hyperthermia
Introduction
Interest in hyperthermia for clinical cancer treatment has been increasing recently, especially in combination use with existing treatments, i.e. chemotherapy, radiotherapy and immunotherapy, to increase the efficiency of those treatments; however, the mechanism of hyperthermal cell killing or sensitization to other stresses/treatments has been unclear. The relation of reactive oxygen species (ROS) with the effect of hyperthermia has been widely alleged and/or reported.(1–4)
Nitroxyl radicals have been conventionally used as chemical redox probes in in vitro and in vivo experiments in electron paramagnetic resonance (EPR) spectroscopy.(5–7) Nitroxyl radicals have been highlighted recently as redox-sensitive MR contrast agents.(8–10) Paramagnetic nitroxyl radical can be directly detected using EPR or indirectly detected through a proton T1 shortening effect using MRI; however, diamagnetic hydroxylamines, which are a one-electron reduced form of nitroxyl radicals, can not be detected by either method. Changing spectroscopic behavior through redox transformation of nitroxyl radicals has been utilized to detect free radical reactions in a sample. A nitroxyl radical can be oxidized to the corresponding oxoammonium cation by several oxidants, such as •OH, O2•−, and/or Fe3+ ion (Eq. 1).
| (1) |
The oxoammonium cation was reduced to the corresponding hydroxylamine by receiving hydrogen from hydrogen donors, such as dihydronicotinamide adenine dinucleotide (NADH), dihydronicotinamide adenine dinucleotide phosphate (NADPH), and/or a reduced form of glutathione (GSH) (Eq. 2).
| (2) |
A recent study(11) also found that oxoammonium may irreversibly react with GSH to make a redox-stable complex (Eq. 3), while the structure of this redox-stable complex is still unclear.
| (3) |
In this paper, temperature-dependent free radical reactions were investigated using nitroxyl radicals as redox probes to examine whether ROS generation can be anticipated for the effect of hyperthermia. The results suggested the temperature-dependent induction of ROS formation in an aqueous solution. The possible mechanisms of temperature-dependent free radical reactions in water are discussed.
Materials and Methods
Chemicals
3-Carbamoyl-2,2,5,5-tetramethylpyrrolidine-N-oxyl (CmP, which is also known as 3CP or carbamoyl-PROXYL) and 4-hydroxyl-2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPOL) were purchased from Sigma-Aldrich (St. Louis, MO). 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) was purchased from LABOTEC Co. (Tokyo, Japan). 5-(2,2-dimethyl-1,3-propoxycyclophosphoryl)-5-methyl-1-pyrroline-N-oxide (CYPMPO) was synthesized as reported elsewhere.(12) The hydroxylamine forms of TEMPOL and CmP, i.e. TEMPOL-H and CmP-H, were a gift from Dr. Murali C. Krishna of the National Cancer Institute (NIH, Bethesda, MD). Other chemicals used in this study were of analytical grade. As the basic solvent of reaction mixtures, 100 mM phosphate buffer (pH 7.0) containing 0.05 mM diethylenetriaminepentaacetic acid (DTPA) (100 mM PB) was prepared and used for all experiments. Deionized water (deionized by the Milli-Q system) was used to prepare 100 mM PB.
EPR signal decay of nitroxyl radicals by heating
A reaction mixture containing 0.1 mM nitroxyl radical (TEMPOL or CmP) and 1 mM GSH was prepared using 100 mM PB. The reaction mixture was kept in a screw-top vial and was incubated in a heat block at various temperatures (0, 24, 37, 50, 70, or 90°C). The time course of the EPR signal of nitroxyl radical in the reaction mixture was measured by an X-band EPR spectrometer (JEOL, Tokyo, Japan) as described below. The experiment at 70°C was repeated with the addition of antioxidants, i.e. 0–20 mM DMPO, 0–20 mM CYPMPO, 600 mM dimethylsulfoxide (DMSO), 600 mM α-mannitol, or 600 mM ethanol. This experiment was also repeated with the 1 mM oxidized form of glutathione (GSSG) instead of GSH, with 1 mM NADH instead of GSH, or with 1 mM NADPH instead of GSH. The experiment at 70°C with 1 mM NADH or NADPH instead of GSH was repeated with the addition of 50 mM DMPO. Again, the experiment at 70°C with GSH was repeated under N2 bubbling. To test the effect of superoxide dismutase (SOD) and catalase (CAT), the experiment with GSH was repeated at 37°C and 70°C.
EPR signal decay of nitroxyl radicals by ROS
To generate •OH in the reaction mixture, a reaction mixture containing 0.1 mM nitroxyl radical (TEMPOL or CmP), 1 mM GSH, and 1 mM H2O2 was prepared using 100 mM PB, and then the reaction mixture was irradiated by ultraviolet B (290 nm) at room temperature. To generate O2•− in the reaction mixture, a reaction mixture containing 0.1 mM nitroxyl radical (TEMPOL or CmP), 1 mM GSH, 0.05 mM hypoxanthine (HPX), and 0.01 U/mL xanthine oxidase (XOD) was prepared using 100 mM PB, and then the reaction mixture was incubated at room temperature. The time course of the EPR signal of nitroxyl radical in the reaction mixture was measured by an X-band EPR spectrometer (JEOL, Tokyo, Japan) as described below.
Oxidation of hydroxylamines with ROS
To see the reaction of hydroxylamines with •OH, a reaction mixture containing 0.1 mM TEMPOL-H or CmP-H and 1 mM H2O2 was prepared using 100 mM PB. The reaction mixture was irradiated by ultraviolet B (290 nm). The time course of EPR signal intensity in the reaction mixture after starting UV irradiation was recorded. To see the reaction of hydroxylamines with O2•−, a reaction mixture containing 0.1 mM TEMPOL-H or CmP-H, 0.05 mM HPX, and 0.01 U/ml XOD was prepared using 100 mM PB, and then the reaction mixture was incubated at room temperature. The time course of EPR signal intensity in the reaction mixture after starting the reaction (adding HPX) was recorded.
Oxidation of hydroxylamines by heating
A reaction mixture containing 0.1 mM TEMPOL-H or CmP-H was prepared using 100 mM PB. The reaction mixture was heated at 70°C. The experiments were repeated under N2 bubbling. The experiments were also repeated adding 1.6 U/ml SOD. The time course of EPR signal intensity in the reaction mixture after heating was recorded.
Induction of OH-spin adduct of spin trapping agents by heating
DMPO was added to 100 mM PB containing 0.05 mM DTPA (pH 7.0) to make the final concentration of 225 mM. The reaction mixture was kept in a screw-top vial and was incubated in a water bath at various temperatures (37, 50, 70, or 90°C). The time course of DMPO-OH formed in the reaction mixture was measured by an X-band EPR spectrometer (JEOL) as follows. Using CYPMPO instead of DMPO, the same procedures were tested again. The experiment was repeated with the addition of several concentrations of a •OH scavenger, such as DMSO or mannitol, at 70°C.
X-band EPR Measurement
An aliquot (120–130 µl) of the reaction mixture was sampled in a quartz flat cell, set in a TE-mode cavity using a special cell holder, and was measured as soon as possible. The sample solution in the flat cell was put back into the vial immediately after the measurement. The EPR conditions were as follows: microwave frequency was 9.4 GHz, microwave power was 4 mW, center field was 334 mT, sweep width was 10 mT, sweep speed was 5 mT/min, modulation frequency was 100 kHz, modulation amplitude was 0.0079 mT, and time constant was 0.03 s.
Results and Discussion
When the aqueous solution of nitroxyl radicals was heated with coexisting GSH, the EPR signal of nitroxyl radicals decreased time- and temperature-dependently (Fig. 1). This EPR signal decay was not observed without GSH. The EPR signal decay of TEMPOL showed the delay time of starting the reaction. The delay time was shortened with increasing temperature, and the reaction rate also became faster temperature-dependently (Fig. 1A). The delay time to start the reaction at room temperature was around 3–4 h. The EPR signal was stable for more than 24 h when the reaction mixture was kept on ice, as shown in a previous report.(11) The reaction of CmP, however, did not show a delay to the initiation of EPR signal loss. The reaction rate of CmP became faster temperature-dependently (Fig. 1B).
Fig. 1.
Temperature-dependent decay of nitroxyl radicals under coexisting GSH. The reaction mixture containing 0.1 mM nitroxyl radical (TEMPOL or CmP) and 1 mM GSH was incubated in a water bath at various temperatures. The reaction mixture was prepared with 100 mM PB, containing 0.05 mM DTPA. Time course of EPR signal in an aliquot of the reaction mixture was measured with X-band EPR spectrometer. Experiments were carried out at various temperatures (0, 24, 37, 50, 70, or 90°C). (A) Time course of TEMPOL in the reaction mixture. (B) Time course of CmP in the reaction mixture. Marks and bars indicate average and SD of at least 3 experiments.
Fig. 2 shows the effect of various antioxidants on the EPR signal loss of nitroxyl radicals at 70°C in the reaction mixture containing GSH. A relatively high concentration (600 mM) of antioxidants did not have any notable effects on the reaction of TEMPOL, except that DMSO showed very slight suppression of the reaction. The reaction of CmP, however, could be suppressed by a high concentration of antioxidants.
Fig. 2.
Inhibition of heat-induced decay of nitroxyl radicals by anti-oxidants. The reaction mixture containing 0.1 mM nitroxyl radical (TEMPOL or CmP), 1 mM GSH, and 600 mM of an anti-oxidant (α-mannitol, DMSO, or ethanol) was incubated in a heat block at 70°C. (A) Time course of TEMPOL in the reaction mixture. (B) Time course of CmP in the reaction mixture. Marks and bars indicate average and SD of at least 3 experiments.
Fig. 3 shows the effect of a spin trapping agent, DMPO, on the EPR signal loss of nitroxyl radicals in the reaction mixture at 70°C. Adding DMPO to the reaction mixture of TEMPOL suppressed the EPR signal loss of TEMPOL depending on the concentration of DMPO; however, DMPO did not have an effect on the delay time to the initiation of EPR signal loss (Fig. 3A). DMPO also suppressed the reaction of CmP. Another spin trapping agent, CYPMPO, showed stronger suppression of the reactions of both TEMPOL and CmP (Fig. 4).
Fig. 3.
Inhibition of heat-induced decay of nitroxyl radicals by a spin trapping agent, DMPO. The reaction mixture containing 0.1 mM nitroxyl radical (TEMPOL or CmP) and 1 mM GSH and several concentration of DMPO (0, 1, 10, or 20 mM), which was prepared with 100 mM PB, was incubated in a water bath at 70°C. (A) Time course of TEMPOL in the reaction mixture. (B) Time course of CmP in the reaction mixture. Marks and bars indicate average and SD of at least 3 experiments.
Fig. 4.
Inhibition of heat-induced decay of nitroxyl radicals by a spin trapping agent, CYPMPO. The reaction mixture containing 0.1 mM nitroxyl radical (TEMPOL or CmP) and 1 mM GSH and several concentrations of CYPMPO (0, 1, 10, or 20 mM), which was prepared with 100 mM PB, was incubated in a water bath at 70°C. (A) Time course of TEMPOL in the reaction mixture. (B) Time course of CmP in the reaction mixture. Marks and bars indicate average and SD of at least 3 experiments.
EPR signal loss of TEMPOL when heating the reaction mixture was not obtained when GSSG was used instead of GSH (Fig. 5A). The EPR signal, however, temporarily decreased and again recovered when NAD(P)H was used instead of GSH (Fig. 5B). This recovering EPR signal intensity may due to the re-oxidation of hydroxylamine to the corresponding nitroxyl radical. This temporary decrease of the EPR signal was eliminated by adding DMPO to the reaction mixture (Fig. 5C). The same behavior was observed for the reaction of CmP (Fig. 6).
Fig. 5.
Reaction of TEMPOL in the heated reaction mixture containing GSSG, NADH, or NADPH instead of GSH. The reaction mixture containing 0.1 mM TEMPOL and 1 mM GSSG, NADH, or NADPH was incubated at 70°C. (A) Time courses of TEMPOL in the reaction mixture. (B) Detailed time course of TEMPOL in the reaction mixture containing NAD(P)H. (C) Effect of DMPO on the reaction of TEMPOL. Marks and bars indicate average and SD of at least 3 experiments.
Fig. 6.
Reaction of CmP in the heated reaction mixture containing GSSG, NADH, or NADPH instead of GSH. The reaction mixture containing 0.1 mM CmP and 1 mM GSSG, NADH, or NADPH was incubated at 70°C. (A) Time courses of CmP in the reaction mixture. (B) Detailed time course of CmP in the reaction mixture containing NAD(P)H. (C) Effect of DMPO on the reaction of CmP. Marks and bars indicate average and SD of at least 3 experiments.
The EPR signal loss of TEMPOL by heating in the reaction mixture containing GSH was suppressed by bubbling the reaction mixture with N2 gas (Fig. 7A). The start of the decrease of the EPR signal was markedly delayed by N2 gas bubbling, although the reaction could not be stopped completely. On the other hand, the EPR signal loss of CmP by heating with coexisting GSH increased with N2 gas bubbling compared with air, and the EPR signal of CmP gradually recovered (Fig. 7B). DMPO could restrict this EPR signal decay of CmP under N2 bubbling conditions (data not shown). The results in Fig. 7 suggest that the oxygen dissolved in the reaction mixture is related with the temperature-dependent free radical formation in the reaction mixture, while the reaction mechanisms were different between TEMPOL and CmP.
Fig. 7.
Effect of N2 bubbling on the heat-induced decay of nitroxyl radicals. The reaction mixture containing 0.1 mM nitroxyl radical (TEMPOL or CmP) and 1 mM GSH prepared with 100 mM PB, was incubated in a water bath at 70°C with bubbling N2 gas. Bubbling was started 10 min before starting incubation. (A) Time course of TEMPOL in the reaction mixture. (B) Time course of CmP in the reaction mixture. Marks and bars indicate average and SD of at least 3 experiments.
Fig. 8 shows EPR signal losses of nitroxyl radicals in the reaction mixture containing GSH induced by chemically generated •OH or O2•−. •OH was generated by irradiating UVB to H2O2. O2•− was generated by reacting with HPX and XOD. The GSH-dependent reduction of nitroxyl radicals can be caused by either •OH or O2•−. Both TEMPOL and CmP lost most of the EPR signal relatively quickly when the reaction was induced by •OH. O2•− could also reduce the EPR signal of both nitroxyl radicals; however, the reaction of CmP under generating O2•− appeared smaller than that with TEMPOL, which lost almost all EPR signal under generating O2•−. TEMPOL has been reported as a SOD mimicking reagent.(13,14) The results in Fig. 8 show that TEMPOL may sensitively react with O2•− compared with CmP.
Fig. 8.
EPR signal decay of nitroxyl radicals by chemically generated •OH (H2O2/UVB) and O2•− (HPX/XOD). EPR spectrum of an aliquot of the reaction mixture was measured with an X-band EPR spectrometer and the time course of the EPR signal of (A) TEMPOL and (B) CmP was plotted. A reaction mixture containing 0.1 mM nitroxide, 1 mM GSH, and 1 mM H2O2 was irradiated by UV (290 nm) at room temperature (open circle). A reaction mixture containing 0.1 mM nitroxide, 0.05 mM HPX, and 0.01 U/mL XOD was incubated at room temperature (open diamond). Marks and bars indicate average and SD of at least 3 experiments.
Fig. 9 shows the effect of SOD and CAT on the heat-induced EPR signal decay of TEMPOL. SOD could restrict the EPR signal decay of TEMPOL at both 37°C and 70°C, while CAT could not, suggesting that the O2•− generated in the reaction mixture is related with the heat-induced EPR signal loss of TEMPOL, while the mechanism of O2•− generation is still in progress. SOD was deactivated at 70°C; therefore, SOD could not stop the reaction at 70°C but delayed the reaction (Fig. 9B); however, the reaction at 37°C was almost stopped during 120 min experimentation (Fig. 9A).
Fig. 9.
Effect of superoxide dismutase (SOD) and catalase (CAT) on the heat-induced EPR signal decay of TEMPOL. A reaction mixture containing 0.1 mM TEMPOL, 1 mM GSH, and 1.6 U/mL SOD was incubated at 37°C or 70°C. The experiment was repeated using CAT instead of SOD. EPR spectrum of an aliquot of the reaction mixture was measured with an X-band EPR spectrometer and the time course of the EPR signal of (A) TEMPOL and (B) CmP was plotted. Marks and bars indicate average and SD of at least 3 experiments.
Fig. 10 shows the results of oxidizing hydroxylamine forms of TEMPOL or CmP (TEMPOL-H or CmP-H) to the corresponding nitroxyl radicals in several ways, such as exposing hydroxylamine to chemically generated •OH, O2•−, or simply heating the aqueous solution of a hydroxylamine. EPR signals increased quickly when the hydroxylamines were exposed to •OH (open circles). EPR signal intensities increased gradually when the hydroxylamines were exposed to O2•− (open diamonds). The oxidation of hydroxylamines can be caused also by either •OH or O2•−. When the hydroxylamines were heated at 70°C, the EPR signal intensities gradually increased, similar to the reaction with O2•− (triangle). This heat-induced EPR signal growth was restricted by N2 gas bubbling (square). Both TEMPOL-H and CmP-H showed similar time course patterns of reactions, while the reactions were greater for CmP-H than TEMPOL-H.
Fig. 10.
Oxidation of hydroxylamines to nitroxyl radicals. Hydroxylamine form of TEMPOL or CmP (TEMPOL-H or CmP-H) dissolved in 100 mM PB containing 0.05 mM DTPA (pH 7.0) was oxidized under several conditions, i.e. by •OH, O2•−, or heating. EPR spectrum of an aliquot of the reaction mixture was measured with an X-band EPR spectrometer and the time course of the EPR signal of (A) TEMPOL and (B) CmP was plotted. A reaction mixture containing 0.1 mM hydroxylamine and 1 mM H2O2 was irradiated by UV (290 nm) (circle). A reaction mixture containing 0.1 mM hydroxylamine, 0.05 mM HPX, and 0.01 U/mL XOD was incubated at room temperature (diamond). A reaction mixture containing 0.1 mM hydroxylamine was heated at 70°C under air (triangle) or N2 bubbling (square). Marks and bars indicate average and SD of at least 3 experiments.
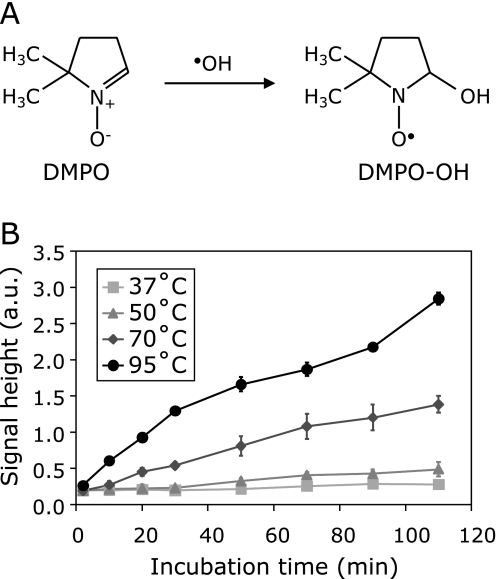
When the aqueous reaction mixture containing DMPO was heated, the EPR signal of DMPO-OH radical was observed (Fig. 11A). The EPR signal intensity of DMPO-OH increased time- and temperature-dependently (Fig. 11B). The temperature-dependent formation of DMPO-OH was inhibited by the addition of α-mannitol and/or DMSO (Fig. 12 A and B). This observation did not coincide with a previous report by Shoji et al.(15) who reported that the formation of DMPO-OH was suppressed under an argon atmosphere, using ultra-pure water, or the addition of EDTA; however, the addition of α-mannitol or DMSO did not affect the formation of DMPO-OH. In contrast to the previous report, the formation of DMPO-OH was suppressed by the relatively high concentration of •OH scavengers in this paper, in which the experimental system contained 0.05 mM DTPA. Therefore, most DMPO-OH formation in water by heating was not via scavenging •OH; however, part of it was via scavenging •OH. The •OH-independent formation of DMPO-OH was suppressed by a metal ion chelator, such as DTPA. The •OH-dependent formation of DMPO-OH may not suppressed by such metal ion chelators. The EPR signal of DMPO-OOH was not observed in this experiment. DMPO-OOH may develop and be transformed to DMPO-OH quickly, since N2 gas bubbling could stop the appearance of the DMPO-OH signal (data not shown).
Fig. 11.
Temperature-dependent formation of DMPO-OH in PBS. DMPO was dissolved in 100 mM PBS containing 0.05 mM DTPA (pH 7.0). The final concentration of DMPO in the reaction mixture was 225 mM. The reaction mixture was incubated in a water bath at an identical temperature for 120 min. EPR spectrum of an aliquot of the reaction mixture was measured at X-band EPR spectrometer. (A) Time course of EPR spectrum in the reaction mixture at 70°C. (B) Time course of EPR signal height of lowest line of DMPO-OH was plotted.
Fig. 12.
Inhibition of temperature-dependent DMPO-OH formation by several antioxidants. An anti-oxidant (α-mannitol or DMSO) was added to the reaction mixture containing 225 mM DMPO with several concentrations. The reaction mixture was incubated in water bath at 70°C. (A) Effect of α-mannitol. (B) Effect of DMSO. Marks and bars indicate average and SD of at least 3 experiments.
Heating a solution containing GSH and nitroxyl radical caused EPR signal decay of the nitroxyl radical temperature-dependently. This heat-induced EPR signal decay of nitroxyl radicals was suppressed by adding EPR spin trapping agents, such as DMPO and CYPMPO, or a relatively high concentration of free radical scavengers, such as DMSO, α-mannitol, and ethanol, although the reaction of TEMPOL was only slightly weakened by DMSO. This suggests that the generation of ROS in the reaction mixture related to the EPR signal decay.
Heating a solution containing NAD(P)H and a nitroxyl radical caused temporary EPR signal decay of the nitroxyl radical, which recovered subsequently to the initial level. This temporary EPR signal decay of nitroxyl radical reacting with NAD(P)H was eliminated by adding a spin trapping agent, DMPO. Since NAD(P)H can cause temporary EPR signal decay, nitroxyl radicals were oxidized to oxoammonium by ROS.
The EPR signal loss of TEMPOL by heating in a reaction mixture containing GSH was suppressed by bubbling N2 gas, while the EPR signal loss of CmP by heating with coexisting GSH increased with N2 gas bubbling. DMPO can restrict this EPR signal decay of CmP under N2 bubbling conditions (data not shown). Both TEMPOL and CmP lost most of the EPR signal relatively quickly when the reaction was induced by chemically generated •OH. The chemically generated O2•− also reduced the EPR signal of both nitroxyl radicals with coexisting GSH; however, the reaction of CmP was less than that of TEMPOL. SOD could restrict the EPR signal decay of TEMPOL at both 37°C and 70°C, while CAT could not. Since SOD could restrict the GSH-dependent EPR signal decay of TEMPOL, it was suggested that O2•− generated in the reaction mixture was related to this reaction. EPR signals increased quickly when the hydroxylamines were exposed to photo-chemically generated •OH. EPR signal intensities increased gradually when the hydroxylamines were exposed to chemically generated O2•−. Increasing the nitroxyl EPR signal by heating a hydroxylamine was suppressed by N2 bubbling. Heating a DMPO solution showed the temperature-dependent generation of DMPO-OH spin adducts. This heat-induced generation of DMPO-OH could be suppressed by adding a high concentration of anti-oxidants to the reaction mixture. N2 gas bubbling could stop the appearance of the DMPO-OH signal by heating. This suggests that the generation of ROS in the reaction mixture.
Conclusion
The results in this paper suggest that heating an aqueous solution containing oxygen can generate ROS. Since SOD could restrict the GSH-dependent EPR signal decay of TEMPOL, the main ROS generated in reaction mixtures was estimated as O2•−. Since bubbling N2 gas restricted this reaction, O2•− was probably generated from dissolved O2 in the reaction mixture while the mechanism of O2•− generation was still in progress. O2•− was partly transformed to •OH, since antioxidants can slightly restrict the GSH-dependent EPR signal decay of both TEMPOL and CmP.
Abbreviations
- CAT
catalase
- CmP
3-carbamoyl-2,2,5,5-tetramethylpyrrolidine-N-oxyl
- CYPMPO
5-(2,2-dimethyl-1,3-propoxycyclophosphoryl)-5-methyl-1-pyrroline-N-oxide
- DMPO
5,5-dimethyl-1-pyrroline-N-oxide
- DMSO
dimethylsulfoxide
- DTPA
diethylenetriaminepentaacetic acid
- GSH
reduced form of glutathione
- GSSG
oxidized form of glutathione
- HPX
hypoxanthine
- NADH
dihydronicotinamide adenine dinucleotide
- NADPH
dihydronicotinamide adenine dinucleotide phosphate
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TEMPOL
4-hydroxyl-2,2,6,6-tetramethylpiperidine-N-oxyl
- XOD
xanthine oxidase
References
- 1.Bettaieb A, Averill-Bates DA. Thermotolerance induced at a fever temperature of 40 degrees C protects cells against hyperthermia-induced apoptosis mediated by death receptor signalling. Biochem Cell Biol. 2008;86:521–538. doi: 10.1139/O08-136. [DOI] [PubMed] [Google Scholar]
- 2.Cui ZG, Kondo T, Feril LB, Jr., Waki K, Inanami O, Kuwabara M. Effects of anitoxidants on X-ray- or hyperthermia-induced apoptosis in human lymphoma U937 cells. Apoptosis. 2004;9:757–763. doi: 10.1023/B:APPT.0000045782.56480.6b. [DOI] [PubMed] [Google Scholar]
- 3.Chen F, Wang CC, Kim E, Harrison LE. Hyperthermia in combination with oxidative stress induces autophagic cell deth in HT-29 colon cancer cells. Cell Biol Int. 2008;32:715–723. doi: 10.1016/j.cellbi.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Wang CC, Chen F, Kim E, Harrison LE. Thermal sensitization through ROS modulation: a strategy to improve the efficacy of hyperthermic intraperitoneal chemotherapy. Surgery. 2007;142:384–392. doi: 10.1016/j.surg.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Swartz HM, Khan N, Khramtsov VV. Use of electron paramagnetic resonance spectroscopy to evaluate the redox state in vivo. Antioxid Redox Signal. 2007;9:1757–1771. doi: 10.1089/ars.2007.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeshita K, Ozawa T. Recent progress in in vivo ESR spectroscopy. J Radiat Res (Tokyo) 2004;45:373–384. doi: 10.1269/jrr.45.373. [DOI] [PubMed] [Google Scholar]
- 7.Utsumi H, Yamada K. In vivo electron spin resonance-computed tomography/nitroxyl probe technique for non-invasive analysis of oxidative injuries. Arch Biochem Biophys. 2003;416:1–8. doi: 10.1016/s0003-9861(03)00285-6. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto K, Hyodo F, Matsumoto A, et al. High-resolution mapping of tumor redox status by magnetic resonance imaging using nitroxides as redox-sensitive contrast agents. Clin Cancer Res. 2006;12:2455–2462. doi: 10.1158/1078-0432.CCR-05-2747. [DOI] [PubMed] [Google Scholar]
- 9.Hyodo F, Matsumoto K, Matsumoto A, Mitchell JB, Krishna MC. Probing the intracellular redox status of tumors with magnetic resonance imaging and redox-sensitive contrast agents. Cancer Res. 2006;66:9921–9928. doi: 10.1158/0008-5472.CAN-06-0879. [DOI] [PubMed] [Google Scholar]
- 10.Cotrim AP, Hyodo F, Matsumoto K, et al. Differential radiation protection of salivary glands versus tumor by tempol with accompanying tissue assessment of tempol by MR functional imaging. Clin Cancer Res. 2007;13:4928–4933. doi: 10.1158/1078-0432.CCR-07-0662. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto K, Okajo A, Nagata K, et al. Detection of free radical reactions in an aqueous sample induced by low linear-energy-transfer irradiation. Biol Pharm Bull. 2009;32:542–547. doi: 10.1248/bpb.32.542. [DOI] [PubMed] [Google Scholar]
- 12.Kamibayashi M, Oowada S, Kameda H, et al. Synthesis and characterization of a practically better DEPMPO-type spin trap, 5-(2,2-dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1-pyrroline N-oxide (CYPMPO) Free Radic Res. 2006;40:1166–1172. doi: 10.1080/10715760600883254. [DOI] [PubMed] [Google Scholar]
- 13.Samuni A, Krishna CM, Riesz P, Finkelstein E, Russo A. A novel metal-free low molecular weight superoxide dismutase mimic. J Biol Chem. 1988;263:17921–17924. [PubMed] [Google Scholar]
- 14.Krishna MC, Grahame DA, Samuni A, Mitchell JB, Russo A. Oxoammonium cation intermediate in the nitroxide-catalyzed dismutation of superoxide. Proc Natl Acad Sci USA. 1992;89:5537–5541. doi: 10.1073/pnas.89.12.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shoji T, Li L, Abe Y, et al. DMPO-OH radical formation from 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) in hot water. Anal Sci. 2007;23:219–221. doi: 10.2116/analsci.23.219. [DOI] [PubMed] [Google Scholar]