Abstract
In inflammatory bowel diseases, interleukin-1β production is accelerated. Butyrate, a short chain fatty acid, plays an important role in inflammatory bowel diseases. We investigated the effect of butyrate on interleukin-1β production in macrophage and elucidated its underlying mechanism. We stimulated THP-1 cells, a human premonocytic cell line, by lipopolysaccharide alone and by butyrate with lipopolysaccharide. Butyrate with lipopolysaccharide increased interleukin-1β production more than lipopolysaccharide alone. Butyrate with lipopolysaccharide increased caspase-1 activity more than lipopolysaccharide alone. As for the phosphorylation pathway, PD98059 (ERK1/2 inhibitor), SB203580 (p38 MAPK inhibitor), SP600125 (JNK1/2 inhibitor) decreased caspase-1 activity and interleukin-1β production to approximately 50% of the controls. Pertussis toxin (G protein-coupled signal transduction pathway inhibitor) also reduced interleukin-1β production to approximately 50%. Butyrate with lipopolysaccharide increased reactive oxygen species levels more than lipopolysaccharide alone. The addition of N-acetyl L-cysteine reduced reactive oxygen species levels to a level similar to that of lipopolysaccharide alone. Butyrate with lipopolysaccharide increased nitric oxide production more than lipopolysaccharide alone, and the addition of N-acetyl L-cysteine reduced the elevated amount of nitric oxide. In conclusions, butyrate enhances interleukin-1β production by activating caspase-1, via reactive oxygen species, the phosphorylation of MAPK, and G protein mediated pathways in lipopolysaccharide stimulated THP-1 cells.
Keywords: butyrate, macrophage, interleukin-1β (IL-1β), caspase-1, reactive oxygen species (ROS)
Introduction
Inflammatory bowel diseases (IBD), such as ulcerative colitis (UC) and Crohn’s disease, are characterized by the increased migration of leukocytes into intestinal mucosal lesions, necrosis of epithelial cells, and large amounts of enteric ulceration.(1) At the onset or the active phase in IBD, the production of pro-inflammatory cytokines and chemokines, particularly interleukin (IL)-1β, has been observed in these lesions, as has the activation of caspase-1 in macrophages.(1–3)
Ohkusa et al.(4) have successfully induced UC-like intestinal tracts in mice by administering enemas using conditioned media from the obligate anaerobe Fusobacterium varium, isolated from the intestinal mucosa of UC patients. They suggest that short chain fatty acids (SCFAs) generated by Fusobacterium varium, particularly butyrate, played important roles in this induction. Eftimiadi et al.(5) reported that butyrate enhances IL-1β production from monocytes. These studies suggest that butyrate induced IL-1β may be closely and critically associated with the pathogenesis of the disease. Therefore, we hypothesize that butyrate promotes the inflammation in mucosa by producing IL-1β from activated macrophages.
Although IL-1β is regulated by p38 mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinases (ERKs) in human smooth muscle cells,(6) the mechanism by which butyrate induces IL-1β production in monocyte-macrophages is not yet understood. In the present study, we investigated the effect of butyrate on the expression of IL-1β and caspase-1 activity in THP-1 cells, a human premonocytic cell line, and characterized the signal transduction pathways by which butyrate induces IL-1β production.
Materials and Methods
Materials
Lipopolysaccharide (LPS; Salmonella typhosa 0901) was purchased from Difco (Detroit, MI); PD98059, SB203580, SP600125, and GF109203X from Calbiochem (La Jolla, CA); Diphenyleneiodonium chloride from Sigma Chem. (St. Louis, MO); Ac-YVAD-CHO from BIOMOL (Plymouth, PA); and pertussis toxin (PTX) from Wako Pure Chem. (Osaka, Japan). Oligonucleotide primers were synthesized and purified by BEX (Tokyo, Japan). Total cell protein concentrations were determined using a DC protein assay kit (Bio-Rad Lab., Hercules, CA) with bovine serum albumin (Bio-Rad) as the standard. All other chemicals and materials (except those described below) were obtained from Nacalai Tesque (Kyoto, Japan).
Cells
THP-1 cells (ATCC, Manassas, VA) were cultured in RPMI 1640 or HAM’s F12 medium (Nissui Pharmaceutical Co. Ltd., Tokyo, Japan) with 292 µg/ml L-glutamine, 100 units/ml penicillin, 100 µg/ml streptomycin, and 10% fetal bovine serum (FBS) at 37°C and under 5% CO2. Differentiation to macrophages was induced with 100 nmol/l phorbol 12-myristate 13-acetate (PMA, Sigma) for 48 h.(7) Cell viability was examined using an MTT cell viability assay kit (R&D Systems, Minneapolis, MN). The value for cell viability of the positive control cells, which were treated with 0.1 µg/ml LPS with or without 1 mmol/l butyrate in 0.5% dimethylsulfoxide (DMSO, v/v), were standardized as 100%. Incubation medium with or without substrates in all experiments did not significantly affect cell viability (data not shown).
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from THP-1 cells (5.0 × 106) using TRIzol reagent (Invitrogen, Carlsbad, CA). Single-strand cDNA was synthesized from 1 µg of total RNA using an RT-PCR reverse transcription kit (Maxim Biotech, San Francisco, CA). Incubation was carried out at 37°C for 60 min. The temperature of the reaction was then raised to 94°C for 5 min in order to make the enzyme inactive and was then reduced to 4°C. PCR amplification was performed using a Gene Amp PCR System 9700 (Applied Biosystems, Carlsbad, CA). For semi-quantitative analysis, the linearity of amplification of IL-1β and GAPDH cDNA, depending on the PCR cycle number, was established in preliminary experiments. A total of 30 cycles for IL-1β and GAPDH were performed. Products were analyzed by Kodak 1-D Image Analysis software (Eastman Kodak Co. Ltd., NY). The GAPDH primers used were 5'-CCACCCATGGCAAATTCCATGGCA-3' and 5'-TCTAGACGGCAGGTCAGGTCCACC-3'. These were designed to yield a 696-bp product. The IL-1β primers used were 5'-AAACAGATGAAGTGCTCCTTCCA-3' and 5'-GAGAACACCACTTGTTGCTCCA-3'. These were designed to yield a 389-bp product.
IL-1β protein production
THP-1 cells were seeded onto a 24-well plate at density of 1.0 × 106 cells/well in RPMI 1640 medium with 292 µg/ml L-glutamine, 100 units/ml penicillin, 100 µg/ml streptomycin, and 10% FBS and cultured at 37°C for 48 h under a humidified atmosphere of 5% CO2. After differentiation with 100 nmol/l PMA, cells were washed with PBS and treated with PBS or 0.1 µg/ml LPS alone, 1 mmol/l butyrate with 0.1 µg/ml LPS for 3, 6, 12, and 24 h.(8) The control cells were treated only with phosphate buffered saline (PBS), and positive control cells were treated with LPS alone. IL-1β protein concentration in the medium was examined by IL-1β Human ELISA Kit (R&D Systems). The optical density for each specimen were determined at λex 450 nm, λem 550 nm using a Molecular Devices microplate reader (Wako).
Caspase-1 activity
Caspase-1 activity was determined using a caspase-1 fluorometric assay kit (R&D Systems). Cells treated with or with not chemicals for 6 h were washed in cold PBS at 3 times, then re-suspended in 400 µl of cold lysis buffer and incubated on ice for 10 min. The cell lysates were pelleted, followed by transfer of the supernatants to microcentrifuge tubes. 50 µl of 2× reaction buffer with 1 M dithiothreitol (DTT) and 5 µl of Caspase-1 fluorogenic substrate (WEHD-AFC) were added to each well, followed by 37°C for 2 h incubation. A control reaction of treated cells without WEHD-AFC was incubated. The fluorometrical density for each specimen were determined at λex 400 nm, λem 505 nm using a GENios microplate reader (Wako). The results are expressed as fold increase, with caspase-1 activity seen in 0.5% DMSO treated cells (the vehicle control) standardized as 1-fold.
Western blotting
For Western blot analysis, 5 × 106 cells were lysed in M-PER Mammalian Protein Extraction reagent (Pierce, Rockford, IL). Denature proteins were separated using SDS-polyacrylamide gel electrophoresis on a 10 or 15% polyacrylamide gel and then transferred onto Immobilon-P membranes (Millipore, Billerica, MA).(9) We used rabbit anti-human phospho-p38 MAPK, rabbit anti-human p38 MAPK, rabbit anti-human phospho-extracellular signal-regulated kinase1/2 (ERK1/2), rabbit anti-human ERK1/2, rabbit anti-human phospho-c-Jun NH2-terminal kinase1/2 (JNK1/2), rabbit anti-human JNK1/2, rabbit anti-human phospho-mitogen-activated protein kinase kinase1/2 (MEK1/2), rabbit anti-human MEK1/2 (R&D Systems, Minneapolis, MN), rabbit anti-human Caspase-1 (BIOMOL International), rabbit anti-human actin (Santa Cruz Biotechnology, Santa Cruz, SA), anti-rabbit antibody horseradish peroxidase-linked immunoglobulin G (IgG) antibodies (GE Healthcare, Bucks, UK), or non-labeled rabbit IgG antibodies (Wako Pure Chem.). The blots were developed using an ECL (GE Healthcare) and the images were then analysed by Kodak 1-D Image Analysis software. The ratios of protein expression levels of protein were determined by dividing the band intensity of the product of interest by that of the corresponding actin band.
Effects of specific inhibitors
Differentiated cells were treated with 0.5% (v/v) DMSO alone (vehicle), 1 mmol/l butyrate with 0.1 µg/ml LPS, or 1 mmol/l butyrate with 0.1 µg/ml LPS in 50 mmol/l of PD98059 (PD), 50 mmol/l of SB203580 (SB), 10 mmol/l of SP600125 (SP), 10 mmol/l of GF109203X (GF), 10 mmol/l of diphenyleneiodonium (DPI), 20 mmol/l of N-acetyl L-cysteine (NAC), or 100 mmol/l of Ac-YVAD-CHO (YVAD), which were dissolved in DMSO.(8,9) The positive control cells were treated with 0.1 µg/ml LPS alone for 6 h (caspase-1 assay) or 24 h (IL-1β assay).
Effects of PTX
THP-1 cells were treated with 100 ng/ml PTX at 37°C for 60 min. They were then treated with 0.1 µg/ml LPS alone or 1 mmol/l butyrate with LPS in RPMI 1640 medium for 24 h.
Detection of reactive oxygen species products
The intracellular production of reactive oxygen species (ROS) was assessed using a 5-(and-6)-carboxy-2',7'-dichlorodihydrofluorescein diacetate fluorescein probe (carboxy-H2DCF-DA, Invitrogen, Carlsbad, CA).(9) THP-1 cells were treated with 0.1 mg/ml LPS for 60 min with or without 1 mmol/l butyrate, or with or without 20 mmol/l NAC for 60 min. Then, cells were exposed to 10 mmol/l carboxy-H2DCFDA in 1 ml PBS for 30 min at 37°C. Cells were washed twice, re-suspended in 1 ml PBS, and analyzed by fluorescence on a FACSCanto (Becton Dickson, Mountain View, CA). The intensity of DCF in the cells was analyzed by WinMDI ver 2.8 software (http://facs.scripps.edu/software.html).
Nitrite/nitrate quantification
THP-1 cells were seeded onto a 24-well plate at density of 1.0 × 106 cells/well in HAM’s F12 medium. Differentiation cells were treated with 0.5 µg/ml LPS alone or 1 mM butyrate with LPS, and vehicle (0.5% DMSO, v/v) alone, butyrate with LPS and 20 mM NAC, and LPS with NAC for 6, 12, 24 h in HAM’s F12 medium. Nitric oxide (NO) production from incubate medium was determined by measuring the amount of nitrite/nitrate (NO2–/NO3–), a stable oxidation end product of NO, NO2/NO3 Assay Kit-C2 (DOJINDO Lab., Kumamoto, Japan). The optical density for each specimen were determined at 540 nm using a Molecular Devices microplate reader (Wako).
Protein determination
Total protein concentrations in lysed THP-1 cells were determined using a DC Protein Assay kit (Bio-Rad Lab.), according to the protocol of the manufacturer, with bovine serum albumin (Bio-Rad Lab.) employed as the standard.
Statistical analysis
Data are expressed as the mean ± SD. Date were assessed by Kruskall-Wallis variance analysis and Dunnet’s multiple comparison test or the Mann-Whitney U test. Statistical analysis was performed by GraphPad Prism ver 4.0 (GraphPad Software Inc., San Diego, CA). Two-tailed values of p<0.05 were considered to indicate statistical significance.
Results
Butyrate with LPS increases expression of IL-1β mRNA and protein production
Preliminary experiments using >2 mmol/l butyrate induced excessive toxicity and apoptosis after 24 h (data not shown). A 1 mmol/l butyrate concentration was therefore used in all subsequent experiments. The expression of IL-1β m-RNA was not clearly observed in either the controls or the butyrate alone-treated cells. Both LPS alone and butyrate with LPS stimulations elevated the expression of IL-1β m-RNA levels (Fig. 1). After 24 h, IL-1β m-RNA levels in both stimulations remained elevated to a similar level (data not shown).
Fig. 1.
Effects of butyrate and lipopolysaccharide (LPS) on interleukin-1β (IL-1β) mRNA expression in THP-1 cells. THP-1cells were incubated with 1 mmol/l butyrate alone, 0.1 µg/ml LPS alone, or 1 mmol/l butyrate with 0.1 µg/ml LPS for 3 h. IL-1β mRNA expressions were detected by reverse transcription-polymerase chain reaction (RT-PCR) as described in Materials and Methods. Representative pictures show mRNA expression of IL-1β (upper panel) and GAPDH (lower panel).
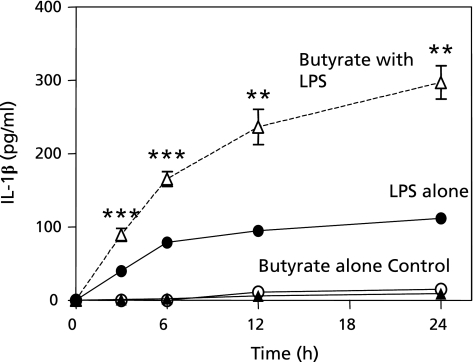
LPS alone increased IL-1β protein production in a time-dependent manner compared to those in the controls or in butyrate alone-treated cells. Butyrate with LPS significantly increased IL-1β protein production compared to those in LPS alone-treated cells (Fig. 2).
Fig. 2.
Effects of butyrate and lipopolysaccharide (LPS) on interleukin-1β (IL-1β) protein expression in THP-1 cells. THP-1 cells were seeded onto a 24-well plate at a density of 1.0 × 106 cells/well in RPMI 1640 medium with 100 nmol/l phorbol 12-myristate 13-acetate (PMA) and then cultured at 37°C for 48 h under a humidified atmosphere of 5% CO2. After washing, cells were incubated with medium alone (open circle), 1 mmol/l butyrate alone (closed triangle), 0.1 µg/ml LPS alone (closed circle), or 1 mmol/l butyrate with 0.1 µg/ml LPS (open triangle) for 0, 3, 6, 12, and 24 h. IL-1β protein in medium was examined by ELISA. Data are expressed as the mean ± SD (n = 5). **p<0.01, ***p<0.001 vs the controls, by Kruskall-Wallis variance analysis and Dunnet’s multiple comparison test.
Butyrate with LPS enhances caspase-1 expression
Next, we determined the caspase-1 protein expression. There was no difference of caspase-1 expression in the controls or the butyrate alone-treated cells. LPS alone significantly increased caspase-1 expression: more than 3-fold above the controls. Butyrate with LPS also increased caspase-1 expression to a similar level, which seems to match an earlier trend compared to LPS alone (Fig. 3 (a) and (b)). These results suggested that IL-1β production in THP-1 cells by LPS alone or butyrate with LPS was mediated by caspase-1 activation.
Fig. 3.
Effects of butyrate and lipopolysaccharide (LPS) on caspase-1 expression in THP-1 cells. After differentiation, cells were incubated with phosphate buffered saline (PBS); control, 1 mmol/l butyrate alone, 0.1 µg/ml LPS alone, or 1 mmol/l butyrate with 0.1 µg/ml LPS for 0, 3, 6, 12, and 24 h. Expression of caspase-1 protein and actin was determined by Western blotting. Upper panels show the representative pictures of caspase-1 and actin protein expressions. In lower panels, the values in three independent experiments are expressed as fold increase (the mean ± SD) relative to that of the controls. **p<0.01 vs the controls, by Kruskall-Wallis variance analysis and Dunnet’s multiple comparison test.
Effect of kinase inhibitors and antioxidants on caspase-1 activity and IL-1β expression
First, to determine whether butyrate affects the early activation of the MAPKs pathways to induce IL-1β release, we examined phosphorylation of p38 MAPK, JNK1/2, MEK1/2 and ERK1/2 proteins. As shown in Fig. 4, butyrate with LPS stimulation accelerated the phosphorylation of these proteins, which also occurred to a lesser extent in LPS alone.
Fig. 4.
Effects of lipopolysaccharide (LPS) and butyrate on mitogen-activated protein kinase (MAPK) cascade pathways. Cells were seeded onto a 60-mm tissue culture dish at a density of 5 × 106 cells/dish and then cultured at 37°C for 48 h under a humidified atmosphere of 5% CO2. After differentiation, cells were treated with phosphate buffered saline (PBS); control, 1 mmol/l butyrate alone, 0.1 µg/ml LPS alone, or 1 mmol/l butyrate with 0.1 µg/ml LPS for 5, 10, 20, 30, 60, 120, and 180 min. The phosphorylation of p38 MAPK, c-Jun NH2-terminal kinase1/2 (JNK1/2), mitogen-activated protein kinase kinase1/2 (MEK1/2), and extracellular signal-regulated kinase1/2 (ERK1/2) was analyzed by Western blotting as described in Materials and Methods. Actin was used as the internal control. The pictures are representative results from three independent experiments.
Next, to elucidate the mechanisms by which butyrate enhances IL-1β production in LPS-stimulated cells, we examined the effect of various protein kinase inhibitors or anti-oxidants on IL-1β protein production and caspase-1 activity. Butyrate treatment increased caspase-1 activity 3.7-fold and IL-1β protein production 110-fold (mean, 352.5 pg/ml) compared to the control cells (DMSO only). Ac-YVAD-CHO (caspase-1 inhibitor) decreased caspase-1 activity by 78% and IL-1β protein production by 88%. Similarly: PD98059 (ERK1/2 inhibitor) by 46 and 49%; SB203580 (p38 MAPK inhibitor) by 54 and 63%; SP600125 (JNK1/2 inhibitor) by 43 and 57%; NAC (anti-oxidant) by 46 and 42%; DPI [Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibitor] by 38 and 44%; and GF109203X (protein kinase C inhibitor) by 11 and 6% (Fig. 5 (a) and (b)).
Fig. 5.
Effects of kinase inhibitors and an antioxidant on caspase-1 activity and interleukin-1β (IL-1β) in THP-1 cells treated with butyrate and lipopolysaccharide (LPS). After differentiation, cells were seeded onto a 60-mm dish (caspase-1) or a 24-well plate (IL-1β) at a density of 1 × 106 cells/well and 5 × 106 cells/dish, respectively, in RPMI 1640 medium. After washing, the cells were treated with the vehicle (0.5% v/v dimethylsulfoxide (DMSO)), 100 mmol/l Ac-YVAD-CHO (YVAD), 50 mmol/l PD98059 (PD), 50 mmol/l SB203580 (SB), 10 mmol/l SP600125 (SP), 20 mmol/l N-acetyl L-cysteine (NAC), 10 mmol/l diphenyleneiodonium (DPI), and 10 mmol/l GF109203X (GF). Additional inhibitors or antioxidants cells were incubated at 37°C for 20 min, and then were treated with 0.1 µg/mL LPS alone or 1 mmol/l butyrate with 0.1 µg/ml LPS for 24 h. (a) shows the caspase-1 activity by fluorometric assay. The values in five independent experiments are expressed as fold increase (the mean ± SD) relative to that of the controls. (b) shows IL-1β concentrations measured by ELISA kit. The values in five independent experiments are expressed as the mean ± SD. *p<0.05 vs LPS alone. #p<0.05, ##p<0.01, ###p<0.001 vs butyrate with LPS, by Kruskall-Wallis variance analysis and Dunnet’s multiple comparison test.
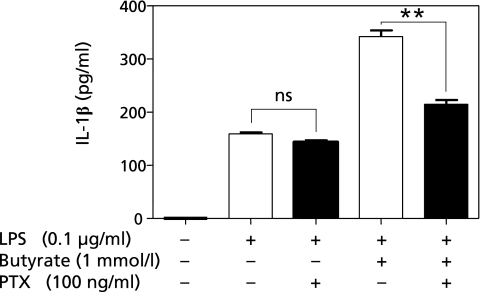
Moreover, we found that pre-treatment with PTX reduced IL-1β protein production by approximately 50% compared to cells without PTX (Fig. 6). These results suggest that butyrate with LPS accelerates IL-1β protein production via oxidative mechanisms and the protein phosphorylation pathways involving MAP kinase and Gi protein-mediated signaling.
Fig. 6.
Effects of pertussis toxin (PTX) on interleukin-1β (IL-1β) production in THP-1 cells. Cells were seeded onto a 24-well plate at a density of 1 × 106 cells/well, and then cultured at 37°C for 24 h under a humidified atmosphere of 5% CO2. After differentiation, cells were treated with the 100 ng/ml PTX, and incubating at 37°C for 1 h. They were then treated with 0.1 µg/ml lipopolysaccharide (LPS) alone or 1 mmol/l butyrate with 0.1 µg/ml LPS for 24 h. IL-1β protein concentrations in the medium were examined by ELISA kit. The values in five independent experiments are expressed as the mean ± SD. Statistical analysis was performed using the Mann-Whitney U test, **p<0.01, vs without PTX treatment.
Butyrate with LPS enhances nitrite/nitrate production via ROS generation
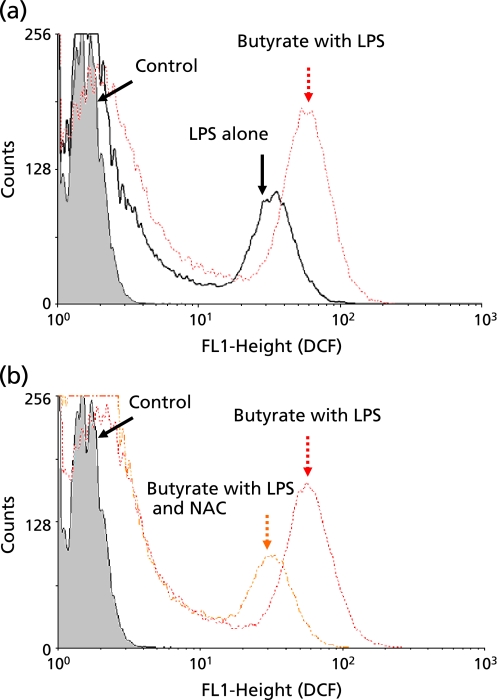
Next, to determine influence of butyrate on ROS production, we used flow cytometry to measure intracellular ROS in THP-1 cells treated with LPS alone and butyrate with LPS. As shown in Fig. 7(a), the control cells exhibited constitutive basal levels of ROS, and the LPS increased these. Butyrate with LPS increased ROS levels to an even greater extent. The addition of NAC, however, reduced ROS levels to a level similar to that seen in LPS alone-treated cells (Fig. 7(b)). We also examined the effects of nitrite/nitrate production. Butyrate with LPS significantly increased nitrite/nitrate production compared to LPS alone (Fig. 8). The addition of NAC resulted in a significant reduction in the elevated amount of nitrite/nitrate by butyrate with LPS.
Fig. 7.
Effects of butyrate and N-acetyl L-cysteine (NAC) on reactive oxygen species (ROS) production in THP-1 cells. After differentiation, cells were incubated with 0.1 µg/ml lipopolysaccharide (LPS) alone or 1 mmol/l butyrate with 0.1 µg/ml LPS, or 1 mmol/l butyrate with 0.1 µg/ml LPS and 20 mmol/l NAC, for 1 h. Then, cells were stained with carboxy-H2DCFDA for 30 min. The amount of ROS was determined by FACS analysis. (a) shows the effect of LPS alone and LPS with butyrate. (b) shows the effects of butyrate with LPS, and butyrate with LPS and NAC. Each result is a representative one from three independent experiments.
Fig. 8.
Effects of butyrate and N-acetyl L-cysteine (NAC) on nitric oxide (NO) production in THP-1 cells. After differentiation, cells were treated with 0.5% v/v dimethylsulfoxide; DMSO (the controls, closed circle), 1 mmol/l butyrate (open square), 0.5 µg/ml lipopolysaccharide; LPS alone (open circle), 1 mmol/l butyrate with 0.5 µg/ml LPS (open triangle), 0.5 µg/ml LPS with 20 mmol/l NAC (closed square), or 1 mmol/l butyrate with 0.5 µg/ml LPS and 20 mmol/l NAC (closed triangle) for 6, 12, and 24 h. NO production from THP-1 cells was determined by measuring the amount of nitrite/nitrate as described in Materials and Methods. The values from five independent experiments are expressed as the mean ± SD. *p<0.05 vs LPS alone, ##p<0.01 vs LPS with butyrate, by Kruskall-Wallis variance analysis and Dunnet’s multiple comparison test.
Discussion
In this study, we demonstrated that LPS induced IL-1β production in THP-1 cells and that butyrate enhanced this production. Butyrate also enhanced caspase-1 activation, ROS production, the phosphorylation pathways involving MAP kinase, and Gi protein-mediated signaling. The PKC inhibitor used in this study did not show any effects. These results seem to demonstrate that butyrate enhances IL-1β production by activating caspase-1 via ROS and the phosphorylation of MAPK pathways and the Gi protein-mediated pathways.
G protein-coupled receptors (GPCRs) constitute one type of receptor localized in the cell membrane, and are also known as seven trans-membrane domain receptors. In recent years, it has been reported that two GPCRs (GPR41 and GPR43) have an affinity for SCFAs.(10–13)
Le Poul et al.(11) reported that GPR41 and 43 bound to a short-chain fatty acid acts as a chemo-attractant signal for peripheral blood mononuclear cells. They showed that stimulation of GPR41 and 43 increased the intracellular Ca2+ level by SCFA and that PTX abolished the response of GPR41 but not of GPR43. Although details regarding the GPCR signal cascade are still not well understood, signal pathways involving non-receptor tyrosine kinases,(12) such as phosphatidylinositol 3-kinase (PI3K)(13) and protein kinase C (PKC),(14) may play a significant role in immune reactions. It has been shown that GPR41 receptors are coupled with Gi/o and that their activation induces the formation of inositol 1,4,5-trisphosphate, increases intracellular Ca2+ concentration and ERK1/2 activation, and decreases intracellular cyclic adenosine monophosphate (cAMP).(11) In this study, we were able to identify the association of Gi protein, although we did not examine the direct association of GPR41 in IL-1β production by butyrate. In the future, we aim to clarify the detailed mechanisms involved in GPR41 in IL-1β production by butyrate.
NADPH oxidase is the primary enzyme for ROS generation in phagocytic cells.(15) Excessive ROS stimulate MAPK pathway activation(16) and activate MAPKs promote casapse-1 and IL-1β expression.(17) Our results seem to be consistent with these reports. Therefore, we postulated that butyrate may lead to accelerated NADPH oxidase activity resulting in increased activation of p38 MAPK, JNK 1/2, MEK1/2, and ERK1/2 pathways, the activation of caspase-1, and finally more mature efficient IL-1β production. However, there is also a possibility that ROS may directly activate caspase-1 through a redox-sensitive cysteine residue.(18) Because p38 MAPK, JNK, MEK inhibitors are not fully effective and because an increase in caspase-1 expression was observed earlier in the butyrate-treated cells than in cells given LPS alone, it appears that partial intervention of the direct path needs to be considered.
SCFAs such as acetate, propionate, and butyrate are produced by anaerobic bacterial fermentation of undigested carbohydrates in the colon,(19) and they are rapidly absorbed to provide energy for the colorectal epithelium by means of increased blood flow inrectal mucosa.(20) SCFAs also induce apoptosis in a number of colorectal tumor cell lines.(21) On the other hand, butyrate has anti-inflammatory effects in the colon,(22) and it also has a prominent immunity regulation effect by modulating IL-8(23) and macrophage inflammatory protein 2 (MIP-2) activities(24) and suppressing the NF-κB activity,(25,26) leading to the regulation of inflammatory cytokines such as TNF-α, IL-6, IL-8, and others.(27,28)
However, the results of a controlled clinical trial that used enemas containing sodium butyrate showed that butyrate was not effective in the treatment of UC,(29,30) suggesting that butyrate’s immune control mechanisms use pathways that may lead to both suppression and aggravation. As shown in this study, butyrate may have a significant influence on the production of IL-1β. Our data can be applied to strategies for preventing anaerobic bacteria from producing butyrate, which may be an effective treatment of IBD. Pharmacological inhibition of p38 MAPK, JNK 1/2, MEK1/2, and ERK1/2 seems to be beneficial effects for the treatment of inflammatory responses based on experiments with animal models,(31,32) and IL-1β has been reduced by the inhibition of p38 MAPK phosphorylation in the rat dentate gyrus in vitro.(33)
This study has some limitations. For example, we found that butyrate-mediated induction of activated p38 MAPK occurred within 10 min, nitrite detection outside the cell occurred after 6 h, and intracellular detection of ROS occurred after 60 min. These time lags may be due to the reaction time for NO detection in the medium and the reagent sensitivity of the DCFH-DA fluorescent probe for intracellular ROS detection. Moreover, the LPS concentration (0.1 µg/ml) used to alter NO production in the culture medium was below the lower detection limit. A higher concentration (0.5 µg/ml) should be used to examine these problems.
In conclusion, butyrate enhances IL-1β production by activating caspase-1 via ROS and the phosphorylation of MAPK pathways and the Gi protein mediated pathway in LPS stimulated THP-1 cells.
Acknowledgments
This study was supported by Grant-in-Aid for Health-Science Research of Kobe Gakuin University.
Abbreviations
- carboxy-H2DCF-DA
5-(and-6)-carboxy-2',7'-dichlorodihydrofluorescein diacetate
- DMSO
Dimethylsulfoxide
- DPI
Diphenyleneiodonium
- ERKs
Extracellular signal-regulated kinases
- ERK1/2
Extracellular signal-regulated kinase1/2
- FBS
Fetal bovine serum
- GF
GF109203X
- GPCRs
G protein-coupled receptors
- IBD
Inflammatory bowel diseases
- IL-1β
Interleukin-1β
- JNK1/2
c-Jun NH2-terminal kinase1/2
- LPS
Lipopolysaccharide
- MAPK
Mitogen-activated protein kinase
- MEK1/2
Mitogen-activated protein kinase kinase1/2
- NAC
N-acetyl L-cysteine
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NO
Nitric oxide
- NO2–
Nitrite
- NO3–
Nitrate
- PBS
Phosphate buffered saline
- PD
PD98059
- PMA
Phorbol 12-myristate 13-acetate
- PTX
Pertussis toxin
- ROS
Reactive oxygen species
- RT-PCR
Reverse transcription-polymerase chain reaction
- SB
SB203580
- SCFAs
Short chain fatty acids
- SP
SP600125
- UC
Ulcerative colitis
- YVAD
Ac-YVAD-CHO
References
- 1.Danese S, Fiocchi C. Etiopathogenesis of inflammatory bowel diseases. World J Gastroenterol. 2006;12:4807–4812. doi: 10.3748/wjg.v12.i30.4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dionne S, Hiscott J, D’Agata I, Duhaime A, Seidman EG. Quantitative PCR analysis of TNF-alpha and IL-1 beta mRNA levels in pediatric IBD mucosal biopsies. Dig Dis Sci. 1997;42:1557–1566. doi: 10.1023/a:1018895500721. [DOI] [PubMed] [Google Scholar]
- 3.McAlindon ME, Hawkey CJ, Mahida YR. Expression of interleukin 1 beta and interleukin 1 beta converting enzyme by intestinal macrophages in health and inflammatory bowel disease. Gut. 1998;42:214–219. doi: 10.1136/gut.42.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohkusa T, Okayasu I, Ogihara T, Morita K, Ogawa M, Sato N. Induction of experimental ulcerative colitis by Fusobacterium varium isolated from colonic mucosa of patients with ulcerative colitis. Gut. 2003;52:79–83. doi: 10.1136/gut.52.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eftimiadi C, Stashenko P, Tonetti M, et al. Divergent effect of the anaerobic bacteria by-product butyric acid on the immune response: suppression of T-lymphocyte proliferation and stimulation of interleukin-1 beta production. Oral Microbiol Immunol. 1991;6:17–23. doi: 10.1111/j.1399-302x.1991.tb00446.x. [DOI] [PubMed] [Google Scholar]
- 6.Jung YD, Liu W, Reinmuth N, et al. Vascular endothelial growth factor is upregulated by interleukin-1 beta in human vascular smooth muscle cells via the P38 mitogen-activated protein kinase pathway. Angiogenesis. 2001;4:155–162. doi: 10.1023/a:1012291524723. [DOI] [PubMed] [Google Scholar]
- 7.Fujioka Y, Cooper AD, Fong LG. Multiple processes are involved in the uptake of chylomicron remnants by mouse peritoneal macrophages. J Lipid Res. 1998;39:2339–2349. [PubMed] [Google Scholar]
- 8.Okumura T, Fujioka Y, Morimoto S, et al. Chylomicron remnants stimulate release of interleukin-1beta by THP-1 cells. J Atheroscler Thromb. 2006;13:38–45. doi: 10.5551/jat.13.38. [DOI] [PubMed] [Google Scholar]
- 9.Kamemura K, Fujioka Y, Takaishi H, et al. Chylomicron remnants upregulate CD40 expression via the ERK pathway and a redox-sensitive mechanism in THP-1 cells. Atherosclerosis. 2006;187:257–264. doi: 10.1016/j.atherosclerosis.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Hirasawa A, Hara T, Katsuma S, Adachi T, Tsujimoto G. Free fatty acid receptors and drug discovery. Biol Pharm Bull. 2008;31:1847–1851. doi: 10.1248/bpb.31.1847. [DOI] [PubMed] [Google Scholar]
- 11.Le Poul E, Loison C, Struyf S, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 12.Sanborn BM, Ku CY, Shlykov S, Babich L. Molecular signaling through G-protein-coupled receptors and the control of intracellular calcium in myometrium. J Soc Gynecol Investig. 2005;12:479–487. doi: 10.1016/j.jsgi.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Radeff-Huang J, Seasholtz TM, Matteo RG, Brown JH. G protein mediated signaling pathways in lysophospholipid induced cell proliferation and survival. J Cell Biochem. 2004;92:949–966. doi: 10.1002/jcb.20094. [DOI] [PubMed] [Google Scholar]
- 14.Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23:7906–7909. doi: 10.1038/sj.onc.1208160. [DOI] [PubMed] [Google Scholar]
- 15.Park JB. Phagocytosis induces superoxide formation and apoptosis in macrophages. Exp Mol Med. 2003;35:325–335. doi: 10.1038/emm.2003.44. [DOI] [PubMed] [Google Scholar]
- 16.Forman HJ, Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am J Respir Crit Care Med. 2002;166:S4–S8. doi: 10.1164/rccm.2206007. [DOI] [PubMed] [Google Scholar]
- 17.Fubini B, Hubbard A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in Inflammation and fibrosis. Free Radic Biol Med. 2003;34:1507–1516. doi: 10.1016/s0891-5849(03)00149-7. [DOI] [PubMed] [Google Scholar]
- 18.Miller DK, Myerson J, Becker JW. The interleukin-1 beta converting enzyme family of cysteine proteases. J Cell Biochem. 1997;64:2–10. doi: 10.1002/(sici)1097-4644(199701)64:1<2::aid-jcb2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 19.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 20.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heerdt BG, Houston MA, Augenlicht LH. Potentiation by specific short-chain fatty acids of differentiation and apoptosis in human colonic carcinoma cell lines. Cancer Res. 1994;54:3288–3293. [PubMed] [Google Scholar]
- 22.Andoh A, Tsujikawa T, Fujiyama Y. Role of dietary fiber and short-chain fatty acids in the colon. Current Pharm Design. 2003;9:347–358. doi: 10.2174/1381612033391973. [DOI] [PubMed] [Google Scholar]
- 23.Gibson P, Rosella O. Interleukin 8 secretion by colonic crypt cells in vitro: response to injury suppressed by butyrate and enhanced in inflammatory bowel disease. Gut. 1995;37:536–543. doi: 10.1136/gut.37.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohno Y, Lee J, Fusunyan RD, MacDermott RP, Sanderson IR. Macrophage inflammatory protein-2: chromosomal regulation in rat small intestinal epithelial cells. Proc Natl Acad Sci USA. 1997;94:10279–10284. doi: 10.1073/pnas.94.19.10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Segain JP, Raingeard de la Blétière D, Bourreille A, et al. Butyrate inhibits inflammatory responses through NFkappa B inhibition: implications for Crohn’s disease. Gut. 2000;47:397–403. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park JS, Lee EJ, Lee JC, Kim WK, Kim HS. Anti-inflammatory effects of short chain fatty acids in IFN-gamma-stimulated RAW 264.7 murine macrophage cells: involvement of NF-kappa B and ERK signaling pathways. Int Immunopharmacol. 2007;7:70–77. doi: 10.1016/j.intimp.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 27.Yin L, Laevsky G, Giardina C. Butyrate suppression of colonocyte NF-kappa B activation and cellular proteasome activity. J Biol Chem. 2001;276:44641–44646. doi: 10.1074/jbc.M105170200. [DOI] [PubMed] [Google Scholar]
- 28.Lührs H, Gerke T, Schauber J, et al. Cytokine-activated degradation of inhibitory kappaB protein alpha is inhibited by the short-chain fatty acid butyrate. Int J Colorectal Dis. 2001;16:195–201. doi: 10.1007/s003840100295. [DOI] [PubMed] [Google Scholar]
- 29.Steinhart AH, Hiruki T, Brzezinski A, Baker JP. Treatment of left-sided ulcerative colitis with butyrate enemas: a controlled trial. Aliment Pharmacol Ther. 1996;10:729–736. doi: 10.1046/j.1365-2036.1996.d01-509.x. [DOI] [PubMed] [Google Scholar]
- 30.Breuer RI, Soergel KH, Lashner BA, et al. Short chain fatty acid rectal irrigation for left-sided ulcerative colitis: a randomised, placebo controlled trial. Gut. 1997;40:485–491. doi: 10.1136/gut.40.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ten Hove T, van den Blink B, Pronk I, Drillenburg P, Peppelenbosch MP, van Deventer SJ. Dichotomal role of inhibition of p38 MAPK with SB203580 in experimental colitis. Gut. 2002;50:507–512. doi: 10.1136/gut.50.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaffee BD, Manos EJ, Collins RJ, et al. Inhibition of MAP kinase kinase (MEK) results in an anti-inflammatory response in vivo. Biochem Biophys Res Commun. 2000;268:647–651. doi: 10.1006/bbrc.2000.2184. [DOI] [PubMed] [Google Scholar]
- 33.Coogan AN, O’Neill LA, O’Connor JJ. The P38 mitogen-activated protein kinase inhibitor SB203580 antagonizes the inhibitory effects of interleukin-1 beta on long-term potentiation in the rat dentate gyrus in vitro. Neuroscience. 1999;93:57–69. doi: 10.1016/s0306-4522(99)00100-1. [DOI] [PubMed] [Google Scholar]