Abstract
This study aimed to evaluate the relationship between gut probiotic flora and nonalcoholic fatty liver disease in a diet-induced rat model, and to compare the effects of two different probiotic strains on nonalcoholic fatty liver disease. Forty male Sprague-Dawley rats were randomized into 4 groups for 12 weeks: control (standard rat chow), model (fat-rich diet), Lactobacillus (fat-rich diet plus Lactobacillus acidophilus), and Bifidobacterium (fat-rich diet plus Bifidobacterium longum) groups. Probiotics were provided to rats in drinking water (1010/ml). Gut bifidobacteria and lactobacilli were obviously lower at weeks 8 and 10, respectively, in the model group compared with the control group. Supplementation with Bifidobacterium significantly attenuated hepatic fat accumulation (0.10 ± 0.03 g/g liver tissue) compared with the model group (0.16 ± 0.03 g/g liver tissue). However, there was no improvement in intestinal permeability in either the Lactobacillus or the Bifidobacterium group compared with the model group. In all 40 rats, the hepatic total lipid content was negatively correlated with gut Lactobacillus (r = −0.623, p = 0.004) and Bifidobacterium (r = −0.591, p = 0.008). Oral supplementation with probiotics attenuates hepatic fat accumulation. Further, Bifidobacterium longum is superior in terms of attenuating liver fat accumulation than is Lactobacillus acidophilus.
Keywords: fat-rich diet, Lactobacillus, Bifidobacterium, nonalcoholic fatty liver disease (NAFLD), intestinal permeability
Introduction
Hepatic steatosis or nonalcoholic fatty liver disease (NAFLD) is an infiltration of fat inside hepatocytes, usually exceeding 5% of the liver wet weight.(1) NAFLD comprises a wide spectrum of hepatic damage, from simple steatosis alone, to inflammatory changes found in nonalcoholic steatohepatitis (NASH) and advanced fibrosis and cirrhosis of the liver in the absence of alcohol intake. The prevalence of NAFLD has apparently increased in proportion to the increasing incidence of obesity in both adults and children.(2,3) The ”two-hit hypothesis” is currently widely used to explain the development of NAFLD.(4) However, the pathogenesis of NAFLD remains unclear. Closely associated with obesity and insulin resistance, NAFLD is commoner in the obese, diabetics, and patients with features of metabolic syndrome than it is in normal healthy controls.(5,6)
The hypothesis that gut-derived bacteria and their products could play a role in NAFLD is based on several studies. Wigg(7) and Sabaté(8) found that the prevalence of small intestinal bacterial overgrowth is higher in patients with NASH and morbid obesity than it is in control subjects, and that it is associated with severe hepatic steatosis. The association between bariatric surgery for NASH and liver cirrhosis provided clinical evidence for the hypothesis.(9,10) Furthermore, NAFLD and alcohol-induced liver injury share similar pathogenic mechanisms. Rats administered antibiotics and Lactobacillus are protected from ethanol-induced liver damage,(11,12) which may be additional evidence for the hypothesis. Intestinal bacteria may be involved in the mechanism of NAFLD by means of an increased endogenous production of ethanol, and direct activation of inflammatory cytokines in luminal epithelial cells, non-parenchymal liver cells, or both via release of lipopolysaccharide (LPS).(13)
Probiotics are inexpensive, safe, have no known long-term adverse effects, and are widely accepted by the public. Supplementation with probiotics for the management NAFLD would seem to be an attractive idea. Li et al.(14) found that probiotic-treated ob/ob mice had significantly decreased hepatic inflammation, serum alanine aminotransferase (ALT), and hepatic oleic acid compared to the controls. A further animal study showed that oral probiotic treatment significantly improved high-fat diet-induced hepatic natural killer T cell depletion, insulin resistance, and hepatic steatosis.(15) The beneficial effects of a probiotic VSL#3 (a mixture containing 450 billion bacteria of different strains) were also observed in a small-scale human study.(16) Unfortunately, all of the above studies failed to evaluate gut probiotic flora longitudinally during the development of NAFLD. Therefore, the aims of the present study were to evaluate gut probiotic flora longitudinally and examine its association with liver fat accumulation in a diet-induced rat model of NAFLD, and to compare the effects of two different probiotic strains on NAFLD.
Materials and Methods
Animals and experimental protocol
Forty male Sprague-Dawley rats (weighing 150–180 g), obtained from Shanghai Slac Laboratory Animal LTD, were maintained on a 12-h light-dark cycle at a constant room temperature of 20–22°C and humidity of 60–70%. After a 1-week adaptation, the rats were randomly assigned to 4 groups (each group n = 10): control (C), model (M), Lactobacillus (L), and Bifidobacterium (B). The rats in the groups were maintained for 12 weeks with free access to food and water. The control group received standard rat chow (M01-F; Shanghai Slac Laboratory Animal Ltd.), the model group received a fat-rich diet (weight ratio: standard rat chow, 60%; lard, 10%; sucrose, 9%; sesame oil, 1%; egg, 10%; milk powder, 5%; peanut, 5%),(17) the Lactobacillus group received the same fat-rich diet plus Lactobacillus acidophilus (CGMCC 2106), and the Bifidobacterium group received the fat-rich diet plus Bifidobacterium longum (CGMCC 2107). These two strains are common in human bowel flora, the other one is they are acid resistance and were proved to be effective in the treatment of human alcoholic cirrhosis.(14,18) The probiotics were administered to rats in drinking water (1010/ml) and prepared fresh each day.(19) Body weight was recorded once a week. The animal study was approved by the Animal Ethics Committee of Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University.
Biochemical assays
Serum was separated from whole blood and frozen at −80°C. Serum ALT, triglycerides (TG), and total cholesterol (TC) were measured using an automatic biochemical analytical system.
Liver histology and total lipids
Rats were anesthetized by intraperitoneal sodium pentobarbital (60 mg/kg body weight) at the end of the experimental period after overnight fasting. Livers were rapidly excised and thin slices of liver were fixed in 10% neutral-buffered formalin. Formalin-fixed, paraffin-embedded liver sections were stained with H&E (hematoxylin and eosin) and Sudan red IV. A further slice of liver tissue was quickly frozen in liquid nitrogen and stored at −80°C for total lipid analysis. Total lipids were extracted from liver tissue according to a published method.(20)
Intestinal permeability
Intestinal permeability was determined at the end of the experiment using the method of Weaver et al.(21) This procedure is based upon the differential absorption of lactulose and mannitol; the greater the permeability, the higher the ratio of lactulose to mannitol. After an overnight fast, each rat received an intragastric permeability marker (2 ml) containing 100 mg lactulose (Wako Pure Chem., Osaka, Japan) and 50 mg mannitol (Wako Pure Chem). Urine samples were collected for 24 h and stored at −80°C for analysis. The procedure used for urine analysis was briefly as follows: Urine samples were boiled and centrifuged at 1,000 rpm for 10 min. Five milliliters of the resulting supernatant was transferred to a fresh microcentrifuge tube. To this was added 10 µl acetic acid, followed by boiling for 5 min, and then, when cooled, centrifugation at 10,000 rpm for 10 min. Prior to analysis, the samples were initially filtered through a 0.45-µm filter and then through a Microsep centrifugal device (Pall Life Sciences, Washington DC) according to the manufacturer’s instructions. The pretreated samples were analyzed using a Dionex ICS 3000 ion chromatography system (Sunnyvale, CA) as described previously.(22)
Gut probiotic flora
Fresh stool samples were collected every 2 weeks on Wednesday morning. One milliliter of PBS buffer was added to each fecal specimen (180–220 mg), followed by continuous vortexing for 5 min, and then centrifugation at 500 rpm for 5 min. The resulting supernatant was transferred to a fresh microcentrifuge tube, centrifuged at 14,000 × g for 5 min, and the supernatant was discard. The bacterial pellets were used for DNA extraction using a QIAamp DNA Stool Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. DNA samples were stored at −80°C for PCR amplification.
The specific 16S rRNA-targeted primer sets used for quantitative real-time PCR are listed in Table 1. The primer sets for Bifidobacterium and Lactobacillus were designed according to the sequences obtained from public databases (www.ncbi.nlm.nih.gov) and have been validated by other researchers.(23,24)
Table 1.
Specific primer sets used in the study
| Target groups | Primer | Sequence (5'-3') | PCR Products |
|---|---|---|---|
| Lactobacillus | L159-f | GGA AAC AGA TGC TAA TAC CG | 600 bp |
| L677-r | CAC CGC TAC ACA TGG AG | ||
| Bifidobacterium | g-Bifid-f | CTC CTG GAA ACG GGT GG | 520 bp |
| g-Bifid-r | GGT GTT CTT CCC GAT ATC TAC A |
Each sample was measured in triplicate. Real-time PCR was performed using the SYBR Green method. PCRs were performed in 10-µl final volumes in a LightCycler 480 instrument (Roche Diagnostic, Mannheim, Germany). Reaction mixtures contained 2× SYBR Premix Ex TaqTM (Takara Co., Kyoto, Japan), 5 µ1; primer, 0.6 µl; DNA template, 1 µl; and ddH2O, 3.4 µl. Briefly, after pre-denaturation of the DNA template at 95°C for 10 s, the amplification program comprised 40 cycles (10 s at 95°C, 10 s at 60°C, and 60 s at 72°C for Bifidobacterium; and 10 s at 95°C, 10 s at 56°C, and 60 s at 72°C for Lactobacillus). The temperature transition rate was 20°C/s for all steps. The double-stranded PCR products were measured during the 72°C extension step by detection of the fluorescence associated with the binding of SYBR Green I to the products. No amplification was detected in the negative control.
Melting curves were used to determine the specificity of the PCR.(25) Melting curve analysis was performed immediately after the amplification procedure under the following conditions: 95°C, 10 s (4.8°C/s); 65°C, 10 s (2.5°C/s); 95°C, 0 s (hold time, 0.21°C/s); and 40°C, 1 s (2.5°C/s). Standard curves for Bifidobacterium and Lactobacillus were also generated in the LightCycler 480 instrument. Quantification of samples was achieved using the standard curve prepared from a dilution series of known concentrations of plasmid DNA.
Statistical analysis
Data were analyzed using SPSS version 11.0 for Windows. Copy numbers of the 16S rRNA genes of Bifidobacterium and Lactobacillus per gram of stool sample were logarithmically transformed. Data are presented as the mean ± SD for normally distributed data, and differences among the groups were determined using a one-way ANOVA. If the data were non-normally distributed, they are presented as the median plus range, and were analyzed using a nonparametric test for K independent samples. A two-sided p value of 0.05 was considered significant. A Spearman linear correlation analysis was used to assess the possible relationship among intestinal Bifidobacterium and Lactobacillus and total hepatic lipids.
Results
Body weight
None of the rats died during the experiment. The basal body weight of rats was comparable before the start of the study. Body weight showed an obvious increase in the three high-fat-fed groups compared with the control group from week 4 to the end of the study (all p<0.05). There was no significant difference in body weight among the three high-fat-fed groups.
Serum ALT, TC, and TG
Serum ALT levels were similar among the four groups (F = 0.876, p = 0.468). In contrast, serum TC (F = 8.513, p = 0.001) and TG (F = 4.882, p = 0.011) were markedly higher in the rats fed a fat-rich diet than in the control rats. No significant differences were found among the three fat-rich diet-fed groups with regard to TC and TG levels (Table 2).
Table 2.
Serum ALT, TC, TG, hepatic lipids and intestinal permeability
| Groups | ALT (IU/L) | TC (mmol/L) | TG (mmol/L) | Hepatic lipids (g/g liver tissue) | L/M# |
|---|---|---|---|---|---|
| Control | 58.9 ± 18.6 | 1.4 ± 0.1* | 0.3 ± 0.1* | 0.04 ± 0.01* | 0.260 (0.082–0.329)* |
| Model | 59.8 ± 6.8 | 1.7 ± 0.3 | 0.7 ± 0.1 | 0.16 ± 0.03 | 0.774 (0.630–1.243) |
| Lactobacillus | 65.2 ± 8.1 | 1.6 ± 0.1 | 0.8 ± 0.3 | 0.14 ± 0.02 | 1.125 (0.920–1.344) |
| Bifidobacterium | 52.3 ± 11.3 | 1.8 ± 0.2 | 0.7 ± 0.1 | 0.10 ± 0.03+ | 0.788 (0.492–2.164) |
Note: *p<0.05 vs other three groups respectively; +p<0.01 vs Model and Lactobacillus group; #abnormal distribution.
Liver histology
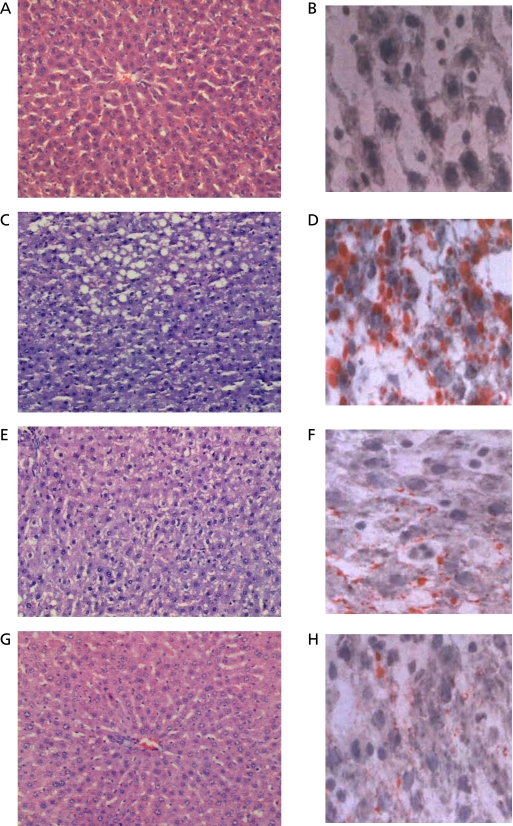
Left panels were stained with H&E at 200× and right panels with Sudan red IV at 400× in Fig. 1. The liver in the Control group showed no signs of lipid accumulation with a normal structure of hepatic lobules (Fig. 1 A and B). Fat-rich diet induced lipid accumulation mainly in the central vein area of liver. The livers from the Model group showed microvesicular and macrovesicular steatosis around the central vein area of liver. The structure of hepatic lobule and cell cords were also destroyed (Fig. 1 C and D). The livers showed mainly microvesicular steatosis in the Lactobacillus group with a destroyed structure of liver lobule (Fig. 1 E and F) while scattered microvesicular steatosis in the Bifidobacterium group with a basically normal hepatic lobule (Fig. 1 G and H).
Fig. 1.
Effect of probiotics on rat hepatic histology. (Left panels H&E staining at 200× and right panels Sudan red IV staining at 400×). (A, B) Control; (C, D) Model; (E, F) fat-rich diet plus Lactobacillus acidophilus; (G, H) fat-rich diet plus Bifidobacterium longum.
Total lipid content and intestinal permeability
Hepatic lipid content was markedly higher in fat-rich diet-fed rats than in the control rats. Although oral supplementation with Lactobacillus failed to reduce hepatic lipid content (0.14 ± 0.02 g/g liver tissue) compared with the model group (0.16 ± 0.03 g/g liver tissue), that with Bifidobacterium significantly attenuated hepatic fat accumulation (0.10 ± 0.03 g/g liver tissue) (Table 2). However, there was no improvement in the intestinal permeability of either the Lactobacillus (median: 1.125, range: 0.920–1.344) or the Bifidobacterium group (0.788, 0.492–2.164) compared with the model group (0.774, 0.630–1.243) (Table 2).
Stool probiotic flora
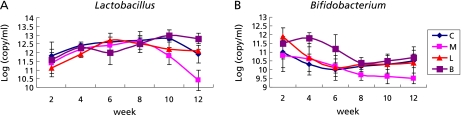
Stool Bifidobacterium content at week 8 (Table 3) and Lactobacillus content at week 10 (Table 4) in the model group were clearly lower than those in the control group, and this phenomenon persisted until the end of the study (Fig. 2). Oral supplementation with Bifidobacterium significantly increased stool Bifidobacterium at week 2 compared with the model group. Similarly, oral supplementation with Lactobacillus increased stool Lactobacillus at week 12 (Fig. 2). In all 40 rats, hepatic total lipid contents were positively correlated (r = 0.580, p = 0.010) with body weight, and negatively correlated with stool Lactobacillus (r = −0.623, p = 0.004) and stool Bifidobacterium (r = −0.591, p = 0.008).
Table 3.
The concentration of stool lactobacillus in the study (Log copy/ml)
| Group Time | Control | Model | Lactobacillus | Bifidobacterium | F value | p value |
|---|---|---|---|---|---|---|
| 2w | 11.8 ± 0.8 | 11.4 ± 0.6 | 11.1 ± 0.5 | 11.6 ± 0.5 | 1.123 | 0.361 |
| 4w | 12.4 ± 0.2 | 12.2 ± 0.4 | 11.9 ± 0.2 | 12.3 ± 0.2 | 2.004 | 0.140 |
| 6w | 12.6 ± 0.5 | 12.4 ± 0.3 | 12.7 ± 0.2 | 12.0 ± 0.7 | 2.514 | 0.088 |
| 8w | 12.7 ± 0.2 | 12.6 ± 0.6 | 12.5 ± 0.5 | 12.5 ± 0.3 | 0.594 | 0.626 |
| 10w | 12.8 ± 0.5 | 11.8 ± 0.5 | 12.2 ± 0.4 | 13.0 ± 0.3 | 6.799 | 0.004 |
| 12w | 11.9 ± 0.5 | 10.4 ± 0.6 | 12.1 ± 0.1* | 12.8 ± 0.3 | 25.814 | 0.000 |
Note: The difference among the groups was made by one-way ANOVA. *p<0.001 vs model group.
Table 4.
The concentration of stool bifidobacterium in the study (Log copy/ml)
| Group Time | Control | Model | Lactobacillus | Bifidobacterium | F value | p value |
|---|---|---|---|---|---|---|
| 2w | 11.0 ± 0.9 | 10.8 ± 0.9 | 11.9 ± 0.5 | 11.5 ± 0.9 | 1.782 | 0.181 |
| 4w | 10.3 ± 0.6 | 10.6 ± 0.7 | 10.7 ± 0.8 | 11.8 ± 0.3* | 7.543 | 0.001 |
| 6w | 10.0 ± 0.4 | 10.2 ± 0.4 | 10.1 ± 0.7 | 11.2 ± 0.8* | 5.180 | 0.008 |
| 8w | 10.2 ± 0.3 | 9.7 ± 0.3 | 10.3 ± 0.2 | 10.4 ± 0.3* | 6.872 | 0.002 |
| 10w | 10.3 ± 0.5 | 9.6 ± 0.4 | 10.3 ± 0.6 | 10.5 ± 0.1* | 6.748 | 0.004 |
| 12w | 10.5 ± 0.4 | 9.5 ± 0.3 | 10.4 ± 0.9 | 10.7 ± 0.4* | 7.793 | 0.002 |
Note: The difference among the groups was made by one-way ANOVA. *p<0.05 vs model group.
Fig. 2.
Trends of Lactobacillus (A) and Bifidobacterium (B) in the study. Data were shown as mean ± SD. Note: C: control; M: model group; L: Lactobacillus group; B: Bifidobacterium group. The difference among the groups was made by one-way ANOVA.
Discussion
Not unexpectedly, the results of our study demonstrated that gut probiotics are closely associated with NAFLD. Stool Bifidobacterium were obviously lower at week 8, and Lactobacillus lower at week 10, in NAFLD model rats. Furthermore, in all 40 rats, hepatic total lipid contents were negatively correlated with stool Lactobacillus (r = −0.623, p = 0.004) and stool Bifidobacterium (r = −0.591, p = 0.008). These results are consistent with those obtained in previous studies. Post-gastric bypass, which is closely associated with NASH and liver cirrhosis,(9,10) causes a large bacterial population shift.(26) Wigg et al.(7) assessed small intestinal bacterial overgrowths in 22 patients with nonalcoholic steatohepatitis and 23 control subjects using a combined 14C-D-xylose and lactulose breath test. Small intestinal bacterial overgrowth was present in 50% of patients with nonalcoholic steatosis and 22% of the control subjects. A further study, conducted in 139 obese subjects, found that the prevalence of small intestinal bacterial overgrowth was obviously higher in obese subjects than in healthy controls (17.1% vs 2.5%). A multivariate regression showed that hepatic steatosis was associated with small intestinal bacterial overgrowth,(8) and a human study by Yao et al.(27) showed that presentation of gut microflora dysfunction was seen in 12 of 40 patients with fatty liver.
Increased intestinal permeability and gut-derived endotoxin are considered to be important clues for the hypothesis of ”liver and lumen interaction”.(13) An increase in intestinal permeability, reflected by the ratio of lactulose to mannitol, was also found in NAFLD model rats compared with controls in our study. Interestingly, however, we found that oral supplementation with probiotics attenuated hepatic fat accumulation, although intestinal permeability failed to improve. Accordingly, it would appear that intestinal permeability does not play a role in the pathogenesis of NAFLD. To date, we have not assessed whether there is an increase in intestinal permeability in NAFLD subjects, as has been suggested by recent studies. First, although the beneficial effects of probiotic supplementation have been identified in both animal and human studies, intestinal permeability has, unfortunately, not been evaluated.(14,16,28) Second, in the studies in which intestinal permeability was evaluated, the results were unclear. Wigg et al.(7) were unable to demonstrate either an increase in small intestine permeability (reflected by the lactulose-rhamnose sugar test) or serum endotoxin level, whereas Jin et al.(29) found a progressive increase of intestinal permeability (reflected by plasma d-lactate and diamine oxidase levels) and endotoxin-induced intestinal destruction in the development of nonalcoholic steatohepatitis. Some plausible explanations for this paradox have been suggested, including limitations of the limulus assay, binding of endotoxin to plasma proteins, and the possibility that other bacterial products such as peptidoglycan-polysaccharide polymers rather than endotoxin could stimulate the release of TNF-α.(30) Another possible explanation is that probiotic treatment plays a role by reducing inflammatory signaling or inhibiting the production of endogenous ethanol, which is thought to contribute to the pathogenesis of NAFLD.(13–15)
Although there are numerous probiotics in nature, we still do not know which are the most beneficial for humans. In studies on probiotics, a mixture of probiotics is usually used,(14,16,28,31) and few studies have compared the effects of single probiotics. Shima et al.(32) found the Lactobacillus casei Shirota enhances intestinal gene expression more strongly than Bifidobacterium breve Yakult. Osmana et al.(33) compared the different effects of Lactobacillus plantarum DSM 15313 and Bifidobacterium infantis DSM 15159 on endotoxin- and d-galactosamine-induced liver injury. DSM 15159 significantly decreased the release of ALT compared to the DSM 15313 group, whereas short chain fatty acids (acetic acid and propionic acid) were decreased significantly in the DSM 15313 group compared to the DSM 15159 group. In our study, we found that oral supplementation with Bifidobacterium significantly reversed the decrease in stool Bifidobacterium at week 2 compared with that in the model group, and that of Lactobacillus at week 12. Bifidobacterium longum was superior in attenuating liver fat accumulation, and there was a non-significant tendency to decrease serum ALT and improve intestinal permeability compared to Lactobacillus acidophilus (CGMCC 2106). There were no significant differences between the two probiotic strains in terms of body weight gain, serum TC, and serum TG. The limitation of the study was that we didn’t detect the gene expression in the liver. Ten of genes are proved to be associated with the development of NAFLD and we didn’t know which gene play a key role in the NAFLD. A gene chip may be used to reveal the relationship between the gut flora and the liver.
In conclusion, hepatic total lipid content is closely associated with gut probiotic flora. Oral supplementation with probiotics attenuates hepatic fat accumulation without improving body weight and intestinal permeability. Bifidobacterium longum has a superior ability to attenuate liver fat accumulation compared to Lactobacillus acidophilus.
Acknowledgments
We thank Dr. Hang XM (Institute of Bio-medicine, Shanghai Jiao Da Onlly Co., Ltd. Shanghai) for kindly supporting Bifidobacterium longum and Lactobacillus acidophilus.
Abbreviations
- ALT
alanine aminotransferase
- B
Bifidobacterium
- C
control
- L
Lactobacillus
- LPS
lipopolysaccharide
- M
model
- NAFLD
nonalcoholic fatty liver disease
- TC
total cholesterol
- TG
triglycerides
Author Contribution
Dr. Ren-ying Xu, the major designer and completer of the study.
Dr. Yan-ping Wan, designer of the study.
Bachelor Qi-yu Fang, assistant of the animal study.
Bachelor Wei Lu, assistant of data analysis.
Dr. Wei Cai, giving important instructions for the paper.
References
- 1.Sherlock S, Dooley J. Diseases of the Liver and Biliary System. Oxford: Blackwell Science; 2002. pp. 81–92. [Google Scholar]
- 2.Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40:S5–S10. doi: 10.1097/01.mcg.0000168638.84840.ff. [DOI] [PubMed] [Google Scholar]
- 3.Sathya P, Martin S, Alvarez F. Nonalcoholic fatty liver disease (NAFLD) in children. Curr Opin Pediatr. 2002;14:593–600. doi: 10.1097/00008480-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Day CP, James OF. Steatohepatitis: a tale of two ”hits” ? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 5.Rector RS, Thyfault JP, Wei Y, Ibdah JA. Non-alcoholic fatty liver disease and the metabolic syndrome: an update. World J Gastroenterol. 2008;114:185–192. doi: 10.3748/wjg.14.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotronen A, Yki-Järvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:27–38. doi: 10.1161/ATVBAHA.107.147538. [DOI] [PubMed] [Google Scholar]
- 7.Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206–211. doi: 10.1136/gut.48.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabaté JM, Jouët P, Harnois F, et al. High prevalence of small intestinal bacterial overgrowth in patients with morbid obesity: a contributor to severe hepatic steatosis. Obes Surg. 2008;18:371–377. doi: 10.1007/s11695-007-9398-2. [DOI] [PubMed] [Google Scholar]
- 9.Nazim M, Stamp G, Hodgson HJ. Non-alcoholic steatohepatitis associated with small intestinal diverticulosis and bacterial overgrowth. Hepatogastroenterology. 1989;36:349–351. [PubMed] [Google Scholar]
- 10.McFarland RJ, Gazet JC, Pilkington TR. A 13-year review of jejunoileal bypass. Br J Surg. 1985;72:81–87. doi: 10.1002/bjs.1800720202. [DOI] [PubMed] [Google Scholar]
- 11.Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218–224. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 12.Segawa S, Wakita Y, Hirata H, Watari J. Oral administration of heat-killed Lactobacillus brevis SBC8803 ameliorates alcoholic liver disease in ethanol-containing diet-fed C57BL/6N mice. Int J Food Microbiology. 2008;128:371–377. doi: 10.1016/j.ijfoodmicro.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 13.Solga SF, Diehl AM. Non-alcoholic fatty liver disease: lumen-liver interactions and possible role for probiotics. J Hepatol. 2003;38:681–687. doi: 10.1016/s0168-8278(03)00097-7. [DOI] [PubMed] [Google Scholar]
- 14.Li Z, Yang S, Lin H, et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343–350. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 15.Ma X, Hua J, Li Z. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J Hepatol. 2008;49:821–830. doi: 10.1016/j.jhep.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loguercio C, Federico A, Tuccillo C, et al. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol. 2005;39:540–543. doi: 10.1097/01.mcg.0000165671.25272.0f. [DOI] [PubMed] [Google Scholar]
- 17.Shen X, Tang Q, Wu J, Feng Y, Huang J, Cai W. Effect of vitamin E supplementation on oxidative stress in a rat model of diet-induced obesity. Int J Vitam Nutr Res. 2009;79:255–263. doi: 10.1024/0300-9831.79.4.255. [DOI] [PubMed] [Google Scholar]
- 18.Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39:1441–1449. doi: 10.1002/hep.20194. [DOI] [PubMed] [Google Scholar]
- 19.Velayudham A, Dolganiuc A, Ellis M, et al. VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice. Hepatology. 2009;49:989–997. doi: 10.1002/hep.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folch J, Lees M, Sloane-Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:457–509. [PubMed] [Google Scholar]
- 21.Weaver LT, Laker MF, Nelson R. Intestinal permeability in the newborn. Arch Dis Child. 1984;59:236–241. doi: 10.1136/adc.59.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jayalakshmi K, Ghoshal UC, Kumar S, Misra A, Roy R, Khetrapal CL. Assessment of small intestinal permeability using 1H-NMR spectroscopy. J Gastrointestin Liver Dis. 2009;18:27–32. [PubMed] [Google Scholar]
- 23.Chen J, Cai W, Feng Y. Development of intestinal bifidobacteria and lactobacilli in breast-fed neonates. Clin Nutr. 2007;26:559–566. doi: 10.1016/j.clnu.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Malinen E, Kassinen A, Rinttilä T, Palva A. Comparison of real-time PCR with SYBR Green I or 5'-nuclease assays and dot-blot hybridization with rDNA-targeted oligonucleotide probes in quantification of selected faecal bacteria. Microbiology. 2003;149:269–277. doi: 10.1099/mic.0.25975-0. [DOI] [PubMed] [Google Scholar]
- 25.Ririe KM, Rasmussen RP, Wittwer CT. Product differentiation by analysis of DNA melting curves during the polymerase chain reaction. Anal Biochem. 1997;245:154–160. doi: 10.1006/abio.1996.9916. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, DiBaise JK, Zuccolo A, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA. 2009;106:2365–2370. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao YC, Feng TJ, Wang JY, et al. The analysis of stool bacterial colony under a microscope in forty fatty liver subjects. Chin J Microecology. 2007;19:96. [Google Scholar]
- 28.Velayudham A, Dolganiuc A, Ellis M, et al. VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice. Hepatology. 2009;49:989–997. doi: 10.1002/hep.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin X, Yu CH, Lv GC, Li YM. Increased intestinal permeability in pathogenesis and progress of nonalcoholic steatohepatitis in rats. World J Gastroenterol. 2007;13:1732–1736. doi: 10.3748/wjg.v13.i11.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farrell GC. Is bacterial ash the flash that ignites NASH? Gut. 2001;48:148–149. doi: 10.1136/gut.48.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukushima M, Yamada A, Endo T, Nakano M. Effects of a mixture of organisms, Lactobacillus acidophilus or Streptococcus faecalis on Δ6-desaturase activity in the livers of rats fed a fat- and cholesterol-enriched diet. Nutrition. 1999;15:373–378. doi: 10.1016/s0899-9007(99)00030-1. [DOI] [PubMed] [Google Scholar]
- 32.Shima T, Fukushima K, Setoyama H, et al. Differential effects of two probiotic strains with different bacteriological properties on intestinal gene expression, with special reference to indigenous bacteria. FEMS Immunol Med Microbiol. 2008;52:69–77. doi: 10.1111/j.1574-695X.2007.00344.x. [DOI] [PubMed] [Google Scholar]
- 33.Osman N, Adawi D, Ahrné S, Jeppsson B, Molin G. Endotoxin- and D-galactosamine-induced liver injury improved by the administration of Lactobacillus, Bifidobacterium and blueberry. Dig Liver Dis. 2007;39:849–856. doi: 10.1016/j.dld.2007.06.001. [DOI] [PubMed] [Google Scholar]