Abstract
Background
Patients with heart failure (HF) experience depressive symptoms which contribute to poorer outcomes. We tested the effects of a brief cognitive therapy intervention on depressive symptoms, negative thinking, health-related quality of life, and cardiac event-free survival.
Methods and Results
Hospitalized patients with depressive symptoms (N=41, 66±11 yrs, 45% female, 81% NYHA Class III/IV) were randomly assigned to control group or a brief, nurse-delivered cognitive therapy intervention, delivered during hospitalization and followed by a one week booster phone call. Depressive symptoms, negative thinking and health-related quality of life were measured at one week and three months. Cardiac event-free survival was assessed at three months. Mixed models repeated measures ANOVA, Kaplan-Meier, and Cox regression were used for data analysis.
Results
There were significant improvements in depressive symptoms and health-related quality of life in both groups but no interactions between group and time. The control group had shorter three-month cardiac event-free survival (40% vs 80%, p<.05) and a 3.5 greater hazard of experiencing a cardiac event (p=.04) than the intervention group.
Conclusion
Nurses can deliver a brief intervention to hospitalized patients with HF that may improve short-term event-free survival. Future research is needed to verify these results with a larger sample size.
Keywords: Cardiovascular, Depression, Cognitive behavioral therapy
Introduction
The purpose of this study was to test the short-term effects of a brief cognitive therapy (CT) intervention for hospitalized patients with HF on depressive symptoms and health outcomes. One-third of hospitalized patients with heart failure (HF) suffer from major depressive disorder.1 The adverse effects of major depressive disorder and depressive symptoms on mortality and morbidity in patients with HF are well documented2–6—patients with HF and depressive symptoms are twice as likely to die or experience a cardiac event compared to HF patients without depressive symptoms.5 Furthermore, a dose-response relationship exists between depressive symptoms and long-term survival in patients with HF. In a 7-year follow-up of patients with HF, those with depressive symptoms at baseline were more likely to die than those without depressive symptoms, and the risk increased by level of depressive symptoms: mild (21%), moderate (53%), and severe depressive symptoms (83%).4 Furthermore, depressive symptoms have a negative impact on health-related quality of life (HRQOL).6
The most recent guidelines for HF management from the American College of Cardiology and the American Heart Association provide little direction for the treatment of depression in patients with HF. The guidelines state, “Good evidence exists for the critical importance of delivering comprehensive supportive care to these patients, including the assessment and treatment of… depression.”7 The lack of specificity in these guidelines reflects the state of the science regarding the treatment of depression in patients with HF. There has been insufficient research on treatments for depressive symptoms in patients with HF.8 Furthermore, in 2013, the Centers for Medicare and Medicaid Services will begin withholding reimbursement for patient with HF readmitted within 30 days.9 Given the increased risk of hospital readmission among patients with HF who have depressive symptoms, as well as the impact of depressive symptoms on health-related quality of life, it is imperative that researchers test interventions for the treatment of depressive symptoms in hospitalized patients with HF.
Recent results from the SADHART-HF trial10 suggest that non-pharmacological interventions carried out by nurses may be effective for the treatment of depressive symptoms in patients with HF. In this randomized controlled trial, antidepressants did not result in significant improvement in depression compared to placebo. However, both the intervention and placebo groups received a nursing intervention, and both experienced considerable reductions in depressive symptoms. The nursing intervention consisted of 1-hour telephone counseling sessions delivered by a psychiatric nurse every other week for 16 weeks (Personal communication, C. O’Connor, March 3, 2010). The investigators suggested that the nursing intervention was so powerful that it obscured any effect of the antidepressant.
Negative thinking is a potential target for intervention in patients with HF. Aaron Beck first described negative thinking in 1967.11 In his cognitive model of depression, Beck theorized that negative thinking influences the emotional, behavioral, and somatic symptoms of depression. Negative thinking consists of automatic and persistent negative cognitions about the self, world, future, and interpersonal relationships.11, 12 In prior studies conducted by our team, negative thinking was a risk factor for depressive symptoms in patients with HF 13 and worsened the depressed mood.14
Cognitive therapy (CT) is a psychotherapeutic intervention based on the cognitive model, and has been used successfully to treat depression in multiple populations.15, 16 In particular, brief CT interventions focused on thought-stopping and affirmations reduced depressive symptoms in women at risk for depression.17, 18 In a qualitative study, patients with HF and depressive symptoms described managing their depressive symptoms using similar cognitive techniques, including a refusal to dwell on negative thoughts and the replacement of negative thoughts with positive affirmations.14
Based on our preliminary work, we proposed that a brief CT intervention, focused on reducing negative thinking using thought-stopping and affirmations, would be a clinically feasible intervention for delivery by nurses during hospitalization. The purpose of this preliminary study was to test the short-term effects of a brief CT intervention for hospitalized patients with HF and depressive symptoms on depressive symptoms and health outcomes. We hypothesized that patients who received the brief nurse-delivered CT intervention would experience lower levels of depressive symptoms at one week and three months compared to a control group, as well as lower levels of negative thinking, improved HRQOL, and longer cardiac-event free survival.
Methods
Design
A 2-group randomized controlled trial with repeated measures was used to determine the short-term effects of the brief CT intervention. We followed the CONSORT (Consolidated Standards of Reporting Trials) guidelines for the reporting of clinical trials.19 Institutional Review Board approval was obtained, and all patients provided informed consent. Enrollment began February 2009 and ended December 2009; follow-up was completed in March 2010.
A sample size of 21 patients per group was determined by an a priori power analysis estimate for depressive symptoms (the primary outcome) in a two-group univariate repeated measures ANOVA algorithm with Greenhouse-Geisser correction.20 A large effect size was chosen based on data from a prior study in which a large group difference in the short-term effect of a similar brief intervention was reported.21 With 21 subjects per group and an alpha level of .05, the power of the ANOVA F tests would be at least 90% to detect a main effect for group, a main effect for time, and the group by time interaction. The large effect size in this context is one such that the ratio of the standard deviation of the group means to the standard deviation of the observations within the populations is at least 0.4.22 In addition, it was also assumed that observations from the same individual would have at least a modest level of correlation over time (r = .3).
Sample
Patients age 21 and older who were hospitalized with a primary or secondary diagnosis of HF at two regional hospitals in Lexington, KY, were screened for study eligibility. Patients were candidates for inclusion if they had a confirmed diagnosis of preserved or non-preserved systolic function HF. We excluded patients with (a) co-existing terminal illness, (b) end-stage HF (defined as mechanical pump support, continuous at home inotropic infusions, referral for heart transplant, or hospice care), (c) cognitive impairment (determined via chart review or nursing report of cognitive status), and (d) self-reported severe depression or suicidal ideation.
Eligible patients were approached on the second day of hospitalization or later. Recruitment was delayed if the patient was experiencing hypotension (blood pressure <80 mm Hg), a pain level of ≥ 5 on the 0–10 pain scale, or admission to an intensive care unit. Patients who agreed to participate and provided written consent were screened for depressive symptoms with the Beck Depression Inventory version II (BDI-II). Those patients with mild to moderate symptoms of depression (BDI-II score 10–28) were enrolled in the trial. Patients without depressive symptoms or those with severe depressive symptoms ended participation after the baseline assessment. Two items on the BDI-II related to low energy and fatigue were not included in the upper cut-off score because patients admitted to the hospital with HF often report HF-related fatigue.23
Randomization
Random allocation was conducted using a true random number generator.24, 25 Group assignments were placed in sealed, numbered envelopes. The investigators and any research staff were blinded to the random allocation sequence. After the patient was enrolled and completed the baseline assessment, the baseline BDI-II score was calculated. If the BDI-II score fell within the mild-moderate depressive symptom range, the next consecutive envelope was opened to determine group assignment. The patients and the principal investigator (R.L.D.) were not blinded to group assignment; however, the co-authors were blinded to patient assignment.
Procedure
After written informed consent was obtained, baseline data collection took place at the patient’s bedside. Privacy was maintained throughout data collection by allowing patients to determine which family members or significant others they would like to have in the hospital room during study procedures. A medical record review was also conducted to obtain clinical and sociodemographic variables.
Both groups completed baseline questionnaires to measure depressive symptoms, negative thinking, and HRQOL. The questionnaires were read out loud for patients who needed assistance due to vision impairment, fatigue, or difficulty reading. Patients with severe depressive symptoms were encouraged to talk to their healthcare provider about their depressive symptoms, and the staff nurse, charge nurse, attending physician, and primary care physician were notified of the patient’s high level of depressive symptoms. Patients in the intervention group received the intervention prior to discharge, at a time convenient to the patient. Patients in the control group received usual care.
One week after discharge, all patients received a phone call reminder to complete and return the one-week questionnaires by mail. Patients who needed assistance were given the option of having the questionnaires read out loud over the phone. At three months, patients completed the questionnaires again by phone or mail, and patients were asked about hospitalizations and emergency department visits that occurred since the baseline assessment.
Intervention
The intervention consisted of a single, 30-minute, one-on-one CT session that took place in the hospital and a 5–10 minute telephone booster one week post-discharge. See Box 1 for an outline of the 30-minute CT session. Both the brief CT session and booster were delivered by the first author, a Master’s-prepared adult health clinical nurse specialist and advanced practice nurse, in collaboration with a PhD-prepared psychiatric clinical nurse specialist (A.R.P.), who had tested a similar intervention with women at risk for depression in randomized controlled trials.17, 18 Previous qualitative work guided the design of the intervention.14 A colorful, 13 page flip chart with photos was used to guide the written script of the intervention. Patients also received a color booklet with the content of the intervention to take home.
Box 1. Summary of the six steps of the brief cognitive therapy intervention.
| Step 1: Depression and heart failure |
| Description of symptoms of depression Depression is common in people with heart failure Bad news: Depression is bad for the heart Good news: There are treatments for depression Discussion question: “Do any of these symptoms of depression sound familiar to you?” |
| Step 2: Thoughts, feelings, and behaviors |
| The connection between thoughts, feelings and behaviors Thoughts can be false and negative, and these thoughts contribute to painful feelings and negative behaviors of depression Two real-life patient stories are told that demonstrate the link between a stressful situation and negative thinking, feelings, and behaviors Discussion question: “Do these stories sound familiar to you? Do you see how the person’s negative thinking led to painful feelings and negative behaviors?” |
| Step 3: Your story |
|
Interactive component: Patient is asked to describe a recent stressful situation Discussion questions: “What kind of thoughts were going through your head? How did these thoughts make you feel? Did you notice any behaviors?” |
| Step 4: Thought-stopping |
| Notice you have a negative thought Snap your fingers or clap your hands and say, “Stop!” By stopping the negative thought you help take away the painful feelings that go with the thought Interactive component: Patient practices the stop technique with the nurse |
| Step 5: Affirmations |
| An affirmation is a short, simple phrase that states how life is at its very best Use first person, present tense, and positive words; if you have a religious faith you may use it in your affirmation Interactive component: The patient comes up with 3–4 affirmations with the help of the nurse The nurse writes the affirmations on 2 brightly colored sticky notes: one for the hospital room and one for the patient to take home |
| Step 6: Homework |
| Practice the stop technique every time you hear a negative thought Hang the list of affirmations somewhere you will see it every day For every negative thought, think at least 2 positive thoughts |
The script of the intervention followed six simple steps focused on reducing negative thinking using thought-stopping and affirmations. One week post-discharge, patients in the intervention group received a booster phone call session. During the booster, the nurse talked with patients about negative thoughts they had experienced and about their experiences with the techniques of thought-stopping and affirmations. The nurse reinforced the techniques and reminded patients of the importance of using thought-stopping and affirmations.
Usual care
Patients in the control group received usual care which consisted of standard evidence-based care for HF according to the American Heart Association/American College of Cardiology 2005 guidelines7 and the Joint Commission of Healthcare Organizations.26 All patients received individual discharge teaching about HF care which included brief written instructions about the emotional consequences of living with HF. Patients were offered the assistance of a chaplain if desired. Patients in the control group also received 40–60 minutes of continuous attention during recruitment, the informed consent process, and the baseline assessment from the same nurse who delivered the intervention to the intervention group. However, although these patients received 40–60 minutes of attention from a nurse, we feel that this group can still be considered a “true” usual care group—the patients in this group did not receive any additional attention other than what would be expected during the enrollment process of a research study.
Outcome measures
Depressive symptoms
The primary endpoint was depressive symptoms, which were defined as self-reported symptoms that exist with or without the presence of major depression.27 The Beck Depression Inventory version II (BDI-II) was used to measure depressive symptoms at baseline, one week post-discharge, and three months after initiation of the intervention. The BDI-II contains 21 items. Total scores range from 0–63; a score of 0–13 indicates none to minimal depressive symptoms, 14 to 28 indicates mild to moderate symptoms, and 29–63 indicates severe symptoms.28 The reliability and validity of the BDI-II has been supported in both medical and non-medical populations.29, 30
Negative thinking
Negative thinking was defined as negative cognitions about the self, world, future, and withdrawal from others, and was measured using the Crandell Cognitions Inventory (CCI).12 The CCI is a 45-item scale that consists of 34 negative items and 11 positive, non-scored, buffer items. Patients were asked to self-report how frequently they experienced each thought. Total scores were calculated by summing the item responses to the 34 negative items; possible scores range from 0 to 170. Higher scores indicate a greater frequency of negative thinking. The reliability and validity of the CCI were supported in psychiatric patients, healthy controls,12 and patients with HF31
Health-related quality of life
Health-related quality of life (HRQOL) is characterized by patient’s self-report of how HF has impacted the following domains of daily life: physical functioning, emotional status, health perception, and symptom burden. The Minnesota Living with HF Questionnaire (MLHFQ), a heart failure-specific questionnaire, was used to measure HRQOL. This 21-item instrument has strong evidence supporting reliability and validity in patients with HF.32 Patients responded to items with the stem question: “Did your HF prevent you from living as you wanted during the past month by.” The statements are rated from 0 (no impact) to 5 (most negative impact). Total scores range from 0 to 105. Higher scores indicate worse HRQOL.
Event-free survival
Three-month cardiac event-free survival was defined as time to a combined end point of cardiovascular mortality, cardiovascular hospitalization, or cardiovascular emergency department (ED) visit. A written protocol for event coding was developed by the investigators prior to enrollment. Dates and reasons for death, hospitalizations, and ED visits were determined by medical record review and patient interview. A cardiovascular rehospitalization or ED visit was pre-defined as an unanticipated admission for the following reasons: HF exacerbation, shortness of breath, cardiovascular-related chest pain, myocardial infarction, syncope, dysrhythmias (including internal cardiac defibrillator [ICD] firing), ICD or biventricular pacemaker placement due to worsening HF, coronary artery disease, hypertension, and palpitations. All hospitalizations and ED visits were verified with hospital records; dates and causes of death were determined by hospital records. Events were initially coded by the PI, who was not blinded to group assignment. The data coding was then examined independently by two co-authors, who were blinded to group assignment. Any discrepancies or disagreements were resolved by the 3 investigators together, in order to come to a final coding decision.
Demographic and clinical characteristics
To completely characterize the sample and describe potential confounding variables, the following demographic information was collected prior to randomization via patient interview: age, sex, race/ethnicity, marital status, education level, and months since HF diagnosis. The following clinical characteristics were determined by medical record review by the PI who was not blinded to randomization status: height, weight, smoking status, most recent left ventricular ejection fraction, comorbidities, laboratory values on admission, and medications at discharge (i.e. drugs, doses, frequencies).
New York Heart Association (NYHA) functional class was determined to further characterize the sample. NYHA functional class is a subjective indicator of functional status and was determined by patient interview at baseline and three months. Patients were assigned a classification of I (ordinary physical activity causes no symptoms of fatigue, dyspnea, angina or palpitations), II (symptoms with ordinary physical activity), III (symptoms occur with less than ordinary physical activity) or IV (symptoms occur even at rest).33
Data analysis
Data analysis was conducted using SPSS v. 18 (SPSS Inc, Chicago). Demographic and clinical differences between the groups were assessed using t-tests or chi-square tests of association. The longitudinal effects of the intervention on depressive symptoms, negative thinking, and health-related quality of life were tested with repeated measures analysis of variance models suitable for mixed models. Time, treatment, and the interaction between time and treatment were entered as fixed effects; subject was included as a random effect. This method is appropriate for use in longitudinal studies where missing data occur34 and allowed us to use data from all subjects, thus employing a conservative intent-to-treat convention. We specified a covariance structure based on the spatial power law which assumes decreasing pairwise correlation with increasing time between time periods. Fisher’s least significant difference procedure was used for post-hoc comparisons. For the mixed model analyses, p-values of .01 or less were deemed significant, as a multiple comparisons adjustment to the usual .05 value.
Cardiac event-free survival was calculated using Kaplan-Meier survival curves with the log-rank test. We also conducted a Cox proportional hazard regression to determine the hazard ratio, controlling for baseline depressive symptoms. Differences in the median number of cardiac events between groups were determined with the independent samples Mann-Whitney U test. A p-value of less than .05 was considered significant.
Results
Sample characteristics
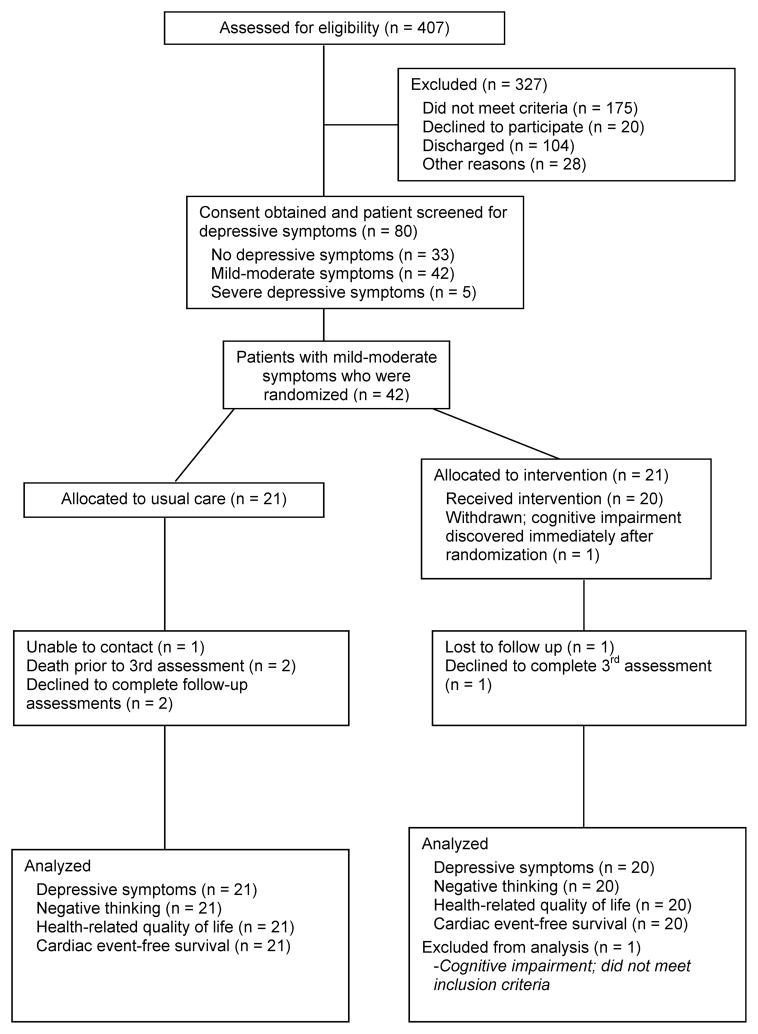
The CONSORT flow diagram is provided in Figure 1. Of the 407 patients who were screened for eligibility, 203 were excluded. The most common reasons for exclusion were cognitive impairment (n = 95), co-existing terminal illness (n = 34), and end-stage heart failure (n = 17). An additional 104 eligible patients were discharged before they could be approached, and 20 declined to participate.
Figure 1.
Study flow diagram
Eighty patients provided informed consent and were screened for depressive symptoms. Of these 80 patients, 42 (53%) had mild-moderate depressive symptoms and were retained in the study, while 33 patients (41%) did not have depressive symptoms and 5 patients (6%) with severe depressive symptoms were excluded. One patient was withdrawn after randomization to the intervention group because of cognitive impairment that was not detected until after the baseline assessment.
The characteristics of the final sample are provided in Table 1. The mean depressive symptom score was 16.7 ± 6.7. One-third of the patients had a history of depression noted in the medical record, 36% were prescribed antidepressants on hospital admission, 41% were prescribed antidepressants at hospital discharge, and 36% self-reported taking antidepressants three months later. There were no differences between the two groups with regard to the proportion of patients who were taking antidepressants at hospital admission, discharge, and 3 months after enrollment.
Table 1.
Baseline characteristics of entire sample and patients randomly assigned to the brief cognitive therapy intervention versus control*
| Characteristic | Overall sample (N = 41) | Intervention group (N = 20) | Control group (N = 21) | p value |
|---|---|---|---|---|
| Female | 19 (45%) | 10 (48%) | 9 (43%) | .8 |
| Age | 66 ± 11 | 68 ± 10 | 64 ± 12 | .2 |
| Married | 24 (57%) | 14 (67%) | 10 (48%) | .2 |
| Minority | 4 (10%) | 2 (10%) | 2 (10%) | 1.0 |
| Education level | ||||
| Less than high school | 15 (36%) | 10 (48%) | 5 (25%) | .3 |
| High school graduate | 12 (29%) | 6 (30%) | 6 (30%) | |
| Some college or greater | 14 (33%) | 5 (24%) | 9 (45%) | |
| Employment status | ||||
| Employed | 3 (7%) | 2 (10%) | 1 (5%) | .5 |
| Sick leave or disability | 15 (36%) | 9 (43%) | 6 (30%) | |
| Retired | 21 (50%) | 10 (48%) | 13 (65%) | |
| Body Mass Index | 31± 8.6 | 28.2 ± 6.4 | 33.8 ± 9.7 | .03 |
| NYHA functional class | ||||
| Class II | 8 (19%) | 6 (29%) | 2 (10%) | .08 |
| Class III | 31 (74%) | 15 (71%) | 16 (76%) | |
| Class IV | 3 (7%) | 0 (0%) | 3 (14%) | |
| Left ventricular ejection fraction | 39.5± 16.4 | 40.8 ± 17 | 38.1 ± 16.1 | .6 |
| Etiology of HF | ||||
| Ischemic | 26 (62%) | 13 (62%) | 13 (62%) | .5 |
| Hypertensive | 8 (19%) | 4 (19%) | 4 (19%) | |
| Idiopathic | 6 (14%) | 2 (10%) | 4 (19%) | |
| Other | 2 (10%) | 2 (10%) | 0 (0%) | |
| Smoking status | ||||
| Never smoked | 17 (41%) | 7 (33%) | 10 (50%) | .4 |
| Former smoker | 14 (33%) | 9 (43%) | 8 (40%) | |
| Current smoker | 7 (17%) | 5 (24%) | 2 (10%) | |
| Second hand smoke exposure | 12 (29%) | 6 (29%) | 6 (29%) | .6 |
| Months since diagnosed with HF | 39 ± 56 | 45 ± 68 | 32 ± 37 | .4 |
| Newly diagnosed with HF in the past month | 13 (31%) | 8 (38%) | 5 (24%) | .3 |
| History of depression | 14 (33%) | 9 (43%) | 5 (24%) | .2 |
| History of anxiety | 13 (31%) | 8 (38%) | 5 (24%) | .3 |
| Comorbidities | ||||
| Prior myocardial infarction | 17 (41%) | 8 (38%) | 9 (43%) | .8 |
| Atrial fibrillation | 19 (45%) | 8 (38%) | 11 (52%) | .4 |
| CABG surgery | 12 (29%) | 10 (48%) | 2 (9.5%) | .006 |
| Biventricular pacemaker | 17 (41%) | 8 (38%) | 9 (43%) | .8 |
| Implanted cardiac defibrillator | 19 (45%) | 8 (38%) | 11 (52%) | .4 |
| Prior stroke | 8 (19%) | 4 (19%) | 4 (19%) | 1.0 |
| Chronic obstructive pulmonary disease | 17 (41%) | 12 (57%) | 5 (25%) | .04 |
| Diabetes | 19 (45%) | 9 (43%) | 10 (48%) | .8 |
| Renal dysfunction | 16 (38%) | 9 (43%) | 7 (33%) | .5 |
| Medications on discharge | ||||
| ACE inhibitor | 19 (45%) | 10 (48%) | 13 (62%) | .4 |
| Antiogensin receptor blocker | 6 (14%) | 3 (14%) | 3 (14%) | 1.0 |
| Beta blocker | 36 (86%) | 17 (81%) | 19 (91%) | .4 |
| Digoxin | 17 (41%) | 9 (43%) | 8 (38%) | .8 |
| Diuretic | 26 (62%) | 16 (76%) | 10 (48%) | .06 |
| Antidepressant use | ||||
| Hospital admission | 15 (36%) | 7 (33%) | 8 (38%) | .7 |
| Hospital discharge | 17 (41%) | 8 (38%) | 9 (43%) | .8 |
| 3 months after enrollment | 15 (37%) | 8 (44%) | 7 (39%) | .7 |
| Laboratory values on admission | ||||
| B-type natriuretic peptide | 746 ± 914 | 963 ± 1171 | 529 ± 496 | .2 |
| BUN | 26.2 ± 18.9 | 31.7 ± 23.6 | 20.5 ± 9.8 | .06 |
| Creatinine | 1.3 ± 1.2 | 1.5 ± 1.6 | 1.1 ± 0.43 | .3 |
| Sodium | 138.5 ± 6.2 | 138 ± 5.3 | 139 ± 7.2 | .7 |
| Beck Depression Inventory-II | 16.7 ± 6.7 | 15.8 ± 6.5 | 17.6 ± 6.7 | .4 |
| Minnesota Living with Heart Failure Questionnaire | 54 ± 25 | 50 ± 25 | 58± 25 | .3 |
| Crandell Cognitions Inventory | 61 ± 19 | 59 ± 19 | 64 ± 18 | .5 |
Data are given as n (%) or mean ± standard deviation unless otherwise indicated.
Differences between groups were assessed using the independent t-test or Pearson chi-square, as appropriate to the level of measurement
There were no differences at baseline between intervention and control groups with regard to sex, age, marital status, and several clinical variables including NYHA Class, ejection fraction, and medications (see Table 1). Patients in the control group had a higher mean body mass index, and patients in the intervention group were more likely to have prior coronary bypass graft surgery and comorbid chronic pulmonary obstructive disease. There were no baseline differences between groups on depressive symptoms, negative thinking, and HRQOL.
Of the 41 patients, 16 (40%) experienced at least one cardiovascular event during the three month follow-up period. Table 2 displays the number and characteristics of first cardiovascular events among the two groups. Thirteen patients had a first event of a HF or cardiovascular hospitalization, and three patients had a first event of an ED visit for HF or cardiovascular reasons. There were only 2 deaths, both due to HF, and both of these deaths occurred in the control group after re-admission to the hospital. The intervention group did not experience any adverse events related to study procedures.
Table 2.
Description of first cardiovascular events during the 3 month follow-up period
| Intervention group (n = 20) | Control group (n = 21) | Mantel-haenszel odds ratio estimate | 95% Confidence interval | P value | |
|---|---|---|---|---|---|
| Cardiovascular hospitalization | Total = 4 (20%) 3 HF exacerbations, 1 other cardiovascular |
Total = 9 (43%) 3 HF exacerbations, 5 other cardiovascular |
3.0 | 0.7 – 12.1 | 0.1 |
| Cardiovascular emergency department visit | Total = 0 (0%) | Total = 3 (14%) 1 HF exacerbation, 2 other cardiovascular |
N/A | N/A | N/A |
| Alive without any cardiovascular events | Total = 16 (80%) | Total = 9 (43%) | 0.2 | 0.1 – 0.8 | .04 |
The two HF deaths that occurred during the study (both in the control group) were not categorized as the first event, since they occurred during a HF hospitalization that was already counted as a first event.
Primary outcome
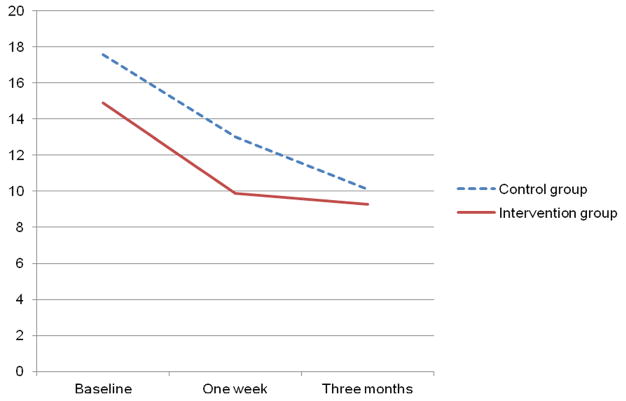
As shown in Table 3, BDI-II scores improved over time in both groups (p < .001); however, there were no significant differences between groups. Figure 3 suggests a trend for patients in the intervention group to have a faster decline in depressive symptoms compared to patients in the control group, but this trend was not statistically significant.
Table 3.
Outcomes for depressive symptoms, negative thinking, and health-related quality of life
| Least-Squares Mean (SE) | Group | Time | Group x Time | |||||
|---|---|---|---|---|---|---|---|---|
| Measure | Brief CT Intervention | Control | F test | p value | F test | p value | F test | p value |
| Beck Depression Inventory-II (N = 41)
| ||||||||
| Baseline | 14.9 (1.6) | 17.6 (1.5) | ||||||
| 1 week | 9.9 (1.6) | 13 (1.6) | 1.4 | .24 | 14.5 | < .0001 | .6 | .56 |
| 3 months | 9.3 (1.6) | 10.1 (1.7) | ||||||
|
| ||||||||
| Crandell Cognitions Inventory (N = 41)
| ||||||||
| Baseline | 60 (4.7) | 64 (4.7) | ||||||
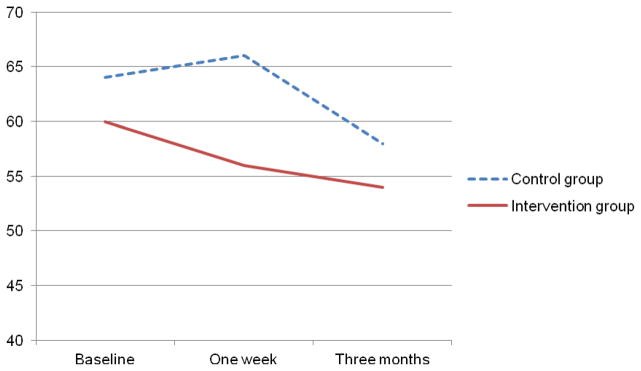
| 1 week | 56 (4.9) | 66 (4.8) | 1.2 | .28 | 1.6 | .22 | 1.1 | .35 |
| 3 months | 54 (5.0) | 58 (5.2) | ||||||
| Minnesota Living with Heart Failure Questionnaire (N = 41) | ||||||||
| Baseline | 52 (5) | 58 (5) | ||||||
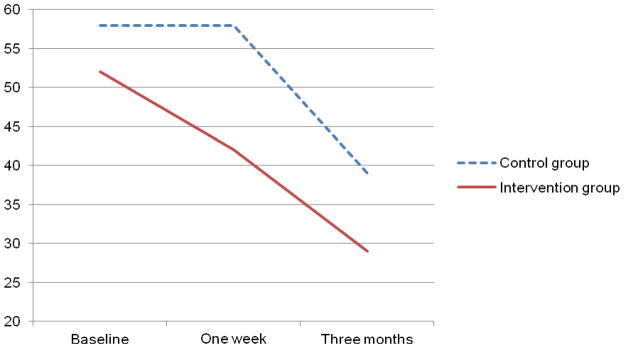
| 1 week | 42 (6) | 58 (5) | 3.3 | .08 | 9.4 | <.001 | 0.8 | .45 |
| 3 months | 29 (6) | 39 (6) | ||||||
Figure 3.
Change in depressive symptoms over time in both groups (N = 41)
Secondary outcomes
Table 3 presents the data on negative thinking and HRQOL. There were no significant changes in negative thinking over time in either group. Both groups experienced significant improvements in HRQOL, but there were no significant group by time interactions. Figures 4 and 5 appear to show trends toward faster decrease in negative thinking and improvement in HRQOL in the intervention group, but these trends were not significant.
Figure 4.
Changes in negative thinking over time in patients with heart failure (N = 41)
Figure 5.
Changes in health-related quality of life over time in patients with heart failure (N = 41)
Patients in the intervention group had longer cardiac event-free survival compared to the control group (Figure 2). At three months, 80% of patients in the intervention group were alive without a cardiac event, compared to 40% of patients in the control group (p = .048). The median number of cardiovascular events (both hospitalizations and ED visits) over the 3-month period was higher in the control group (median = 1 [25th% = 0, 75th% = 1]) compared to the intervention group (median = 0 [25th% = 0, 75th% = 0], p = .017). After controlling for baseline depressive symptoms, patients in the control group had a 3.5 times greater hazard of experiencing a cardiac event (p = .04, 95% Confidence Interval 1.1 – 11.1) compared to the intervention group (Table 4).
Figure 2.
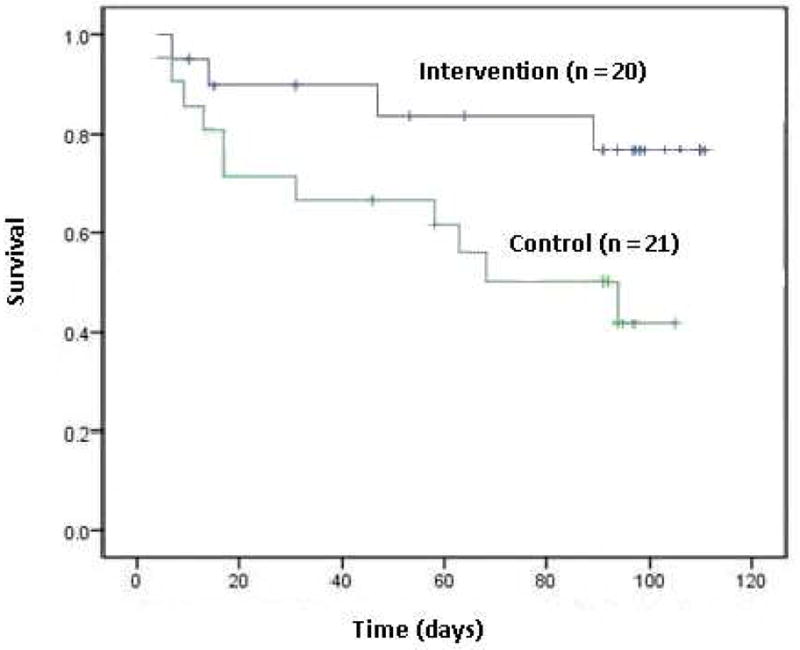
Kaplan-Meier cardiac event-free survival curve for patients with heart failure (N = 41; log rank test p = .048).
Table 4.
Final Cox Regression Model
| Variables | β | Exp (β) | 95% Confidence Interval for Exp (β) | P value |
|---|---|---|---|---|
| Control group | 1.242 | 3.463 | 1.1 – 11.1 | .037 |
| Depressive symptoms (Beck Depression Inventory-II) | .016 | 1.017 | 0.9 – 1.1 | .676 |
Discussion
Researchers have established that depressive symptoms contribute to an increased risk of hospital readmissions in patients with HF.5 The results of this preliminary study suggest that a brief, clinically feasible CT intervention delivered by a nurse may impact cardiac event-free survival in patients with HF. In our study, this survival advantage appeared within the first two weeks after hospital discharge and remained significant across the 3-month follow-up period. If our results can be replicated in a larger randomized controlled trial, this nurse-delivered intervention may be an innovative way to approach the problem of hospital readmissions among patients with HF and depressive symptoms.
Our major finding that a brief CT intervention may improve event free survival is promising, but a lack of differences in depressive symptoms and other endpoints makes it difficult to elucidate the mechanism. It is possible that a larger sample size may have revealed significant improvement in depressive symptoms that would explain the improved event-free survival. Nonetheless, it is unclear at present whether the intervention was the underlying mechanism for the better event-free survival. Therefore, we are conducting a study (Helping Others toward Positive Emotions in Patients with Heart Failure [HOPE-HF]) that is testing the same intervention in a larger sample and will be utilizing biomarkers to provide greater understanding regarding potential underlying mechanisms—including b-type natriuretic peptide, c-reactive protein, inflammatory cytokines, and salivary cortisol (Clinicaltrials.gov identifier: NCT01275742).
One potential mechanism for improved outcomes in the intervention group may be the verbal disclosure of depressive symptoms that occurred during the nurse-delivered intervention. Researchers have demonstrated that shared, written disclosure improves emotional symptoms and physical outcomes in healthy adults,35 while results from a meta-analysis suggest that patients with chronic health conditions who write emotional disclosures experience improved health outcomes.36 In our study, we informally observed that many patients in the intervention group stated that this was the first time that he or she had ever talked with a healthcare provider (or in some cases, anyone at all) about their depressive symptoms.
In the SADHART-HF study, O’Connor et al.10 found that patients with HF who received sertraline or placebo both experienced considerable reductions in depressive symptoms over a 12-week follow-up period. However, both groups received intensive nurse-facilitated support designed to develop rapport, build trust, encourage adherence, and monitor depressive symptoms. The results of our study, in light of the findings of the SADHART-HF investigators, suggest that future research is needed to determine what dosage and type of nursing support for depression can improve outcomes in patients with HF.
Other investigators have not observed a survival advantage when testing CT in a much larger sample of patients with cardiovascular disease. The ENRICHD37 investigators tested the effects of CT on survival in 2,481 patients with cardiovascular disease who had recently experienced a myocardial infarction and were diagnosed with clinical depression or self-reported poor social support. Although patients in the intervention group experienced a significant reduction in depressive symptoms as compared to the control group, there were no differences in cardiac event-free survival between groups.
However, our study differs from the ENRICHD study with regard to sample characteristics, timing of the intervention, and length of follow-up. The ENRICHD investigators recruited patients who had recently experienced a myocardial infarction, while ours was a population of patients with HF. The ENRICHD investigators initiated the intervention several weeks after hospital discharge, while we delivered the intervention prior to hospital discharge, before the patients could have any index cardiac events. Finally, the ENRICHD investigators followed patients for an average of 29 months, while we measured cardiac event-free survival for three months.
Although the 3-month event-free survival outcome is a comparatively brief time period, we feel that 3-month window is important for several reasons. First, patients with HF who are newly discharged from the hospital are highly vulnerable to readmission because of multiple risk factors, including un-addressed emotional distress.38 Second, both 30-day mortality and morbidity rates after HF hospital discharge are high (13% and 22%, respectively).39 Third, by the year 2013, Medicare reimbursement will be denied for HF readmissions that take place within 30 days after discharge. Although a brief, 30-minute intervention might not be expected to have effects lasting longer than 3 months, we feel that it is still important to determine the effects of the brief CT intervention on longer-term cardiac event-free survival. Therefore, the current, ongoing HOPE-HF study was designed with a longer follow-up period.
Patients in our sample had a relatively low level of depressive symptoms at baseline which could explain why both groups experienced a large, natural improvement in depressive symptoms within one week after discharge from a HF hospitalization. Few researchers have described the natural trajectory of depressive symptoms in patients with HF. Koenig et al.40 found that although some patients with HF and depression experienced spontaneous remission within several months after hospital discharge, 36% and 52% of patients continue to have symptoms of mild and major depression, respectively. Koenig et al.40 also demonstrated that the primary predictor of shorter time to remission from depression in hospitalized patients with HF is lower levels of depressive symptoms. In our study, the mean level of depressive symptoms was 16, indicating mild depressive symptoms. This was partially due to the fact that we excluded patients with severe depressive symptoms. The low level of depressive symptoms in our study likely contributed to our inability to detect a difference between groups. Therefore, in the HOPE-HF study, we have chosen to evaluate the effects of the intervention on patients with higher levels of depressive symptoms (Patient Health Questionnaire-9 scores of 10 or greater), who are less likely to improve on their own.
Finally, our finding that patients in both groups experienced improvement in HRQOL after discharge is consistent with the findings of Riegel et al.,41 who demonstrated that HRQOL naturally improves by an average 13 points in the three months after hospital discharge. Riegel et al.41 also suggested that only high-intensity interventions have an effect on HRQOL as measured by the Minnesota Living with HF Questionnaire. A more intense dose of the CT intervention offered in this study may be needed to improve HRQOL after discharge. It is also possible that the Minnesota Living with Heart Failure Questionnaire is not sensitive to smaller changes in HRQOL as a result of a brief, low-intensity intervention.
Limitations
Several limitations should be noted. First, it is possible that the findings might be due to chance. The intervention group appeared to have somewhat less severe depressive symptoms and negative thinking at baseline, and thus the intervention group might have been at slightly lower risk than the control group. However, the two groups had statistically similar clinical and psychological characteristics at baseline, and we controlled for baseline depressive symptoms in the Cox regression. Nonetheless, the promising results of this study warrant replication with a larger sample size before definitive conclusions can be drawn. Second, to facilitate patient retention, the follow-up questionnaires were administered by the same nurse who also administered the intervention. However, several steps were taken to reduce the potential for social desirability or researcher bias. The nurse was blinded to group assignment during the first assessment, follow-up questionnaires were administered by mail when possible, and the nurse did not discuss the intervention with the patient during the follow-up assessments.
Conclusions
Our findings indicate that although depressive symptoms improved rapidly after discharge, patients benefited from a nurse-delivered, brief CT intervention while hospitalized for HF. This intervention is replicable, practical for the acute care setting, and may improve short-term cardiac event-free survival in patients with HF. The findings from this preliminary study have been used to design a larger randomized controlled trial that is powered to detect differences in both depressive symptoms and cardiac event-free survival, includes patients with more severe symptoms of depression, incorporates a longer follow-up period, follows a non-depressed comparison group, and measures possible biobehavioral mediators such as inflammatory cytokines. If the results of this preliminary study are replicable, we will demonstrate that a brief, 30-minute CT for depressive symptoms can be delivered by nurses in a cost-effective way to patients with HF who are at high risk for hospital readmission. The results could have a dramatic impact on the state of the science of depression in patients with HF by shifting researchers’ and clinicians’ focus from pharmacologic interventions toward nurse-delivered, non-pharmacological interventions.
Acknowledgments
This study would not have been possible without the support and collaboration of the administrators, nurses, physicians, and patients at the Central Baptist Heart and Vascular Institute and the Saint Joseph Heart Institute in Lexington, KY. In particular, the authors acknowledge Karen S. Hill, RN, DNP, Dorothy Y. Brockopp, PhD, RN, Debbie Griffith, RN, MN, and Aaron B. Hesselson, MD, FACC, for their support. We would also like to acknowledge Lynne A. Hall, DrPH, RN, for editing assistance, Mary K. Rayens, PhD, for assistance with data analysis, and Sherry Warden, PhD, RN, and Faika Zanjani, PhD, for contributions made on the first author’s dissertation committee.
Funding Sources: There are no industry relationships to disclose. This research was supported by a Philanthropic Educational Organization International Scholar Award. The intellectual contributions of the authors were supported by the University of Kentucky College of Nursing Center for Biobehavioral Research on Self-Management of Cardiopulmonary Disease, NIH, NINR, P20 NR010679. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health. This article was written in partial fulfillment of the requirements for the PhD degree in Nursing at the University of Kentucky.
Footnotes
Disclosures
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Koenig HG. Depression in hospitalized older patients with congestive heart failure. Gen Hosp Psychiatry. 1998;20(1):29–43. doi: 10.1016/s0163-8343(98)80001-7. [DOI] [PubMed] [Google Scholar]
- 2.Jiang W, Alexander J, Christopher E, Kuchibhatla M, Gaulden LH, Cuffe MS, et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med. 2001;161(15):1849–1856. doi: 10.1001/archinte.161.15.1849. [DOI] [PubMed] [Google Scholar]
- 3.Faris R, Purcell H, Henein MY, Coats AJ. Clinical depression is common and significantly associated with reduced survival in patients with non-ischaemic heart failure. Eur J Heart Fail. 2002;4(4):541–551. doi: 10.1016/s1388-9842(02)00101-0. [DOI] [PubMed] [Google Scholar]
- 4.Jiang W, Kuchibhatla M, Clary GL, Cuffe MS, Christopher EJ, Alexander JD, et al. Relationship between depressive symptoms and long-term mortality in patients with heart failure. Am Heart J. 2007;154(1):102–108. doi: 10.1016/j.ahj.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 5.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure: A meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48(8):1527–1537. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 6.Gottlieb SS, Khatta M, Friedmann E, Einbinder L, Katzen S, Baker B, et al. The influence of age, gender, and race on the prevalence of depression in heart failure patients. J Am Coll Cardiol. 2004;43(9):1542–1549. doi: 10.1016/j.jacc.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 7.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53(15):e1–e90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Lane DA, Chong AY, Lip GY. Psychological interventions for depression in heart failure. Cochrane Database Syst Rev. 2005;(1):CD003329. doi: 10.1002/14651858.CD003329.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Axon RN, Williams MV. Hospital readmission as an accountability measure. JAMA. 2011;305(5):504–505. doi: 10.1001/jama.2011.72. [DOI] [PubMed] [Google Scholar]
- 10.O’Connor CM, Jiang W, Kuchibhatla M, Silva SG, Cuffe MS, Callwood DD, et al. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56(9):692–699. doi: 10.1016/j.jacc.2010.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beck AT. Depression: Clinical, experimental, and theoretical aspects. New York: Harper & Row; 1967. [Google Scholar]
- 12.Crandell CJ, Chambless DL. The validation of an inventory for measuring depressive thoughts: the Crandell Cognitions Inventory. Behav Res Ther. 1986;24(4):403–411. doi: 10.1016/0005-7967(86)90005-7. [DOI] [PubMed] [Google Scholar]
- 13.Dekker RL, Lennie TA, Peden AR, Chung ML, Wu JR, Moser DK. Negative thinking: A modifiable target for the treatment of depressive symptoms in patients with heart failure. Circulation. 2008;118(S):976. [Google Scholar]
- 14.Dekker RL, Peden AR, Lennie TA, Schooler MP, Moser DK. Living with depressive symptoms: Patients with heart failure. Am J Crit Care. 2009;18(4):310–318. doi: 10.4037/ajcc2009672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck AT. The current state of cognitive therapy: A 40-year retrospective. Arch Gen Psychiatry. 2005;62(9):953–959. doi: 10.1001/archpsyc.62.9.953. [DOI] [PubMed] [Google Scholar]
- 16.Beck JS. Cognitive therapy: basics and beyond. New York: Guilford Press; 1995. [Google Scholar]
- 17.Peden AR, Hall LA, Rayens MK, Beebe LL. Reducing negative thinking and depressive symptoms in college women. J Nurs Scholarsh. 2000;32(2):145–151. doi: 10.1111/j.1547-5069.2000.00145.x. [DOI] [PubMed] [Google Scholar]
- 18.Peden AR, Rayens MK, Hall LA, Grant E. Testing an intervention to reduce negative thinking, depressive symptoms, and chronic stressors in low-income single mothers. J Nurs Scholarsh. 2005;37(3):268–274. doi: 10.1111/j.1547-5069.2005.00046.x. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. 2001;285(15):1987–1991. doi: 10.1001/jama.285.15.1987. [DOI] [PubMed] [Google Scholar]
- 20.nQuery Advisor: Statistical Solutions. [computer program]. Version 6.02. Boston: Sangus; 2005. [Google Scholar]
- 21.Thompson DR. A randomized controlled trial of in-hospital nursing support for first time myocardial infarction patients and their partners: Effects on anxiety and depression. J Adv Nurs. 1989;14(4):291–297. doi: 10.1111/j.1365-2648.1989.tb03416.x. [DOI] [PubMed] [Google Scholar]
- 22.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 23.Friedman MM, Griffin JA. Relationship of physical symptoms and physical functioning to depression in patients with heart failure. Heart Lung. 2001;30(2):98–104. doi: 10.1067/mhl.2001.114180. [DOI] [PubMed] [Google Scholar]
- 24.Haahr M. Random numbers. In: Salkind NJ, editor. Encyclopedia of Measurement and Statistics. Vol. 3. Thousand Oaks, CA: Sage; 2007. pp. 815–816. [Google Scholar]
- 25.Haahr M. [Accessed January 3, 2009.];Random.org: True random number service. Available at: www.random.org.
- 26.Specifications Manual for National Implementation of Hospital Quality Measures. Joint Commission on Accreditation of Healthcare Organizations; [Accessed January 21, 2009.]. Available at: http://www.jointcommission.org/performancemeasurement/performancemeasurement/current+nhqm+manual.htm. [Google Scholar]
- 27.Judd LL, Akiskal HS, Zeller PJ, Paulus M, Leon AC, Maser JD, et al. Psychosocial disability during the long-term course of unipolar major depressive disorder. Arch Gen Psychiatry. 2000;57(4):375–380. doi: 10.1001/archpsyc.57.4.375. [DOI] [PubMed] [Google Scholar]
- 28.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 29.Grothe KB, Dutton GR, Jones GN, Bodenlos J, Ancona M, Brantley PJ. Validation of the Beck Depression Inventory-II in a low-income African American sample of medical outpatients. Psychol Assess. 2005;17(1):110–114. doi: 10.1037/1040-3590.17.1.110. [DOI] [PubMed] [Google Scholar]
- 30.Whisman MA, Perez JE, Ramel W. Factor structure of the Beck Depression Inventory-Second Edition (BDI-II) in a student sample. J Clin Psychol. 2000;56(4):545–551. doi: 10.1002/(sici)1097-4679(200004)56:4<545::aid-jclp7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 31.Dekker RL, Lennie TA, Hall LA, Peden AR, Chung ML, Moser DK. Developing a shortened measure of negative thinking for use in patients with heart failure. Heart Lung. 2011;40(3):e60–9. doi: 10.1016/j.hrtlng.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heo S, Moser DK, Riegel B, Hall LA, Christman N. Testing the psychometric properties of the Minnesota Living with Heart Failure questionnaire. Nurs Res. 2005;54(4):265–272. doi: 10.1097/00006199-200507000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Mills RM, Jr, Haught WH. Evaluation of heart failure patients: Objective parameters to assess functional capacity. Clin Cardiol. 1996;19(6):455–460. doi: 10.1002/clc.4960190603. [DOI] [PubMed] [Google Scholar]
- 34.Littel RC, Milliken GA, Stroup WW, Wolfinger RD. SAS system for mixed models. Cary, NC: SAS Institute, Inc; 1996. [Google Scholar]
- 35.Radcliffe AM, Lumley MA, Kendall J, Stevenson JK, Beltran J. Written emotional disclosure: testing whether social disclosure matters. J Soc Clin Psychol. 2010;26(3):362–384. doi: 10.1521/jscp.2007.26.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frisina PG, Borod JC, Lepore SJ. A meta-analysis of the effects of written emotional disclosure on the health outcomes of clinical populations. J Nerv Ment Dis. 2004;192(9):629–634. doi: 10.1097/01.nmd.0000138317.30764.63. [DOI] [PubMed] [Google Scholar]
- 37.Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA. 2003;289(23):3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 38.Moser DK, Doering LV, Chung ML. Vulnerabilities of patients recovering from an exacerbation of chronic heart failure. Am Heart J. 2005;150(5):984. doi: 10.1016/j.ahj.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 39.Bueno H, Ross JS, Wang Y, Chen J, Vidán MT, Normand SL, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA. 2010;303(21):2141–2147. doi: 10.1001/jama.2010.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koenig HG. Depression outcome in inpatients with congestive heart failure. Arch Intern Med. 2006;166(9):991–996. doi: 10.1001/archinte.166.9.991. [DOI] [PubMed] [Google Scholar]
- 41.Riegel B, Moser DK, Glaser D, Carlson B, Deaton C, Armola R, et al. The Minnesota Living With Heart Failure Questionnaire: Sensitivity to differences and responsiveness to intervention intensity in a clinical population. Nurs Res. 2002;51(4):209–218. doi: 10.1097/00006199-200207000-00001. [DOI] [PubMed] [Google Scholar]