Abstract
The long-term effects of ocean warming on prokaryotic communities are unknown because of lack of historical data. We overcame this gap by applying a retrospective molecular analysis to the bacterial community on formalin-fixed samples from the historical Continuous Plankton Recorder archive, which is one of the longest and most geographically extensive collections of marine biological samples in the world. We showed that during the last half century, ubiquitous marine bacteria of the Vibrio genus, including Vibrio cholerae, increased in dominance within the plankton-associated bacterial community of the North Sea, where an unprecedented increase in bathing infections related to these bacteria was recently reported. Among environmental variables, increased sea surface temperature explained 45% of the variance in Vibrio data, supporting the view that ocean warming is favouring the spread of vibrios and may be the cause of the globally increasing trend in their associated diseases.
Keywords: climate change, North Sea, vibrios, Vibrio cholerae, plankton
Introduction
The most immediate and direct effect of climate change at a global ocean scale will be the increment in the sea surface temperature (SST) that is estimated to increase by a few degrees during this century (Harvell et al., 2002). Although much evidence has been accumulated on the long-term effects of ocean warming on eukaryotic populations (for example, animals and plants, Edwards and Richardson, 2004; Sala and Knowlton, 2006), no experimental information exists for the effects this may have on marine prokaryotic abundance and diversity (Sarmento et al., 2010). An explanation for this gap is the lack of historical data and the belief that lower trophic levels, such as the primary producers (phytoplankton) and decomposers (heterotrophic prokaryotes), are considered less sensitive to environmental change than their consumers or predators, because sensitivity to climate is considered to increase with trophic level (Voigt et al., 2003; Raffaelli, 2004).
Vibrios are Gram-negative, curved, rod-shaped bacteria belonging to the class Gammaproteobacteria and are still regarded by most marine microbiologists as the dominant culturable bacteria in the ocean (Pruzzo et al., 2005). They are found on a number of biotic and abiotic substrates, notably associated with chitinous plankton, which is considered to be an important reservoir of these bacteria in nature (Colwell, 1996; Turner et al., 2009). Undoubtedly, the most well-known member of the genus is Vibrio cholerae, the aetiological agent of epidemic cholera (Kaper et al., 1995). Other vibrios capable of causing disease in humans include V. parahaemolyticus, the agent of seafood-associated gastroenteritis worldwide (Levin, 2006), and V. vulnificus, the cause of septicaemia and serious wound infections, as well as the leading cause of shellfish-associated deaths in the United States (Shapiro et al., 1998). Several Vibrio species are also pathogenic towards marine animals, including molluscs (Paillard et al., 2004), corals (Vezzulli et al., 2010) and fish (Austin, 2005), with major economic and environmental impacts.
There is substantial evidence that Vibrio-associated diseases are increasing worldwide with climate warming (Harvell et al., 2002). For example, increased SST linked to El Niño events have been shown to pre-date increases in cholera incidence in both Asia and South America (Pascual et al., 2000). Similarly, climate anomalies have been implicated in the expansion of the geographical and seasonal range of seafood-borne illnesses caused by V. parahaemolyticus and V. vulnificus (Martinez-Urtaza et al., 2010). Evidence has also been gathered linking Vibrio infections to the increasing mass mortality of marine life in the coastal marine environment (Paillard et al., 2004; Vezzulli et al., 2010).
The 2010–2011 MCCIP Annual Report Card (http://www.mccip.org.uk/arc), which provides an up-to-date assessment of how climate change is affecting UK seas, considered, for the first time, the potential future increases in marine vibrios as an emergent issue (Marine Climate Change Impacts Partnership Annual Report Card, 2010). In recent years, in a number of Northwest European countries, there has been an unprecedented increase in the number of bathing infections that have been associated with warm water Vibrio species. For example, during the hot summer of 2006 wound infections linked to contact with Baltic and North Sea waters were reported from Germany (V. vulnificus, Frank et al., 2006), southeast Sweden (V. cholerae non-O1/O139, Andersson and Ekdahl, 2006), The Netherlands (V. alginolyticus, Schets et al., 2006) and Denmark (V. alginolyticus and V. parahaemolyticus, Andersen, 2006). These occurrences have led to increased concern over the contribution that climate change may be making to the abundance of vibrios in coastal seas (Marine Climate Change Impacts Partnership Annual Report Card, 2010). However, despite the volume of indirect evidence, it is not clear whether vibrios, which are known to be thermodependent, are increasing within the complex and ecologically regulated bacterial communities in coastal marine waters. This is mainly because of a lack of historical data.
To assess a possible linkage between the occurrence of Vibrio and SST over a decadal scale, we applied molecular and pyrosequencing analysis to the microbial community on formalin-fixed samples from the historical archive of the Continuous Plankton Recorder (CPR) survey (Figure 1). This survey has produced one of the longest and most widespread time series covering the abundance and distribution of marine organisms in the world (http://www.sahfos.ac.uk). The CPR was designed as a zooplankton sampler, but also samples, in a semi-quantitative way, smaller components of the plankton, including mucilage and detrital particles trapped by the fibrils of the filtering silk and the reduced filtration mesh due to trapped zooplankton (Reid et al., 2003). Several studies have been undertaken that compare plankton abundances obtained with the CPR with those obtained using the standard plankton nets. Although catches by the CPR are almost always lower, seasonal cycles are replicated in each comparison and interannual variability generally agrees between time series (Batten et al., 2003).
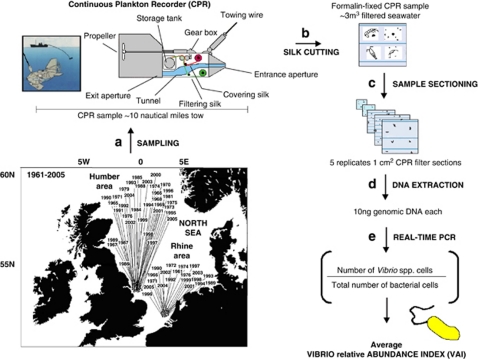
Figure 1.
Retrospective assessment of relative abundance of vibrios, Vibrio relative abundance index (VAI), in the North Sea. Methods used to calculate the relative abundance of vibrios (VAI index) from formalin-fixed plankton samples collected by the CPR survey off the Rhine and Humber estuaries, in August, from 1961 to 2005. (a) CPR samples were collected in the North Sea from 1961 to 2005 by the CPR survey. (b) Back in the laboratory, the silk containing the entrapped plankton (under 4–10% buffered formalin) is cut into blocks, each representing 10 nautical miles of tow (3 m3 of filtered seawater). (c) Five replicate 1-cm2 sections are prepared from each CPR block for microbiological molecular analyses. (d) Genomic DNA is extracted from each section and purified. (e) The ratio of Vibrio spp. cells to total bacterial cells is assessed by real-time PCR of 10 ng of genomic DNA from each section using genus-specific and universal primers, respectively, producing small amplicons of similar size (113 vs 98 bp) to avoid age- and formalin-induced bias (see main text). The VAI is then calculated for each sample by average values of this ratio for the five replicate measurements.
Plankton represents a nutrient-rich reservoir capable of enriching Vibrio species, which may be present, especially during the warmer months, at high densities (Turner et al., 2009). For this reason, it is likely that the CPR system captures a substantial fraction of these bacteria and can provide a long-term record for Vibrio and other particle-associated bacteria present in the water filtered during a tow.
We provide evidence that vibrios, including the species V. cholerae, increased in dominance within the plankton-associated bacterial community of the North Sea during the past 44 years and that this increase is correlated significantly with climate-induced sea surface warming during the same period.
Materials and methods
CPR samples
The CPR is a high-speed plankton sampler designed to be towed from commercially operated ships of opportunity over long distances (Reid et al., 2003, Figure 1). Sampling takes place in the surface layer (∼7 m) and plankton is collected on a band of silk (mesh size 270 μm) that moves across the sampling aperture at a rate proportional to the speed of the towing ship (Reid et al., 2003). The CPR mesh width of 270 μm not only retains larger zooplankton with a high efficiency but also collects small planktonic organisms, such as nauplii, microzooplankton and phytoplankton (Batten et al., 2003). Over 500 phytoplankton and zooplankton taxa are routinely collected, identified and counted on CPR samples. Some are identified to the level of species, some to genus and some to a higher taxonomic group (see Vezzulli and Reid (2003) for a list of major plankton taxa identified in CPR samples collected in the North Sea). On return to the laboratory, the silk is removed from the device and divided into individual samples that are numbered along the route. Only odd samples are analysed, according to the standard procedures. Both analysed and unanalysed samples are stored in plastic boxes in buffered formalin (usually comprising 4–10% buffered formalin) in the CPR archive in Plymouth (England). CPR samples used in this study were collected in two areas located off the Rhine (51.9–52.4°N; 3.3–4.0°E) and Humber (53.5–54.0°N; 0.1–0.9°E) estuaries in the North Sea, in August (corresponding to the seasonal peak of Vibrio counts in seawater), from 1961 to 2005 (Figure 1). The outer limit for sample collection in this study was defined as within 50 nautical miles of the North Sea coast.
Temperature and plankton data
Average SST time series for the Rhine and Humber regions, in summer (August), were calculated from the HadSST data set (Rayner et al., 2006) using the BADC data explorer (http://cdat.badc.nerc.ac.uk/cgi-bin/dxui.py). Yearly mean time series of the phytoplankton colour index (a visual index of chlorophyll, Reid et al., 2003) and abundance of total copepods for the same areas were calculated using the WinCPR database and associated software (Vezzulli et al., 2007).
Nucleic acid extraction and purification from CPR samples
For each CPR sample, the filtering silk was cut into five replicate (1 cm2) sections and each section was placed in a sterile tube. A volume of 25 ml of TE buffer (10 m Tris-HCl, 1 m EDTA, pH 8.0) was added and the sample was vortexed to detach plankton from the silk mesh. Samples were incubated at room temperature for 24 h and vortexed for 30 s. Each plankton suspension was gently centrifuged and the pellet was transferred to a sterile microcentrifuge tube and DNA extraction was performed. Briefly, 50 μl of lysozyme (2 mg ml−1 in 10 m Tris-HCl, pH 8.0) was added to the sample, which was then vortexed vigorously for 1 min. After addition of 180 μl 10% sodium dodecyl sulphate and 25 μl proteinase K (10 mg ml−1), the sample was vortexed for 30 s. The sample was then incubated at 56 °C for 1 h, heated at 90 °C for 1 h in a dry-block heater, vortexed for 10 s and centrifuged at 12 000 g for 3 min. After addition of 200 μl guanidine hydrochloride lysis solution and 200 μl ethanol, the sample was centrifuged (12 000 g for 10 s). The supernatant was then transferred to QIAamp MinElute column (Qiagen, Valencia, CA, USA) and processed according to the manufacturer's recommendation. The retained DNA was purified with QIAquick PCR purification columns (Qiagen) up to a final yield of 1–7 μg ml−1. PCR inhibition tests were conducted on serially 1:2 diluted samples, to which 10 copies per reaction of a genomic reference DNA were added.
Sizing and quantification of genomic DNA
The amount of DNA extracted from the CPR samples was determined fluorimetrically with PicoGreen using a NanoDrop ND-3300 fluorometer (NanoDrop Technologies, Wilmington, DE, USA). Sizing of genomic DNA was conducted in an Agilent Bioanalyzer 2100 (Agilent, Palo Alto, CA, USA) using the High Sensitivity DNA kit (Agilent Technologies).
Real-time PCR
To calculate the Vibrio relative abundance index (Figure 1), 10 ng of genomic DNA extracted from a 1-cm2 section (for a total of five replicate sections for each CPR sample) was analysed by 16S rRNA gene-targeted real-time PCR with SYBR-green detection using a capillary-based LightCycler instrument (Roche Diagnostics, Mannheim, Germany) and a standard curve method for quantification. The oligonucleotide primers used in the PCR reaction were Vib1 f-5′-GGCGTAAAGCGCATGCAGGT-3′ and Vib2 r-5′-GAAATTCTACCCCCCTCTACAG-3′ (Thompson et al., 2004), specific for the genus Vibrio, and 967f-5′-CAACGCGAAGAACCTTACC-3′ and 1046r-5′-CGACAGCCATGCANCACCT-3′ (Sogin et al., 2006), specific for the domain Bacteria, amplifying positions 567–680 and 965–1063 (V6 hyper variable region) of the Escherichia coli numbering of the 16S rRNA, respectively. Each reaction mixture contained 5.0 mmol of MgCl2 and 0.25 μmol of each primer in a final volume of 20 μl. The PCR programme was optimised as follows: initial denaturation at 95 °C for 10 min, subsequent 40 cycles of denaturation at 95 °C for 5 s, annealing at 58 °C (Vibrio spp.) or 57 °C (total bacteria) for 5 s and elongation at 72 °C for 4 s, followed by a final elongation at 72 °C for 10 min. PCR runs were analysed directly in the LightCycler using melting analysis and the software provided with the instrument. For each single real-time PCR assay each DNA template was analysed in triplicate (coefficient of variation <5%). The standards were prepared from 16S rDNA nucleic acid templates of V. cholerae El Tor N16961 at known molar concentrations. Vibrio spp. and the total bacterial concentrations were expressed as number of cells per square centimetre of the CPR sample (cells cm−2) by dividing the total 16SrDNA copy number by the average 16SrDNA copy number in vibrios (n=9, Acinas et al., 2004) and proteobacteria (n=3.5, Kormas, 2011), respectively (Supplementary Table S2).
Pyrosequencing
A PCR amplicon library was generated from genomic DNA extracted and pooled for a total of five replicate sections for each CPR sample using the broad-range bacterial primers, 967f and 1046r, amplifying the V6 hypervariable region of ribosomal RNAs (Sogin et al., 2006). The PCR products were pooled after cycling and cleaned to a total yield of 300 ng using an AMICON Ultra 30K membrane (Millipore, Billerica, MA, USA). Amplicon libraries were bound to beads under conditions that favour one fragment per bead and beads were emulsified in a PCR mixture in oil. After breaking the emulsion, the DNA strands were denatured, and the beads carrying single-stranded DNA clones were deposited into the wells on a PicoTiter-Plate (454 Life Sciences, Branford, CT, USA) for pyrosequencing on a 454 Genome Sequencer FLX Titanium (Roche, Basel, Switzerland). Sequence Reads data are archived at NCBI SRA with the accession number SRA026732.
Bioinformatics analysis
Raw data obtained by the 454 Genome Sequencer FLX were trimmed as described (see Results and Discussion). To assess taxonomic diversity, each trimmed read sequence was BLASTed against a reference database of ∼40 000 unique V6 sequences extracted from the nearly 120 000 published rRNA genes for the Bacteria domain (Sogin et al., 2006) using Blast (version 2.2.18, National Center for Biotechnology Information, Bethesda, MD, USA). We performed MEGAN (Metagenome Analysis Software, version 3.8, University of Tübingen, Tübingen, Germany) analysis, using a bit-score threshold of 35 and retaining only hits whose bit scores were within 10% of the best score. All assignments hit by <20 reads were discarded.
Statistical analysis
The relationship between Vibrio abundance and the predictor variables (SST, phytoplankton colour index and total copepod abundance) was assessed using a non-parametric multiple regression analysis that was based on Euclidean distances calculated on normalised data using the routine DISTLM forward 1.3 (University of Auckland, Auckland, New Zealand) (Anderson, 2003). Forward selection of individual variables was used, where the amounts explained by each variable are added to the model and are conditional on variables already present in the model.
Results and Discussion
Increase over four decades in the relative abundance of Vibrios with rising SST
We analysed a set of 55 samples collected by the CPR survey in the North Sea from off the Rhine and Humber estuaries between 1961 and 2005 (Figure 1, Supplementary Table S1). All samples were collected in August by a CPR machine and each represents a transect of 10 nautical miles (18.5 km) corresponding to 3 m3 of filtered seawater (Reid et al., 2003). The total area sampled equals ∼1400 square miles off the Rhine and ∼1600 square miles off the Humber estuaries over a 44-year period (Figure 1).
We assessed the presence and relative abundance of vibrios on CPR samples by applying a real-time PCR approach that used genomic DNA recovered by an improved extraction and modified purification methodology based on previous studies (Kirby and Reid, 2001; Ripley et al., 2008). We were able to recover environmental DNA from CPR samples that had been stored for up to ∼50 years in a formalin-fixed format, which is suitable for molecular analyses of the associated prokaryotic community (Figure 2). Up to now, reliable molecular quantification of biological targets associated with these samples was considered impossible because of the sample age and storage in formalin, which is believed to hamper molecular analysis principally by causing DNA degradation and crosslinking between adjacent DNA molecules (Ripley et al., 2008). It has also been shown that formalin may interfere with molecular techniques that rely upon enzyme activity such as PCR (De Giorgi et al., 1994). To overcome these concerns, we first assessed DNA fragmentation from historical CPR samples showing that DNA smear in the 200–800-bp size range prevailed in genomic DNA recovered from the older samples, the earliest of which dates back to August 1961 (Supplementary Figure S1). To assess the suitability of the extracted DNA for the molecular study of the bacterial community, a number of PCR trials were then carried out on all samples using (according to DNA fragmentation analysis) primer pairs that amplify a small region (98 bp) of the 16SrDNA of Vibrios (Supplementary Figure S1). Based on these successful trials we finally developed an unbiased index of abundance for Vibrio quantification in CPR samples, termed a ‘Vibrio relative abundance index' (Figure 1). This index measures the relative proportion of the plankton-associated bacteria that are vibrios and is defined as the ratio of Vibrio spp. cells to the total number of bacterial cells assessed by real-time PCR using genus-specific and universal primers, respectively. According to the above results, ∼100-bp amplicons were used, allowing amplification to take place and maximising the reaction yields when the DNA was fragmented. In addition, PCR protocols based on similar-size amplicons (113 vs 98 bp for Vibrio and total Bacteria, respectively) were used assuming that DNA damage over time, including fragmentation, was the same for both amplified fragments. Using this approach we were able to measure and compare relative Vibrio abundances in CPR samples from different years (Figures 3a and c; Supplementary Figure S2).
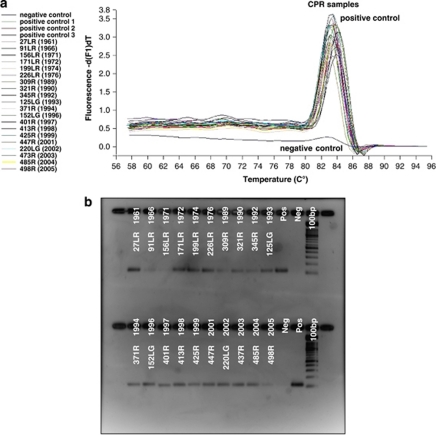
Figure 2.
PCR-based amplification of bacterial DNA extracted from historical CPR samples dating back to August 1961. Melting curve analysis (a) and agarose gel (b) showing the output of real-time PCR amplification of a 113-bp DNA fragment targeting the 16SDNA gene of Vibrio spp. from genomic DNA extracted from archived formalin-fixed CPR samples from the North Sea off the Rhine estuary dating back to August 1961.
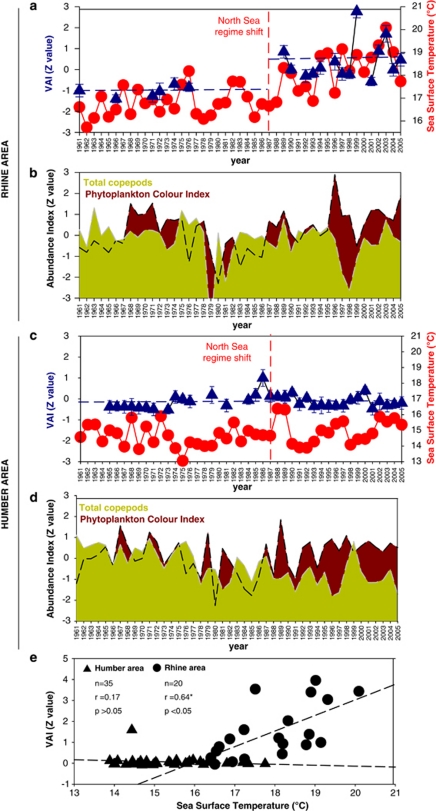
Figure 3.
Relative abundance of Vibrio spp. and levels of environmental variables. Long-term variation in the abundance of Vibrio (a, c) (blue triangles; error bars indicate s.d., n=5), SST (a, c) (red circles), phytoplankton colour index (b, d) and total copepods (b, d) for 1961–2005 off the Rhine and Humber estuaries in the North Sea. Vertical red line=regime shift step change in temperature after 1987 (Kirby et al., 2007). Horizontal blue lines=average standardised Vibrio relative abundance index (VAI) values for the two periods, –0.94 s.d. (1961–1976) and +0.4 s.d. (1989–2005) for the Rhine area and –0.13 s.d. (1965–1987) and −0.13 s.d. (1988–2005) for the Humber area. (e) The Pearson correlation analysis between Vibrio abundance and SST in the North Sea (Pearson's correlation on pooled data; n=55; r=0.27*; P<0.05). Z values are obtained by subtracting the population mean and dividing the difference by the s.d.
Our results show a long-term increase in relative Vibrio abundance coupled to a positive and statistically significant correlation with SST off the Rhine Estuary but not off the Humber Estuary during the past 44 years (Figure 3e). The differences between the two areas may be related to the generally higher summer SST values recorded in the Rhine compared with the Humber area. It is well known that most Vibrio species thrive in seawater, during the seasonal cycle, when temperature exceeds 16–18 °C (Thompson et al., 2004; Vezzulli et al., 2009). In the Rhine area SST in summer generally exceeded 18 °C especially during recent years. In contrast, off the Humber, SST values never exceeded 18 °C during the entire time series (Figure 3e). To investigate the long-term relationship between Vibrio abundance and SST we used data pooled from the two areas (n=55) in the North Sea and applied a non-parametric multiple regression analysis (Anderson, 2003). Because plankton and especially copepods represent important reservoirs of vibrios in the aquatic environment we included the number of total copepods and phytoplankton colour, calculated for the same areas and period (Vezzulli et al., 2007) (Figures 3b and d), as additional predictor variables in the model. Salinity ranging from 33 to 35 p.s.u. was not included as no significant year-to-year change is reported in the analysed period in the study area (Marine Climate Change Impacts Partnership Annual Report Card, 2010).
We showed that in the North Sea, SST and the number of total copepods explained 50% of the variance in the Vibrio data (P<0.05, Table 1). In contrast, phytoplankton colour was redundant in the model (P>0.05). SST alone explains 45% of the variance in Vibrio data supporting evidence from previous studies, that an increase in temperature might enhance not only Vibrio growth rates but also their capability to attach to and multiply on plankton (Huq et al., 1984).
Table 1. Long-term relationship between vibrios and environmental variables in the North Sea.
| Set | %Var | Pseudo-F | P | Cum% |
|---|---|---|---|---|
| (a) Marginal tests, Vibrio abundance vs | ||||
| SST | 45.4 | 42.4 | 0.0001 | |
| Phytoplankton colour | 33.4 | 25.6 | 0.0002 | |
| Total copepods | 20.5 | 13.2 | 0.0004 | |
| (b) Sequential tests, Vibrio abundance vs | ||||
| SST | 45.4 | 42.4 | 0.0001* | 45.4 |
| Total copepods | 4.2 | 4.17 | 0.04* | 50.0 |
| Phytoplankton colour | 1.9 | 1.96 | 0.17 | 51.5 |
Abbreviations: Cum%, cumulative percentage of variance explained; SST, sea surface temperature; %Var, percentage of variance in Vibrio data explained by that individual variable.
*P<0.05.
Results of a multiple regression analysis of the Vibrio relative abundance index data versus SST, the phytoplankton colour index and total copepods in the North Sea. Outputs from the analyses include: (a) the results of the marginal tests (that is, fitting each environmental variable individually, ignoring the others), followed by (b) the results of the forward selection procedure with the conditional tests (that is, fitting each environmental variable one at a time, conditional on the variables that are already included in the model). The multiple regressions were based on Euclidean distances calculated among observations from normalised data. The forward selection of the predictor variables was done with tests by permutation. P-values were obtained using 4999 permutations of raw data for the marginal tests (tests of individual variables), whereas for all conditional tests the programme uses 4999 permutations of residuals under the reduced model.
In the late 1980s an ecological regime shift occurred in the North Sea that was linked to a shift to a positive North Atlantic Oscillation index and coincided with an increased incursion of warm oceanic water from the Atlantic into the northern North Sea (Reid et al., 2001). This event affected all trophic levels, including phytoplankton, zooplankton and benthos to fish (Reid et al., 2001; Kirby et al., 2007). Since approximately this shift (25 years), SST has risen in UK waters, with the largest increases, of between 0.6 and 0.8 °C per decade, in the southern North Sea (Marine Climate Change Impacts Partnership Annual Report Card, 2010). Here we show, for the first time, that warming of seawater temperature over a decadal scale also had a major impact on the prokaryotic community increasing the relative abundance of warm water vibrios associated with plankton in coastal waters. We emphasise, however, that the ratio of vibrios associated with plankton vs free-living vibrios is still an open question. Although some authors (Turner et al., 2009; Stauder et al., 2010) have shown that a high load of vibrios is associated with plankton, the number of free-living vibrios may be even higher. The relative proportion of vibrios associated with plankton compared with free-living concentrations in seawater deserves further investigation, especially in the light of climate change and rising SSTs.
In this study, a marked increase in Vibrio abundance occurred after the late 1980s off the Rhine estuary in the North Sea, matching the ecological regime shift and an associated stepwise increase in SST after 1987 (Kirby et al., 2007, Figure 3a).
Vibrios increased in dominance within the plankton-associated bacterial community
Using pyrosequencing, we provide evidence that bacteria belonging to the genus Vibrio not only increased in relative abundance over the last half century in the southern North Sea, but also became dominant within the plankton-associated bacterial community of coastal marine waters. The bacterial community composition of five selected CPR samples collected off the Rhine Estuary in 1961, 1972 and 1976 (before the regime shift) and in 1998 and 2004 (after the regime shift) was examined. We sequenced ∼44 000 PCR amplicons spanning the V6 hypervariable region of the 16S ribosomal RNA gene from the genomic DNA of the CPR samples. The number of reads per sample ranged from 6037 to nearly 13 000 sequences. To minimise random sequencing errors, a stringent trimming procedure was followed, by eliminating reads that contained one or more ambiguous bases, had errors in the barcode or primer sequence, were atypically short (<70 bp), and had an average quality score <30 (Sogin et al., 2006). On average this step reduced the size of the data set by 23%, resulting in a total of 10.066 (year 1961), 6.745 (year 1972), 6.014 (year 1976), 5.173 (year 1998) and 8.206 (year 2004) trimmed read counts available for bioinformatics analysis. To assess taxonomic diversity, each trimmed read sequence was BLASTed against a reference database of ∼40 000 unique V6 sequences extracted from the nearly 120 000 published rRNA genes for the bacterial domain (Sogin et al., 2006).
The results of BLASTN were used to estimate the taxonomic content of the data set, using NCBI taxonomy with MEGAN (Huson et al., 2007). We had to consider that external contamination of CPR samples would be likely to occur during silk manufacture and removal and cutting from the sampling cassette. Risks associated with laboratory handling were minimised by using un-analysed samples. To further reduce bias, we restricted analysis of the 454 sequences to the Alpha- and Gammaproteobacteria that are known to be abundant in seawater (DeLong, 1996; Venter et al., 2004) and not usually associated with human or laboratory contamination. In addition, only the most abundant reads (for example, those occurring at least 20 times in the trimmed data set) were assigned to bacterial taxa and included in the results (Figure 4). The lowest common ancestor algorithm assigned 35 063 reads to taxa, while 1138 remained unassigned, either because the bit-score of their matches or the minimum number of reads for taxon assignment fell below the threshold (see methods). A total of 33 786 reads were assigned to the domain Bacteria, of which 3207 were assigned to the family Vibrionaceae.
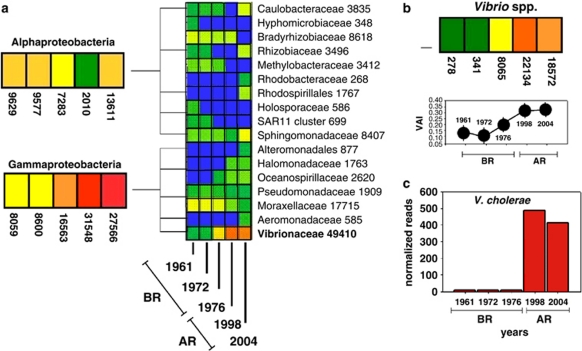
Figure 4.
16S rDNA pyrosequencing-based comparative analysis of the microbial community from historical CPR samples. Comparative analysis of 16S rDNA pyrosequencing data for dominant Alpha- and Gammaproteobacteria groups are shown for CPR samples collected in 1961, 1972, 1976, 1998 and 2004 off the Rhine Estuary. A heat map is shown, where the numbers of normalised reads taken by each taxon in each year are represented as colours (cold-to-hot colour representing low to high number of reads). The cumulative number of normalised reads across the different years is also shown for each taxon (Mitra et al., 2009). AR=after regime shift; BR=before regime shift. (a) The tree is collapsed to the ‘family' level; (b) results for the Vibrio genus, Vibrio relative abundance index (VAI) index is reported for comparison; (c) number of normalised read sequences showing >95% identity to V. cholerae (see also Supplementary Figure S3).
We used a multiple-comparative analysis in MEGAN to compare CPR pyrosequencing data from the different years with taxon data normalised over all reads, such that each data set had 100 000 reads (Mitra et al., 2009). This minimised potential bias arising from differences in absolute read counts, for example, loss of sequences because of degradation, which could be related to age and formalin storage of the samples. We showed that among microorganisms that correspond to the most abundant Operational Taxonomic Units (OTUs), a major shift in bacterial community composition occurred, that would be attributed to an increase in the family Vibrionaceae including the human pathogen V. cholerae (Figure 4, Supplementary Figure S3). This detailed taxonomic analysis showed the presence of a number of bacterial taxa in assemblages associated with coastal CPR samples, dating back to August 1961. These data provide a proof of concept for the assessment of microbial diversity from the large and ocean basin-wide collection of formalin preserved marine biological samples obtained by the CPR survey. Our results open up a novel window for the long-term and retrospective study of microbial biodiversity and the global ecology of marine bacterial communities.
Conclusions
In conclusion, our analyses have demonstrated that a major change in the structure of the bacterial community that is associated with plankton occurred in the southern North Sea in response to increasing SST over the last four decades. This finding is intriguing as it provides a long-term pattern for environmental microbial communities, including abundance and diversity of bacterial species that, despite being dependent on a complex interplay between biotic and abiotic factors in both large and local-scale processes, appears to be driven largely by sea surface warming. Based on this evidence, the increasing dominance of marine Vibrio spp., including pathogenic bacterial species, among plankton-associated bacterial communities of coastal seawater may very likely occur in other areas around the world as a response to climate change. Potential effects on human and animal health and ecosystem functioning are at present unpredictable.
Acknowledgments
We thank all past and present members and supporters of the CPR survey whose efforts have enabled the establishment and long-term maintenance of the CPR data set and the archived samples used in this study. We also acknowledge the voluntary contribution of the owners, masters and crews of the ships that tow the CPRs without whose help the survey would not be possible. The Sir Alister Hardy Foundation for Ocean Science currently operates the CPR survey. Support of the National Institutes of Health (Grant no. 2RO1A1039129-11A2-NIH) and National Oceanic and Atmospheric Administration, Oceans and Human Health Initiative (Grant no. S0660009) is gratefully acknowledged. This work was also supported by the Ministero dell'Università e della Ricerca Scientifica e Tecnologica (PRIN) and by grants from Genoa University.
Author contributions
LV, CP, IB, MGH, PR and RC designed the research; LV, IB and EP performed the research; LV, CP, EP, IB, MGH and RC analysed the data; and LV and CP wrote the paper.
Footnotes
Supplementary Information accompanies the paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Acinas SG, Marcelino LA, Klepac-Ceraj V, Polz MF. Divergence and redundancy of 16S rRNA sequences in genomes with multiple rrn operons. J Bacteriol. 2004;186:2629–2635. doi: 10.1128/JB.186.9.2629-2635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MJ. DISTLM Forward: A FORTRAN Computer Program to Calculate a Distance-Based Multivariate Analysis for a Linear Model Using Forward Selection. Department of Statistics, University of Auckland: New Zealand; 2003. [Google Scholar]
- Andersen PH. Infections with seawater bacteria. EPI-NEWS. 2006;1:26–32. [Google Scholar]
- Andersson Y, Ekdahl K. Wound infections due to Vibrio cholerae in Sweden after swimming in the Baltic Sea, summer 2006. Euro Surveill. 2006;11:E060803.2. doi: 10.2807/esw.11.31.03013-en. [DOI] [PubMed] [Google Scholar]
- Austin B.2005Bacteria pathogens of marine fishIn: Belkin S, Colwell RR (eds).Ocean and Health Pathogens in the Marine Environment Springer-Verlag: New York; 391–413. [Google Scholar]
- Batten SD, Clark R, Flinkman J, Hays GC, John E, John AWG, et al. CPR sampling: the technical background, materials and methods, consistency and comparability. Prog Oceanogr. 2003;58:193–215. [Google Scholar]
- Colwell RR. Global climate and infectious disease: the cholera paradigm. Science. 1996;274:2031–2025. doi: 10.1126/science.274.5295.2025. [DOI] [PubMed] [Google Scholar]
- De Giorgi C, Finetti Sialer M, Lamberti F. Formalin-induced infidelity in PCR-amplified DNA fragments. Mol Cell Probes. 1994;8:459–462. doi: 10.1006/mcpr.1994.1065. [DOI] [PubMed] [Google Scholar]
- DeLong EF.1996Diversity of naturally occurring prokaryotesIn: Colwell RR, Simidu U, Ohwada K (eds).Microbial Diversity in Time and Space Plenum Press: New York; 125–132. [Google Scholar]
- Edwards M, Richardson AJ. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature. 2004;430:881–884. doi: 10.1038/nature02808. [DOI] [PubMed] [Google Scholar]
- Frank C, Littman M, Alpers K, Hallauer J. Vibrio vulnificus wound infections after contact with the Baltic Sea, Germany. Euro Surveill. 2006;11:E060817.1. doi: 10.2807/esw.11.33.03024-en. [DOI] [PubMed] [Google Scholar]
- Harvell CD, Mitchell CE, Ward JR, Altizer S, Dobson AP, Ostfeld RS, et al. Climate warming and disease risks for terrestrial and marine biota. Science. 2002;296:2158–2162. doi: 10.1126/science.1063699. [DOI] [PubMed] [Google Scholar]
- Huq A, West PA, Small EB, Huq MI, Colwell RR. Influence of water temperature, salinity, and Ph on survival and growth of toxigenic Vibrio cholerae serovar O1 associated with live copepods in laboratory microcosms. Appl Environ Microbiol. 1984;48:420–424. doi: 10.1128/aem.48.2.420-424.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson DH, Auch AF, Qi J, Schuster SC. MEGAN analysis of metagenomic data. Genome Res. 2007;17:377–386. doi: 10.1101/gr.5969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper JB, Morris JGJ, Levine MM. Cholera. Clin Microbiol Rev. 1995;8:48–86. doi: 10.1128/cmr.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby RR, Reid PC. PCR from the CPR offers a historical perspective on marine population ecology. J Mar Biol Assoc UK. 2001;81:539–540. [Google Scholar]
- Kirby RR, Beaugrand G, Lindley JA, Richardson AJ, Edwards M, Reid PC. Climate effects and benthic–pelagic coupling in the North Sea. Mar Ecol Prog Ser. 2007;330:31–38. [Google Scholar]
- Kormas KA.2011Interpreting diversity of Proteobacteria based on 16S rRNA gene copy numberIn: Sezenna ML (ed.).Proteobacteria: Phylogeny, Metabolic Diversity and Ecological Effects Nova Publishers, Hauppauge: New York; 73–89. [Google Scholar]
- Levin RE. Vibrio parahaemolyticus, a notably lethal human pathogen derived from seafood: a review of its pathogenicity, characteristics, subspecies characterization, and molecular methods of detection. Food Biotechnol. 2006;20:93–128. [Google Scholar]
- Marine Climate Change Impacts Partnership 2010Marine climate change impacts annual report card 2010–2011In: Baxter JM, Buckley PJ, Wallace CJ (eds).Summary Report MCCIP: Lowestoft; Downloadable from http://www.mccip.org.uk/arc . [Google Scholar]
- Martinez-Urtaza J, Bowers JC, Trinanes J, DePaola A. Climate anomalies and the increasing risk of Vibrio parahaemolyticus and Vibrio vulnificus illnesses. Food Res Int. 2010;43:1780–1790. [Google Scholar]
- Mitra S, Klar B, Huson DH. Visual and statistical comparison of metagenomes. Genome Anal. 2009;25:1849–1855. doi: 10.1093/bioinformatics/btp341. [DOI] [PubMed] [Google Scholar]
- Paillard C, Le Roux F, Borreg JJ. Bacterial disease in marine bivalves, a review of recent studies: trends and evolution. Aquat Living Resour. 2004;17:477–498. [Google Scholar]
- Pascual M, Rodó X, Ellner SP, Colwell RR, Bouma MJ. Cholera dynamics and El Niño-Southern oscillation. Science. 2000;289:1766–1769. doi: 10.1126/science.289.5485.1766. [DOI] [PubMed] [Google Scholar]
- Pruzzo C, Huq A, Colwell RR, Donelli G.2005Pathogenic Vibrio species in the marine and estuarine environmentIn: Belkin S, Colwell RR (eds).Ocean and Health Pathogens in the Marine Environment Springer-Verlag: New York; 217–252. [Google Scholar]
- Raffaelli DG. How extinction patterns affect ecosystems. Science. 2004;306:1141–1142. doi: 10.1126/science.1106365. [DOI] [PubMed] [Google Scholar]
- Rayner NA, Brohan P, Parker DE, Folland CK, Kennedy JJ, Vanicek M. Improved analyses of changes and uncertainties in sea surface temperature measured in situ since the mid-nineteenth century: the HadSST2 data set. J Climate. 2006;19:446–469. [Google Scholar]
- Reid PC, Borges MD, Svendsen EA. Regime shift in the North Sea circa 1988 linked to changes in the North Sea fishery. Fish Res. 2001;50:163–171. [Google Scholar]
- Reid PC, Colebrook JM, Matthews JBL, Aiken J. The Continuous Plankton Recorder: concepts and history, from Plankton Indicator to undulating recorders. Prog Oceanogr. 2003;58:117–173. [Google Scholar]
- Ripley SJ, Baker AC, Miller PI, Walne AW, Schroeder DC. Development and validation of a molecular technique for the analysis of archived formalin-preserved phytoplankton samples permits retrospective assessment of Emiliania huxleyi communities. J Microbiol Meth. 2008;73:118–124. doi: 10.1016/j.mimet.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Sala E, Knowlton N. Global marine biodiversity trends. Annu Rev Env Resour. 2006;31:93–122. [Google Scholar]
- Sarmento H, Montoya JM, Vazquez-Dominguez E, Vaque D, Gasol JM. Warming effects on marine microbial food web processes: how far can we go when it comes to predictions. Philos Trans R Soc B. 2010;365:2137–2149. doi: 10.1098/rstb.2010.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schets FM, Van den Berg HHJL, Demeulmeester AA, Van Dijk E, Rutjes SA, Van Hooijdonk HJP, et al. Vibrio alginolyticus infections in the Netherlands after swimming in the North Sea. Euro Surveill. 2006;11:E061109.3. doi: 10.2807/esw.11.45.03077-en. [DOI] [PubMed] [Google Scholar]
- Shapiro RL, Altekruse S, Hutwagner L, Bishop R, Hammond R, Wilson S, et al. The role of Gulf Coast oysters harvested in warmer months in Vibrio vulnificus infections in the United States, 1988–1996. J Infect Dis. 1998;178:752–759. doi: 10.1086/515367. [DOI] [PubMed] [Google Scholar]
- Sogin ML, Morrison HG, Huber JA, Mark Welch D, Huse SM, Neal PR. Microbial diversity in the deep sea and the underexplored ‘rare biosphere'. Proc Natl Acad Sci USA. 2006;103:12115–12120. doi: 10.1073/pnas.0605127103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauder M, Vezzulli L, Pezzati E, Repetto B, Pruzzo C. Temperature affects Vibrio cholerae O1 El Tor persistence in the aquatic environment via an enhanced expression of GbpA and MSHA adhesins. Environ Microbiol Rep. 2010;2:140–144. doi: 10.1111/j.1758-2229.2009.00121.x. [DOI] [PubMed] [Google Scholar]
- Thompson JR, Randa MA, Marcelino LA, Tomita-Mitchell A, Lim E, Polz MF. Diversity and dynamics of a North Atlantic coastal Vibrio community. Appl Environ Microbiol. 2004;70:4103–4110. doi: 10.1128/AEM.70.7.4103-4110.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JW, Good B, Cole D, Lipp EK. Plankton composition and environmental factors contribute to Vibrio seasonality. ISME J. 2009;3:1082–1092. doi: 10.1038/ismej.2009.50. [DOI] [PubMed] [Google Scholar]
- Venter JC, Remington K, Heidelberg JF, Halpern AL, Rusch D, Eisen JA, et al. Environmental genome shotgun sequencing of the Sargasso Sea. Science. 2004;304:66–74. doi: 10.1126/science.1093857. [DOI] [PubMed] [Google Scholar]
- Vezzulli L, Dowland PS, Reid PC, Hylton EK.2007Gridded Database Browser of North Sea Plankton, Version 1.1: Fifty Four Years (1948–2001) of Monthly Plankton Abundance from the Continuous Plankton Recorder (CPR) Survey Sir Alister Hardy Foundation: Plymouth, UK; , http://cpr.cscan.org/ . [Google Scholar]
- Vezzulli L, Previati M, Pruzzo C, Marchese A, Bourne DG, Cerrano C. Vibrio infections triggering mass mortality events in a warming Mediterranean Sea. Environ Microbiol. 2010;12:2007–2019. doi: 10.1111/j.1462-2920.2010.02209.x. [DOI] [PubMed] [Google Scholar]
- Vezzulli L, Pezzati E, Moreno M, Fabiano M, Pane L, Pruzzo C. Benthic ecology of Vibrio spp. and pathogenic Vibrio species in a coastal Mediterranean environment (La Spezia Gulf, Italy) Microb Ecol. 2009;58:808–818. doi: 10.1007/s00248-009-9542-8. [DOI] [PubMed] [Google Scholar]
- Vezzulli L, Reid PC. The CPR survey (1948–1997): a gridded database browser of plankton abundance in the North Sea. Progr Oceanogr. 2003;58:327–336. [Google Scholar]
- Voigt W, Perner J, Davis AJ, Eggers T, Schumacher J, Bahrmann R, et al. Trophic levels are differentially sensitive to climate. Ecology. 2003;84:2444–2453. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.