Abstract
NADPH oxidase is a superoxide (O2•−)-generating enzyme first identified in phagocytes, essential for their bactericidal activities. Later, in non-phagocytes, production of O2•− was also demonstrated in an NADPH-dependent manner. In the last decade, several non-phagocyte-type NADPH oxidases have been identified. The catalytic subunit of these oxidases, NOX, constitutes the NOX family. There are five homologs in the family, NOX1 to NOX5, and two related enzymes, DUOX1 and DUOX2. Transgenic or gene-disrupted mice of the NOX family have also been established. NOX/DUOX proteins possess distinct features in the dependency on other components for their enzymatic activities, tissue distributions, and physiological functions. This review summarized the characteristics of the NOX family proteins, especially focused on their functions clarified through studies using gene-modified mice.
Keywords: NADPH oxidase, NOX, superoxide, oxidative stress
Introduction
Reactive oxygen species (ROS) including hydrogen peroxide (H2O2), hydroxyl radical, singlet oxygen, peroxynitrite, and superoxide (O2•−) have been documented to possess both beneficial and detrimental properties at the same time. On one hand, ROS exert bactericidal activities by oxidizing infected microorganisms. On the other hand, ROS elicit lipid peroxidation and inactivate nitric oxide (NO) that dilates blood vessels.
Major sources of O2•− generated in the body are NADPH oxidases. These oxidases produce O2•− from oxygen using NADPH as an electron donor. Studies on NADPH oxidases have been energetically performed mainly in phagocytes, neutrophils. This is because mutations in the gene encoding the phagocyte NADPH oxidase were found in patients with chronic granulomatous disease (CGD).(1) In CGD patients, generation of O2•− and bactericidal activity are attenuated in phagocytes due to the defects in NOX2/NADPH oxidase. This leads to the formation of granulomas in the lung, liver, lymph nodes, and gastrointestinal tracts. The phagocyte NADPH oxidase is composed of two membrane subunits gp91phox (cytochrome b558 heavy chain; later designated as NOX2) and p22phox, three cytosolic subunits p67phox, p47phox, and p40phox, and a small GTP-binding protein Rac. NOX2 is the catalytic subunit of this enzyme complex. Bacterial infection induces association and translocation of the cytosolic components to the phagosome membrane, which elicits activation of the enzyme.
In recent years it has been reported that O2•− is produced in non-phagocytes such as vascular smooth muscle cells (VSMC) and gastrointestinal epithelial cells in an NADPH-dependent manner. Since NOX2 is not expressed in these cells, the presence of non-phagocyte-type NOX homologs has been assumed. A novel homolog, highly expressed in colon epithelial cells (CEC), was first reported by Suh et al.(2) in 1999 and by Banfi et al.(3) in 2000. This homolog was designated as NOX1. To date, five NOX isoforms (NOX1, NOX2, NOX3, NOX4, and NOX5) and two related enzymes, DUOX1 and DUOX2, were identified.
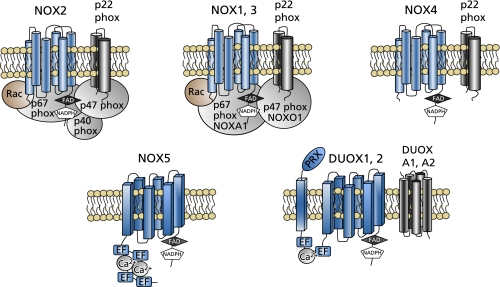
NOX enzymes have six transmembrane domains at the N-terminal half. In the third and fifth transmembrane domains, four heme-binding histidines were conserved. In the C-terminal cytosolic region, they have an FAD-binding domain and an NADPH-binding domain. As for NOX5, it has additional EF-hands, the Ca2+-binding domains, at the N-terminus. In addition to EF-hands, DUOX enzymes have a membrane-spanning region and a peroxidase-like domain at the N-terminus (Fig. 1).
Table 1.
Characteristics of NOX isoforms
| Isoform | Human gene locus | Coupling components | Major distribution sites | Related disorders |
|---|---|---|---|---|
| NOX1 | Xq22 | p22phox | colon epithelium | hypertension, aortic dissection (aneurysm), neointima formation |
| NOXA1/p67phox | VSMC | inflammatory pain, cerebral ischemia, neuroinflammation | ||
| NOXO1/p47phox | hyperoxia-induced acute lung injury | |||
| Rac | colorectal cancer | |||
| NOX2 | Xp21.1 | p22phox | phagocyte | cardiac hypertrophy, fibrosis, heart failure |
| p67phox | myocardial infarction, neovascularization (ischemic cardiovascular diseases) | |||
| p47phox | Alzheimer’s disease, Parkinson’s disease, ischemic stroke | |||
| p40phox | neuropathic pain (peripheral nerve injury) | |||
| Rac | glutamate release (schizophrenia) | |||
| liver fibrosis, Liver ischemia and reperfusion injury | ||||
| amyotrophic lateral sclerosis (ALS) | ||||
| NOX3 | 6q25.3 | p22phox | inner ear | development of the otoconia |
| NOXA1/p67phox | hearing loss | |||
| NOXO1/p47phox | insulin resistance (diabetes) | |||
| Rac | ||||
| NOX4 | 11q14.2-q21 | p22phox | kidney (many other organs) | mitochondrial dysfunction (cardiac hypertrophy, interstitial fibrosis) |
| sympathetic nerve activity (heart failure, myocardial infarction) | ||||
| pulmonary fibrosis, pulmonary hypertension | ||||
| diabetic nephropathy, renal cancer | ||||
| NOX5 | 15q22.31 | spleen, testis, lymph node | Barrett’s esophagus, prostate cancer | |
| DUOX1 | 15q21 | DUOXA1 (maturation factor) | thyroid | host defense |
| DUOX2 | 15q15.3 | DUOXA2 (maturation factor) | thyroid | hypothyroidism, host defense |
Fig. 1.
Schematic diagrams of NOX/NADPH oxidases. EF, EF-hands. PRX, peroxidase-like domain.
NOX2
Activation of NOX2/NADPH oxidase by cytosolic components
As mentioned above, NOX2 was first designated as gp91phox (cytochrome b558 heavy chain), the catalytic subunit of the phagocyte NADPH oxidase. The NOX2 gene was identified as a responsible gene for CGD.(1) NOX2/NADPH oxidase is composed of NOX2, p22phox, p67phox, p47phox, p40phox, and a small GTP-binding protein Rac (Fig. 1). Activation mechanisms of NOX2/NADPH oxidase have been studied by many groups and documented.(4,5) NOX2 and p22phox are located at the plasma membrane and stabilize each other. Through its C-terminal Src homology 3 (SH3) domain, p67phox is associated with the C-terminal proline-rich region (PRR) of p47phox. The cytosolic subunit p47phox has two SH3 domains necessary for its association with the C-terminal PRR of p22phox. In the resting state, the SH3 domains of p47phox bind intramolecularly to the autoinhibitory region (AIR) in the C-terminal half, interrupting binding to p22phox. The N-terminal phagocyte oxidase (PX) domain of p47phox is necessary for binding to membrane phosphoinositides. Another cytoplasmic component p40phox is associated with p67phox via mutual Phox/Bem1p (PB1) domains. Phagocytosis of bacteria or stimulation with phorbol ester (TPA) induces phosphorylation and conformational change in p47phox, enabling its binding to p22phox. Rac is associated with GDP nucleotides dissociation inhibitor for Rho (RhoGDI) in the cytosol. With stimulation, Rac translocates to the membrane independently of p47phox and p67phox. GTP-bound Rac interacts with the N-terminal region of p67phox containing four tetratricopeptide repeat (TPR) motifs. Thus, translocation of cytosolic components to the phagosome membrane and association with the membrane subunits lead to the activation of the enzyme.
Transcriptional regulation of NOX2
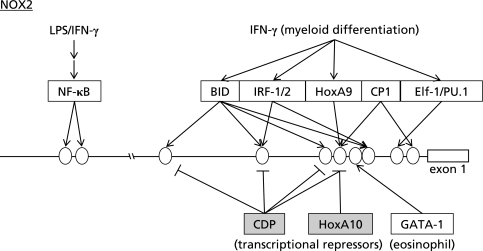
In addition to the regulation of the enzymatic activity by cytosolic components, the expression level of NOX2 is regulated at the level of transcription. Point mutations at −57, −55, −53, and −52 of the NOX2 gene have been detected in patients with CGD.(6,7) The transcription of NOX2 is restricted to myeloid cells differentiated beyond the promyelocyte stage. Interferon (IFN)-γ and lipopolysaccharide (LPS) were shown to increase the expression level of NOX2 in human monocyte-derived macrophages and neutrophils.(8) TPA or retinoic acid/dimethylformamide (DMF) was also reported to induce NOX2 expression in myeloid cells.(9) A number of DNA-binding proteins were shown to interact with the NOX2 promoter region, including BID (binding increased during differentiation), IFN regulatory factor (IRF)-1, IRF-2, the CCAAT box binding protein CP1, and the transcriptional repressor CDP (CCAAT displacement protein) (Fig. 2).(9–13) Voo and Skalnik reported that Elf-1 and PU.1, both of which are Ets family transcription factors highly expressed in myeloid cells, bound to the region containing −57/−52 of the NOX2 gene (Fig. 2). Point mutation at –57 or –55 found in CGD patients was shown to reduce the binding affinity and activity of Elf-1 and PU.1.(14) Protein kinase C (PKC)-dependent phosphorylation of PU.1 was reported to be involved in IFN-γ-induced expression of NOX2 in human monocytes.(15) Bei et al.(16) reported that the transcription of NOX2 during myeloid differentiation was activated by HoxA9 but repressed by HoxA10, both of which are homeodomain transcription factors (Fig. 2). They also found HoxA10-dependent transcriptional repression of p67phox.(17) Tyrosine phosphorylation by Janus tyrosine kinase 2 (JAK2), which is activated by stimulation with IFN-γ, was shown to decrease DNA-binding activity of HoxA10 that represses NOX2 transcription.(18) On the other hand, activation of SHP-2 protein-tyrosine phosphatase was shown to increase HoxA10-induced transcriptional repression of NOX2 and p67phox.(19) Yang et al.(20) reported that GATA-1 was an activator, while GATA-2 was a relative competitive inhibitor of GATA-1 in the eosinophil-specific expression of NOX2 (Fig. 2). NF-κB-dependent transcription of NOX2 was also reported, suggesting the presence of a positive feedback loop in which NF-κB activation by oxidative stress leads to further oxidative burst via NOX2/NADPH oxidase.(21)
Fig. 2.
Transcription factors involved in the expression of NOX2. Ovals indicate binding sites for transcription factors on the NOX2 gene. Transcription factors were expressed as open boxes. Transcriptional repressors were expressed as shaded squares.
Physiological roles of NOX2/NADPH oxidase
Numerous reports using NOX2-deficient mice have been published as an animal model of CGD.(22–24) In this review, only recent findings on the roles NOX2/NADPH oxidase are summarized. Many of the previous studies focused on the function of NOX2 in vascular tissues. Recent findings further showed that NOX2 expressed in bone marrow (BM) and endothelial progenitor cells (EPC) takes part in neovascularization induced by hindlimb ischemia.(25) After hindlimb ischemia induced by ligation of femoral artery, the expression of NOX2 and ROS production were significantly increased in BM-mononuclear cells (BMC). Increased circulation of EPC-like cells was observed in wild type mice, but blunted in NOX2-deficient mice. Impaired neovascularization following hindlimb ischemia in NOX2-deficient mice was rescued by transplantation of BM from wild type mice. Intravenous infusion of BMC derived from NOX2-deficient mice to wild type mice revealed that the neovascularization and homing capacity are impaired in BMC derived from NOX2-deficient mice. Migration and invasion capacity as well as polarization of actins induced by stromal derived factor were significantly suppressed in BM stem/progenitor cells of NOX2-deficient mice. Accordingly, these findings suggest that NOX2-derived ROS regulate EPC mobilization from BM as well as their homing to promote neovascularization following ischemic cardiovascular injuries.
In the prefrontal cortex, a novel role of NOX2 in glutamate release was reported.(26) When the locomotor activity was measured by the frequency of crossings, rearings, groomings, and sniffings in mice administered with ketamine, marked increases in these parameters were observed in wild type mice with the elevation of the oxidative stress maker, 8-OHdG, and c-fos mRNA. In contrast, these behavioral alterations like schizophrenia were not observed in NOX2-deficient mice treated with ketamine. Microdialysis in freely moving animals revealed that the ketamine-induced increase in the extracellular concentration of glutamate and dopamine in the brain was abolished in NOX2-deficient mice. However, NOX2-deficiency did not affect the release of these neurotransmitters induced by amphetamine. After repeated injections of ketamine, a reduced expression of the subunit 2A of the NMDA receptor was demonstrated in wild type mice but not in NOX2-deficient mice. These findings suggest that NOX2 is involved in the development of psychotic symptoms induced by ketamine by elevating glutamate levels and down-regulating the NMDA receptor subunit.(26)
In the spinal cord microglia, NOX2-derived ROS were documented to take part in nerve injury-induced neuropathic pain.(27) Following spinal nerve transection (SNT), increased levels of ROS and NOX2 mRNA were demonstrated in the spinal cord microglia. Mechanical allodynia and thermal hyperalgesia induced by SNT were significantly attenuated in NOX2-deficient mice. SNT induced activation of microglia and subsequent expression of pro-inflammatory cytokines in the spinal cord of wild type mice. These responses were significantly suppressed in NOX2-deficient mice. Furthermore, intrathecal administration of an antioxidant to wild type mice reproduced similar effects observed in NOX2-deficient mice. NOX2 in the spinal cord microglia may therefore play a significant role in the development of neuropathic pain, possibly by regulating proinflammatory cytokines.
In the liver, a crucial role of NOX2 in stellate cell activation and liver fibrosis has been demonstrated.(28) In patients with hepatitis C virus or primary biliary cirrhosis, NOX2 was expressed in α-SMA-positive hepatic stellate cells (HSC). In bile duct ligation (BDL)-induced liver fibrosis, increased expression of NOX2 was in fact demonstrated in HSC isolated from mice. Treatment with apoptotic bodies (AB) also induced NOX2 expression in isolated HSC, indicating that the expression of NOX2 was induced following phagocytosis. In primary cultured HSC, an anti-NOX2 small interfering RNA (siRNA) suppressed production of ROS and induction of collagen synthesis following phagocytosis of AB. Interestingly, phagocytotic activity was significantly decreased in HSC isolated from NOX2-deficient mice. Phagocytosis of apoptotic hepatocytes induced by injection of lentiviral-green fluorescence protein with the hepatocyte-specific α1 antitrypsin promoter and adeno-tumor necrosis factor-related apoptosis-inducing ligand (ad-TRAIL), activated HSC and increased profibrogenic genes in wild type mice. In contrast, these responses were significantly attenuated in NOX2-deficient mice. The fact that NOX2-deficiency significantly suppressed liver fibrosis in BDL model suggests that NOX2 may play a central role in liver fibrogenesis possibly by mediating activation of HSC following phagocytosis of hepatocytes.(28)
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease that can be caused by dominant mutations in superoxide dismutase-1 (SOD1). Oxidative stress is thought to be an important component of disease progression in ALS. Marden et al.(29) reported that NOX2-derived ROS was an exacerbating factor of ALS. They bred NOX2-deficient mice crossed with hemizygous SOD1G93A transgenic mice, a mouse model of ALS, and evaluated the development of motor neuron disorders. Deficiency of NOX2 significantly delayed the death of hemizygous SOD1G93A ALS mice. Production of O2•− in the spinal cord, which was significantly increased in SOD1G93A ALS mice, was almost completely abolished in NOX2-deficient mice. In addition, the death of motor neurons in the lumbar region of the spinal cord and the expression level of the activated microglial marker, CD11b, were significantly reduced in NOX2-deficient mice. In SOD1G93A ALS mice, a decline in rotarod performance and stride length was observed due to hindlimb muscle atrophy. In contrast, these symptoms were alleviated in NOX2-deficient mice. These findings suggest that targeted inhibition of NOX2 using pharmacologic-based approaches may provide substantial benefits for ALS patients.
NOX1
Identification of NOX1 and its coupling components
NOX1 was originally isolated from colon epithelial cells (CEC).(2,3) It is also expressed in vascular smooth muscle cells (VSMC) and other cell lineages. Its expression is induced by vasoactive factors such as angiotensin II (Ang II) and platelet-derived growth factor (PDGF) that elicit proliferation and hypertrophy of VSMC.(2,30) NOX1 is also expressed in macrophages, where it was suggested to be involved in foam cell formation.(31) In mast cells, NOX1 was reported to take part in interleukin (IL)-1β-induced IL-8 secretion(32) and in antigen-induced production of Th2 cytokines.(33)
Like NOX2, NOX1 requires other components for its enzyme activity. In addition to the membrane subunit p22phox, p67phox, p47phox, and Rac1 could compose NOX1/NADPH oxidase.(34,35) Later, the p67phox homolog NOXA1 (NOX activator 1) and the p47phox homolog NOXO1 (NOX organizer 1) were identified. Unlike p67phox and p47phox, NOXA1 and NOXO1 were constitutively associated with NOX1 and p22phox, respectively (Fig. 1).(36–38) Subcellular localization of NOX1 has been documented in caveola and in early endosomes.(39,40)
Transcriptional regulation of NOX1 in colon epithelial cells
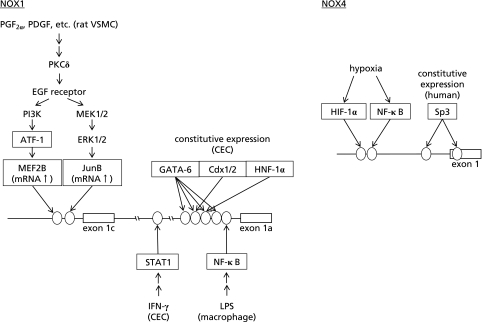
The expression level of NOX1 is very high in the colon compared to other organs.(2,3) The human NOX1 gene is located on Xq22, and the mRNA is encoded by 13 exons. The initiation codon exists in the first exon. Several groups reported the mechanisms of transcriptional regulation of NOX1 in the colon. Brewer et al.(41) reported that a GATA binding site at –135/–130 to be essential for the basal transcriptional activity using a human CEC line Caco-2. Valente et al.(42) reported that the four regions at –201/–181, –177/–157, –149/–124, and –107/–88, to which GATA-4/5/6 could bind, are essential for the basal transcriptional activity in Caco-2 cells, and that Cdx1/2 and HNF-1α could bind to –177/–157 and –149/–124, respectively (Fig. 3). The expression pattern of NOX1, which is highly expressed in the lower part of large intestine, coincides with the patterns of GATA-6, HNF-1α, and Cdx1. These transcription factors may therefore contribute to the expression of NOX1 in the colon. Involvement of GATA-6 in the expression of NOX1 in Caco-2 cells was also reported, in that up-regulation of NOX1 by oncogenic Ras was mediated by MEK-ERK-dependent phosphorylation of GATA-6.(43) On the other hand, Kuwano et al.(44) found that NOX1 is induced in the human CEC line T84 stimulated with IFN-γ. The γ-activated sequence (GAS) at –3818/–3810 was essential for this induction, and phosphorylated STAT1 could bind to this sequence upon stimulation with IFN-γ (Fig. 3). They also reported the mechanism of expressional regulation of NOXO1 in colon epithelial cells. While IL-1β, flagellin, IFN-γ, and tumor necrosis factor (TNF)-α similarly induced NOX1 expression in T84 cells, only TNF-α fully induced NOXO1 expression. They identified three putative binding sites for Sp1, and an AP-1 binding site as crucial elements for the TNF-α-dependent expression of NOXO1 in the colon.(45) Recently, NOX1/NADPH oxidase in the colon was reported to play a key role in the balance between goblet and absorptive cell types by regulating cell proliferation and post-mitotic differentiation.(46)
Fig. 3.
Signaling pathways and transcription factors involved in the expression of NOX1 and NOX4. Ovals indicate binding sites for transcription factors on NOX genes. Transcription factors were expressed as boxes.
Identification of novel NOX1 transcripts induced with phenotype modulation of VSMC
While the expression level of NOX1 in VSMC is increased by stimulation with Ang II, PDGF, prostaglandin (PG) F2α, aldosterone/high salt, serum, or TPA, the level is extremely low compared to that in CEC.(30,47,48) It was therefore assumed that the expression mechanism of the NOX1 gene is different between VSMC and CEC. When we analyzed the 5'-end of the NOX1 mRNA, cDNAs with longer 5'-ends were identified by 5'-RACE in a mouse VSMC line, P53LMACO1.(49) Sequence analyses of cDNA obtained from mouse cecum revealed that the first exon of the cecum cDNA contained the start codon similar to the human NOX1 mRNA. On the other hand, two novel cDNAs with additional exons at the 5'-end were isolated from P53LMACO1 cells. We named the exon containing the start codon as exon 1a, and additional 5'-exons as exon 1b to 1f. Consequently, three types of mRNAs are generated from these 5'-exons, a-type, c-type, and f-type, respectively. The c-type and f-type contained exon 1b, and ATG in this exon was used as the start codon, making the N terminus 28-residues longer than that of the a-type. The a-type was expressed not only in the cecum but also in the intact mouse aorta. On the other hand, the c-type was not expressed in the intact aorta, but its expression was induced by wire injury, Ang II administration, or by culturing aortic VSMC in the medium containing serum. As the expression pattern of the c-type coincided with the pattern of markers indicating phenotypic modulation of VSMC, the expression of the c-type NOX1 might be related to dedifferentiation of VSMC.(49) Although a sequence homologous to the rodent exon 1c was found in the human gene, it did not seem to encode an exon. Indeed, the c-type-like cDNA has not been isolated from human VSMC (Katsuyama et al., unpublished data). The transcriptional regulation of the NOX1 gene in VSMC therefore seems to be species-specific.
Signaling pathways involved in induction of NOX1 in VSMC
PGF2α, one of the primary prostanoids generated in the vascular tissue, was known to cause hypertrophy of VSMC. We found that this is mediated by O2•− generated by NOX1.(47) We next examined signaling pathways involved in the up-regulation of NOX1.
Diphenyleneiodonium (DPI), an inhibitor of flavoproteins including NADPH oxidase itself, almost completely suppressed PGF2α- or PDGF-induced up-regulation of NOX1 mRNA in a rat VSMC line, A7r5.(50) Exploration into the site of action for DPI using various inhibitors suggested the involvement of mitochondrial oxidative phosphorylation. In a luciferase reporter assay, activation of the cAMP response element (CRE)-dependent gene transcription by PGF2α was attenuated by oligomycin, an inhibitor of mitochondrial FoF1-ATPase. Oligomycin and other mitochondrial inhibitors also suppressed PGF2α-induced phosphorylation of activating transcription factor (ATF)-1, a transcription factor of the CREB/ATF family. Silencing of ATF-1 gene by RNA interference significantly reduced the induction of NOX1 by PGF2α or PDGF, while overexpression of ATF-1 recovered the level of NOX1 mRNA suppressed by oligomycin. Thus, ATF-1 was shown to play a pivotal role in the up-regulation of NOX1 in VSMC.(50)
We next demonstrated that PGF2α enhanced the phosphorylation of the epidermal growth factor (EGF) receptor, and a selective inhibitor of EGF receptor kinase, tyrphostin AG1478, significantly suppressed PGF2α-induced NOX1 expression in A7r5 cells.(51) AG1478 also blunted the PGF2α-induced phosphorylation of ERK1/2 and Akt. PI3 kinase inhibitors not only reduced PGF2α-induced NOX1 expression, but also suppressed the phosphorylation of ATF-1. Accordingly, the transactivation of the EGF receptor and the activation of ERK1/2, PI3 kinase, and ATF-1 were shown to constitute the signaling pathways involved in the up-regulation of NOX1 (Fig. 3).(51) On the other hand, an oxidized extracellular oxidation-reduction state (E(h)) increased the expression of NOX1.(52) When aortic segments and cultured VSMC were exposed to E(h) between −150 mV (reduced) and 0 mV (oxidized) by altering the concentration of cysteine and its disulfide, cystine, a more oxidized E(h) increased the expression of NOX1 and resulted in NOX1-dependent proliferation of VSMC. The induction of NOX1 was mediated by the EGF receptor, ERK1/2, and ATF-1 as reported previously.(51)
Since the receptor for PGF2α is known to activate PKC, involvement of PKC in the up-regulation of NOX1 expression was investigated in A7r5 cells.(53) Among isoform-selective inhibitors, an inhibitor of PKCδ, rottlerin, significantly attenuated the PGF2α-induced increase in NOX1 mRNA. Gene silencing of PKCδ by RNA interference significantly suppressed the PGF2α-induced increase in NOX1 mRNA as well as phosphorylation of the EGF receptor, ERK1/2, and ATF-1. Silencing of the PKCδ gene also attenuated the PDGF-induced increase in NOX1 mRNA and transactivation of the EGF receptor. Thus, PKCδ was shown to mediate the transactivation of the EGF receptor elicited not only by PGF2α, but also by PDGF, and the subsequent activation of ERK1/2 and ATF-1 leads to the up-regulation of NOX1 expression (Fig. 3).(53)
To further clarify the mechanisms underlying the transcriptional regulation of NOX1 by vasoactive factors, we analyzed the 5'-end of the rat NOX1 mRNA isolated from cultured VSMC and A7r5 cells.(54) NOX1 mRNA expressed in rat VSMC contained an exon homologous to the exon 1c of the mouse NOX1 gene at the 5'-end. However, neither the counterpart of mouse exon 1b, nor the sequence encoding the additional N-terminal peptide was identified. Reporter assays revealed that the binding site of myocyte enhancer factor 2 (MEF2) at −139/−130 was essential for the up-regulation of NOX1 by PGF2α. Stimulation of A7r5 cells with PGF2α increased binding of MEF2 to this sequence. Among four subtypes of MEF2, the expression level of MEF2B was extremely low, but was elevated by stimulation with PGF2α or PDGF. RNA interference against MEF2B suppressed the induction of NOX1 by PGF2α or PDGF. In addition, RNA interference against ATF-1 suppressed the induction of MEF2B by PGF2α or PDGF. Thus, the induction of NOX1 in VSMC was mediated by MEF2B, of which induction was dependent on the activation of ATF-1 (Fig. 3).(54)
We also identified an AP-1 binding site at −98/−92 essential for NOX1 expression in rat VSMC. PGF2α and PDGF augmented the binding of JunB to this sequence, and an inhibitor of MAPK/ERK kinase suppressed the expression of JunB induced by PGF2α or PDGF.(55) Thus, not only the ATF-1-MEF2B pathway but also the ERK1/2-JunB pathway seems to regulate the inducible expression of NOX1 in VSMC (Fig. 3).
Crosstalk between NOX1 and NF-κB
Stimulation with a TLR4 agonist LPS induced the expression of NOX1 in mouse macrophage, and O2•− derived from NOX1 was thought to take part in foam cell formation.(31) The NOX1 mRNA species expressed in the macrophage was the a-type. The induction was mediated by NF-κB, and a NF-κB binding site was found at −91/−82 of the mouse a-type NOX1 promoter.(31) Induction of NOX1 by LPS was also reported in gastric mucosal cells.(34) Thus, NF-κB might be a crucial transcription factor for the inducible expression of NOX1 in various cell types (Fig. 3). On the other hand, Kim et al.(32) reported that NOX1-derived O2•− is involved in IL-1β-induced activation of NF-κB and transcriptional activation of IL-8 gene in mast cells. IL-1β was shown to induce NOX1 expression via leukotriene (LT) B4-BLT2 receptor signaling. Accordingly, there seems to be a positive feedback loop in which NF-κB activation by NOX1-derived O2•− leads to induction of NOX1 and further amplifies O2•− production.
Physiological roles of NOX1/NADPH oxidase in cardiovascular system
The analyses of NOX1 gene-modified mice have been mainly focused on cardiovascular biology. This is because NOX1 is up-regulated by various vasoactive factors such as Ang II and PGDF. We generated NOX1 gene-disrupted mice and reported a pivotal role for NOX1-derived ROS in the pressor response to Ang II.(56) NOX1 deficiency did not affect baseline blood pressure. After Ang II infusion, a significant increase was observed in mean blood pressure, accompanied by augmented expression of NOX1 mRNA and production of O2•− in the aorta of wild-type mice. In contrast, the elevation in blood pressure and production of O2•− were significantly blunted in NOX1-deficient mice. Ang II-mediated elevation of blood pressure was not affected by administration of a NO synthase inhibitor L-NAME to wild-type mice, but the reduced pressor response to Ang II in NOX1-deficient mice was abolished by L-NAME. Gavazzi et al.(57) also investigated the role of NOX1 in blood pressure regulation, and reported similar results. ROS derived from NOX1/NADPH oxidase were hence suggested to play a pivotal role in the pressor response to Ang II.
In chronic hypertension model, on the other hand, Yogi et al.(58) showed no relationship between NOX1 and blood pressure regulation. When NOX1-deficient mice were crossed with transgenic mice expressing human renin (TTRhRen), four genotypes were generated: control, TTRhRen, NOX1-deficient, and TTRhRen/NOX1-deficient mice. Unlike in the hypertension model induced by short-term Ang II-infusion, NOX1 deficiency had no effect on the development of hypertension in TTRhRen mice despite a significant reduction in oxidative stress. The phosphorylation of redox-sensitive signaling molecules and expression of proinflammatory mediators in the kidney were significantly attenuated in TTRhRen/NOX1-deficient mice. These alterations, however, affected neither the blood pressure nor structural findings associated with renal fibrosis. NOX1/NADPH oxidase may thus play a key role in the modulation of specific redox signaling pathways, but not in the development of hypertension when the endogenous renin-angiotensin system is chronically elevated. These studies indicated that NOX1-derived ROS may play different roles dependent on the model of hypertension.
The implication of NOX1 in neointimal formation was shown by Lee et al.(59) When neointimal formation was induced by wire-injury in femoral artery of NOX1-deficient mice and smooth muscle-specific NOX1-overexpressing mice (SM-NOX1-Tg), neointimal formation was significantly reduced in NOX1-deficient mice, but not in SM-NOX1-Tg. The proliferation and migration of VSMC were reduced in NOX1-deficient mice, while they were enhanced in SM-NOX1-Tg. In NOX1-deficient cells altered phosphorylation of cofilin and expression of mDia1 and PAK1, important regulators of the actin cytoskeleton, were demonstrated. Overexpression of S3A, a constitutively active cofilin mutant, partially recovered reduced migration of NOX1-deficient cells. Taken together, NOX1 appears to enhance neointimal formation by promoting cell migration via regulation of cofilin activity.
A different line of study indicated the involvement of NOX1-derived ROS in aortic dissection. Gavazzi et al.(60) showed that Ang II-induced dissection of aorta was significantly suppressed in NOX1-deficient mice. The underling mechanism was suggested to be NOX1-dependent regulation of tissue inhibitor of metalloproteinase 1 (TIMP-1) expression, which could lead to tissue damage through an altered protease/inhibitor balance. NOX1-derived ROS may be an important factor not only in aortic dissection but also in aneurysm formation.(60)
Physiological roles of NOX1/NADPH oxidase in the nervous system
We previously reported the involvement of NOX1 in inflammatory pain.(61) While there was no difference in nociceptive responses to heat or mechanical stimuli between wild type and NOX1-deficient mice, thermal and mechanical hyperalgesia was significantly attenuated in NOX1-deficient mice. To delineate the molecular mechanisms underlying the reduced hyperalgesia observed in NOX1-deficient mice, we isolated dorsal root ganglia (DRG) neurons and examined the activity of TRPV1, a cation channel implicated in the development of thermal hyperalgesia. In DRG neurons derived from wild type mice, pretreatment with a PKC activator TPA augmented the elevation of intracellular calcium by capsaicin, an agonist of TRPV1. In contrast, this elevation was significantly attenuated in NOX1-deficient mice. Interestingly, NOX1-deficiency or treatment with ROS-scavenging agents perturbed TPA-induced translocation of PKCε. In cells transfected with tagged PKCε, H2O2 induced translocation and a reduction in free sulfhydryls of full-length PKCε, but not of the deletion mutant lacking the C1A domain. These findings suggest that NOX1-derived ROS oxidize the C1A domain of PKCε and accelerate the translocation of PKCε to plasma membranes in DRG neurons, thereby enhancing the TRPV1 activity and the sensitivity to painful stimuli.(61)
In brain microglia, a novel function of NOX1 was documented.(62) LPS, which elicits proinflammatory and neurotoxic activation of microglia, stimulated NOX1-derived O2•− production and enhanced the expression of inducible NO synthase (iNOS). By injecting LPS in the striatum, microglial production of cytotoxic nitrite species and loss of presynaptic proteins in striatal neurons were demonstrated in wild type mice, but not in NOX1-deficient mice. Thus, microglial NOX1 may contribute to immune defenses and the neural tissue damage that occurs in neurodegenerative and inflammatory diseases.(62)
The involvement of NOX1 in the pathophysiology of cerebral ischemia was documented, although underling molecular mechanisms were not fully elucidated.(63) The expression of NOX1 was detected in all cell types cultured from the brain with a relatively similar expression level. In the middle cerebral artery occlusion model, smaller brain infarcts were observed in NOX1-deficient mice, accompanied by the preservation of blood-brain barrier and reduced cerebral edema. These beneficial effects of NOX1-deficiency were, however, blunted as the period of ischemia extended (2 h and more). The apoptotic responses in the ischemic core, the boundary, and the contralateral striatum were similar between the two genotypes. Infusion of an antioxidant TEMPOL or a NO synthase inhibitor L-NAME did not affect the ischemic tissue damage between wild type and NOX1-deficient mice. These results suggested that NOX1-derived ROS may be involved in ischemia/reperfusion-associated tissue damage and blood-brain barrier disruption in experimental stroke. ROS-mediated direct cellular injury was, however, unlikely to explain the protective effect achieved by genetic deletion of the enzyme.(63)
Physiological roles of NOX1/NADPH oxidase in the lung
A crucial role of NOX1 was demonstrated in hyperoxia-induced acute lung injury.(64) When exposed to a high concentration of oxygen, wild type mice exhibited features of severe lung damage. In contrast, hyperoxia-induced lung injury was significantly attenuated in NOX1-deficient mice. Increased NOX1 expression and NOX1-derived ROS production were observed in alveolar epithelial type II cells and endothelial cells isolated from adult mouse lung exposed to hyperoxia. Hyperoxia-induced cellular apoptosis was markedly attenuated in NOX1-deficient mice. Furthermore, NOX1-deficiency decreased hyperoxia-induced phosphorylation of Jun-N-terminal kinase (JNK) and ERK in alveolar cells. These findings suggest an important role of NOX1 in hyperoxia-induced lung injury through regulation of apoptosis in alveolar epithelial and endothelial cells via JNK- and ERK-dependent pathways.(64)
Physiological roles of NOX1/NADPH oxidase in the colon
Although NOX1 is abundantly expressed in the colon, there are few reports concerning its function in the gastrointestinal tract. Recently, Coant et al.(46) reported that the fate of proliferative progenitor cells in the colon was controlled by the pathway dependent on NOX1. In the colon of NOX1-deficient mice, a significant increase in the number of differentiated goblet cells was observed. The absence or presence of NOX1 in cultured colon cancer cells affected the expression of goblet- or enterocyte-specific differentiation factors, respectively. NOX1-deficiency perturbed cell cycle entry of progenitor cells and influenced cell positioning in the crypts. In accordance with these findings, PI3 kinase/Akt/Wnt/β-catenin and Notch1 signaling was repressed in NOX1-deficient mice. Concomitantly, reduced expression of MMP2/9 known to take part in Notch1 activation was demonstrated. The activity of PTEN, a dual-specificity phosphatase that antagonizes PI3 kinase/Akt signaling, was enhanced in NOX1-deficient mice with altered oxidation and phosphorylation status. This led to the inactivation of the Wnt pathway effector β-catenin that promotes cell proliferation. Thus, NOX1 was suggested to control the balance between goblet and absorptive cell types in the colon by coordinately modulating PI3 kinase/Akt/Wnt/β-catenin and Notch1 signaling.(46)
NOX3
NOX3 gene was identified in the otoconia-deficient head tilt mice. In these mice, an allelic series of mutations in the otoconia-deficient head tilt (het) locus was found, causing null mutation or point mutations in the NADPH binding site of NOX3.(65) Thus, NOX3 appears to be essential for gravity perception.
The expression of NOX3 is almost restricted to the inner ear. In situ hybridization demonstrated that NOX3 mRNA was expressed in the vestibular and cochlear sensory epithelia and in the spiral ganglions.(66) To date there is no report on the mechanism underlying the inner ear-specific expression of NOX3.
The activity of NOX3 has been documented to depend on p22phox and NOX organizers (p47phox or NOXO1), activators (p67phox or NOXA1), and Rac1 (Fig. 1).(35,66–68)
Molecular evolution study by Kawahara et al.(69) revealed that NOX3 emerged relatively late in evolution, corresponding to the permanent transition of vertebrates from water to land. Although fish and amphibians have otoconia, they do not have NOX3 gene, suggesting a unique function of NOX3 in land vertebrates.
Recently, the involvement of NOX3 in cisplatin-induced hearing loss was reported. Knockdown of NOX3 using siRNA abrogated cisplatin ototoxicity, as evidenced by protection of outer hair cells from damage and reduced threshold shifts in auditory brainstem responses in the rat. Transtympanic administration of NOX3 siRNA reduced the expression of TRPV1 and kidney injury molecule-1 (KIM-1), biomarkers of cochlear damage. Concomitantly, NOX3 siRNA reduced the expression of Bax, recovered the decreased expression of Bcl2, and attenuated apoptosis induced by cisplatin in the cochlea.(70) Thus, selective inhibitors of NOX3, of which expression is almost restricted to the inner ear, would be useful for preventing cisplatin ototoxicity.
Another line of study demonstrated a novel role of NOX3 in insulin resistance.(71) In db/db mice, increased expression of NOX3 and generation of ROS were demonstrated in the liver, which was accompanied by increased accumulation of lipid compared with wild type mice. Treatment of a human hepatocellular carcinoma cell line HepG2 with palmitate, the main component of plasma free fatty acids, significantly elevated ROS production, NOX3 expression, and gluconeogenesis. Hepatic insulin resistance induced by palmitate was mediated by JNK and p38 MAPK pathways, and rescued by RNA interference against NOX3. A different line of investigation demonstrated that an anti-NOX3 siRNA prevented the TNF-α-induced decrease in cellular glycogen. In TNF-α-treated HepG2 cells, NOX3-dependent activation of JNK, inhibition of IRS-1, and phosphorylation of Akt and glycogen synthase kinase (GSK) were observed.(72) These studies were, however, carried out in cultured cells and could not verify whether NOX3 is a source of ROS in vivo. Further investigations using NOX3 gene-modified mice may be required to clarify the role of NOX3 in insulin resistance.
NOX4
Identification of NOX4
NOX4 was first identified as ”renal NOX” and later found in various tissues.(73–75) It was reported that NOX4 requires only p22phox to exert its enzymatic activity (Fig. 1). NOX4 was shown to generate H2O2, rather than O2•−, constitutively without other cytosolic components. Recently, it was reported that the C-terminal half (dehydrogenase domain) of NOX4/NOX2 determines the dependency on the cytosolic components for the enzyme activation.(76,77) Another report demonstrated that the activity of NOX4 is determined by mRNA levels.(78) Thus, the expression level of NOX4 seems to imply its enzymatic activity. Recently, polymerase (DNA-directed) delta-interacting protein 2 (Poldip2) was reported to interact with p22phox and enhance the activity of NOX4/NADPH oxidase.(79) Subcellular localization of NOX4 was demonstrated in focal adhesions, stress fibers, and nucleus of VSMC.(79) NOX4 was also detected in perinuclear region of pulmonary arterial smooth muscle cells (PASMC),(80) endoplasmic reticulum (ER) of exogenously transfected cells,(76) and in mitochondria of mesangial cells and cardiomyocytes.(81,82)
Constitutive and ubiquitous expression of NOX4
In contrast to NOX1, of which expression is induced by various bioactive factors in specific cell types, NOX4 is constitutively expressed in various cell lineages. Ubiquitous expression of NOX4 suggests it as a housekeeping gene. Indeed, the promoter region of the NOX4 gene contains multiple GC bases, characteristics of housekeeping genes. Zhang et al.(83) reported that E2F1 transcription factor is involved in the expression of NOX4 in vascular smooth muscle cells in rodents. We recently found that Sp3 transcription factor is crucial for transcriptional activation of the human NOX4 gene.(84) In the 5'-flanking and non-coding regions of the human NOX4 gene, three GC-boxes containing putative Sp/Klf binding sites were found, which were not conserved in rodent genes. Reporter assays using SH-SY5Y and HEK293 cells demonstrated that these GC-boxes were essential for the basal expression of the NOX4 gene. Electrophoresis mobility shift assays and chromatin immunoprecipitation assays demonstrated that Sp1 and Sp3 could bind to GC-boxes at −239/−227 and +69/+81 in these cells. By transfection of an anti-Sp3 short hairpin RNA-expression plasmid, promoter activity of the NOX4 gene was reduced in SH-SY5Y and HEK293 cells. Thus, Sp3, which is expressed ubiquitously and involved in the expression of various genes, seems to play a key role in the expression of NOX4 in various cell lineages in humans (Fig. 3).(84)
Inducible expression of NOX4
On the other hand, inducible expression of NOX4 was also reported. Hypoxia has been reported to induce NOX4 expression in PASMC.(80,85) Transforming growth factor (TGF)-β has also been known to induce the expression of NOX4 in various cell types.(86–89) Cannabidiol, a nonpsychoactive cannabinoid, was reported to induce the expression of NOX4 with p22phox, and cellular apoptosis in human leukemia cells. This effect was mediated by cannabinoid receptor 2 (CB2).(90) A major oxysterol contained in oxidized low-density lipoprotein, 7-ketocholesterol, was reported to induce the expression of NOX4 in human aortic SMC. This induction was mediated by IRE-1 (an ER-membrane kinase), JNK, and AP-1. Since NOX4 was shown to induce ER stress and apoptosis, these findings suggest that NOX4 is involved in oxysterol-induced SMC death and the pathogenesis of atherosclerosis.(91) On the other hand, ursolic acid, an active constituent of natural herbs, was reported to suppress the expression of NOX4 in endothelial cells. These natural compounds might therefore exert their beneficial effects by suppressing NOX4 expression that leads to the increase in bioactive NO.(92)
Induction of NOX4 expression by hypoxia
NADPH oxidases have been suggested to serve as oxygen sensors in the lung. Chronic hypoxia induces vascular remodeling with medial hypertrophy and leads to the development of pulmonary hypertension. Mittal et al.(80) reported that NOX4 is expressed in the media of small pulmonary arteries and the expression level was increased in mice exposed to chronic hypoxia. Induction of NOX4 was also observed in isolated PASMC exposed to hypoxia. Also in patients with idiopathic pulmonary arterial hypertension, the level of NOX4 expression was up-regulated in the lung. Treatment of PASMC with an anti-NOX4 siRNA significantly reduced the proliferation of PASMC. Diebold et al.(85) found a putative hypoxia responsive element in the human NOX4 promoter and demonstrated that this sequence is indispensable for the binding of hypoxia-inducible factor-1α (HIF-1α). In fact, RNA interference against HIF-1α suppressed hypoxia-induced expression of NOX4. The involvement of NF-κB in the hypoxia-induced expression of NOX4 was further reported in PASMC, in that hypoxia increased binding of NF-κB p65 to the putative NF-κB binding site adjacent to the putative hypoxia responsive element. Interestingly, rosiglitazone, a PPARγ agonist, suppressed the binding of NF-κB and the promoter activity of NOX4.(93) Thus, NOX4 appears to be a target gene of HIF-1α and NF-κB under hypoxic condition (Fig. 3). NOX4 induced by hypoxia might be involved in the pathogenesis of various cardiovascular diseases through vascular remodeling.
Induction of NOX4 expression by TGF-β
The expression of NOX4 was induced by TGF-β in cardiac fibroblasts, smooth muscle cells of the pulmonary artery and airway, and in hepatocytes.(86–89) TGF-β up-regulated the expression of NOX4 in Hep3B cells but not in HepG2 cells, both of which are human hepatocellular carcinoma cell lines.(89) Treatment with PD98059 recovered the reactivity of HepG2 cells to TGF-β, suggesting that the MEK/ERK pathway is involved in the regulation of NOX4 expression.(94)
A role for NOX4 in tissue repair and fibrogenesis has been documented. TGF-β1 induced NOX4 expression in lung mesenchymal cells via Smad3, a receptor-regulated protein that modulates gene transcription. NOX4-derived H2O2 was required for TGF-β1-induced myofibroblast differentiation, extracellular matrix production and contractility. NOX4 was up-regulated in mice subjected to bleomycin- or hapten-induced pulmonary injury, as well as in patients with idiopathic pulmonary fibrosis (IPF). RNA interference against NOX4 abrogated fibrogenesis in two murine models of lung injury. Thus, selective inhibitors of NOX4 may be useful for the treatment of fibrotic disorders, if appropriate drug delivery systems were established.(95) In the kidney, NOX4 seems to be also involved in the pathogenesis of fibrotic diseases. Increased expression of NOX4 in a rat model of type 1 diabetes induced by streptozotocin was reported, and the involvement of NOX4 in glomerular hypertrophy and fibronectin expression, features of diabetic nephropathy, was suggested.(81)
In vivo analyses using NOX4 gene-modified mice and gene transfer
While NOX4 is ubiquitously expressed in various tissues, the roles for NOX4-derived ROS are somewhat controversial. NOX4 acts as a regulator of proliferation, hypertrophy, and cell survival,(80,87,88,96,97) whereas it acts as a regulator of apoptosis and differentiation in some cell types.(82,89–91,94,98) The reason for these discrepancies has not been clarified yet.
On the other hand, in vivo functions of NOX4 were mainly investigated in cardiac tissue. Ago et al.(82) generated two types of NOX4-transgenic mice. One was with cardiac-specific overexpression of NOX4 (Tg-NOX4), and the other was with catalytically-inactive NOX4 (Tg-NOX4-P437H). The catalytically-inactive NOX4 cannot form a heterodimer with p22phox and thereby inhibits production of O2•− by endogenous NOX4. Expression of NOX4 in cardiac tissues was up-regulated by aging as well as by hypertrophic stimulation such as pressure overload. During young age, the cardiac phenotype of Tg-NOX4 or Tg-NOX4-P437H was not much different from that of wild type mice. However, at 13 to 14 months old, Tg-NOX4 mice showed severe cardiac dysfunction with increased ROS production, apoptosis, and fibrosis in cardiac tissues. In agreement with the finding in the kidney,(99) NOX4 was identified in mitochondria, and NOX4-derived ROS was shown to elicit mitochondrial dysfunction by oxidation of mitochondrial proteins involved in the TCA cycle and electron transport chain.
The same group generated cardiac-specific NOX4 deficient mice (c-NOX4−/−) and elucidated the function of NOX4 in cardiac tissue.(100) When pressure overload was induced by transverse aortic constriction (TAC), a significant increase in NOX4 expression and ROS production in mitochondria was observed in wild type mice but not in c-NOX4−/−. Cardiac hypertrophy, interstitial fibrosis, apoptosis, and cardiac dysfunction induced by TAC were significantly attenuated in c-NOX4−/−. Furthermore, TAC-induced mitochondrial swelling, cytochrome c release, and decreased mitochondrial DNA and aconitase activity were significantly attenuated in c-NOX4−/−. These studies clearly indicated that NOX4 in cardiac myocytes is a major source of mitochondrial oxidative stress, and NOX4-derived ROS amplify mitochondrial and cardiac dysfunction by oxidation of mitochondrial proteins.
As opposed to these findings, a protective role for NOX4 in pressure overload-induced cardiac remodeling was demonstrated.(101) In this study, NOX4-null mice (systemic NOX4 deficiency) and cardiomyocyte-targeted NOX4 transgenic mice were utilized. No difference in basal cardiac functions was observed between the two genotypes. When suprarenal aortic constriction was performed to generate chronic pressure overload, expression of NOX4 was markedly increased in cardiac myocytes, along with contractile impairment and ventricular dilatation in wild type mice. Interestingly, NOX4-null mice developed greater cardiac dilatation, contractile impairment, and cardiac hypertrophy compared with wild type mice. In accordance with these findings, cardiomyocyte-targeted NOX4-transgenic mice were protected against chronic pressure overload-induced stress. Taken together, these results obtained through both loss-of-function and gain-of-function approaches indicate that an increase in myocardial NOX4 expression is protective against chronic pressure overload-induced cardiac dysfunction. As a major mechanism underlying the beneficial effects of NOX4, preservation of capillary density in myocardium, which was decreased in wild type mice and in NOX4-null mice after chronic pressure overload, was suggested in NOX4-transgenic mice. Indeed, decreased levels of HIF-1α and vascular endothelial growth factor (VEGF) in NOX4-null mice were recovered in NOX4-transgenic mice. NOX4-derived ROS thus appear to regulate paracrine angiogenic activity by enhancement of HIF-1α and VEGF expression to protect the heart from chronic pressure overload-associated pathologies.
Hence the effects of NOX4-derived ROS in the heart have been variable in the hands of different workers. It should be noted, however, that NOX4 gene-modified mice and experimental models of pressure overload applied in their studies were dissimilar. The functional roles for NOX4 in cardiac tissues still remain controversial and further investigations seem to be required.
In the paraventricular nucleus (PVN) of the hypothalamus, a key regulator of sympathetic nerve activity, NOX4 was reported to be an important mediator in myocardial infarction (MI)-induced cardiac dysfunction.(102) After induction of MI by coronary arterial ligation, the expression of NOX4 was up-regulated in PVN with increased ROS production. To clarify the role of NOX4 in PVN, adenoviral gene transfer of siRNA targeted against NOX4 (AdsiNOX4) to PVN was performed. PVN-targeted AdsiNOX4 suppressed the MI-induced ROS production in PVN, and improved MI-induced cardiac dysfunction by attenuating chronic sympathoexcitation, recovering left ventricle β-adrenergic receptor responsiveness, and reducing periinfarct apoptosis. Targeted inhibition of NOX4 in PVN might thus provide a novel treatment strategy for MI-induced heart failure.
NOX5
NOX5 was identified as a Ca2+-dependent NADPH oxidase.(103) It has four EF-hands, the Ca2+-binding domains, at the N-terminus (Fig. 1). According to the evolutional analyses, Ca2+-regulated, EF-hand-containing NOXes appeared very early during the evolution of eukaryotes.(69)
Unlike other NOX proteins, NOX5 does not require other components for its activation (Fig. 1). Therefore, its activation is dependent on Ca2+ levels.(104) Kawahara et al.(105) reported that phosphatidylinositol (4,5)-bisphosphate [PtdIns(4,5)P2] plays a key role in the translocation of NOX5 to the plasma membrane. The polybasic region between the fourth EF-hand and the first transmembrane region was shown to bind PtdIns(4,5)P2 and be essential for membrane localization of NOX5. Jamali et al.,(106) on the other hand, reported that H2O2 induced production of O2•− in K562 cells expressing NOX5. This activation was mediated by Ca2+-dependent translocation of c-Abl non-receptor tyrosine kinase to the membrane, where the association of NOX5 with c-Abl was suggested.
Expression of NOX5 was demonstrated in the testis, spleen, lymph nodes, and in VSMC.(103) Since NOX5 gene is absent in rodents, analyses using gene-disrupted mice are not feasible. Involvement of NOX5 in PDGF-induced proliferation of VSMC(107) and in the regulation of growth and apoptosis of prostate cancer cells(108) has been suggested. Expression of NOX5 was also demonstrated in hairy cells (mature malignant B cells), but not in circulating normal B cells.(109) NOX5-derived oxidants were shown to inhibit SHP-1 phosphatase, suggesting that NOX5 is involved in the constitutive phosphorylation signals in hairy cells.(109) In Barrett's esophageal adenocarcinoma, overexpression of NOX5 and the involvement in carcinogenesis were suggested. Platelet-activating factor (PAF) was shown to induce NOX5 expression in esophageal adenocarcinoma cells, and this induction was mediated by the binding of STAT5 to an element in the NOX5 promoter.(110)
DUOX1 and DUOX2
DUOX1 and DUOX2 were initially identified as homologs of NOX2 in the thyroid gland.(111,112) The structure of DUOX is composed of the NOX-like region at the C-terminal half, two EF-hands, a membrane-spanning region, and a peroxidase-like domain at the N-terminus (Fig. 1). Therefore they were designated as dual-oxidase, DUOX. H2O2 derived from DUOX2 is essential for the synthesis of thyroid hormones by thyroid peroxidase. Mutations in the DUOX2 gene were reported in congenital hypothyroidism.(113,114)
DUOX does not require other components of NADPH oxidase for its activity. However, DUOX maturation factors named DUOXA1 and DUOXA2 were identified as transmembrane proteins essential for ER-to-Golgi transition, maturation, and targeting DUOX1 and DUOX2, respectively, to the plasma membrane as functional complexes.(115,116) DUOXA1 was originally identified as numb-interacting protein 1 (NIP1). DUOXA1 and DUOXA2 genes were arranged in a head-to-head orientation and co-expressed with DUOX1 and DUOX2 genes, respectively. A mutation in the DUOXA2 gene was also reported in congenital hypothyroidism.(117) Rigutto et al.(118) reported differential activation of DUOX1 and DUOX2 by protein kinases. DUOX1 but not DUOX2 activity was stimulated by forskolin via protein kinase A-mediated phosphorylation of DUOX1 on serine 955, while TPA induced DUOX2 phosphorylation via PKC activation.
Both DUOX1 and DUOX2 were highly expressed in the thyroid gland. DUOX1 is also expressed in the airway epithelia, placenta, prostate, testis, pancreas, and in the heart.(119,120) DUOX2 is expressed in epithelial cells in salivary excretory ducts and rectal glands.(120,121) As lactoperoxidase (LPO), an enzyme with antimicrobial properties, was detected in airway secretions and saliva, DUOX enzymes may play essential roles in host defense by supplying H2O2 to LPO.(120) The expression of DUOX1 in the airway epithelia was increased by the treatment with Th2 cytokines IL-4 and IL-13, whereas the expression of DUOX2 was highly induced by the treatment with the Th1 cytokine IFN-γ. Differential regulation of DUOX1 and DUOX2 by Th1 and Th2 cytokines might be important for host defense and inflammatory responses. The expression of DUOX2 was also up-regulated by polyinosine-polycytidylic acid and by rhinovirus infection.(122) In lung cancer cells, the expression of DUOX1 and DUOX2 is often suppressed by hypermethylation of the CpG-rich promoter regions in the genes.(123)
Inhibitors of NOX/NADPH Oxidases
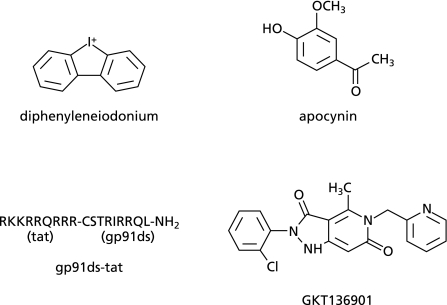
Diphenyleneiodonium (DPI) is the compound that has been most frequently used as an inhibitor of NOX/NADPH oxidases (Fig. 4). DPI is, however, a nonselective inhibitor of flavin-containing enzymes including nitric oxide synthases, cytochrome P450s, and NADH-ubiquinone oxidoreductase (complex I of the mitochondrial respiratory chain). In fact, as mentioned before, DPI inhibits an inducible expression of NOX1 in VSMC probably via inhibition of NADH-ubiquinone oxidoreductase.(50) Thus, due to its selectivity and cytotoxicity, the usefulness of DPI may be limited to in vitro experiments.
Fig. 4.
Structure of available NOX/NADPH oxidase inhibitors.
Apocynin (acetovanillone, 4-hydroxy-3-methoxyacetophenone) has been also used as an inhibitor of NOX/NADPH oxidases (Fig. 4). Apocynin was thought to inhibit the binding of p47phox to NOX2. Recently, however, apocynin was reported to act as an antioxidant rather than an inhibitor of NADPH oxidase. In leukocytes, apocynin is a prodrug that is activated by myeloperoxidase, a process that results in the formation of apocynin dimers. Cells lacking myeloperoxidase failed to generate these dimers and, therefore, are unable to activate apocynin. Thus, apocynin seems to inhibit NADPH oxidase in leukocytes, while it could act as an antioxidant in other cell lineages.(124)
A specific peptide inhibitor of NADPH oxidase, gp91ds-tat, was developed by Pagano and his colleagues.(125) It is a chimeric peptide consisting of 9 amino acids derived from a HIV-coat protein (tat) and a 9-amino acid sequence of NOX2 known to interact with p47phox (docking sequence) (Fig. 4). The 9 amino acids derived from HIV tat protein give the peptide cell permeability. Thus, gp91ds-tat interferes with the assembly and activation of NOX2-containing NADPH oxidase. As gp91ds-tat binds to p47phox, it could inhibit the activity of NOX1-containing NADPH oxidase. Oral administration of gp91ds-tat is basically unsuitable due to its low bioavailability as a peptide. Besides, potential immunogenicity of the chimeric peptide containing tat protein may limit the use of this inhibitor in animal studies.
Recently, high-throughput screenings for selective NOX4 inhibitors were performed. Cells transfected with NOX4 or the membrane fractions of the transfectants were utilized for the screening. Vichem Chemie Research (Hungary) identified three compounds that inhibit NOX4 activity for further drug development. One is a phenantridinone derivative, another is an isoflavone derivative, and the other is an oxalylamide derivative.(126) Genkyotex (Switzerland) developed a selective NOX4/1 inhibitor, GKT136901, which has low affinity to other NOX enzymes (Fig. 4). High plasma concentration of the inhibitor could be achieved by oral administration in rats.(127,128) Thus, GKT136901 is currently expected for further clinical trials for the treatment of NOX4/1-related disorders such as pulmonary fibrosis, cancers, and cardiovascular and metabolic diseases.
Conclusions
We summarized isoform-specific activation mechanisms, transcriptional regulation, and physiological roles of NOX/DUOX-containing NADPH oxidases. Studies using gene-modified animals may further clarify the entire picture of their unidentified roles. In the meantime, development of isoform-selective NOX inhibitors is expected for the treatment of a wide range of acute and chronic diseases.
Abbreviations
- AB
apoptotic bodies
- ad-TRAIL
adeno-tumor necrosis factor-related apoptosis-inducing ligand
- AIR
autoinhibitory region
- ALS
amyotrophic lateral sclerosis
- Ang II
angiotensin II
- ATF
activating transcription factor
- BDL
bile duct ligation
- BID
binding increased during differentiation
- BM
bone marrow
- BMC
bone marrow-mononuclear cells
- CB2
cannabinoid receptor 2
- CEC
colon epithelial cells
- CDP
CCAAT displacement protein
- CGD
chronic granulomatous disease
- CRE
cAMP response element
- DMF
dimethylformamide
- DPI
diphenyleneiodonium
- DRG
dorsal root ganglia
- E(h)
extracellular oxidation-reduction state
- EGF
epidermal growth factor
- EPC
endothelial progenitor cells
- ER
endoplasmic reticulum
- GAS
γ-activated sequence
- GSK
glycogen synthase kinase
- HIF-1α
hypoxia-inducible factor-1α
- H2O2
hydrogen peroxide
- HSC
hepatic stellate cells
- IFN
interferon
- IL
interleukin
- iNOS
inducible NO synthase
- IPF
idiopathic pulmonary fibrosis
- IRF
IFN regulatory factor
- JAK2
Janus tyrosine kinase 2
- JNK
Jun-N-terminal kinase
- KIM-1
kidney injury molecule-1
- LPO
lactoperoxidase
- LPS
lipopolysaccharide
- LT
leukotriene
- MEF2
myocyte enhancer factor 2
- MI
myocardial infarction
- NIP1
numb-interacting protein 1
- NO
nitric oxide
- NOXA1
NOX activator 1
- NOXO1
NOX organizer 1
- O2•−
superoxide
- PAF
platelet-activating factor
- PASMC
pulmonary arterial smooth muscle cells
- PB1
Phox/Bem1p
- PDGF
platelet-derived growth factor
- PG
prostaglandin
- PKC
protein kinase C
- Poldip2
polymerase (DNA-directed) delta-interacting protein 2
- PRR
proline-rich region
- PtdIns(4,5)P2
phosphatidylinositol (4,5)-bisphosphate
- PVN
paraventricular nucleus
- PX
phagocyte oxidase
- RhoGDI
GDP nucleotides dissociation inhibitor for Rho
- ROS
reactive oxygen species
- SH3
Src homology 3
- siRNA
small interfering RNA
- SNT
spinal nerve transection
- SOD1
superoxide dismutase-1
- TAC
transverse aortic constriction
- TGF
transforming growth factor
- TIMP
tissue inhibitor of metalloproteinase
- TNF
tumor necrosis factor
- TPA
phorbol ester
- TPR
tetratricopeptide repeat
- VEGF
vascular endothelial growth factor
- VSMC
vascular smooth muscle cells
References
- 1.Royer-Pokora B, Kunkel LM, Monaco AP, et al. Cloning the gene for an inherited human disorder—chronic granulomatous disease—on the basis of its chromosomal location. Nature. 1986;322:32–38. doi: 10.1038/322032a0. [DOI] [PubMed] [Google Scholar]
- 2.Suh YA, Arnold RS, Lassegue B, et al. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 3.Bánfi B, Maturana A, Jaconi S, et al. A mammalian H+ channel generated through alternative splicing of the NADPH oxidase homolog NOH-1. Science. 2000;287:138–142. doi: 10.1126/science.287.5450.138. [DOI] [PubMed] [Google Scholar]
- 4.Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J. 2008;275:3249–3277. doi: 10.1111/j.1742-4658.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- 5.Leto TL, Morand S, Hurt D, Ueyama T. Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid Redox Signal. 2009;11:2607–2619. doi: 10.1089/ars.2009.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newburger PE, Skalnik DG, Hopkins PJ, Eklund EA, Curnutte JT. Mutations in the promoter region of the gene for gp91-phox in X-linked chronic granulomatous disease with decreased expression of cytochrome b558. J Clin Invest. 1994;94:1205–1211. doi: 10.1172/JCI117437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki S, Kumatori A, Haagen IA, et al. PU.1 as an essential activator for the expression of gp91(phox) gene in human peripheral neutrophils, monocytes, and B lymphocytes. Proc Natl Acad Sci USA. 1998;95:6085–6090. doi: 10.1073/pnas.95.11.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassatella MA, Bazzoni F, Flynn RM, Dusi S, Trinchieri G, Rossi F. Molecular basis of interferon-gamma and lipopolysaccharide enhancement of phagocyte respiratory burst capability. Studies on the gene expression of several NADPH oxidase components. J Biol Chem. 1990;265:20241–20246. [PubMed] [Google Scholar]
- 9.Eklund EA, Luo W, Skalnik DG. Characterization of three promoter elements and cognate DNA binding protein(s) necessary for IFN-gamma induction of gp91-phox transcription. J Immunol. 1996;157:2418–2429. [PubMed] [Google Scholar]
- 10.Eklund EA, Skalnik DG. Characterization of a gp91-phox promoter element that is required for interferon gamma-induced transcription. J Biol Chem. 1995;270:8267–8273. doi: 10.1074/jbc.270.14.8267. [DOI] [PubMed] [Google Scholar]
- 11.Luo W, Skalnik DG. CCAAT displacement protein competes with multiple transcriptional activators for binding to four sites in the proximal gp91phox promoter. J Biol Chem. 1996;271:18203–18210. doi: 10.1074/jbc.271.30.18203. [DOI] [PubMed] [Google Scholar]
- 12.Luo W, Skalnik DG. Interferon regulatory factor-2 directs transcription from the gp91phox promoter. J Biol Chem. 1996;271:23445–23451. doi: 10.1074/jbc.271.38.23445. [DOI] [PubMed] [Google Scholar]
- 13.Skalnik DG, Strauss EC, Orkin SH. CCAAT displacement protein as a repressor of the myelomonocytic-specific gp91-phox gene promoter. J Biol Chem. 1991;266:16736–16744. [PubMed] [Google Scholar]
- 14.Voo KS, Skalnik DG. Elf-1 and PU.1 induce expression of gp91(phox) via a promoter element mutated in a subset of chronic granulomatous disease patients. Blood. 1999;93:3512–3520. [PubMed] [Google Scholar]
- 15.Mazzi P, Donini M, Margotto D, Wientjes F, Dusi S. IFN-gamma induces gp91phox expression in human monocytes via protein kinase C-dependent phosphorylation of PU.1. J Immunol. 2004;172:4941–4947. doi: 10.4049/jimmunol.172.8.4941. [DOI] [PubMed] [Google Scholar]
- 16.Bei L, Lu Y, Eklund EA. HOXA9 activates transcription of the gene encoding gp91Phox during myeloid differentiation. J Biol Chem. 2005;280:12359–12370. doi: 10.1074/jbc.M408138200. [DOI] [PubMed] [Google Scholar]
- 17.Lindsey S, Zhu C, Lu YF, Eklund EA. HoxA10 represses transcription of the gene encoding p67phox in phagocytic cells. J Immunol. 2005;175:5269–5279. doi: 10.4049/jimmunol.175.8.5269. [DOI] [PubMed] [Google Scholar]
- 18.Kakar R, Kautz B, Eklund EA. JAK2 is necessary and sufficient for interferon-gamma-induced transcription of the gene encoding gp91PHOX. J Leukoc Biol. 2005;77:120–127. doi: 10.1189/jlb.0704429. [DOI] [PubMed] [Google Scholar]
- 19.Lindsey S, Huang W, Wang H, Horvath E, Zhu C, Eklund EA. Activation of SHP2 protein-tyrosine phosphatase increases HoxA10-induced repression of the genes encoding gp91(PHOX) and p67(PHOX) J Biol Chem. 2007;282:2237–2249. doi: 10.1074/jbc.M608642200. [DOI] [PubMed] [Google Scholar]
- 20.Yang D, Suzuki S, Hao LJ, et al. Eosinophil-specific regulation of gp91(phox) gene expression by transcription factors GATA-1 and GATA-2. J Biol Chem. 2000;275:9425–9432. doi: 10.1074/jbc.275.13.9425. [DOI] [PubMed] [Google Scholar]
- 21.Anrather J, Racchumi G, Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem. 2006;281:5657–5667. doi: 10.1074/jbc.M506172200. [DOI] [PubMed] [Google Scholar]
- 22.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 23.Lambeth JD, Krause KH, Clark RA. NOX enzymes as novel targets for drug development. Semin Immunopathol. 2008;30:339–363. doi: 10.1007/s00281-008-0123-6. [DOI] [PubMed] [Google Scholar]
- 24.Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urao N, Inomata H, Razvi M, et al. Role of nox2-based NADPH oxidase in bone marrow and progenitor cell function involved in neovascularization induced by hindlimb ischemia. Circ Res. 2008;103:212–220. doi: 10.1161/CIRCRESAHA.108.176230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorce S, Schiavone S, Tucci P, et al. The NADPH oxidase NOX2 controls glutamate release: a novel mechanism involved in psychosis-like ketamine responses. J Neurosci. 2010;30:11317–11325. doi: 10.1523/JNEUROSCI.1491-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim D, You B, Jo EK, Han SK, Simon MI, Lee SJ. NADPH oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain. Proc Natl Acad Sci USA. 2010;107:14851–14856. doi: 10.1073/pnas.1009926107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang JX, Venugopal S, Serizawa N, et al. Reduced nicotinamide adenine dinucleotide phosphate oxidase 2 plays a key role in stellate cell activation and liver fibrogenesis in vivo. Gastroenterology. 2010;139:1375–1384. doi: 10.1053/j.gastro.2010.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marden JJ, Harraz MM, Williams AJ, et al. Redox modifier genes in amyotrophic lateral sclerosis in mice. J Clin Invest. 2007;117:2913–2919. doi: 10.1172/JCI31265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lassègue B, Sorescu D, Szöcs K, et al. Novel gp91(phox) homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 31.Lee SH, Park DW, Park SC, et al. Calcium-independent phospholipase A2beta-Akt signaling is involved in lipopolysaccharide-induced NADPH oxidase 1 expression and foam cell formation. J Immunol. 2009;183:7497–7504. doi: 10.4049/jimmunol.0900503. [DOI] [PubMed] [Google Scholar]
- 32.Kim GY, Lee JW, Ryu HC, Wei JD, Seong CM, Kim JH. Proinflammatory cytokine IL-1beta stimulates IL-8 synthesis in mast cells via a leukotriene B4 receptor 2-linked pathway, contributing to angiogenesis. J Immunol. 2010;184:3946–3954. doi: 10.4049/jimmunol.0901735. [DOI] [PubMed] [Google Scholar]
- 33.Cho KJ, Seo JM, Lee MG, Kim JH. BLT2 is upregulated in allergen-stimulated mast cells and mediates the synthesis of Th2 cytokines. J Immunol. 2010;185:6329–6337. doi: 10.4049/jimmunol.1001213. [DOI] [PubMed] [Google Scholar]
- 34.Kawahara T, Kohjima M, Kuwano Y, et al. Helicobacter pylori lipopolysaccharide activates Rac1 and transcription of NADPH oxidase Nox1 and its organizer NOXO1 in guinea pig gastric mucosal cells. Am J Physiol Cell Physiol. 2005;288:C450–C457. doi: 10.1152/ajpcell.00319.2004. [DOI] [PubMed] [Google Scholar]
- 35.Ueyama T, Geiszt M, Leto TL. Involvement of Rac1 in activation of multicomponent Nox1- and Nox3-based NADPH oxidases. Mol Cell Biol. 2006;26:2160–2174. doi: 10.1128/MCB.26.6.2160-2174.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bánfi B, Clark RA, Steger K, Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem. 2003;278:3510–3513. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- 37.Geiszt M, Lekstrom K, Witta J, Leto TL. Proteins homologous to p47phox and p67phox support superoxide production by NAD(P)H oxidase 1 in colon epithelial cells. J Biol Chem. 2003;278:20006–20012. doi: 10.1074/jbc.M301289200. [DOI] [PubMed] [Google Scholar]
- 38.Takeya R, Ueno N, Kami K, et al. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J Biol Chem. 2003;278:25234–25246. doi: 10.1074/jbc.M212856200. [DOI] [PubMed] [Google Scholar]
- 39.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 40.Miller FJ, Jr., Filali M, Huss GJ, et al. Cytokine activation of nuclear factor kappa B in vascular smooth muscle cells requires signaling endosomes containing Nox1 and ClC-3. Circ Res. 2007;101:663–671. doi: 10.1161/CIRCRESAHA.107.151076. [DOI] [PubMed] [Google Scholar]
- 41.Brewer AC, Sparks EC, Shah AM. Transcriptional regulation of the NADPH oxidase isoform, Nox1, in colon epithelial cells: role of GATA-binding factor(s) Free Radic Biol Med. 2006;40:260–274. doi: 10.1016/j.freeradbiomed.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 42.Valente AJ, Zhou Q, Lu Z, et al. Regulation of NOX1 expression by GATA, HNF-1alpha, and Cdx transcription factors. Free Radic Biol Med. 2008;44:430–443. doi: 10.1016/j.freeradbiomed.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 43.Adachi Y, Shibai Y, Mitsushita J, Shang WH, Hirose K, Kamata T. Oncogenic Ras upregulates NADPH oxidase 1 gene expression through MEK-ERK-dependent phosphorylation of GATA-6. Oncogene. 2008;27:4921–4932. doi: 10.1038/onc.2008.133. [DOI] [PubMed] [Google Scholar]
- 44.Kuwano Y, Kawahara T, Yamamoto H, et al. Interferon-gamma activates transcription of NADPH oxidase 1 gene and upregulates production of superoxide anion by human large intestinal epithelial cells. Am J Physiol Cell Physiol. 2006;290:C433–C443. doi: 10.1152/ajpcell.00135.2005. [DOI] [PubMed] [Google Scholar]
- 45.Kuwano Y, Tominaga K, Kawahara T, et al. Tumor necrosis factor alpha activates transcription of the NADPH oxidase organizer 1 (NOXO1) gene and upregulates superoxide production in colon epithelial cells. Free Radic Biol Med. 2008;45:1642–1652. doi: 10.1016/j.freeradbiomed.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 46.Coant N, Ben Mkaddem S, Pedruzzi E, et al. NADPH oxidase 1 modulates WNT and NOTCH1 signaling to control the fate of proliferative progenitor cells in the colon. Mol Cell Biol. 2010;30:2636–2650. doi: 10.1128/MCB.01194-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katsuyama M, Fan C, Yabe-Nishimura C. NADPH oxidase is involved in prostaglandin F2alpha-induced hypertrophy of vascular smooth muscle cells: induction of NOX1 by PGF2alpha. J Biol Chem. 2002;277:13438–13442. doi: 10.1074/jbc.M111634200. [DOI] [PubMed] [Google Scholar]
- 48.Fan C, Kawai Y, Inaba S, et al. Synergy of aldosterone and high salt induces vascular smooth muscle hypertrophy through up-regulation of NOX1. J Steroid Biochem Mol Biol. 2008;111:29–36. doi: 10.1016/j.jsbmb.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 49.Arakawa N, Katsuyama M, Matsuno K, et al. Novel transcripts of Nox1 are regulated by alternative promoters and expressed under phenotypic modulation of vascular smooth muscle cells. Biochem J. 2006;398:303–310. doi: 10.1042/BJ20060300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katsuyama M, Fan C, Arakawa N, et al. Essential role of ATF-1 in induction of NOX1, a catalytic subunit of NADPH oxidase: involvement of mitochondrial respiratory chain. Biochem J. 2005;386:255–261. doi: 10.1042/BJ20041180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan C, Katsuyama M, Nishinaka T, Yabe-Nishimura C. Transactivation of the EGF receptor and a PI3 kinase-ATF-1 pathway is involved in the upregulation of NOX1, a catalytic subunit of NADPH oxidase. FEBS Lett. 2005;579:1301–1305. doi: 10.1016/j.febslet.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 52.Stanic B, Katsuyama M, Miller FJ., Jr An oxidized extracellular oxidation-reduction state increases Nox1 expression and proliferation in vascular smooth muscle cells via epidermal growth factor receptor activation. Arterioscler Thromb Vasc Biol. 2010;30:2234–2241. doi: 10.1161/ATVBAHA.110.207639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fan CY, Katsuyama M, Yabe-Nishimura C. PKCdelta mediates up-regulation of NOX1, a catalytic subunit of NADPH oxidase, via transactivation of the EGF receptor: possible involvement of PKCdelta in vascular hypertrophy. Biochem J. 2005;390:761–767. doi: 10.1042/BJ20050287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katsuyama M, Cevik MO, Arakawa N, et al. Myocyte enhancer factor 2B is involved in the inducible expression of NOX1/NADPH oxidase, a vascular superoxide-producing enzyme. FEBS J. 2007;274:5128–5136. doi: 10.1111/j.1742-4658.2007.06034.x. [DOI] [PubMed] [Google Scholar]
- 55.Cevik MO, Katsuyama M, Kanda S, et al. The AP-1 site is essential for the promoter activity of NOX1/NADPH oxidase, a vascular superoxide-producing enzyme: possible involvement of the ERK1/2-JunB pathway. Biochem Biophys Res Commun. 2008;374:351–355. doi: 10.1016/j.bbrc.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 56.Matsuno K, Yamada H, Iwata K, et al. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation. 2005;112:2677–2685. doi: 10.1161/CIRCULATIONAHA.105.573709. [DOI] [PubMed] [Google Scholar]
- 57.Gavazzi G, Banfi B, Deffert C, et al. Decreased blood pressure in NOX1-deficient mice. FEBS Lett. 2006;580:497–504. doi: 10.1016/j.febslet.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 58.Yogi A, Mercure C, Touyz J, et al. Renal redox-sensitive signaling, but not blood pressure, is attenuated by Nox1 knockout in angiotensin II-dependent chronic hypertension. Hypertension. 2008;51:500–506. doi: 10.1161/HYPERTENSIONAHA.107.103192. [DOI] [PubMed] [Google Scholar]
- 59.Lee MY, San Martin A, Mehta PK, et al. Mechanisms of vascular smooth muscle NADPH oxidase 1 (Nox1) contribution to injury-induced neointimal formation. Arterioscler Thromb Vasc Biol. 2009;29:480–487. doi: 10.1161/ATVBAHA.108.181925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gavazzi G, Deffert C, Trocme C, Schappi M, Herrmann FR, Krause KH. NOX1 deficiency protects from aortic dissection in response to angiotensin II. Hypertension. 2007;50:189–196. doi: 10.1161/HYPERTENSIONAHA.107.089706. [DOI] [PubMed] [Google Scholar]
- 61.Ibi M, Matsuno K, Shiba D, et al. Reactive oxygen species derived from NOX1/NADPH oxidase enhance inflammatory pain. J Neurosci. 2008;28:9486–9494. doi: 10.1523/JNEUROSCI.1857-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chéret C, Gervais A, Lelli A, et al. Neurotoxic activation of microglia is promoted by a nox1-dependent NADPH oxidase. J Neurosci. 2008;28:12039–12051. doi: 10.1523/JNEUROSCI.3568-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kahles T, Kohnen A, Heumueller S, et al. NADPH oxidase Nox1 contributes to ischemic injury in experimental stroke in mice. Neurobiol Dis. 2010;40:185–192. doi: 10.1016/j.nbd.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 64.Carnesecchi S, Deffert C, Pagano A, et al. NADPH oxidase-1 plays a crucial role in hyperoxia-induced acute lung injury in mice. Am J Respir Crit Care Med. 2009;180:972–981. doi: 10.1164/rccm.200902-0296OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paffenholz R, Bergstrom RA, Pasutto F, et al. Vestibular defects in head-tilt mice result from mutations in Nox3, encoding an NADPH oxidase. Genes Dev. 2004;18:486–491. doi: 10.1101/gad.1172504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bánfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem. 2004;279:46065–46072. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- 67.Ueno N, Takeya R, Miyano K, Kikuchi H, Sumimoto H. The NADPH oxidase Nox3 constitutively produces superoxide in a p22phox-dependent manner: its regulation by oxidase organizers and activators. J Biol Chem. 2005;280:23328–23339. doi: 10.1074/jbc.M414548200. [DOI] [PubMed] [Google Scholar]
- 68.Nakano Y, Banfi B, Jesaitis AJ, Dinauer MC, Allen LA, Nauseef WM. Critical roles for p22phox in the structural maturation and subcellular targeting of Nox3. Biochem J. 2007;403:97–108. doi: 10.1042/BJ20060819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kawahara T, Quinn MT, Lambeth JD. Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes. BMC Evol Biol. 2007;7:109. doi: 10.1186/1471-2148-7-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mukherjea D, Jajoo S, Kaur T, Sheehan KE, Ramkumar V, Rybak LP. Transtympanic administration of short interfering (si)RNA for the NOX3 isoform of NADPH oxidase protects against cisplatin-induced hearing loss in the rat. Antioxid Redox Signal. 2010;13:589–598. doi: 10.1089/ars.2010.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gao D, Nong S, Huang X, et al. The effects of palmitate on hepatic insulin resistance are mediated by NADPH Oxidase 3-derived reactive oxygen species through JNK and p38MAPK pathways. J Biol Chem. 2010;285:29965–29973. doi: 10.1074/jbc.M110.128694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li L, He Q, Huang X, et al. NOX3-derived reactive oxygen species promote TNF-alpha-induced reductions in hepatocyte glycogen levels via a JNK pathway. FEBS Lett. 2010;584:995–1000. doi: 10.1016/j.febslet.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 73.Geiszt M, Kopp JB, Varnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci USA. 2000;97:8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shiose A, Kuroda J, Tsuruya K, et al. A novel superoxide-producing NAD(P)H oxidase in kidney. J Biol Chem. 2001;276:1417–1423. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]
- 75.Yang S, Madyastha P, Bingel S, Ries W, Key L. A new superoxide-generating oxidase in murine osteoclasts. J Biol Chem. 2001;276:5452–5458. doi: 10.1074/jbc.M001004200. [DOI] [PubMed] [Google Scholar]
- 76.von Löhneysen, Noack D, Wood MR, Friedman JS, Knaus UG. Structural insights into Nox4 and Nox2: motifs involved in function and cellular localization. Mol Cell Biol. 2010;30:961–975. doi: 10.1128/MCB.01393-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nisimoto Y, Jackson HM, Ogawa H, Kawahara T, Lambeth JD. Constitutive NADPH-dependent electron transferase activity of the Nox4 dehydrogenase domain. Biochemistry. 2010;49:2433–2442. doi: 10.1021/bi9022285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Serrander L, Cartier L, Bedard K, et al. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lyle AN, Deshpande NN, Taniyama Y, et al. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res. 2009;105:249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mittal M, Roth M, König P, et al. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res. 2007;101:258–267. doi: 10.1161/CIRCRESAHA.107.148015. [DOI] [PubMed] [Google Scholar]
- 81.Block K, Gorin Y, Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci USA. 2009;106:14385–14390. doi: 10.1073/pnas.0906805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang L, Sheppard OR, Shah AM, Brewer AC. Positive regulation of the NADPH oxidase NOX4 promoter in vascular smooth muscle cells by E2F. Free Radic Biol Med. 2008;45:679–685. doi: 10.1016/j.freeradbiomed.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 84.Katsuyama M, Hirai H, Iwata K, et al. Sp3 transcription factor is crucial for transcriptional activation of the human NOX4 gene. The FEBS journal. 2011;278:964–972. doi: 10.1111/j.1742-4658.2011.08018.x. [DOI] [PubMed] [Google Scholar]
- 85.Diebold I, Petry A, Hess J, Görlach A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol Biol Cell. 2010;21:2087–2096. doi: 10.1091/mbc.E09-12-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 87.Sturrock A, Cahill B, Norman K, et al. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- 88.Sturrock A, Huecksteadt TP, Norman K, et al. Nox4 mediates TGF-beta1-induced retinoblastoma protein phosphorylation, proliferation, and hypertrophy in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1543–L1555. doi: 10.1152/ajplung.00430.2006. [DOI] [PubMed] [Google Scholar]
- 89.Carmona-Cuenca I, Roncero C, Sancho P, et al. Upregulation of the NADPH oxidase NOX4 by TGF-beta in hepatocytes is required for its pro-apoptotic activity. J Hepatol. 2008;49:965–976. doi: 10.1016/j.jhep.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 90.McKallip RJ, Jia W, Schlomer J, Warren JW, Nagarkatti PS, Nagarkatti M. Cannabidiol-induced apoptosis in human leukemia cells: A novel role of cannabidiol in the regulation of p22phox and Nox4 expression. Mol Pharmacol. 2006;70:897–908. doi: 10.1124/mol.106.023937. [DOI] [PubMed] [Google Scholar]
- 91.Pedruzzi E, Guichard C, Ollivier V, et al. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol Cell Biol. 2004;24:10703–10717. doi: 10.1128/MCB.24.24.10703-10717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Steinkamp-Fenske K, Bollinger L, Völler N, et al. Ursolic acid from the Chinese herb danshen (Salvia miltiorrhiza L.) upregulates eNOS and downregulates Nox4 expression in human endothelial cells. Atherosclerosis. 2007;195:e104–e111. doi: 10.1016/j.atherosclerosis.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 93.Lu X, Murphy TC, Nanes MS, Hart CM. PPAR{gamma} regulates hypoxia-induced Nox4 expression in human pulmonary artery smooth muscle cells through NF-{kappa}B. Am J Physiol Lung Cell Mol Physiol. 2010;299:L559–L566. doi: 10.1152/ajplung.00090.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Caja L, Sancho P, Bertran E, Iglesias-Serret D, Gil J, Fabregat I. Overactivation of the MEK/ERK pathway in liver tumor cells confers resistance to TGF-{beta}-induced cell death through impairing up-regulation of the NADPH oxidase NOX4. Cancer Res. 2009;69:7595–7602. doi: 10.1158/0008-5472.CAN-09-1482. [DOI] [PubMed] [Google Scholar]
- 95.Hecker L, Vittal R, Jones T, et al. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mochizuki T, Furuta S, Mitsushita J, et al. Inhibition of NADPH oxidase 4 activates apoptosis via the AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic cancer PANC-1 cells. Oncogene. 2006;25:3699–3707. doi: 10.1038/sj.onc.1209406. [DOI] [PubMed] [Google Scholar]
- 97.Block K, Eid A, Griendling KK, Lee DY, Wittrant Y, Gorin Y. Nox4 NAD(P)H oxidase mediates Src-dependent tyrosine phosphorylation of PDK-1 in response to angiotensin II: role in mesangial cell hypertrophy and fibronectin expression. J Biol Chem. 2008;283:24061–24076. doi: 10.1074/jbc.M803964200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Clempus RE, Sorescu D, Dikalova AE, et al. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2007;27:42–48. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gorin Y, Block K, Hernandez J, et al. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem. 2005;280:39616–39626. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]
- 100.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci USA. 2010;107:15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang M, Brewer AC, Schröder K, et al. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci USA. 2010;107:18121–18126. doi: 10.1073/pnas.1009700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Infanger DW, Cao X, Butler SD, et al. Silencing nox4 in the paraventricular nucleus improves myocardial infarction-induced cardiac dysfunction by attenuating sympathoexcitation and periinfarct apoptosis. Circ Res. 2010;106:1763–1774. doi: 10.1161/CIRCRESAHA.109.213025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bánfi B, Molnár G, Maturana A, et al. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem. 2001;276:37594–37601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- 104.Banfi B, Tirone F, Durussel I, et al. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5) J Biol Chem. 2004;279:18583–18591. doi: 10.1074/jbc.M310268200. [DOI] [PubMed] [Google Scholar]
- 105.Kawahara T, Lambeth JD. Phosphatidylinositol (4,5)-bisphosphate modulates Nox5 localization via an N-terminal polybasic region. Mol Biol Cell. 2008;19:4020–4031. doi: 10.1091/mbc.E07-12-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.El Jamali, Valente AJ, Lechleiter JD, et al. Novel redox-dependent regulation of NOX5 by the tyrosine kinase c-Abl. Free Radic Biol Med. 2008;44:868–881. doi: 10.1016/j.freeradbiomed.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jay DB, Papaharalambus CA, Seidel-Rogol B, Dikalova AE, Lassègue B, Griendling KK. Nox5 mediates PDGF-induced proliferation in human aortic smooth muscle cells. Free Radic Biol Med. 2008;45:329–335. doi: 10.1016/j.freeradbiomed.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brar SS, Corbin Z, Kennedy TP, et al. NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. Am J Physiol Cell Physiol. 2003;285:C353–369. doi: 10.1152/ajpcell.00525.2002. [DOI] [PubMed] [Google Scholar]
- 109.Kamiguti AS, Serrander L, Lin K, et al. Expression and activity of NOX5 in the circulating malignant B cells of hairy cell leukemia. J Immunol. 2005;175:8424–8430. doi: 10.4049/jimmunol.175.12.8424. [DOI] [PubMed] [Google Scholar]
- 110.Si J, Behar J, Wands J, et al. STAT5 mediates PAF-induced NADPH oxidase NOX5-S expression in Barrett’s esophageal adenocarcinoma cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G174–G183. doi: 10.1152/ajpgi.00291.2007. [DOI] [PubMed] [Google Scholar]
- 111.Dupuy C, Ohayon R, Valent A, Nöel-Hudson MS, Dème D, Virion A. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Cloning of the porcine and human cdnas. J Biol Chem. 1999;274:37265–37269. doi: 10.1074/jbc.274.52.37265. [DOI] [PubMed] [Google Scholar]
- 112.De Deken X, Wang D, Many MC, et al. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem. 2000;275:23227–23233. doi: 10.1074/jbc.M000916200. [DOI] [PubMed] [Google Scholar]
- 113.Moreno JC, Bikker H, Kempers MJ, et al. Inactivating mutations in the gene for thyroid oxidase 2 (THOX2) and congenital hypothyroidism. N Engl J Med. 2002;347:95–102. doi: 10.1056/NEJMoa012752. [DOI] [PubMed] [Google Scholar]
- 114.Vigone MC, Fugazzola L, Zamproni I, et al. Persistent mild hypothyroidism associated with novel sequence variants of the DUOX2 gene in two siblings. Hum Mutat. 2005;26:395. doi: 10.1002/humu.9372. [DOI] [PubMed] [Google Scholar]
- 115.Grasberger H, Refetoff S. Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J Biol Chem. 2006;281:18269–18272. doi: 10.1074/jbc.C600095200. [DOI] [PubMed] [Google Scholar]
- 116.Morand S, Ueyama T, Tsujibe S, Saito N, Korzeniowska A, Leto TL. Duox maturation factors form cell surface complexes with Duox affecting the specificity of reactive oxygen species generation. FASEB J. 2009;23:1205–1218. doi: 10.1096/fj.08-120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zamproni I, Grasberger H, Cortinovis F, et al. Biallelic inactivation of the dual oxidase maturation factor 2 (DUOXA2) gene as a novel cause of congenital hypothyroidism. J Clin Endocrinol Metab. 2008;93:605–610. doi: 10.1210/jc.2007-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rigutto S, Hoste C, Grasberger H, et al. Activation of dual oxidases Duox1 and Duox2: differential regulation mediated by camp-dependent protein kinase and protein kinase C-dependent phosphorylation. J Biol Chem. 2009;284:6725–6734. doi: 10.1074/jbc.M806893200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Edens WA, Sharling L, Cheng G, et al. Tyrosine cross-linking of extracellular matrix is catalyzed by Duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. J Cell Biol. 2001;154:879–891. doi: 10.1083/jcb.200103132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Geiszt M, Witta J, Baffi J, Lekstrom K, Leto TL. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 2003;17:1502–1504. doi: 10.1096/fj.02-1104fje. [DOI] [PubMed] [Google Scholar]
- 121.El Hassani RA, Benfares N, Caillou B, et al. Dual oxidase2 is expressed all along the digestive tract. Am J Physiol Gastrointest Liver Physiol. 2005;288:G933–G942. doi: 10.1152/ajpgi.00198.2004. [DOI] [PubMed] [Google Scholar]
- 122.Harper RW, Xu C, Eiserich JP, et al. Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Lett. 2005;579:4911–4917. doi: 10.1016/j.febslet.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 123.Luxen S, Belinsky SA, Knaus UG. Silencing of DUOX NADPH oxidases by promoter hypermethylation in lung cancer. Cancer Res. 2008;68:1037–1045. doi: 10.1158/0008-5472.CAN-07-5782. [DOI] [PubMed] [Google Scholar]
- 124.Heumüller S, Wind S, Barbosa-Sicard E, et al. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 125.Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(–) and systolic blood pressure in mice. Circ Res. 2001;89:408–414. doi: 10.1161/hh1701.096037. [DOI] [PubMed] [Google Scholar]
- 126.Borbély G, Szabadkai I, Horváth Z, et al. Small-molecule inhibitors of NADPH oxidase 4. J Med Chem. 2010;53:6758–6762. doi: 10.1021/jm1004368. [DOI] [PubMed] [Google Scholar]
- 127.Vendrov AE, Madamanchi NR, Niu XL, et al. NADPH oxidases regulate CD44 and hyaluronic acid expression in thrombin-treated vascular smooth muscle cells and in atherosclerosis. J Biol Chem. 2010;285:26545–26557. doi: 10.1074/jbc.M110.143917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Laleu B, Gaggini F, Orchard M, et al. First in class, potent, and orally bioavailable NADPH oxidase isoform 4 (Nox4) inhibitors for the treatment of idiopathic pulmonary fibrosis. J Med Chem. 2010;53:7715–7730. doi: 10.1021/jm100773e. [DOI] [PubMed] [Google Scholar]