Abstract
Background
Autonomic abnormalities have been implicated in both diastolic dysfunction and abnormal heart rate (HR) recovery; however, few studies have assessed whether diastolic dysfunction is associated with abnormal HR recovery, and whether both modify exercise capacity.
Methods and Results
Exercise echocardiography with diastolic assessment was performed in 2826 patients with normal wall motion responses to symptom-limited exercise testing. HR recovery was defined as the difference in HR from peak exercise to 1 minute in recovery; abnormal HR recovery was defined as the lowest quartile. Mean HR recovery was 32±14 beats per minute. Patients with diastolic dysfunction or abnormal HR recovery had lower exercise capacity, and those with both had the lowest exercise capacity (p<0.0001 for comparison to normal responses). Indices of abnormal diastolic function were correlated with abnormal HR recovery. In multivariable analysis, after age, diastolic dysfunction (referent: normal diastolic function) was the strongest predictor of abnormal HR recovery (adjusted OR 1.47; 95% CI 1.20-1.80) and incrementally predictive of chronotropic incompetence (adjusted OR 1.42; 95% CI 1.16-1.74).
Conclusions
Diastolic dysfunction is independently associated with abnormal HR recovery after symptom-limited exercise. Further studies are needed to determine if diastolic function modifies the adverse outcomes observed in those with abnormal HR recovery.
Introduction
In health, heart rate (HR) drops rapidly after cessation of exercise as parasympathetic tone to the sinoatrial node is enhanced and catecholamine levels drop. Abnormal HR recovery is defined by a blunted reduction in HR after exercise and is independently associated with increased morbidity and mortality1-5. HR recovery is considered to be a robust marker of fitness, mediated in part by the autonomic nervous system1, 2, 5-7. In addition, HR recovery has been shown to be similar to other traditional risk factors in its association with clinical outcomes6, even among patients without overt cardiovascular disease3, and the association of HR recovery with increased mortality is independent of systolic function, angiographic disease severity, or exercise capacity4, 8. However, the mechanisms and clinical correlates of abnormal HR recovery are incompletely understood.
Normal diastolic function allows for left ventricular filling at rest and during exercise without abnormal elevation of left ventricular filling pressures. Patients with heart failure have impaired autonomic function and abnormal HR recovery, which are associated with reduced exercise capacity9, 10. The autonomic nervous system affects cardiac myocyte calcium handling11 and studies of HR variability have suggested that cardiac autonomic abnormalities, as may be seen in patients with abnormal HR recovery9, are associated with diastolic dysfunction12, 13. We hypothesized that diastolic dysfunction and abnormal HR recovery are related, and sought to explore their relationship to one another and to exercise capacity among patients undergoing symptom-limited testing by exercise echocardiography who did not have evidence of myocardial ischemia.
Methods
We studied patients who underwent a clinically indicated stress echocardiogram and did not have evidence of stress-induced wall motion abnormalities (i.e., negative stress imaging) at the Mayo Clinic, Rochester, MN from January 2006 - December 2006. During this time period, our stress echocardiography laboratory had implemented the Diastolic Function Initiative, whereby in addition to assessment of wall motion response, we routinely assessed left ventricular diastolic function and left ventricular filling pressures non-invasively in patients undergoing an exercise echocardiography protocol. All patients in our analysis performed symptom-limited treadmill exercise utilizing the standard Bruce protocol. The study cohort comprised patients who were referred for exercise testing based on the following reasons: 1380 (48.9%) for chest pain or shortness of breath, 625 (22.1%) with multiple cardiovascular risk factors and suspected coronary artery disease, 272 (9.6%) for an abnormal electrocardiogram, 239 (8.5%) for further evaluation of known coronary artery disease, 238 (8.3%) for pre-operative risk stratification, and 72 (2.6%) for abnormal coronary calcium study on computed tomography. Patients with missing HR data (n = 140), non-sinus rhythm (n = 120), valvular heart disease (n = 76), poor image quality (n = 7), reduced ejection fraction (<50%, n=88), or echocardiographic evidence of stress-induced myocardial ischemia (n = 790) were excluded, leaving a final study population of 2826 patients.
The Mayo Clinic institutional review board approved this retrospective study. The study was supported by a grant from the Mayo Foundation. The authors are solely responsible for the design and conduct of this study, all study analyses, editing, drafting, and final contents of the manuscript.
Heart Rate Recovery
HR data was acquired from continuous electrocardiographic monitoring during exercise testing. Blood pressure was measured by trained nurses at the end of each 3-minute stage on the Bruce protocol using a sphygmomanometer with the cuff over the upper extremity and auscultation of the brachial artery. One-minute recovery data for HR and blood pressure were obtained after rapid positioning in the left lateral decubitus position after exercise14, 15. Clinical data were abstracted from the medical record at the time of the stress echocardiogram. HR recovery was defined as peak HR - 1 minute recovery HR. Abnormal HR recovery was defined by the lowest quartile from our cohort (≤24 beats per min (bpm)). Chronotropic response was assessed and derived as the proportion of HR reserve used at peak exercise using the following formula: (HRpeak – HRbaseline)/(220 – age – HRbaseline) × 10016, 17. Chronotropic incompetence was defined as a HR reserve (i.e., chronotropic index) <0.817-19 or <0.62 if on beta-blockers20.
Echocardiographic Assessment
Echocardiographic assessment of diastolic function at rest included pulsed-wave Doppler measurements of the early (E) and late (A) transmitral inflow velocities, deceleration time (DT), early diastolic velocity of the medial (septal) mitral annulus (e') utilizing tissue Doppler, non-invasive assessment of left ventricular filling pressures (E/e'), and left atrial size. E/e' was also measured immediately post-exercise but after regional wall motion assessment; post-exercise left ventricular inflow patterns were not assessed. Tissue Doppler was not performed in patients with dense mitral annular calcification. Elevated left ventricular filling pressure was defined as an E/e' ≥ 1521, 22. Left atrial volume was assessed in biplane views and measured using the area-length method indexed to body surface area. Resting diastolic function was graded as normal, mild dysfunction or moderate/severe dysfunction and classified as such based on previously published definitions22, 23. Normal diastolic function was defined as an E/A between 0.75 and 1.5, normal left atrial volume index (<28ml/m2), and normal left ventricular filling pressure (E/e' <10). Mild diastolic dysfunction included patients with an E/A of less than 0.75 and E/e'<10. Moderate/severe diastolic dysfunction included patients with an E/A>1.5, left atrial volume index ≥28 ml/m2, and E/e'≥10. Patients with a pseudonormal pattern were included in the moderate/severe diastolic dysfunction group as all had left atrial volume indices ≥28 ml/m2. Patients with mild or moderate/severe diastolic dysfunction were grouped as abnormal diastolic function and compared to patients with normal diastolic function for the purposes of this study.
Statistics
Summary data for continuous variables are expressed as mean ± SD or median (25th-75th interquartile range) if distributions were not Gaussian and counts/percentages for categorical variables. Patients were stratified into 4 groups: 1) normal HR recovery and normal diastolic function, 2) normal HR recovery and abnormal diastolic function, 3) abnormal HR recovery and normal diastolic function, and 4) abnormal HR recovery and abnormal diastolic function. Univariate analysis was performed using ANOVA and Pearson χ2. Distributions that appeared skewed were tested with the Wilcoxon Rank Sum test. We defined HR recovery as abnormal based on the lowest quartile of HR recovery in our study population, an approach that has previously been used24. In addition, sensitivity analysis was performed using a previously defined HR recovery cutpoint of ≤18 bpm15. We also analyzed HR recovery data as a continuous variable to understand its relationship with diastolic function and exercise capacity. These relationships are presented as the Pearson correlation coefficient (r). Multivariable logistic regression was used to assess the independent association of clinical and echocardiographic variables with abnormal HR recovery. Stepwise backward variable selection techniques were used to assess model stability and potential interactions. Baseline candidate variables significant in univariate analysis at p<0.05 and entered into the model included: age, sex, diastolic dysfunction, hypertension, wall motion score index at baseline, E/e' at baseline, beta blockers, left ventricular hypertrophy, baseline systolic blood pressure, history of coronary artery disease, previous/current smoking, and BMI>30kg/m2. Given their clinical relevance, we also entered ejection fraction, left ventricular end diastolic dimension, and heart rate at baseline in the model. The contribution of each variable to the outcome of abnormal HR recovery was reported using its model χ2 and adjusted odds ratios. All analyses performed were two-sided and p was considered significant at <0.05.
Results
HR Recovery and Diastolic Function
Clinical, echocardiographic, and exercise characteristics are shown by groups (Tables 1 and 2). Mean age was 58±13years and 46% were female; mean HR recovery was 32±14 bpm. Groups differed with regard to several baseline characteristics, including age, body mass index (BMI), and comorbidities. Diastolic indices differed across groups as expected, based on the categorization used in the study. Patients with abnormal HR recovery and/or diastolic dysfunction displayed lower peak HR, lower exercise capacity (measured in metabolic equivalents (METs)), and lower rate pressure product as compared to those with normal responses. The association between exercise capacity and group remained statistically significant (p<0.0001) after adjusting for age. Patients with abnormal diastolic function, as compared to normal diastolic function, included a larger percentage with chronotropic incompetence, even after accounting for beta blocker use (35% vs 23%, respectively, p<0.0001). When patients not taking calcium channel blockers were also excluded, chronotropic incompetence was more frequent in those with abnormal diastolic function as compared to patients with normal diastolic function (33% vs 22%, respectively, p<0.0001). HR recovery correlated with diastolic indices, including mitral DT (per 40 ms) (r=-0.13, p<0.0001), left atrial volume index (r=-0.10, p<0.0001), E/A (r=0.30, p<0.0001), resting E/e' (r=-0.20, p<0.0001), and resting e'(r=0.27, p<0.0001).
Table 1. Baseline Clinical and Echocardiographic Characteristics by Diastolic Function and Heart Rate Recovery†.
| Characteristics | Normal HR recovery and normal diastolic function (n = 1430) | Normal HR recovery and abnormal diastolic function (n = 620) | Abnormal HR recovery and normal diastolic function (n = 326) | Abnormal HR recovery and abnormal diastolic function (n = 449) | p value* |
|---|---|---|---|---|---|
| Clinical Variables | |||||
|
| |||||
| Age (yr) | 52±11 | 65±10 | 59±12 | 70±9 | <0.0001 |
| Female | 658 (46) | 278 (45) | 147 (45) | 217 (48) | 0.69 |
| BMI kg/m2 | 27 (5) | 29 (5) | 28 (5) | 29 (5) | <0.0001 |
| Systolic blood pressure (mm Hg) | 122±18 | 129±19 | 127±19 | 132±21 | <0.0001 |
| Heart rate (bpm) | 75±13 | 74±13 | 75±13 | 75±15 | 0.36 |
| Prior MI | 56 (4) | 42 (7) | 25 (8) | 30 (7) | 0.004 |
| Prior PCI | 77 (5) | 59 (10) | 27 (8) | 53 (12) | <0.0001 |
| Diabetes | 78 (5) | 89 (14) | 27 (8) | 87 (19) | <0.0001 |
| Hypertension | 493 (34) | 376 (61) | 173 (53) | 321 (71) | <0.0001 |
| Previous/current smoker | 584 (41) | 285 (46) | 159 (49) | 220 (49) | 0.002 |
| Beta-blockers | 259 (18) | 211 (34) | 104 (32) | 201 (45) | <0.0001 |
| Calcium channel blockers | 68 (27) | 69 (27) | 31 (12) | 84 (33) | 0.0002 |
| ACE or ARBs | 216 (33) | 184 (28) | 74 (11) | 182 (28) | <0.0001 |
|
| |||||
| Echocardiographic Variables | |||||
|
| |||||
| Ejection fraction (%) | 61±4 | 62±5 | 62±4 | 62±4 | 0.05 |
| WMSI | 1.01±0.57 | 1.03±0.12 | 1.2±0.95 | 1.03±0.12 | <0.0001 |
| E velocity (cm/s) | 73±15 | 64±18 | 74±16 | 68±18 | <0.0001 |
| A velocity (cm/s) | 60±15 | 77±18 | 67±16 | 84±19 | <0.0001 |
| E/A ratio | 1.3±0.4 | 0.9±0.3 | 1.1±0.3 | 0.8±0.3 | <0.0001 |
| e' (medial mitral annulus, m/s) | 0.10±0.02 | 0.07±0.02 | 0.09±0.02 | 0.07±0.02 | <0.0001 |
| Resting E/e' | 8±2 | 11±4 | 9±2 | 11±4 | <0.0001 |
| Deceleration Time (ms) | 198±34 | 226±45 | 199±34 | 230±52 | <0.0001 |
| Left atrial volume index (ml/m2 | 25±6 | 30±9 | 26±7 | 31±10 | <0.0001 |
| Tricuspid regurgitant jet velocity (m/s) | 2.35±0.28 | 2.43±0.26 | 2.43±0.31 | 2.52±0.31 | <0.0001 |
Data presented as mean±SD or n (%).
Heart rate recovery is defined as the difference between heart rate at peak exercise and heart rate at 1 minute in recovery and abnormal heart rate recovery is defined as the lowest quartile for the study sample (≤24 beats per minute).
Categorical variables compared using Pearson χ2 test. Continuous variables compared using ANOVA.
Abbreviations: bpm = beats per minute; m/s = meters per second; MI = myocardial infarction; PCI = percutaneous coronary revascularization; ACE or ARBs = angiotensin converting enzyme inhibitors or angiotensin receptor blockers; WMSI = wall motion score index.
Table 2. Exercise Echocardiographic Characteristics by Heart Rate Recovery†.
| Characteristics | Normal HR recovery and normal diastolic function | Normal HR recovery and abnormal diastolic function | Abnormal HR recovery and normal diastolic function | Abnormal HR recovery and abnormal diastolic function | p value* |
|---|---|---|---|---|---|
| Exercise Variables | |||||
|
| |||||
| METs achieved | 11±3 | 9±2 | 9±2 | 8±2 | <0.0001 |
| Functional aerobic capacity, % | 122±27 | 117±29 | 114±30 | 112±32 | <0.0001 |
| Peak systolic blood pressure (mm Hg) | 166±23 | 168±24 | 165±25 | 164±25 | 0.04 |
| Peak heart rate (bpm) | 159±19 | 143±19 | 142±23 | 130±21 | <0.0001 |
| Rate pressure product, ×103 (mmHg beats/min) | 26.25±4.7 | 24.20±5.1 | 23.56±5.6 | 21.54±5.6 | <0.0001 |
| Heart rate recovery (bpm) | 40±11 | 35±9 | 16±8 | 16±8 | <0.0001 |
|
| |||||
| Exercise echo cardiographic variables | |||||
|
| |||||
| E velocity (cm/s) | 91±22 | 85±24 | 95±24 | 95±26 | <0.0001 |
| E/e' | 8±3 | 10±4 | 9±3 | 12±4 | <0.0001 |
| Ejection fraction (stress, %) | 72±5 | 71±5 | 72±5 | 71±5 | 0.009 |
Data presented as mean±SD or n (%).
Heart rate recovery is defined as the difference between heart rate at peak exercise and heart rate at 1 minute in recovery and abnormal heart rate recovery is defined as the lowest quartile for the study sample (≤24 beats per minute).
Categorical variables compared using Pearson χ2 test. Continuous variables compared using ANOVA.
Abbreviations: METs = metabolic equivalents (1 MET = 3.5 mL/Kg per minu consumption); bpm = beats per minute; cm/s = centimeters per second.
There were 19 patients (0.7%) who had normal filling pressures at rest (E/e'≤10) but had elevated filling pressures (E/e'≥15) with exercise. HR recovery was inversely related to post exercise E/e' (r=-0.20, p<0.0001). Significantly higher filling pressures at rest and post exercise were observed in patients with abnormal HR recovery and/or abnormal diastolic function when compared to patients with normal responses (Figure 1).
Figure 1.
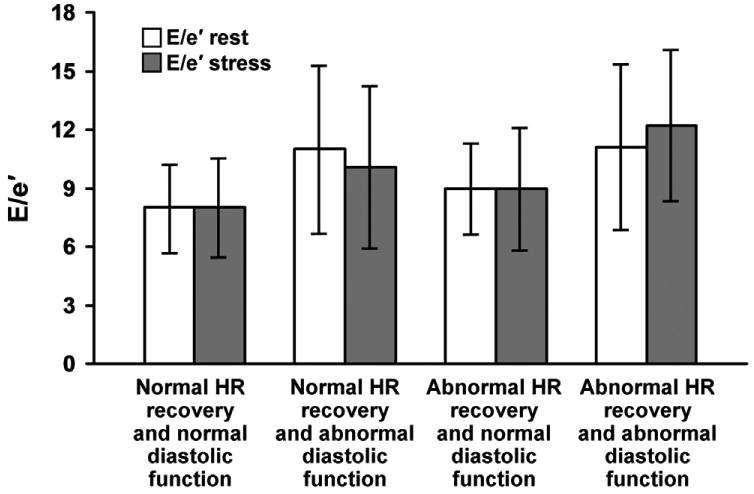
Left ventricular filling pressures (E/e') at rest and stress across groups stratified by HR recovery and diastolic function. Higher resting and stress filling pressures were seen in groups with abnormal HR recovery and/or abnormal diastolic function compared to patients in the group with normal indices for both. E/e' remained the same or decreased with exercise, except in those with diastolic dysfunction and abnormal HR recovery in which case it increased with exercise. Between group comparisons to the group with normal HR recovery and normal diastolic function (referent) for E/e'at rest and E/e' at stress were significant at p≤0.001. Abbreviations: HR = heart rate.
Impact of HR Recovery and Diastolic Function on Exercise Capacity
Exercise capacity and HR recovery were directly correlated overall (r=0.37, p=<0.0001), and within each subgroup of diastolic function (r=0.29, p<0.001 for normal diastolic function; r=0.27, p<0.001 for abnormal diastolic function). The relationship between exercise capacity and diastolic function within successively higher quintiles of HR recovery is shown in Figure 2. As expected, exercise capacity increased with increasing HR recovery; however, patients with diastolic dysfunction had lower exercise capacity, as assessed by METs achieved, as compared to patients with normal diastolic function within each HR recovery quintile. This relationship was similar after accounting for both beta blocker and calcium channel blocker use between diastolic function groups. After excluding those taking beta blockers, patients with normal diastolic function had reduced exercise capacity when HR recovery was abnormal as compared to when HR recovery was normal (9.6±2.5 METs vs 11.2±2.5 METs, p<0.0001, respectively). After excluding those taking calcium channel blockers, patients with normal diastolic function had reduced exercise capacity when HR recovery was abnormal as compared to when HR recovery was normal (9.4±2.4 METs vs 11.1±2.5 METs, p <0.0001, respectively). Among those not taking beta blockers with abnormal diastolic function, patients with normal HR recovery had greater exercise capacity than patients with abnormal HR recovery (8.9±2.3 METs vs 7.9±2.1 METs, p<0.0001, respectively). Similarly, in those not taking calcium channel blockers and having abnormal diastolic function, patients with normal HR recovery had greater exercise capacity than patients with abnormal HR recovery (8.9±2.3 METs vs 7.7±2.1 METs, p<0.0001, respectively). The lowest exercise capacity was observed in patients with diastolic dysfunction and abnormal HR recovery.
Figure 2.
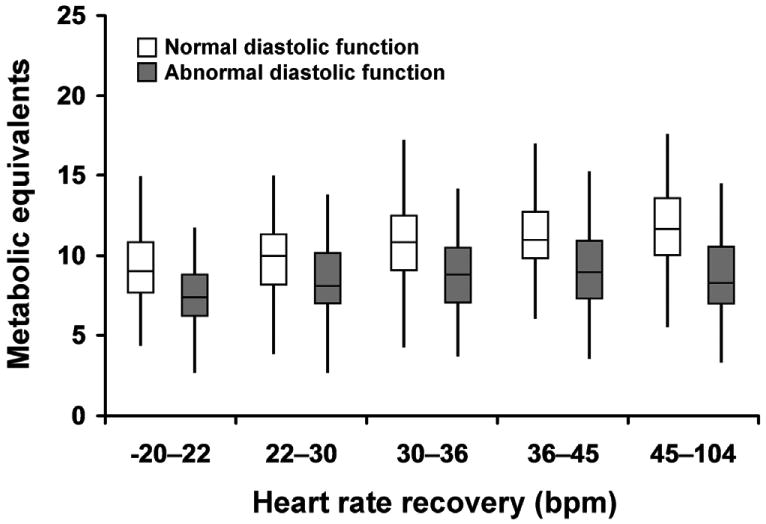
The relationship between exercise capacity, as measured in metabolic equivalents, and diastolic function across quintiles of HR recovery. Patients with normal diastolic function (clear boxes) had greater exercise capacity within higher quartiles of HR recovery as compared to patients with abnormal diastolic function (shaded boxes) who had an attenuated exercise capacity despite increase in HR recovery. Boxes represent 25th-75th percentile and medians are denoted by the line within the boxes. The tails on either end of the boxes denote 10th-90th percentile. All pairwise comparisons between normal and abnormal diastolic function within each HR recovery quintile were significant at p<0.0001. Abbreviations: bpm = beats per minute.
Predictors of Abnormal HR Recovery
Table 3A shows the results of the multivariable logistic regression model evaluating factors associated with abnormal HR recovery. Age (adjusted OR 1.75 per 10 year increment; 95% CI 1.59-1.92) and diastolic dysfunction (adjusted OR 1.47; 95% CI 1.20-1.80) were the strongest predictors of abnormal HR recovery. Other variables independently associated with abnormal HR recovery included hypertension, beta blockers, female sex, previous/current smoker, and BMI > 30kg/m2 (overall model c-index = 0.738). Assessment of models excluding non-significant factors was performed and showed similar results. A sensitivity analysis using previously published cutoffs for abnormal HR recovery in patients undergoing stress echocardiography (≤18 beats per min)4, 15 showed similar results; diastolic dysfunction (referent; normal diastolic function) remained independently associated with abnormal HR recovery (adjusted OR 1.37; 95% CI 1.06-1.79). To eliminate confounding from beta blockers, we restricted the model to only patients not taking beta blockers (n = 2042) to assess the association between diastolic dysfunction and abnormal HR recovery. This model showed a similar magnitude of association between diastolic dysfunction and abnormal HR recovery (adjusted OR 1.64; 95% CI 1.27-2.11) (Table 3B). Similarly, when the model was restricted to those patients not taking calcium channel blockers (n = 2572), there was a similar odds of having abnormal HR recovery in patients with diastolic dysfunction (adjusted OR 1.44; 95% CI 1.16-1.78). Results were similar when the lowest quartile of peak HR (i.e., <133 bpm) was modeled as the outcome of interest; diastolic dysfunction remained an independent predictor of low peak HR. Although HR at baseline was not significant in univariate analysis, when included in the multivariable model as an additional covariate, diastolic dysfunction remained a significant independent predictor of abnormal HR recovery. When diastolic function was added to the baseline clinical model, the overall model chi-square was significantly increased from 310.4 to 325.0 (p<0.001). Finally, given the inter-relationships between age, peak HR, and functional capacity, we modeled the proportion of HR reserve used at peak exercise to determine factors independently associated with chronotropic incompetence. In this multivariable model, diastolic dysfunction was an independent predictor of chronotropic incompetence (adjusted OR 1.42; 95% CI 1.16-1.74).
Table 3A. Multivariable regression of baseline variables associated with abnormal heart rate recovery in patients without exercise echocardiographic evidence of ischemia*†.
| Variables | Model χ2 | Adjusted Odds Ratio (95% Confidence Interval) | p value |
|---|---|---|---|
| Age, per 10 year | 133.1 | 1.75 (1.59-1.92) | <0.0001 |
| Any diastolic dysfunction (vs normal diastolic function) | 13.9 | 1.47 (1.20-1.80) | 0.0002 |
| Hypertension | 8.7 | 1.36 (1.11-1.66) | 0.003 |
| Beta blockers | 7.2 | 1.32 (1.08-1.63) | 0.007 |
| Previous/current smoker | 7.4 | 1.29 (1.07-1.55) | 0.007 |
| Female sex | 6.5 | 1.27 (1.06-1.53) | 0.011 |
| BMI>30kg/m | 4.4 | 1.23 (1.01-1.50) | 0.036 |
| Table 3B. Multivariable regression of baseline variables associated with abnormal heart rate recovery in patients not on beta blockers and without exercise echocardiographic evidence of ischemia† | |||
|---|---|---|---|
| Variables | Model χ2 | Adjusted Odds Ratio (95% Confidence Interval) | p value |
| Age, per 10 year | 69.3 | 1.63 (1.45-1.82) | <0.0001 |
| Any diastolic dysfunction (vs normal diastolic function) | 14.5 | 1.64 (1.27-2.11) | 0.0001 |
| Hypertension | 8.2 | 1.40 (1.11-1.76) | 0.004 |
| Previous/current smoker | 6.1 | 1.32 (1.06-1.66) | 0.01 |
| Female sex | 2.9 | 1.22 (0.97-1.52) | 0.09 |
| BMI>30kg/m | 0.8 | 1.12 (0.88-1.43) | 0.36 |
Wall motion score index at rest, E/e' at rest, left ventricular hypertrophy, baseline systolic blood pressure, history of coronary artery disease, were associated with abnormal heart rate recovery in univariate analysis but not in multivariable analysis.
Ejection fraction, left ventricular end diastolic dimension, and heart rate at baseline were not significant in univariate analysis.
model c-index 0.738
model c-index 0.719
Discussion
We show that in patients without exercise-induced myocardial ischemia, resting diastolic dysfunction is independently associated with abnormal HR recovery. Patients with abnormal HR recovery, as compared to patients with normal HR recovery, were more likely to have higher resting and post-exercise E/e' and other echo Doppler parameters of diastolic dysfunction, including shorter DT, larger left atrial volumes, and lower medial e' values. Diastolic dysfunction remained associated with abnormal HR recovery after adjusting for baseline demographic, clinical, and echocardiographic characteristics. Furthermore, diastolic dysfunction was associated with chronotropic incompetence, as defined using the more rigorous definition of the proportion of HR reserve used during exercise. Moreover, the lowest exercise capacity was observed in patients with both diastolic dysfunction and abnormal HR recovery. These findings suggest that baseline diastolic dysfunction and autonomic abnormalities, as represented by abnormal HR recovery and chronotropic incompetence, are interrelated and likely contributory factors to impairment of exercise capacity.
The current data identifies an independent association between diastolic dysfunction and abnormal autonomic function, as represented by abnormal HR recovery and chronotropic incompetence, in a large cohort of patients without evidence of ischemia by exercise echocardiography. Prior studies have demonstrated an association between heart failure and left ventricular systolic dysfunction to abnormal HR recovery4, 15, 25; however, an association between diastolic function and abnormal HR recovery had remained unknown. Interestingly, we observed that among patients with resting diastolic dysfunction, abnormal HR recovery, as compared to normal HR recovery, was associated with higher E/e'. E/e' usually remains the same or decreases with exercise, and this was observed in all groups except in those with diastolic dysfunction and abnormal HR recovery. Abnormal autonomic function, as represented by abnormal HR recovery, may help explain a subset of patients that develop elevated E/e' with exercise and, consequently, exercise intolerance. The patients in our study all exercised on the Bruce protocol, thereby reducing the heterogeneity of different exercise protocols. In addition, measurements of diastolic function were assessed immediately prior to exercise rather than on a separate occasion. Such an approach is preferable when evaluating diastolic parameters that may be susceptible to change depending on loading conditions or clinical health status.
Considered a blunted return of parasympathetic activation post-exercise, abnormal HR recovery is a marker of autonomic dysfunction1, 5. Similarly, impaired sympathetic/parasympathetic balance has been postulated as the mechanism behind chronotropic incompetence1, which is of prognostic significance in a broad population of patients. In addition, impairment of cardiac vagal tone has been shown to predict mortality in asymptomatic patients and in those undergoing stress testing8, 15, 26. As a prognostic marker, HR recovery is a predictor of mortality after accounting for left ventricular systolic function and angiographically documented coronary artery disease severity4, 15. Autonomic abnormalities have been associated with diastolic dysfunction in a small group of patients with diabetic cardiomyopathy12 and have been identified in patients with heart failure and preserved ejection fraction9, 10, 27. This relationship may be related to the influence of the autonomic nervous system on calcium handling in cardiomyocytes11-13, which can affect pressure relaxation and calcium cycling kinetics28. Our results, which identify an association between diastolic dysfunction and abnormal HR recovery, are notable given that previous studies assessing the relationship between HR recovery as a predictor variable and clinical outcome did not consider diastolic parameters when assessing confounders of HR recovery4, 6, 8, 15. The degree to which the autonomic nervous system contributes to diastolic dysfunction, including whether HR recovery and diastolic dysfunction are modifiable markers or mediators of risk, requires further study.
A negative correlation between resting left ventricular filling pressure (E/e') and HR recovery in patients with heart failure and preserved ejection fraction undergoing cardiopulmonary exercise testing has been shown, although comprehensive diastolic function assessment was not performed29. Fang et al. noted diastolic dysfunction was associated with impaired exercise capacity in diabetic patients while normal HR recovery was associated with preserved diastolic function as measured using tissue Doppler30. Borlaug et al. identified impairment of HR response, specifically HR deceleration and blunted baroreflex response, in previously hospitalized patients with heart failure and preserved ejection fraction, indicating the presence of autonomic dysfunction in these patients when compared to a hypertensive reference group with similar comorbidities9. Given the morbidity of heart failure, particularly the subtype with preserved ejection fraction, cardiac devices that modulate sympathovagal balance using carotid sinus stimulation are being evaluated31. Along similar mechanistic principles, vagus nerve stimulators have been tested in phase II studies in patients with symptomatic heart failure and left ventricular dysfunction32. Our finding that diastolic dysfunction is associated with both abnormal HR recovery and chronotropic incompetence suggests a potential mechanistic link to the impairments in cardiac and autonomic reserve that lead to symptomatic heart failure in patients with diastolic dysfunction27.
The complex relationship between HR control and the parasympathetic nervous system is reflected in numerous modulators of parasympathetic function, second messenger systems, and calcium cycling33. Parasympathetic activation has been associated with cardioprotection and favorable left ventricular remodeling, while alterations of parasympathetic control, mediated primarily by the vagus nerve, have been observed early in the course of left ventricular dysfunction33, 34. Given the challenges of treating diastolic dysfunction and the absence of effective therapies for diastolic heart failure, modulation of parasympathetic and sympathetic balance may indirectly enhance diastolic function, and this merits future study.
Limitations
Exercise capacity was measured by estimated METs, which is less precise compared with expired gas analysis, and duration of exercise was symptom-limited and not maximal, as is often the case in clinical evaluation. Although ethnic information was not available, our study population was mostly white and of European ancestry, thus findings may not be readily generalizable to other populations. Although we report rates of calcium channel blocker use at baseline, we do not have information regarding the relative frequency of nondihydropyridine calcium channel blocker usage in patients; this could confound peak HR and HR recovery values. We excluded patients with stress induced regional wall motion abnormalities as myocardial ischemia could confound the assessment of the relationship between diastolic function and abnormal HR recovery; however, we cannot definitively exclude the possibility that ischemia may have been a factor in some patients given that exercise echocardiography has imperfect sensitivity.
Conclusions
Resting diastolic dysfunction is independently associated with abnormal HR recovery, a marker of autonomic dysfunction and adverse clinical outcome. This suggests that abnormalities in autonomic function may contribute to impairment of diastolic performance in disease states, such as hypertensive heart disease, aging and heart failure. Future studies evaluating factors associated with HR recovery, or HR recovery as a predictor of clinical outcomes, should take baseline diastolic function assessment into account. This would provide a greater understanding as to whether diastolic abnormalities modify or interact with the adverse outcomes observed among patients with abnormal HR recovery. Further investigation is needed to determine if novel treatments currently under investigation to enhance autonomic balance might improve diastolic function.
Acknowledgments
Sources of support: Dr. Gharacholou is a participant in the NIH clinical research loan repayment program (1L30 AG034828-01). The study was supported by a grant from the Mayo Foundation.
Footnotes
Conflict of Interest: none declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kligfield P, Lauer MS. Exercise electrocardiogram testing: beyond the ST segment. Circulation. 2006;114(19):2070–82. doi: 10.1161/CIRCULATIONAHA.105.561944. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher GF, Balady GJ, Amsterdam EA, et al. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation. 2001;104(14):1694–740. doi: 10.1161/hc3901.095960. [DOI] [PubMed] [Google Scholar]
- 3.Cole CR, Foody JM, Blackstone EH, et al. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med. 2000;132(7):552–5. doi: 10.7326/0003-4819-132-7-200004040-00007. [DOI] [PubMed] [Google Scholar]
- 4.Vivekananthan DP, Blackstone EH, Pothier CE, et al. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol. 2003;42(5):831–8. doi: 10.1016/s0735-1097(03)00833-7. [DOI] [PubMed] [Google Scholar]
- 5.Imai K, Sato H, Hori M, et al. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol. 1994;24(6):1529–35. doi: 10.1016/0735-1097(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 6.Shetler K, Marcus R, Froelicher VF, et al. Heart rate recovery: validation and methodologic issues. J Am Coll Cardiol. 2001;38(7):1980–7. doi: 10.1016/s0735-1097(01)01652-7. [DOI] [PubMed] [Google Scholar]
- 7.Arai Y, Saul JP, Albrecht P, et al. Modulation of cardiac autonomic activity during and immediately after exercise. Am J Physiol. 1989;256(1 Pt 2):H132–41. doi: 10.1152/ajpheart.1989.256.1.H132. [DOI] [PubMed] [Google Scholar]
- 8.Cole CR, Blackstone EH, Pashkow FJ, et al. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341(18):1351–7. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 9.Borlaug BA, Melenovsky V, Russell SD, et al. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114(20):2138–47. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 10.Phan TT, Shivu GN, Abozguia K, et al. Impaired heart rate recovery and chronotropic incompetence in patients with heart failure with preserved ejection fraction. Circ Heart Fail. 3(1):29–34. doi: 10.1161/CIRCHEARTFAILURE.109.877720. [DOI] [PubMed] [Google Scholar]
- 11.Flarsheim CE, Grupp IL, Matlib MA. Mitochondrial dysfunction accompanies diastolic dysfunction in diabetic rat heart. Am J Physiol. 1996;271(1 Pt 2):H192–202. doi: 10.1152/ajpheart.1996.271.1.H192. [DOI] [PubMed] [Google Scholar]
- 12.Poirier P, Bogaty P, Philippon F, et al. Preclinical diabetic cardiomyopathy: relation of left ventricular diastolic dysfunction to cardiac autonomic neuropathy in men with uncomplicated well-controlled type 2 diabetes. Metabolism. 2003;52(8):1056–61. doi: 10.1016/s0026-0495(03)00091-x. [DOI] [PubMed] [Google Scholar]
- 13.Willenheimer RB, Erhardt LR, Nilsson H, et al. Parasympathetic neuropathy associated with left ventricular diastolic dysfunction in patients with insulin-dependent diabetes mellitus. Scand Cardiovasc J. 1998;32(1):17–22. doi: 10.1080/14017439850140292. [DOI] [PubMed] [Google Scholar]
- 14.Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) Circulation. 2002;106(14):1883–92. doi: 10.1161/01.cir.0000034670.06526.15. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe J, Thamilarasan M, Blackstone EH, et al. Heart rate recovery immediately after treadmill exercise and left ventricular systolic dysfunction as predictors of mortality: the case of stress echocardiography. Circulation. 2001;104(16):1911–6. [PubMed] [Google Scholar]
- 16.Wilkoff BL, Miller RE. Exercise testing for chronotropic assessment. Cardiol Clin. 1992;10(4):705–17. [PubMed] [Google Scholar]
- 17.Lauer MS, Francis GS, Okin PM, et al. Impaired chronotropic response to exercise stress testing as a predictor of mortality. JAMA. 1999;281(6):524–9. doi: 10.1001/jama.281.6.524. [DOI] [PubMed] [Google Scholar]
- 18.Elhendy A, Mahoney DW, Khandheria BK, et al. Prognostic significance of impairment of heart rate response to exercise: impact of left ventricular function and myocardial ischemia. J Am Coll Cardiol. 2003;42(5):823–30. doi: 10.1016/s0735-1097(03)00832-5. [DOI] [PubMed] [Google Scholar]
- 19.Lauer MS, Mehta R, Pashkow FJ, et al. Association of chronotropic incompetence with echocardiographic ischemia and prognosis. J Am Coll Cardiol. 1998;32(5):1280–6. doi: 10.1016/s0735-1097(98)00377-5. [DOI] [PubMed] [Google Scholar]
- 20.Khan MN, Pothier CE, Lauer MS. Chronotropic incompetence as a predictor of death among patients with normal electrograms taking beta blockers (metoprolol or atenolol) Am J Cardiol. 2005;96(9):1328–33. doi: 10.1016/j.amjcard.2005.06.082. [DOI] [PubMed] [Google Scholar]
- 21.Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102(15):1788–94. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 22.Grewal J, McCully RB, Kane GC, et al. Left ventricular function and exercise capacity. JAMA. 2009;301(3):286–94. doi: 10.1001/jama.2008.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khouri SJ, Maly GT, Suh DD, et al. A practical approach to the echocardiographic evaluation of diastolic function. J Am Soc Echocardiogr. 2004;17(3):290–7. doi: 10.1016/j.echo.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Jouven X, Empana JP, Schwartz PJ, et al. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352:1951–8. doi: 10.1056/NEJMoa043012. [DOI] [PubMed] [Google Scholar]
- 25.Kitaoka H, Takata J, Furuno T, et al. Delayed recovery of postexercise blood pressure in patients with chronic heart failure. Am J Cardiol. 1997;79(12):1701–4. doi: 10.1016/s0002-9149(97)00229-4. [DOI] [PubMed] [Google Scholar]
- 26.Nishime EO, Cole CR, Blackstone EH, et al. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. JAMA. 2000;284(11):1392–8. doi: 10.1001/jama.284.11.1392. [DOI] [PubMed] [Google Scholar]
- 27.Patel H, Ozdemir BA, Patel M, et al. Impairment of autonomic reactivity is a feature of heart failure whether or not the left ventricular ejection fraction is normal. Int J Cardiol. 2010 doi: 10.1016/j.ijcard.2010.04.054. [DOI] [PubMed] [Google Scholar]
- 28.Oh JK, Seward JB, Tajik AJ. The Echo Manual. 3rd. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 29.Guazzi M, Myers J, Peberdy MA, et al. Cardiopulmonary Exercise Testing Variables Reflect the Degree of Diastolic Dysfunction in Patients With Heart Failure-Normal Ejection Fraction. J Cardiopulm Rehabil Prev. 2010;30(3):165–72. doi: 10.1097/HCR.0b013e3181d0c1ad. [DOI] [PubMed] [Google Scholar]
- 30.Fang ZY, Sharman J, Prins JB, et al. Determinants of exercise capacity in patients with type 2 diabetes. Diabetes Care. 2005;28(7):1643–8. doi: 10.2337/diacare.28.7.1643. [DOI] [PubMed] [Google Scholar]
- 31.Georgakopoulos D, Little WC, Abraham WT, et al. Chronic baroreflex activation: a potential therapeutic approach to heart failure with preserved ejection fraction. J Cardiac Fail. 2011;17:167–78. doi: 10.1016/j.cardfail.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 32.De Ferrari GM, Crijns HJ, Borggrefe M, et al. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J. 2011;32:847–55. doi: 10.1093/eurheartj/ehq391. [DOI] [PubMed] [Google Scholar]
- 33.Olshansky B, Sabbah HN, Hauptman PJ, et al. Parasympathetic nervous system and heart failure: pathophysiology and potential implications for therapy. Circulation. 2008;118(8):863–71. doi: 10.1161/CIRCULATIONAHA.107.760405. [DOI] [PubMed] [Google Scholar]
- 34.Kinugawa T, Dibner-Dunlap ME. Altered vagal and sympathetic control of heart rate in left ventricular dysfunction and heart failure. Am J Physiol. 1995;268(2 Pt 2):R317–23. doi: 10.1152/ajpregu.1995.268.2.R310. [DOI] [PubMed] [Google Scholar]


