Abstract
Background
Among older persons, the association between frailty and spirometry-confirmed respiratory impairment has not yet been evaluated.
Methods
Using data on white participants aged 65–80 years (Cardiovascular Health Study, N=3,578), we evaluated cross-sectional and longitudinal associations between frailty and respiratory impairment, including their combined effect on mortality. Baseline assessments included frailty status (Fried-phenotype; non-frail, pre-frail, and frail) and spirometry. Outcomes included development of frailty features (pre-frail or frail) at Year-3 and respiratory impairment (airflow limitation or restrictive-pattern) at Year-4, and death (median follow-up, 13.2 years).
Results
At baseline, 48.3% were pre-frail, 5.8% were frail, 13.8% had airflow limitation, and 9.3% had restrictive-pattern; 46.1% subsequently died. At baseline, pre-frail and frail were cross-sectionally associated with airflow limitation—adjusted odds ratio (OR) (95% confidence interval): 1.62 (1.29, 2.04) and 1.88 (1.15, 3.09), and restrictive-pattern—adjusted OR: 1.80 (1.37, 2.36) and 3.05 (1.91, 4.88), respectively. Longitudinally, participants with baseline frailty features had an increased likelihood of developing respiratory impairment―adjusted OR: 1.42 (1.11, 1.82). Conversely, participants with baseline respiratory impairment had an increased likelihood of developing frailty features—adjusted OR: 1.58 (1.17, 2.13). Mortality was highest among participants who were frail and had respiratory impairment—adjusted hazard ratio: 3.91 (2.93, 5.22), relative to those who were non-frail and had no respiratory impairment.
Conclusion
Frailty and respiratory impairment are strongly associated with one another and substantially increase the risk of death when both are present. Establishing these associations may inform interventions designed to reverse or prevent the progression of either condition and to reduce adverse outcomes.
Keywords: frailty, respiratory impairment, death
INTRODUCTION
Frailty is a geriatric syndrome commonly characterized by the features of slow gait speed, low physical activity, exhaustion, reduced grip strength, or unintentional weight loss.1 Based on the 3-level Fried-phenotype, pre-frail is defined as having 1 or 2 of these features, frail as having 3 or more, while non-frail has none.1 Among community-living older persons aged ≥65-years, the prevalence of pre-frail and frail are 47% and 7%, respectively.1–3 Adverse outcomes associated with pre-frail and frail include falls, disability, institutionalization, and death.1 Importantly, strategies for the prevention and treatment of frailty remain to be elucidated, in part because pathophysiology has not been firmly established. Proposed factors include systemic inflammation, endocrine dysfunction, neuropsychological impairment, cardio- and cerebrovascular disease, and malnutrition.1,4 These factors may share a common pathway that includes sarcopenia and osteopenia which, in turn, could lead to the Fried-phenotype.1
Cumulative exposures to tobacco smoke, respiratory infections, occupational dusts, and air pollution occur across the lifespan.5–7 Depending on host factors, especially advancing age, these exposures can lead to respiratory impairment, most often defined spirometrically as airflow limitation or restrictive-pattern.5–8 The respiratory impairment can, in turn, increase the risk for adverse outcomes but its association with frailty, and vice-versa, has not been evaluated.9–14
Because frailty and respiratory impairment share risk factors (tobacco use and aging) and mechanisms (inflammatory cytokines and endocrine dysfunction), we postulate that these two conditions will be strongly associated, cross-sectionally and longitudinally.1,4–8,15–19 Consequently, using data from a large, population-based sample of community-living older persons, we evaluated the association between frailty and spirometry-confirmed respiratory impairment, and determined their combined effect on mortality. Because respiratory impairment and frailty are remediable, establishing such associations could help to inform interventions designed to reverse or prevent the progression of either condition and to reduce adverse outcomes.1,4–7,15–21
METHODS
Study Population
We used deidentified, publicly-available data from the Cardiovascular Health Study (CHS), a longitudinal study of older persons, assembled in 1989–1990, with follow-up through 2002.22 For the present study, eligibility criteria included age 65–80 years, white race, no self-reported asthma, and completion of at least two American Thoracic Society (ATS) acceptable spirometric maneuvers and a frailty evaluation. Of the 4,047 participants who were eligible based on age, race, and no self-reported asthma, 469 (11.6%) did not meet the stated spirometry criterion, yielding a final sample of 3,578 (88.4%), all of whom completed a frailty evaluation.
Our analyses were limited to whites aged 65–80 years because spirometric reference values for the Lambda-Mu-Sigma (LMS) method are currently unavailable for non-whites and those aged >80-years.23,24 Our rationale for favoring the LMS method in aging populations is described below. To focus on “irreversible” pathology for airflow limitation, participants with current or prior self-reported asthma were excluded (n=283). We did not exclude participants based on spirometric reproducibility criteria, as per current convention,25 or for any other respiratory disease.
Demographic and Clinical Characteristics
These included age, body mass index (BMI, calculated as weight in kilograms divided by height–squared), sex, education, smoking history, health status, chronic conditions, respiratory symptoms, and frailty.22 Health status was assessed based on the participant’s response to— "Would you say your health in general is excellent, very good, good, fair, or poor?" Chronic conditions were ascertained by self-report and, when available, by adjudication (myocardial infarction, heart failure, stroke, and claudication), direct measurements (high blood pressure and diabetes), or validated scale (Center for Epidemiologic Studies-Depression Scale [CES-D]).1,22 Respiratory symptoms included cough or sputum production for ≥3 consecutive months during the year, dyspnea-on-exertion (ATS-grade I: “when hurrying on level ground or walking up a slight hill”), or wheezing in prior year.22 Using procedures published by Fried and colleagues,1 the following five frailty features were evaluated: 1) slow gait speed—slowest quintile during a timed 15-feet walk (sex- and height-adjusted), 2) low physical activity—lowest quintile of kilocalories/week (sex-adjusted), 3) exhaustion—two questions from CES-D; 4) reduced grip strength—lowest quintile of the average of three dynamometer readings (sex- and BMI-adjusted), and 5) unintentional weight loss—at least 10 pounds in the prior year. Based on the Fried-phenotype, a 3-level frailty status was then established: pre-frail had 1 or 2 of the frailty features, frail had 3 or more, while non-frail had none.1
Spirometry
To assess respiratory impairment, participants underwent spirometry using contemporary ATS protocols.22,26 The measured values of interest included the ratio of forced expiratory volume in 1-second to forced vital capacity (FEV1/FVC) and FVC alone. The FEV1/FVC was calculated from the largest set of FEV1 and FVC values that were recorded in any of the spirometric maneuvers meeting ATS-acceptability criteria.25,26
To establish age-appropriate comparisons between measured and predicted values for FEV1/FVC and FVC, we used the LMS method as this is the only available approach that considers all elements of distribution—median (Mu), coefficient-of-variation (Sigma), and skewness (Lambda).7–9,23,27,28 Using this method,23 Z-scores for FEV1/FVC and FVC were calculated for each participant, as follows: [(measured value ÷ predicted median)Lambda minus 1] ÷ (Lambda × Sigma). The predicted values for median, lambda, and skewness were calculated from LMS equations that were based on four pooled reference samples, with ages ranging from 4–80 years.23,24 An LMS-calculated Z-score of −1.64 was subsequently used to define the lower limit of normal as the 5th percentile of distribution (LMS-LLN5). Based on this threshold, participants were classified as having 1) normal pulmonary function, if FEV1/FVC and FVC were both ≥LMS-LLN5, 2) airflow limitation, if FEV1/FVC<LMS-LLN5, or 3) restrictive-pattern, if FEV1/FVC≥LMS-LLN5 but FVC<LMS-LLN5.9,23
Outcomes
The longitudinal outcomes included 1) development of respiratory impairment, as either airflow limitation or restrictive-pattern, 2) development of frailty features, as either pre-frail or frail, and 3) all-cause mortality. Composite measures of respiratory impairment and frailty were evaluated in order to optimize the available number of adverse outcomes for analyses.
The development of respiratory impairment was assessed at the first follow-up spirometry (Year-4). Of 2,749 participants who had normal baseline pulmonary function, 659 (24.0%) had missing spirometry at Year-4, including 158 who died. In comparison with participants who completed spirometry at Year-4, the nondecedents who did not complete spirometry were older, had more chronic conditions, and a greater likelihood of fair-to-poor health status and being pre-frail or frail (all p<0.001).
The development of frailty features was assessed at the first follow-up frailty evaluation (Year-3). Of 1,641 participants who were non-frail at baseline, 178 (10.8%) had missing frailty evaluation at Year-3, including 39 who died. In comparison with participants who completed the frailty evaluation at Year-3, the nondecedents who did not complete the frailty evaluation had a greater likelihood of fair-to-poor health status (p<0.01).
All-cause mortality was recorded via obituaries, medical records, death certificates, and a hospitalization database, over a median follow-up of 13.2 years (interquartile range, 9.0–13.6). Vital status was available on all participants.
Statistical Analysis
We first summarized baseline characteristics according to the 3-level frailty status, with differences evaluated by the chi-square test (categorical variables) and analysis of variance (continuous variables).
We next evaluated cross-sectional associations between the 3-level frailty status and respiratory impairment (airflow limitation and restrictive-pattern), at baseline. In these analyses, we used a generalized logit regression model, adjusted for age, height, gender, smoking history, BMI, reduced health, and chronic conditions. In the multivariable analyses, we evaluated interactions between covariates and frailty status, as well as collinearity and multicollinearity. Model fit was assessed by residual analysis and goodness-of-fit statistics. In secondary analyses, we evaluated cross-sectional associations between each of the five frailty features and respiratory impairment, at baseline, also using a covariate-adjusted generalized logit regression model.
Longitudinal associations between frailty features and respiratory impairment were subsequently evaluated. Specifically, among participants with normal pulmonary function at baseline, we evaluated the association between baseline frailty features (pre-frail or frail versus non-frail) and the development of respiratory impairment (airflow limitation or restrictive-pattern) at Year-4. Similarly, among participants who were non-frail at baseline, we evaluated the association between baseline respiratory status (airflow limitation or restrictive-pattern versus normal pulmonary function) and the development of frailty features (pre-frail or frail) at Year-3. To evaluate the magnitude and significance of these associations, we used logistic regression models, adjusted for covariates as described earlier. Because the amount of missing data for these outcomes was not small (10.2%-to-23.4%), we created indicator variables for missing values, and regressed explanatory variables on these binary (Yes/No) outcomes. Variables related to being missing were then included in a multiple imputation analysis of the longitudinal outcomes, using PROC MI (SAS 9.2). We imputed 5 different datasets using a monotone method and logistic model. PROC MIANALYZE (SAS 9.2) was subsequently used to combine these imputations and rerun the relevant regression analyses to determine if missing data significantly impacted the results obtained from complete case analyses.
Lastly, we evaluated longitudinal associations of the 3-level frailty status and respiratory impairment (airflow limitation or restrictive-pattern), as measured at baseline, with subsequent all-cause mortality. These factors were coded to represent a 3-by-2 factorial design, with the reference group including participants who were non-frail and had normal pulmonary function. To evaluate the magnitude and significance of these associations, we used Cox regression models, adjusted for covariates as described earlier. Goodness-of-fit was assessed by model-fitting procedures and analysis of residuals. The proportional hazards assumption was tested for the time-to-event outcome and each variable in the multivariable model, by using interaction terms, retained if p<0.05 after adjusting for multiplicity of comparisons. Higher-order effects were tested for continuous covariates and were included in the final model if they met the forward selection criterion of p<0.20.29
SAS 9.2 (SAS Institute; Cary, NC) was used for all analyses, with p<0.05 (two-sided) denoting statistical significance.
RESULTS
Among all 3,578 study participants, the mean age was 71.5 years; 2,063 (57.7%) were female, 1,641 (45.9%) were non-frail, 1,728 (48.3%) were pre-frail, and 209 (5.8%) were frail. As shown in Table 1, there were statistically significant increases across the 3-level frailty status (from non-frail to frail) in age and BMI, as well as in the frequency of female sex, lower education, current smokers, fair-to-poor health status, chronic conditions, respiratory symptoms, and spirometric respiratory impairment.
Table 1.
Baseline characteristics according to frailty status a
| Characteristic | Non-Frail n=1,641 |
Pre-Frail n=1,728 |
Frail n=209 |
p-value |
|---|---|---|---|---|
| Age, mean (SD), years | 70.8 (3.8) | 71.9 (4.3) | 73.8 (4.2) | < .001 |
| BMI (kg/m2), mean (SD) | 26.0 (3.7) | 26.6 (4.0) | 27.5 (4.6) | < .001 |
| Females, No. (%) | 875 (53.3) | 1,042 (60.3) | 146 (69.9) | < .001 |
| Education (< High School), No. (%) | 331 (20.2) | 495 (28.7) | 68 (32.7) | < .001 |
| Smoking status, No. (%) | ||||
| Never | 721 (43.9) | 766 (44.4) | 90 (43.1) | .049 |
| Former | 752 (45.8) | 731 (42.3) | 91 (43.5) | |
| Current | 168 (10.2) | 230 (13.3) | 28 (13.4) | |
| Fair-to-poor health, No. (%) | 181 (11.1) | 452 (26.2) | 99 (47.6) | < .001 |
| Chronic conditions, No. (%) b | ||||
| Arthritis | 698 (42.9) | 937 (54.8) | 144 (69.9) | < .001 |
| High blood pressure | 646 (39.4) | 825 (47.8) | 109 (52.2) | < .001 |
| Prior pneumonia | 395 (24.3) | 502 (29.3) | 62 (30.0) | .003 |
| Depression c | 66 (4.0) | 295 (17.1) | 79 (37.8) | < .001 |
| Myocardial infarction | 149 (9.1) | 222 (12.9) | 42 (20.1) | < .001 |
| Diabetes | 129 (7.9) | 209 (12.1) | 39 (18.7) | < .001 |
| COPD d | 96 (5.9) | 141 (8.2) | 25 (12.3) | < .001 |
| Stroke | 38 (2.3) | 84 (4.9) | 18 (8.6) | < .001 |
| Heart failure | 25 (1.5) | 49 (2.8) | 15 (7.2) | < .001 |
| Kidney disease | 22 (1.3) | 46 (2.7) | 11 (5.3) | < .001 |
| Claudication | 13 (0.8) | 20 (1.2) | 4 (1.9) | .250 |
| Respiratory symptoms, No. (%) e | 565 (35.0) | 852 (50.0) | 142 (69.3) | < .001 |
| Spirometric category, No. (%) f | ||||
| Normal pulmonary function | 1,350 (82.3) | 1,266 (73.3) | 133 (63.6) | < .001 |
| Airflow limitation | 189 (11.5) | 272 (15.7) | 34 (16.3) | |
| Restrictive-pattern | 102 (6.2) | 190 (11.0) | 42 (20.1) | |
Abbreviation: SD= standard deviation; CES-D= Center for Epidemiologic Studies Depression Scale; COPD= chronic obstructive pulmonary disease; FEV1/FVC= ratio of forced expiratory volume in 1-second to forced vital capacity; LMS-LLN5= Lambda-Mu-Sigma defined lower limit of normal (i.e. the 5th percentile distribution of LMS-derived Z-scores).
Based on the Fried-phenotype (see methods).
Of 3,578 participants, 107 (3.0%) had at least 1 medical condition missing.
Based on a CES-D score of ≥10 (see methods).
Included emphysema and chronic bronchitis.
Included cough or sputum production, dyspnea-on-exertion, or wheezing (see methods).
Normal pulmonary function was defined by an FEV1/FVC ≥ LMS-LLN5 and FVC ≥ LMS-LLN5; airflow limitation was defined by FEV1/FVC < LMS-LLN5; and restrictive-pattern was defined by FEV1/FVC ≥ LMS-LLN5 and FVC < LMS-LLN5 (see methods).
Table 2 shows cross-sectional associations between the 3-level frailty status and respiratory impairment, as measured at baseline. In adjusted models, relative to non-frail, a status of pre-frail and frail conferred a statistically significant 62% and 88% increase in the odds of having airflow limitation and 80% and 205% increase in the odds of having restrictive-pattern, respectively. In secondary analyses, of the five frailty features, only slow gait speed and physical inactivity were cross-sectionally associated with airflow limitation (adjusted OR: 1.43 [1.12, 1.83] and 1.40 [1.07, 1.82], respectively); whereas slow gait speed, physical inactivity, and grip strength were cross-sectionally associated with restrictive-pattern (adjusted OR: 1.96 [1.51, 2.55], 1.78 [1.35, 2.34], and 1.59 [1.19, 2.12], respectively).
Table 2.
Cross-sectional association between frailty status and respiratory impairment, as measured at baseline (N=3,471) a
| Frailty Status | Respiratory Impairment | |||
|---|---|---|---|---|
| Airflow limitation | Restrictive-pattern | |||
| Odds Ratio (95% CI) b | ||||
| Unadjusted | Adjusted c | Unadjusted | Adjusted c | |
| Non-Frail | 1.00 | |||
| Pre-Frail | 1.54 (1.26, 1.89) | 1.62 (1.29, 2.04) | 2.01 (1.55, 2.60) | 1.80 (1.37, 2.36) |
| Frail | 1.72 (1.12, 2.64) | 1.88 (1.15, 3.09) | 4.28 (2.84,6.46) | 3.05 (1.91, 4.88) |
Abbreviation: CI= confidence interval; FEV1/FVC= ratio of forced expiratory volume in 1-second to forced vital capacity; LMS-LLN5= Lambda-Mu-Sigma defined lower limit of normal.
Of 3,578 participants, 107 (3.0%) had missing covariate information—yielding a sample size of 3,471.
Generalized logit regression model.
Adjusted for age, height, height2, height3, gender, smoking history, BMI, BMI2, health status, and chronic conditions.
Table 3 shows the longitudinal association between baseline frailty features (pre-frail or frail) and the development of respiratory impairment (airflow limitation or restrictive-pattern) at Year-4. In adjusted models, relative to participants who were non-frail at baseline, those with frailty features had a statistically significant 42% increase in the odds of developing respiratory impairment. Conversely, Table 4 shows the longitudinal association between baseline respiratory impairment (airflow limitation or restrictive-pattern) and the development of frailty features (pre-frail or frail) at Year-3. In adjusted models, relative to participants who had normal pulmonary function at baseline, those with respiratory impairment had a statistically significant 58% increase in the odds of developing frailty features. The magnitude and significance of the associations reported in Tables 3 and 4 did not change appreciably when the analyses were rerun with multiple imputation of missing values (data not shown).
Table 3.
Longitudinal association between baseline frailty features (pre-frail or frail) and the development of respiratory impairment (airflow limitation or restrictive-pattern) at Year-4, among participants who had normal pulmonary function at baseline (N=2,033) a
| Frailty features | N | No. (%) of participants who developed respiratory impairment |
Development of respiratory impairment Odds ratio (95% CI) b |
|
|---|---|---|---|---|
| Unadjusted | Adjusted c | |||
| Absent (non-frail) | 1,078 | 157 (14.6) | 1.00 | |
| Present (pre-frail or frail) | 955 | 196 (20.5) | 1.52 (1.20, 1.91) | 1.42 (1.11, 1.82) |
Abbreviation: CI= confidence interval.
Of 2,749 participants who had normal pulmonary function, 74 (2.7%) had missing covariate information and 642 (23.4%) had missing outcome (development of respiratory impairment)—yielding a sample size of 2,033.
Logistic regression model.
Adjusted for age, height, height2, gender, smoking history, BMI, health status, and chronic conditions.
Table 4.
Longitudinal association between baseline respiratory impairment (airflow limitation or restrictive-pattern) and the development of frailty features (pre-frail or frail) at Year-3, among participants who were non-frail at baseline (N=1,435) a
| Respiratory Impairment | N | No. (%) of participants who developed frailty features |
Development of frailty features Odds Ratio (95% CI) b |
|
|---|---|---|---|---|
| Unadjusted | Adjusted c | |||
| Absent (normal pulmonary function) | 1,189 | 458 (38.5) | 1.00 | |
| Present (airflow limitation or restrictive-pattern) | 246 | 121 (49.2) | 1.54 (1.17, 2.04) | 1.58 (1.17, 2.13) |
Abbreviation: CI= confidence interval.
Of 1,641 participants who were non-frail at baseline, 39 (2.4%) had missing covariate information and 167 (10.2%) had missing outcome (development of frailty features)—yielding a sample size of 1,435.
Logistic regression model.
Adjusted for age, height, height2, gender, smoking history, BMI, health status, and chronic conditions.
Table 5 shows the mortality rates according to the 3-level frailty status and respiratory impairment (airflow limitation or restrictive-pattern), as measured at baseline. Participants who were frail and had respiratory impairment had the highest mortality rate, leading to a 2.5-fold increase in mortality relative to those who were non-frail and had normal pulmonary function. For each of the three frailty groups, mortality rates were higher in the presence than in the absence of respiratory impairment.
Table 5.
Mortality rates according to baseline frailty status and respiratory impairment (N = 3,471) a
| Frailty Status | Respiratory impairment | |
|---|---|---|
| Absent (normal pulmonary function) | Present (airflow limitation or restrictive-pattern) | |
| n / N (%) of deaths among participants | ||
| Non-frail | 440/1321 (33.3) | 147/281 (52.3) |
| Pre-frail | 578/1,228 (47.1) | 293/445 (65.8) |
| Frail | 80/126 (63.5) | 58/70 (82.9) |
Of the 3,578 study participants, 107 (3.0%) had missing covariate information.
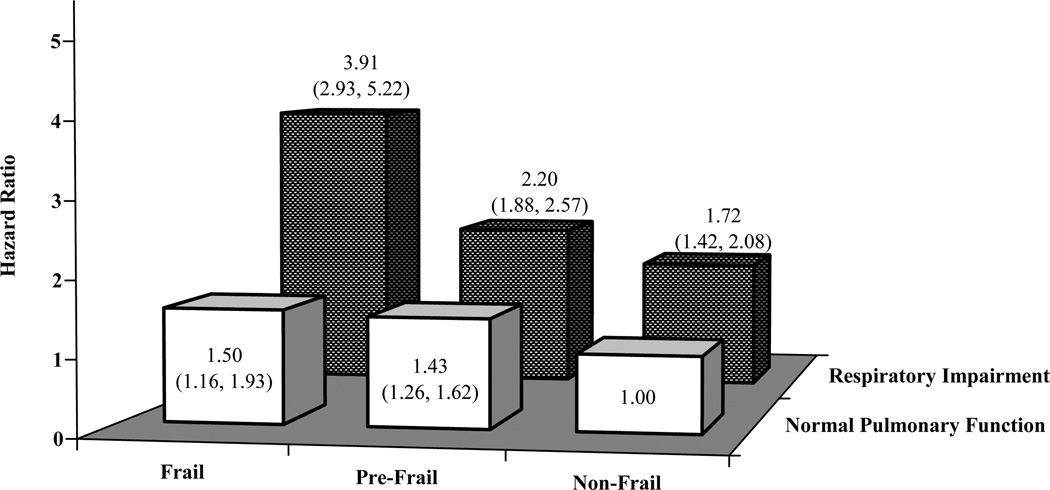
Figure 1 shows hazard ratios (HR) for death, according to the 3-level frailty status and respiratory impairment (airflow limitation or restrictive-pattern), as measured at baseline. In adjusted models, relative to those who were non-frail and had normal pulmonary function, participants who were frail and had respiratory impairment had a statistically significant 291% increase in the risk of death, while those who were pre-frail and had respiratory impairment had a statistically significant 120% increase in the risk of death. Although hazard ratios were not as high, the risk of death was also elevated among participants who were pre-frail or frail but had normal pulmonary function, as well as those who were non-frail but had a respiratory impairment. The effects of frailty and respiratory impairment on mortality were multiplicative, not additive (p=.037).
Figure 1.
Adjusted hazard ratios (95% confidence interval) for all-cause mortality, according to baseline frailty status and respiratory impairment (N = 3,471) a
a Single Cox regression model, adjusted for age, height, gender, smoking history, BMI, BMI2, health status, and chronic conditions. The reference group included non-frail participants who had normal pulmonary function. There was a significant interaction between frailty and respiratory impairment (p=.037). The sample size of each subgroup is provided in Table 5.
DISCUSSION
In a large sample of community-living white older persons, we found that frailty and respiratory impairment were strongly associated with one another, cross-sectionally and longitudinally, and substantially increased the risk of death when both were present, independent of potential confounders. These results suggest that the association between frailty and respiratory impairment is bidirectional and that their combined effects are particularly deleterious.
In cross-sectional analysis, we found that frail participants had an increased likelihood of having a respiratory impairment. For example, frail participants had an increased odds of having airflow limitation and restrictive pattern of 88% and 205%, respectively. Because it was accompanied by high smoking rates and an increased prevalence of self-reported COPD, the airflow limitation in frail participants was likely due to COPD.5,7,30,31 Because it was accompanied by an increased prevalence of heart failure and associated with reduced grip strength, the restrictive-pattern in frail participants may have been due to heart failure or respiratory muscle weakness.1,30,32 Future work, using more comprehensives tests of pulmonary function such as body plethysmography, respiratory muscle pressures, and diffusion capacity, should evaluate the potential mechanisms, including their relative contributions, that underlie respiratory impairment in frail older persons. Given that frailty is associated with systemic inflammation, endocrine dysfunction, cardiovascular disease, sarcopenia, and osteopenia, these mechanisms may involve—in addition to COPD, heart failure, and respiratory muscle weakness— asthma and bronchiectasis for airflow limitation, and interstitial lung disease and thoracic kyphosis for restrictive-pattern.1,4,30–36
Longitudinally, our results suggest a bidirectional relationship between frailty features and respiratory impairment. Specifically, we found that participants with frailty features had an increased likelihood of developing respiratory impairment, and vice-versa. A bidirectional relationship has important therapeutic implications. For example, strategies that target frailty and common associated features (sarcopenia and osteopenia) could also potentially improve pulmonary function (restrictive-pattern) in older persons.4,32,34,37–39 Conversely, strategies that target COPD and common coexisting conditions (cardiovascular disease, malnutrition, depression, and cognitive decline) could also potentially improve frailty in older persons.20,31,40–43
Our results further suggest that adverse outcomes are substantially increased when frailty and respiratory impairment are both present. In fact, the combined effects of frailty and respiratory impairment on mortality were more than additive, such that frail participants who also had respiratory impairment had a nearly 4-fold increased risk of death, relative to those who were non-frail and had normal pulmonary function. Consequently, future work should evaluate interventions that concurrently target frailty and respiratory impairment on a more complete array of outcomes, including injurious falls, disability, and hospitalizations. A pulmonary rehabilitation program, for example, may be especially beneficial if adapted to the unique needs of older persons who are frail and have respiratory impairment. Such a program might offer smoking cessation, exercise and respiratory muscle training, cardiopulmonary pharmacotherapy, osteoporosis management, and nutritional support.44–46 Subsequently, new advances that reverse the systemic inflammation and endocrine dysfunction of frailty and respiratory impairment might also be integrated into the rehabilitation program.1,4,15–19
We acknowledge several potential limitations to our study. First, because participants were all white, our findings may not be applicable to non-whites. Once LMS-derived Z-scores are more broadly available, our results should be confirmed in non-whites.23,24 Second, our analysis of the longitudinal associations between frailty and respiratory impairment did not account for death as a competing risk. Because older decedents are more likely than older nondecedents to be frail and have respiratory impairment at the time of death,1,9 the omission of decedents from our analysis likely attenuated the magnitude of the longitudinal associations between frailty and respiratory impairment. Lastly, to optimize the number of adverse outcomes available for analyses, we used composite measures. This approach prevented us from evaluating longitudinally which respiratory impairment, airflow limitation or restrictive-pattern, was more strongly associated with frailty, including the combined effect on mortality.
In conclusion, among community-living white older persons, frailty and respiratory impairment are strongly associated with one another and substantially increase the risk of death when both are present. Because frailty and respiratory impairment are potentially remediable, establishing these associations could help to inform interventions designed to reverse or prevent the progression of either condition and to reduce adverse outcomes.1,4–7,15–21
Acknowledgments
Funding source: Dr. Vaz Fragoso is currently a recipient of career development awards from the Department of Veterans Affairs and the Yale Pepper Center. Dr. Gill is the recipient of an NIA Midcareer Investigator Award in Patient-Oriented Research (K24AG021507). Dr. Van Ness received support from the Claude D. Pepper Older Americans Independence Center at Yale (2P30AG021342). The work for this report was funded in part by a grant from the National Institute on Aging (R03AG037051).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflicts of interest.
REFERENCES
- 1.Fried L, Tangen M, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol Med Sci. 2001;56A:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 2.Rockwood K, Howlett S, MacKnight C, et al. Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: report from the Canadian study of health and aging. J Gerontol Med Sci. 2004;59A:1310–1317. doi: 10.1093/gerona/59.12.1310. [DOI] [PubMed] [Google Scholar]
- 3.Boyd CM, Xue Q-L, Simpson CF, et al. Frailty, hospitalization, and progression of disability in a cohort of disabled older women. Am J Med. 2005;118:1225–1231. doi: 10.1016/j.amjmed.2005.01.062. [DOI] [PubMed] [Google Scholar]
- 4.Cawthon CM, Ensrud KE, Laughlin GA, et al. Sex hormones and frailty in older men: the osteoporotic fractures in men (MrOS) study. J Clin Endocrinol Metab. 2009;94:3806–3815. doi: 10.1210/jc.2009-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC. Second National Report on Human Exposure to Environmental Chemicals: Tobacco Smoke. Morb Mortal Wkly Rep. 2002;51:300–303.
- 6.Abbey DE, Nishino N, McDonnell WF, et al. Long-term inhalable particles and other air pollutants related to mortality in nonsmokers. Am J Respir Crit Care Med. 1999;159:373–382. doi: 10.1164/ajrccm.159.2.9806020. [DOI] [PubMed] [Google Scholar]
- 7.GOLD executive summary. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 8.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 9.Vaz Fragoso CA, Concato J, McAvay G, et al. The ratio of the forced expiratory volume in 1-second to forced vital capacity in establishing chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:446–451. doi: 10.1164/rccm.200909-1366OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butcher SJPT, Meshke JM, Sheppard SM. Reductions in functional balance, coordination, and mobility measures among patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2004;24(4):274–280. doi: 10.1097/00008483-200407000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Okubadejo AA, O’Shea L, Jones PW, Wedzicha JA. Home assessment of activities of daily living in patients with severe chronic obstructive pulmonary disease on long-term oxygen therapy. Eur Respir J. 1997;10:1572–1575. doi: 10.1183/09031936.97.10071572. [DOI] [PubMed] [Google Scholar]
- 12.Hole DJ, Watt GCM, Davey-Smith G, et al. Impaired lung function and mortality risk in men and women: findings from the renfrew and paisley prospective population study. BMJ. 1996;313:711–715. doi: 10.1136/bmj.313.7059.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sin DD, Wu L, Man SFP. The relationship between reduced lung function and cardiovascular mortality: A population-based study and a systematic review of the literature. Chest. 2005;127:1952–1959. doi: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 14.Buch P, Friberg J, Scharling H, et al. Reduced lung function and risk of atrial fibrillation in the Copenhagen city heart study. Eur Respir J. 2003;21:1012–1016. doi: 10.1183/09031936.03.00051502. [DOI] [PubMed] [Google Scholar]
- 15.Andreassen H, Vestbo J. Chronic obstructive pulmonary disease as a systemic disease: An epidemiological perspective. Eur Respir J. 2003;22 Suppl.46:2s–4s. doi: 10.1183/09031936.03.00000203. [DOI] [PubMed] [Google Scholar]
- 16.Dahl M, Tybjaerg-Hansen A, Vestbo J, Lange P, Nordestgaard BG. Elevated plasma fibrinogen associated with reduced pulmonary function and increased risk of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1008–1011. doi: 10.1164/ajrccm.164.6.2010067. [DOI] [PubMed] [Google Scholar]
- 17.Takabatake N, Nakamura H, Abe S, Hino T, Saito H, et al. Circulating leptin in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159(4):1215–1219. doi: 10.1164/ajrccm.159.4.9806134. [DOI] [PubMed] [Google Scholar]
- 18.Schols AMWJ, Creutzberg EC, Buurman WA, Campfield LA, Saris WHM, Wouters EFM. Plasma leptin is related to proinflammatory status and dietary intake in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:1220–1226. doi: 10.1164/ajrccm.160.4.9811033. [DOI] [PubMed] [Google Scholar]
- 19.Kamischke A, Kemper DE, Castel MA, Luthke M, Rolf C, et al. Testosterone levels in men with chronic obstructive pulmonary disease with or without glucocorticoid therapy. Eur Respir J. 1998;11:41–45. doi: 10.1183/09031936.98.11010041. [DOI] [PubMed] [Google Scholar]
- 20.Sutherland ER, Cherniak RM. Management of chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2689–2697. doi: 10.1056/NEJMra030415. [DOI] [PubMed] [Google Scholar]
- 21.Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166:418–423. doi: 10.1001/archinte.166.4.418. [DOI] [PubMed] [Google Scholar]
- 22.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, et al. The Cardiovascular Health Study: Design and rationale. Ann Epidemiol. 1991;1(3):263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 23.Stanojevic S, Wade A, Stocks J, et al. Reference ranges for spirometry across all ages. Am J Respir Crit Care Med. 2008;177:253–260. doi: 10.1164/rccm.200708-1248OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spirometry (LMS) [Accessed May 17, 2011]; Available at: http://www.lungfunction.org/growinglungs/all-age.html.
- 25.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 26.American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991;144:1202–1218. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 27.Hansen JF, Sun X-G, Wasserman K. Spirometric criteria for airway obstruction. Chest. 2007;131:349–355. doi: 10.1378/chest.06-1349. [DOI] [PubMed] [Google Scholar]
- 28.Vaz Fragoso CA, Gill TM. Defining chronic obstructive pulmonary disease in an aging population. J Am Geriatr Soc. 2010;58:2224–2226. doi: 10.1111/j.1532-5415.2010.03128.x. (Editorial) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48:1503–1510. doi: 10.1016/0895-4356(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 30.Chan ED, Welsh CH. Geriatric respiratory medicine. Chest. 1998;114:1704–1733. doi: 10.1378/chest.114.6.1704. [DOI] [PubMed] [Google Scholar]
- 31.Gooneratne NS, Patel NP, Corcoran A. Chronic obstructive pulmonary disease: diagnosis and management in older adults. J Am Geriatr Soc. 2010;58(6):1153–1162. doi: 10.1111/j.1532-5415.2010.02875.x. [DOI] [PubMed] [Google Scholar]
- 32.Enright PL, Kronmal RA, Manolio TA, et al. Respiratory muscle strength in the elderly. Correlates and reference values. Cardiovascular Health Study Research Group. Am J Respir Crit Care Med. 1994;149:430–438. doi: 10.1164/ajrccm.149.2.8306041. [DOI] [PubMed] [Google Scholar]
- 33.Enright PL, McClelland RL, Newman AB, et al. Underdiagnosis and undertreatment of asthma in the elderly. Chest. 1999;116:603–613. doi: 10.1378/chest.116.3.603. [DOI] [PubMed] [Google Scholar]
- 34.Di Bari M, Chiarlone M, Matteuzzi D, et al. Thoracic kyphosis and ventilatory dysfunction in unselected older persons: an epidemiological study in Dicomano, Italy. J Am Geriatr Soc. 2004;52:909–915. doi: 10.1111/j.1532-5415.2004.52257.x. [DOI] [PubMed] [Google Scholar]
- 35.American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 36.Raghu G, Freudenberger TD, Yang S, et al. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur Respir J. 2006;27:136–142. doi: 10.1183/09031936.06.00037005. [DOI] [PubMed] [Google Scholar]
- 37.Janssens W, Lehouck A, Carremans C, et al. Vitamin D beyond bones in chronic obstructive pulmonary disease time to act. Am J Respir Crit Care Med. 2009;179:630–636. doi: 10.1164/rccm.200810-1576PP. [DOI] [PubMed] [Google Scholar]
- 38.Wilhelm-Leen ER, Hall YN, deBoer IH, Chertow GM. Vitamin D deficiency and frailty in older Americans. J Intern Med. 2010;268:171–180. doi: 10.1111/j.1365-2796.2010.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harrison RA, Siminoski K, Vethanayagam D, Majumdar SR. Osteoporosis-related kyphosis and impairments in pulmonary function: a systematic review. J Bone Miner Res. 2007;22:447–457. doi: 10.1359/jbmr.061202. [DOI] [PubMed] [Google Scholar]
- 40.Hubbard RE, Searle SD, Mitnitski A, Rockwood K. Effect of smoking on the accumulation of deficits, frailty and survival in older adults: a secondary analysis from the Canadian study of health and aging. J Nutrition Health Aging. 2009;13(5):468–472. doi: 10.1007/s12603-009-0085-y. [DOI] [PubMed] [Google Scholar]
- 41.Incalzi RA, Marra C, Giordano A, et al. Cognitive impairment in chronic obstructive pulmonary disease. J Neurol. 2003;250(3):325–332. doi: 10.1007/s00415-003-1005-4. [DOI] [PubMed] [Google Scholar]
- 42.Fletcher C, Peto K. The natural history of chronic airflow obstruction. Brit Med J. 1977;1:1645–1648. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Griffith KA, Sherrill DL, Manolio TA, et al. Predictors of loss of lung function in the elderly of the Cardiovascular Health Study. Am J Respir Crit Care Med. 2001;163:61–68. doi: 10.1164/ajrccm.163.1.9906089. [DOI] [PubMed] [Google Scholar]
- 44.Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest. 2007;131:4S–42S. doi: 10.1378/chest.06-2418. [DOI] [PubMed] [Google Scholar]
- 45.Xue Q-L, Fried LP, Glass TA, et al. Life-space constriction, development of frailty, and the competing risk of mortality: the women's health and aging study I. Am J Epidemiol. 2008;167:240–248. doi: 10.1093/aje/kwm270. [DOI] [PubMed] [Google Scholar]
- 46.Physical Activity and Older Americans: Benefits and Strategies. Agency for Healthcare Research and Quality and the Centers for Disease Control. [Accessed May 7, 2011];2002 June; Available at http://www.ahrq.gov/ppip/activity.htm.