Summary
Fabry disease patients show a deficiency in the activity of the lysosomal enzyme α-galactosidase (α-GAL or α-Gal A). One proposed treatment for Fabry disease is pharmacological chaperone therapy, where a small molecule stabilizes the α-GAL protein, leading to increased enzymatic activity. Using enzyme kinetics, tryptophan fluorescence, circular dichroism, and proteolysis assays, we show that the pharmacological chaperones 1-deoxygalactonojirimycin (DGJ) and galactose stabilize the human α-GAL glycoprotein. Crystal structures of complexes of α-GAL and chaperones explain the molecular basis for the higher potency of DGJ over galactose. Using site directed mutagenesis, we show the higher potency of DGJ results from an ionic interaction with D170. We propose that protonation of D170 in acidic conditions leads to weaker binding of DGJ. The results establish a biochemical basis for pharmacological chaperone therapy applicable to other protein misfolding diseases.
Introduction
α-Galactosidase (α-GAL, also known as α-galactosidase A or α-GAL A; EC 3.2.1.22) is a lysosomal glycosidase that break down complex macromolecules for cellular reuse. α-GAL catalyzes the hydrolysis of terminal α-linked galactosides from macromolecules. In humans, deficiency of the α-GAL enzyme causes Fabry disease, a lysosomal storage disease characterized by the progressive accumulation of metabolites in the cells, leading to tissue damage and eventual organ failure (Brady et al., 1967; Desnick et al., 2001). Many Fabry disease-causing mutations have been identified in the GLA gene encoding the α-GAL protein (Human Gene Mutation Database, www.hgmd.org), most of which disrupt the hydrophobic core of the protein, presumably leading to protein misfolding and degradation in the ER (Eng and Desnick, 1994; Fan et al., 1999; Garman and Garboczi, 2002, 2004; Okumiya et al., 1995; Romeo et al., 1975). Thus Fabry disease is primarily a protein misfolding disease.
The only currently approved treatment for Fabry disease is Enzyme Replacement Therapy (ERT), where recombinant enzyme is intravenously administered into patients to restore the missing enzymatic function. ERT has demonstrated reduction of accumulated substrate in tissues, leading to clinical improvement of Fabry disease patients (Eng et al., 2001; Schiffmann et al., 2001), and has been proposed for many inherited metabolic diseases (Beutler, 2006).
An alternative treatment, pharmacological chaperone (PC) therapy, has been proposed for Fabry disease and other protein misfolding diseases (Fan and Ishii, 2007; Parenti, 2009; Sawkar et al., 2005; Suzuki et al., 2009; Tan et al., 1991). In contrast to using non-specific small molecules for “chemical chaperone therapy,” PC therapy for Fabry disease uses an active-site specific chaperone, such as the catalytic product galactose (Frustaci et al., 2001), or a product analogue, such as the imino sugar 1-deoxygalactonojirimycin (DGJ, currently in Phase III clinical trials) (Asano et al., 2000). In PC therapy, the small molecule is hypothesized to stabilize the folded enzyme, shifting the folding equilibrium towards properly folded protein, and reducing removal of the polypeptide through Endoplasmic Reticulum (ER)-associated degradation (ERAD) (Cohen and Kelly, 2003; Fan et al., 1999; Yam et al., 2006; Yam et al., 2005). PCs such as DGJ and galactose are promising clinical candidates, yet their biochemical mechanism is not well understood: they have been proposed to accelerate the folding of their target, to slow the unfolding of the target, to stabilize the target, to allow for proper folding, to promote post-translational modification, to stabilize the protein, and/or to allow binding of a partner to the target (Fan et al., 1998; Lieberman et al., 2009). Additionally, how competitive enzymatic inhibition leads to increased activity remains unresolved. Because of their potential for treating a wide range of protein misfolding diseases (Cohen and Kelly, 2003), PCs have attracted intense clinical attention.
In this study, we examine the biochemical and biophysical basis for PC binding to human α-GAL. We show by biochemical assays that DGJ binds to and stabilizes α-GAL with higher potency than galactose. We investigate the effect of pH on the binding affinities of DGJ and galactose and show that the chaperones stabilize better at near neutral pH than at acidic pH. Crystal structures of α-GAL in complex with the PCs DGJ and galactose reveal a key ionic interaction critical for the increased potency of DGJ. Finally, we performed biochemical studies on a D170A variant of α-GAL, unambiguously identifying the atomic interaction responsible for the increased potency of DGJ over galactose.
Results and Discussion
Binding of pharmacological chaperones
To measure binding of the PCs DGJ and galactose, we examined the enzymatic activity of α-GAL in the presence of the chaperones. Both DGJ and galactose act as competitive inhibitors of α-GAL. We determined the Ki for DGJ to be 39 nM and for galactose to be 16 mM (Figure S1 & Table S1). The enzymatic assays show that DGJ is 400,000 fold more potent than galactose at inhibiting α-GAL, corresponding to a 7.6 kcal/mol of additional binding energy, a remarkable difference for molecules that differ in only two functional groups.
Resistance to unfolding monitored by Trp fluorescence
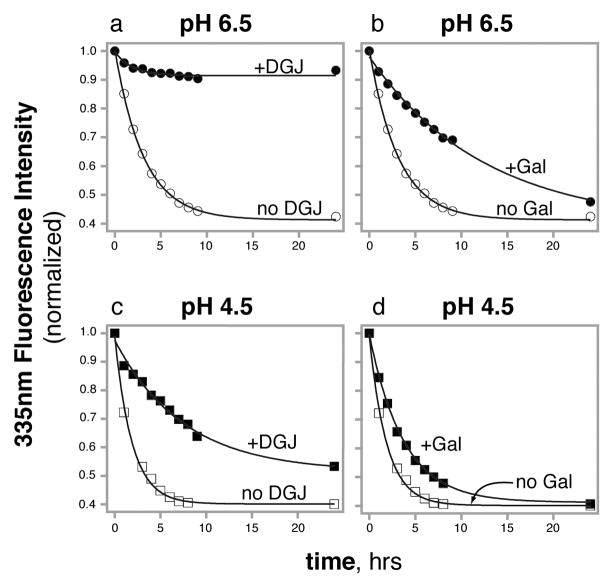
To measure the unfolding rate of α-GAL in 7.5 M urea, we used intrinsic tryptophan fluorescence. The fluorescence signal of α-GAL shows a decrease in fluorescence intensity and a red shift in λmax from 335 nm to 350 nm as the protein denatures. In the absence of chaperone, α-GAL denatures with a t½ of 2.2 hours at pH 6.5 and 1.3 hours at pH 4.5 (Figures 1 & S2), indicating that α-GAL chemically denatures slightly faster at the lower pH.
Figure 1.
Pharmacological chaperones slow the unfolding kinetics of α-GAL (measured by Trp fluorescence). Rate of unfolding of α-GAL at pH 6.5 (a and b) and pH 4.5 (c and d) in the absence (open symbols) and presence (filled symbols) of 50 μM DGJ (a and c) and 50 mM galactose (b and d). See also Figure S2.
Next, to test the effect of DGJ on the unfolding rate, we repeated the fluorescence assay after pre-incubation with DGJ. The rate of unfolding of α-GAL is slowed considerably by the addition of PCs, particularly at pH 6.5. At pH 6.5, the addition of 50 μM DGJ slows the unfolding of α-GAL to a t½ greater than 24 hours, with little change in the fluorescence spectrum over 24 hours. At pH 4.5, the addition of 50 μM DGJ decreases the rate of unfolding of α-GAL from a t½ of 1.3 hours to 6.5 hours.
Third, to test the effect of galactose, we repeated the fluorescence assay with galactose. The addition of 50 mM galactose also slows the urea unfolding of α-GAL, increasing the t½ of unfolding from 2.2 hours to 8.0 hours at pH 6.5 and from 1.3 hours to 2.5 hours at pH 4.5.
We conclude from these experiments that i) PCs are able to slow the rate of unfolding of α-GAL, ii) DGJ is more effective than galactose at preventing unfolding of α-GAL, and iii) both chaperones slow the unfolding more at pH 6.5 than pH 4.5.
Increase in apparent melting temperature [Tm(app)] of α-GAL measured by CD
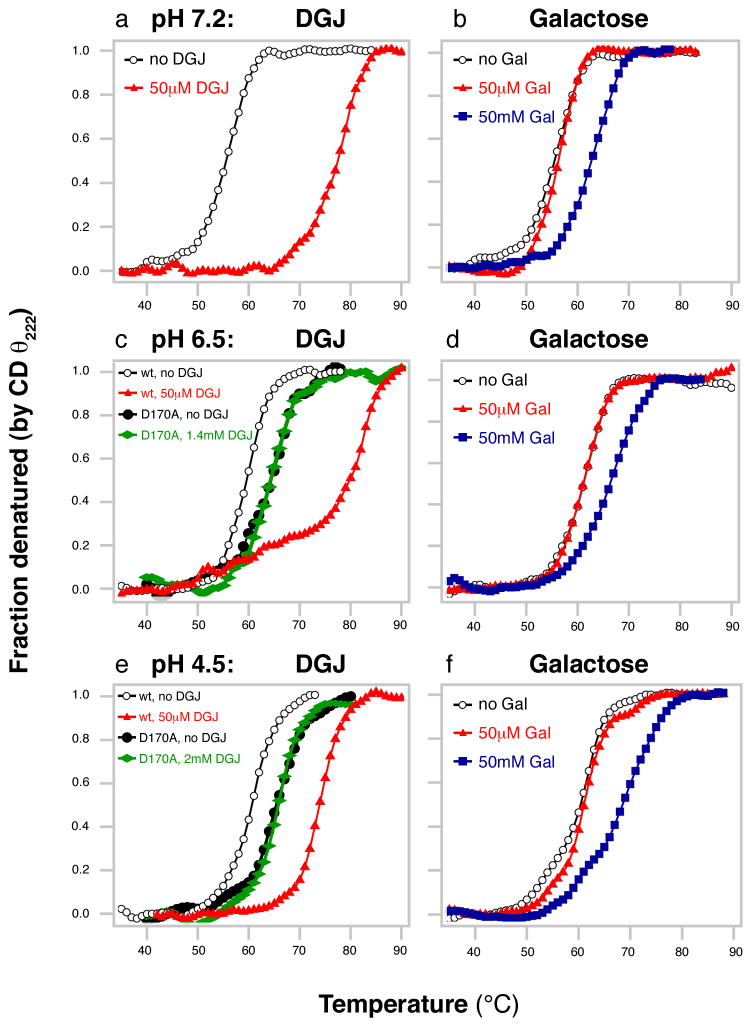
To examine the thermal stability of α-GAL, we measured the apparent melting temperature Tm(app) of α-GAL using the CD signal at 222 nm in thermal denaturation experiments. To assay the effects of the pharmacological chaperones DGJ and galactose and of pH on the stability of α-GAL, we repeated the thermal denaturations in the presence of the chaperones and at three pH values. Upon the addition of 50 μM DGJ, the Tm(app) of α-GAL increases by 13.2–22.0°C, depending on the pH (Figure 2 & Table S2). In contrast, 50 μM galactose has no effect on the Tm(app) of α-GAL. However, upon increasing the concentration of galactose 1000-fold to 50 mM, the Tm(app) of α-GAL increases by 5.3–8.5°C.
Figure 2. Increased apparent melting temperature Tm(app) of α-GAL (monitored by CD).
DGJ (a, c, e) and galactose (b, d, f) were tested at pH 7.2 (a, b), pH 6.5 (c, d), and pH 4.5 (e, f) in the absence (white) and presence of 50 μM (red) or 50 mM (blue) DGJ or galactose. In (c) and (e), the D170A mutant is also shown in the absence (black) and presence (green) of 1.4 or 2 mM DGJ. The D170A mutant does not respond to even 30- or 40- fold higher concentrations of DGJ.
We also compared the Tm(app) as a function of pH. In the absence of pharmacological chaperone, the Tm(app) of α-GAL is unchanged between pH 4.5 and 6.5 (60.7°C and 60.8°C respectively), but is lower at pH 7.2 (56.1°C), indicating that the protein is less stable at higher pH values.
Resistance to protease digestion monitored by proteolysis
To examine the effect of the PCs on the resistance of α-GAL to protease digestion, we performed proteolysis experiments. In the presence of a protease and urea, the amount of undigested α-GAL protein represents a measure of the protein’s stability.
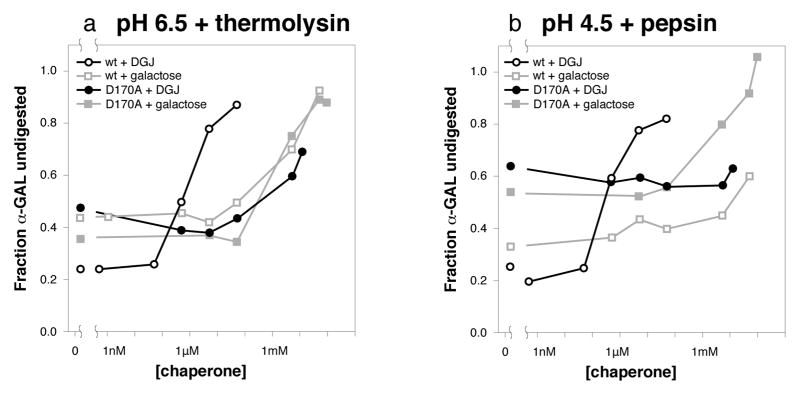
To examine the effect of chaperones at pH 6.5, we digested α-GAL with thermolysin at pH 6.5 and quantitated the amount of α-GAL resistant to the protease. In the presence of DGJ, the α-GAL becomes increasingly resistant to thermolysin digestion starting at 500 nM DGJ (Figures 3 & S3). In the presence of galactose, the α-GAL becomes resistant to protease digestion starting at 10,000-fold higher concentration, approximately 5 mM galactose.
Figure 3. DGJ and galactose confer protease resistance upon α-GAL.
Thermolysin (a) and pepsin (b) digestion of wild type α-GAL (open symbols) and D170A α-GAL (filled symbols) in urea after incubation with DGJ (black) and galactose (grey) respectively. Wild type and D170A α-GAL band intensities were quantitated at multiple chaperone concentrations. The D170A mutant responds only to high concentrations of chaperone. See also Figures S3 and S4, and Table S2.
To examine the effect at pH 4.5, we digested α-GAL with pepsin and quantitated the undigested α-GAL. In the presence of DGJ, the α-GAL becomes increasingly resistant to protease starting at 500 nM DGJ (Figure 3). In the presence of galactose, the α-GAL becomes resistant to protease starting at 10,000-fold higher concentration, approximately 5 mM galactose.
The proteolysis experiments mirror the results of the fluorescence and CD experiments, showing that both DGJ and galactose are able to stabilize the α-GAL protein. In all three assays, the potency of DGJ is much higher than galactose. In general, the stabilizing effects of DGJ are more pronounced at near-neutral pH.
Structural basis for improved potency of DGJ
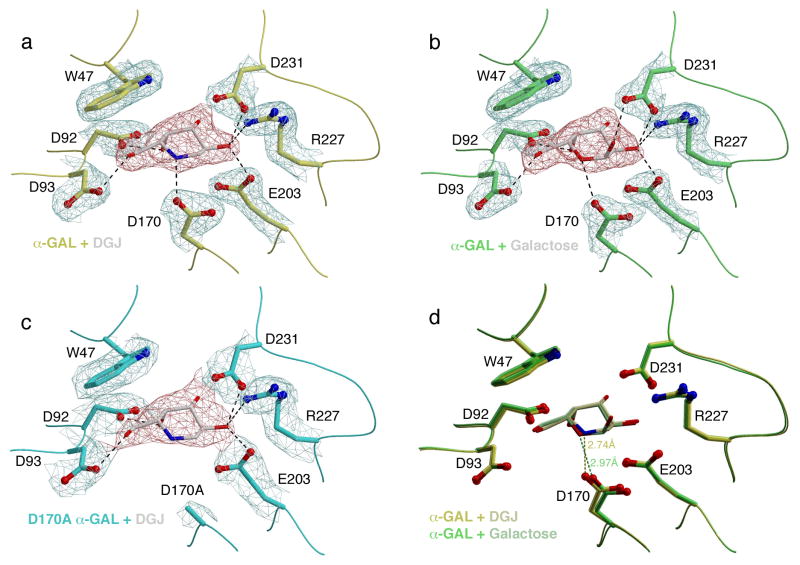
To examine the structural effects of PC binding to α-GAL, we determined high-resolution crystal structures of two complexes: the α-GAL:DGJ complex at 2.1A resolution and the α-GAL:galactose complex at 2.0Å resolution, allowing us to examine the atomic basis for the differences in potency between the chaperones.
The crystal structures show that both DGJ and galactose bind similarly in the active site of α-GAL, as expected for binding of a catalytic product and a product analogue (Figure 4). There is a more favorable interaction between D170 and the DGJ ligand compared to the galactose ligand. In order to act as a nucleophile in the α-GAL reaction mechanism (Guce et al., 2010), the D170 side chain must be deprotonated and negatively charged. The DGJ ligand contains a protonatable heterocyclic nitrogen atom, allowing for an energetically favorable hydrogen bond. Because the pKa of DGJ is 7.1 (Legler and Pohl, 1986), the nitrogen is likely protonated in the pH 5.1 crystals, leading to highly favorable charged interaction between the DGJ and the D170 side chain. Galactose functions as a chaperone by mirroring the binding of the galactoside substrate, and the Ki for the PC is close to the KM for substrate (5–20 mM). The galactose ligand contains an unprotonated heterocyclic oxygen, which makes either weak van der Waals interaction with the deprotonated D170 side chain, or a hydrogen bond if D170 is protonated.
Figure 4. Crystal structures of human α-GAL bound to pharmacological chaperones.
(a) and (b) show σA-weighted 2Fo-Fc electron density maps of DGJ- and galactose-soaked crystals of wild-type human α-GAL, and (c) shows the D170A mutant α-GAL with DGJ bound. All maps are contoured at 1.8σ with the ligand density colored red for clarity. (d) shows a superposition of the (a) and (b), highlighting the key interaction between the ligand and the D170 carboxylate. See also Table S3.
Effect of D170 on the interaction with chaperones
The crystal structures of the complexes of α-GAL with DGJ and galactose led us to hypothesize that the higher potency of DGJ derives entirely from an interaction between the heterocyclic nitrogen of the ligand and the carboxylate of the catalytic nucleophile D170. To test this hypothesis, we made a D170A variant of human α-GAL (lacking the carboxylate) and examined the ability of DGJ to bind and stabilize this variant. Because the D170A variant lacks enzymatic activity, we used biochemical and crystallographic assays to test PC binding.
First, we repeated the CD thermal denaturation experiments at pH 6.5 and 4.5 with the D170A α-GAL variant. Consistent with our hypothesis, the D170A α-GAL shows no increase in Tm(app), even in the presence of 1.4 or 2 mM DGJ (Figure 2), whereas the wild type α-GAL shows a 13–21° increase in Tm(app) with 30- or 40-fold less DGJ. Thus, the D170 carbonyl is critical to the stabilizing effect of DGJ.
Second, we repeated the proteolysis experiments on the D170A mutant with DGJ and galactose. In contrast to wild type α-GAL, the D170A variant requires a much higher concentration of DGJ to protect from digestion. The removal of the D170 carboxylate group increases the DGJ concentration threshold for protection by over 1000-fold (Figures 3, S3, & S4). Thus, the D170 carboxylate group is primarily responsible for the much higher potency of DGJ. Using galactose as a PC in the protease assay shows that in the D170A variant, DGJ is no better than galactose as a PC, with protection occuring at millimolar concentrations of galactose. These results indicate that the D170 carboxylate is more critical to the DGJ interaction than it is to the galactose interaction, and that the increased potency of DGJ is entirely due to interaction with the D170 side chain.
Third, we determined a crystal structure of DGJ bound to the D170A mutant α-GAL, which showed that DGJ binds to the D170A active site identically to wild type α-GAL (Figure 4). Thus the high potency of DGJ for wild type α-GAL derives from its interaction with the D170 side chain.
Conclusions
Our studies show that PC binding confers thermodynamic stability α-GAL and dramatically slows the unfolding of the protein. In the equilibrium between native and unfolded states of α-GAL, PC binding to the native state slows the rate of unfolding and shifts the equilibrium toward the native state. This property is particularly valuable in the ER, where the folding of the nascent polypeptide helps it to avoid the ERAD pathway. Stabilization of the native state of the protein increases the fraction of enzyme that traffics out of the ER and travels to the lysosome.
For a small molecule to be an effective PC, it must be able to selectively bind to the active site of an enzyme but then dissociate, allowing the enzyme to turnover substrate (Fan, 2003, 2008). Different models exist for the force driving low pH dissociation, including protonation of the pharmacological chaperone, protonation of an active site residue, competition by excess substrate, etc. (Fan, 2003, 2008; Fantur et al., 2010; Jo et al., 2010; Suzuki et al., 2009). For lysosomal enzymes, the pH dependence of affinity of the PC is important, as the chaperone must dissociate from the active site at low pH for the enzyme to function. The heterocyclic nitrogen of DGJ has a pKa of 7.1, and the protonation state of PCs has been hypothesized to cause the pH-dependent release (Fantur et al., 2010). We propose an alternative hypothesis for the pH dependence of DGJ binding to α-GAL, that the protonation state of the active site nucleophile D170 causes pH dependence. At pH 6.5 and 7.2, the D170 carboxylate is expected to deprotonate and the DGJ nitrogen to protonate, leading to a highly favorable ionic interaction between them. In contrast, at pH 4.5, the carboxylate of D170 is more likely to protonate, losing its ionic interaction with the nitrogen of DGJ, leading to weaker binding. In our model, the protonation state of the D170 carboxylate affects the affinity of α-GAL for DGJ, and removal of the carboxylate in the D170A α-GAL variant leads to much weaker binding to DGJ. Our experiments do not support an alternative model where protonation of the nitrogen in DGJ leads to weaker binding of the pharmacological chaperone (Fantur et al., 2010).
In conclusion, we have made the following observations about the interaction of DGJ and PCs. First, DGJ binds to the WT α-GAL and stabilizes the enzyme, as shown by the CD, fluorescence, and proteolysis experiments. Second, in all the biochemical experiments, the protective effect of DGJ is greater at neutral pH than at pH 4.5. Third, galactose is capable of PC activity but requires 10,000 to 100,000-fold higher concentrations than DGJ, consistent with the differences in Ki measured in enzymatic assays. Fourth, crystal structures show that the PCs bind exclusively to the active site, and the protective effect of the chaperones derives from specific interactions with active site residues. As a counter example, glucose binds weakly to α-GAL away from the active site (Guce et al., 2010) but does not show the same chaperoning effect as DGJ or galactose. Fifth, the enhanced potency of DGJ results from interactions with the D170 carboxylate. When the carboxylate is removed in the D170A variant, DGJ chaperones no better than galactose. We have identified the key atomic interaction responsible for the increased potency of DGJ. These results can be generalized to the entire family of active site specific chaperones, allowing for development of improved chaperones.
Supplementary Material
Significance.
Using a pharmacological chaperone to treat a protein folding disease presents a molecular paradox: to increase the activity of the enzyme, a competitive inhibitor of the enzyme is used. We probe the molecular mechanism of the paradox using biochemical and biophysical approaches on human α-GAL, including enzyme kinetics, chemical denaturation monitored by fluorescence, thermal denaturation monitored by circular dichroism, protease susceptibility, and x-ray crystallography. Our studies show that 1-deoxygalactonojirimycin (DGJ), which is only two functional groups different from galactose, is a 400,000-fold better binder. We hypothesize that one ionic interaction is responsible for the higher potency of DGJ. We test the hypothesis using a D170A mutant α-GAL lacking the ionic interaction, which loses the high potency of DGJ. We explore the pH dependence of pharmacological chaperone binding, as the chaperones must dissociate from α-GAL in the low pH of the lysosome. In this work, we refute one proposed mechanism of action (that protonation of the small molecule leads to weaker binding in the lysosome), and propose that protonation of the catalytic nucleophile D170 causes weaker DGJ binding at low pH.
Acknowledgments
This work was funded by NIH grant R01 DK76877 to S.C.G. and by NSF Integrative Graduate Education and Research Traineeship 0654128 to N.E.C. We gratefully acknowledge Jean Jankonic, Marc Allaire, and Vivian Stojanoff at the National Synchrotron Light Source X6A beam line, funded by the National Institute of General Medical Sciences, National Institute of Health under agreement GM-0080. We thank Genzyme for the gift of Fabrazyme and Kathy Hanley for the gift of α-GAL. The authors declare no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asano N, Ishii S, Kizu H, Ikeda K, Yasuda K, Kato A, Martin OR, Fan JQ. In vitro inhibition and intracellular enhancement of lysosomal α-galactosidase A activity in Fabry lymphoblasts by 1-deoxygalactonojirimycin and its derivatives. Eur J Biochem. 2000;267:4179–4186. doi: 10.1046/j.1432-1327.2000.01457.x. [DOI] [PubMed] [Google Scholar]
- Beutler E. Lysosomal storage diseases: natural history and ethical and economic aspects. Mol Genet Metab. 2006;88:208–215. doi: 10.1016/j.ymgme.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Brady RO, Gal AE, Bradley RM, Martensson E, Warshaw AL, Laster L. Enzymatic defect in Fabry’s disease. Ceramidetrihexosidase deficiency. N Engl J Med. 1967;276:1163–1167. doi: 10.1056/NEJM196705252762101. [DOI] [PubMed] [Google Scholar]
- Cohen FE, Kelly JW. Therapeutic approaches to protein-misfolding diseases. Nature. 2003;426:905–909. doi: 10.1038/nature02265. [DOI] [PubMed] [Google Scholar]
- Desnick RJ, Ioannou YA, Eng CM. α-Galactosidase A Deficiency: Fabry Disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill; 2001. pp. 3733–3774. [Google Scholar]
- Eng CM, Desnick RJ. Molecular basis of Fabry disease: mutations and polymorphisms in the human α-galactosidase A gene. Hum Mutat. 1994;3:103–111. doi: 10.1002/humu.1380030204. [DOI] [PubMed] [Google Scholar]
- Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldek S, Caplan L, Linthorst GE, Desnick RJ. Safety and efficacy of recombinant human α-galactosidase A—replacement therapy in Fabry’s disease. N Engl J Med. 2001;345:9–16. doi: 10.1056/NEJM200107053450102. [DOI] [PubMed] [Google Scholar]
- Fan JQ. A contradictory treatment for lysosomal storage disorders: inhibitors enhance mutant enzyme activity. Trends Pharmacol Sci. 2003;24:355–360. doi: 10.1016/S0165-6147(03)00158-5. [DOI] [PubMed] [Google Scholar]
- Fan JQ. A counterintuitive approach to treat enzyme deficiencies: use of enzyme inhibitors for restoring mutant enzyme activity. Biol Chem. 2008;389:1–11. doi: 10.1515/BC.2008.009. [DOI] [PubMed] [Google Scholar]
- Fan JQ, Ishii S. Active-site-specific chaperone therapy for Fabry disease. Yin and Yang of enzyme inhibitors. FEBS J. 2007;274:4962–4971. doi: 10.1111/j.1742-4658.2007.06041.x. [DOI] [PubMed] [Google Scholar]
- Fan JQ, Ishii S, Asano N, Suzuki Y. Inhibitors enhance lysosomal α-galactosidase A activity in Fabry lymphoblasts: A possible molecular therapy for a genetic disorder. Glycobiology. 1998;8:1143–1143. [Google Scholar]
- Fan JQ, Ishii S, Asano N, Suzuki Y. Accelerated transport and maturation of lysosomal α-galactosidase A in Fabry lymphoblasts by an enzyme inhibitor. Nat Med. 1999;5:112–115. doi: 10.1038/4801. [DOI] [PubMed] [Google Scholar]
- Fantur K, Hofer D, Schitter G, Steiner AJ, Pabst BM, Wrodnigg TM, Stutz AE, Paschke E. DLHex-DGJ, a novel derivative of 1-deoxygalactonojirimycin with pharmacological chaperone activity in human G(M1)-gangliosidosis fibroblasts. Mol Genet Metab. 2010;100:262–268. doi: 10.1016/j.ymgme.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Frustaci A, Chimenti C, Ricci R, Natale L, Russo MA, Pieroni M, Eng CM, Desnick RJ. Improvement in cardiac function in the cardiac variant of Fabry’s disease with galactose-infusion therapy. N Engl J Med. 2001;345:25–32. doi: 10.1056/NEJM200107053450104. [DOI] [PubMed] [Google Scholar]
- Garman SC, Garboczi DN. Structural basis of Fabry disease. Mol Genet Metab. 2002;77:3–11. doi: 10.1016/s1096-7192(02)00151-8. [DOI] [PubMed] [Google Scholar]
- Garman SC, Garboczi DN. The molecular defect leading to Fabry disease: structure of human α-galactosidase. J Mol Biol. 2004;337:319–335. doi: 10.1016/j.jmb.2004.01.035. [DOI] [PubMed] [Google Scholar]
- Guce AI, Clark NE, Salgado EN, Ivanen DR, Kulminskaya AA, Brumer H, Garman SC. Catalytic mechanism of human α-galactosidase. J Biol Chem. 2010;285:3625–3632. doi: 10.1074/jbc.M109.060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo H, Yugi K, Ogawa S, Suzuki Y, Sakakibara Y. Molecular basis of chemical chaperone effects of N-octyl-β-valienamine on human β-glucosidase in low/neutral pH conditions. J Proteomics Bioinform. 2010;3:104–112. [Google Scholar]
- Legler G, Pohl S. Synthesis of 5-amino-5-deoxy-D-galactopyranose and 1,5-dideoxy-1,5-imino-D-galactitol, and their inhibition of α- and β-D-galactosidases. Carbohydr Res. 1986;155:119–129. doi: 10.1016/s0008-6215(00)90138-1. [DOI] [PubMed] [Google Scholar]
- Lieberman RL, D’Aquino JA, Ringe D, Petsko GA. Effects of pH and iminosugar pharmacological chaperones on lysosomal glycosidase structure and stability. Biochemistry. 2009;48:4816–4827. doi: 10.1021/bi9002265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumiya T, Ishii S, Kase R, Kamei S, Sakuraba H, Suzuki Y. α-galactosidase gene mutations in Fabry disease: heterogeneous expressions of mutant enzyme proteins. Hum Genet. 1995;95:557–561. doi: 10.1007/BF00223869. [DOI] [PubMed] [Google Scholar]
- Parenti G. Treating lysosomal storage diseases with pharmacological chaperones: from concept to clinics. EMBO Mol Med. 2009;1:268–279. doi: 10.1002/emmm.200900036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo G, D’Urso M, Pisacane A, Blum E, De Falco A, Ruffilli A. Residual activity of α-galactosidase A in Fabry’s disease. Biochem Genet. 1975;13:615–628. doi: 10.1007/BF00484919. [DOI] [PubMed] [Google Scholar]
- Sawkar AR, Adamski-Werner SL, Cheng WC, Wong CH, Beutler E, Zimmer KP, Kelly JW. Gaucher disease-associated glucocerebrosidases show mutation-dependent chemical chaperoning profiles. Chem Biol. 2005;12:1235–1244. doi: 10.1016/j.chembiol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Schiffmann R, Kopp JB, Austin HA, 3rd, Sabnis S, Moore DF, Weibel T, Balow JE, Brady RO. Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA. 2001;285:2743–2749. doi: 10.1001/jama.285.21.2743. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Ogawa S, Sakakibara Y. Chaperone therapy for neuronopathic lysosomal diseases: competitive inhibitors as chemical chaperones for enhancement of mutant enzyme activities. Perspect Medicin Chem. 2009;3:7–19. doi: 10.4137/pmc.s2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan A, van den Broek L, van Boeckel S, Ploegh H, Bolscher J. Chemical modification of the glucosidase inhibitor 1-deoxynojirimycin. Structure-activity relationships. J Biol Chem. 1991;266:14504–14510. [PubMed] [Google Scholar]
- Yam GH, Bosshard N, Zuber C, Steinmann B, Roth J. Pharmacological chaperone corrects lysosomal storage in Fabry disease caused by trafficking-incompetent variants. Am J Physiol Cell Physiol. 2006;290:C1076–1082. doi: 10.1152/ajpcell.00426.2005. [DOI] [PubMed] [Google Scholar]
- Yam GH, Zuber C, Roth J. A synthetic chaperone corrects the trafficking defect and disease phenotype in a protein misfolding disorder. Faseb J. 2005;19:12–18. doi: 10.1096/fj.04-2375com. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.