Abstract
The meiosis-specific synaptonemal complex protein SYCP3 has been reported to be aberrantly expressed in tumours. However, in contrast to its well-defined function in meiosis, its possible role in mitotic cells is entirely unknown. Here, we show that SYCP3 is expressed in a range of primary tumours and that it impairs chromosomal integrity in mitotic cells. Expression of SYCP3 inhibits the homologous recombination (HR) pathway mediated by RAD51, inducing hypersensitivity to DNA-damaging agents such as a poly(ADP-ribose) polymerase (PARP) inhibitor and chromosomal instability. SYCP3 forms a complex with BRCA2 and inhibits its role in HR. These findings highlight a new mechanism for chromosomal instability in cancer and extend the range of PARP-inhibitor sensitive tumours to those expressing SYCP3.
Keywords: chromosomal instability, homologous recombination, meiosis-specific protein
Introduction
SYCP3 is a component of the synaptonemal complex, a meiosis-specific supramolecular proteinaceous structure essential for synapsis of the maternal and paternal homologous chromosomes (Page & Hawley, 2004). Although SYCP3 was first considered to be a meiosis-specific protein, it has been reported to be aberrantly expressed in human leukaemia and primary cervical cancers (Niemeyer et al, 2003; Kang et al, 2010), suggesting that SYCP3 is a member of cancer/testis antigens whose expression is normally limited to the germ cells but abnormally activated in cancer (Simpson et al, 2005). However, although the meiotic role of SYCP3 is well characterized, its mitotic role is entirely unknown.
Homologous recombination (HR) is one of the main pathways in the repair of DNA double-strand breaks (DSBs) in mitotic cells (Hartlerode & Scully, 2009). RAD51 has a central role in the early stages of HR. The breast cancer susceptibility protein BRCA2 binds to RAD51 and recruits it to the sites of DSBs and promotes RAD51 filament formation, which is essential for the subsequent homologous DNA pairing and strand-exchange steps of HR (Gudmundsdottir & Ashworth, 2006).
In this study, we investigated the role for SYCP3 in mitotic cells. We show that SYCP3 inhibits the mediator role of BRCA2 in HR and induces chromosomal instability by impairing the intrinsic repair pathway. As SYCP3 is expressed in various tumour types, our finding suggests that inactivation of HR by SYCP3 might be prevalent in cancer.
Results
SYCP3 is expressed in various tumours
We first examined the expression profiles of SYCP3 in two non-cancerous normal human cells and 16 human cancer cell lines by western blot analysis. Although no expression of SYCP3 was detected in the normal retinal pigmented epithelial cell line RPE and the normal human mammary epithelial cell line HME, SYCP3 was aberrantly expressed in various cancer cell lines (supplementary Fig S1A online). The hepatocellular carcinoma cell line HepG2 and the prostate cancer cell line DU145 showed moderate expression levels, whereas most of other cancer cells showed low expression levels compared with the high expression levels observed in normal testis. Although SYCP3 expression was not detected in the colorectal carcinoma cell line DLD1, its expression was induced in DLD1 cells after treatment with the demethylating agent 5-azacytidine (supplementary Fig S1B online), indicating that SYCP3 expression in mitotic cells is regulated by a demethylation-dependent process, which is in agreement with the previous report (Maatouk et al, 2006).
To evaluate the expression pattern of SYCP3 in human primary tumours, we analysed its expression by using an mRNA array that contained two duplicated spots of mRNA from 47 different tumours and 47 normal tissues from unmatched donors (supplementary Fig S2A and Table S1 online). The array was probed for expression of SYCP3 and β-actin, and the signal ratios of SYCP3/β-actin from the two duplicated spots were averaged. Whereas almost all the normal samples showed low levels of signal ratios, significantly increased levels of SYCP3/β-actin signal ratios were observed in one adrenal tumour, three liver tumours, one stomach tumour and one kidney tumour (supplementary Fig S2B online). Although one normal liver sample showed a high level of the signal ratio, its biological significance is unknown because the profile of the donor is not available. These findings indicate that SYCP3 expression is not specific to certain tumour types but is observed in tumours of various tissue origins.
DNA damage is accumulated by expression of SYCP3
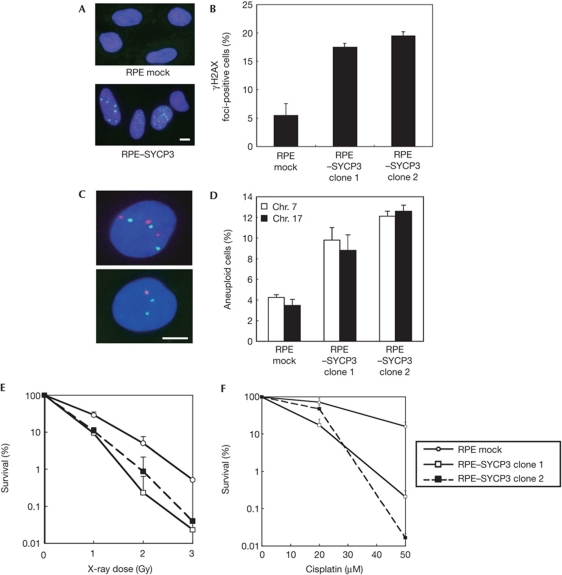
To investigate the role of SYCP3 expression in mitotic cells, we established two independent RPE clones stably expressing SYCP3 at low levels comparable with endogenous levels in cancer such as the fibrosarcoma cell line HT1080 (supplementary Fig S3A online). Immunofluorescence detection of nuclear foci of γH2AX, the phosphorylated form of histone H2AX, which is recruited to DSBs in response to DNA damage, revealed an increase in the frequency of foci-positive cells after forced SYCP3 expression from 5.5±1.5% (mean±s.d.) in mock cells to 17.5±0.5% and 19.5±0.5% in the two SYCP3-expressing RPE clones, respectively (Fig 1A,B). These results indicate an accumulation of DSBs in SYCP3-expressing cells.
Figure 1.
Expression of SYCP3 leads to increased DNA double-strand breaks (DSBs), aneuploidy and hypersensitivity to DNA-damaging agents. (A) Immunofluorescence visualization of γH2AX foci (green) in RPE cells transfected with an empty vector (upper panel) and in RPE cells expressing SYCP3 (lower panel). Scale bar, 10 μm. (B) Percentage of cells containing more than three large γH2AX foci. A total of 100 cells were examined for each cell clone. (C) Microscopy images of interphase fluorescence in situ hybridization in SYCP3-expressing cells using probes for chromosomes 7 (Chr., orange) and 17 (green). Scale bar, 10 μm. (D) Percentage of cells containing one, three or four copies of chromosomes. A total of 500 cells were examined for each cell clone. In B and D, columns and bars represent the mean of three independent experiments and s.d., respectively. (E,F) Sensitivity to ionizing radiation (IR) and cisplatin. The representative result of three independent experiments is shown. The symbols and bars represent mean and s.d. of the triplicate dishes, respectively.
Expression of SYCP3 leads to increased aneuploidy
We next examined the frequency of aneuploidy by fluorescence in situ hybridization analysis using two independent chromosome-specific centromeric probes (Fig 1C,D). Results showed a significant increase in aneuploidy frequency at chromosome 7 from 4.3±0.3% in mock cells to 9.8±1.2% and 12.1±0.5% in SYCP3-expressing RPE cells (P<0.05, Fisher’s exact test). At chromosome 17, the frequency also increased from 3.5±0.6% to 8.8±1.5% and 12.6±0.6% (P<0.05). These results indicate that expression of SYCP3 leads to increased aneuploidy in mitotic cells.
SYCP3 confers hypersensitivity to DNA damage
Next, we examined the sensitivity of SYCP3-expressing RPE cells to DNA damage by measuring their ability to form colonies after exposure to ionizing radiation (IR) or cisplatin, an interstrand crosslinking agent. Results showed a roughly twofold increase in sensitivity to IR and cisplatin after expression of SYCP3 (Fig 1E,F). These findings indicate that expression of SYCP3 regulates sensitivity to DNA damage, which includes DSBs.
SYCP3 decreases IR-induced RAD51 foci formation
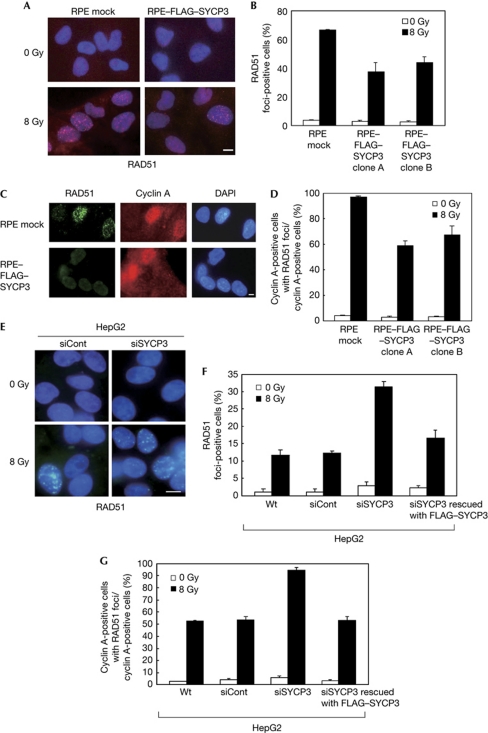
DSBs are repaired by either HR or non-homologous end-joining. As deficiency in HR but not in non-homologous end-joining leads to hypersensitivity to DNA crosslinking agents (Nojima et al, 2005), we assumed that expression of SYCP3 inhibits the HR pathway. RAD51 forms nuclear foci in S and G2 phases in a DNA damage-dependent manner (Tashiro et al, 2000). We therefore assessed the IR-induced foci formation of several recombination molecules including RAD51 in RPE clones stably expressing FLAG–SYCP3 at levels comparable with that of endogenous expression in HepG2 cells (supplementary Fig 3B online).
A decrease in the frequency of IR-induced RAD51 foci-positive cells was observed after stable SYCP3 expression from 66.3±0.6% (mean±s.d.) in mock cells to 37.3±6.3% and 44.0±4.0% in the two FLAG–SYCP3-expressing RPE clones (Fig 2A,B). The protein levels of RAD51 were similar in mock and FLAG–SYCP3-expressing cells (supplementary Fig S4A online), suggesting that expression of SYCP3 inhibits the recruitment but not the expression levels of RAD51 after DNA damage. The cell populations in S phase were almost similar between mock and FLAG–SYCP3-expressing cells (supplementary Fig 4B online), suggesting that impaired IR-induced RAD51 foci formation was not due to a decrease in the number of cells in S phase. The frequency of IR-induced RAD51 foci-positive cells in cells in S and G2 phases was also examined by double staining of RAD51 with cyclin A (Fig 2C), and a robust decrease from 96.7±0.6% in mock cells to 59.0±3.6% and 67.3±6.8% in the two FLAG–SYCP3-expressing RPE clones was observed (Fig 2D).
Figure 2.
The mitotic SYCP3 protein inhibits the IR-induced RAD51 foci formation in S and G2 phases. (A,E) Immunofluorescence visualization of RAD51 foci formation (red (A) and green (E)). Cells were non-treated (upper panels) or treated with 8 Gy X-ray (lower panels) and stained at 2 h after irradiation with an anti-RAD51 antibody. Scale bars, 10 μm. (B,F) Percentage of cells containing more than five damage-induced RAD51 foci. (C) Immunofluorescence visualization of RAD51 and cyclin A in RPE cells, showing ionizing radiation (IR)-induced foci formation of RAD51 in RPE-mock cells in S and G2 phases. The cells were treated with 8 Gy X-ray, followed by staining with the anti-RAD51 antibody (green), an anti-cyclin A antibody (red) and DAPI (4,6-diamidino-2-phenylindole; blue) 2 h later. Scale bar, 10 μm. (D,G) Percentage of cells containing more than five IR-induced RAD51 foci in cells stained with cyclin A. In B, D, F and G, columns and bars represent the mean of three independent experiments and s.d., respectively. A total of 100 cells were examined for each cell line. Wt, wild type.
No differences were observed in the foci formation of NBS1, which functions upstream of RAD51 in a complex with MRE11 and RAD50 in response to DNA damage, or in foci formation of BRCA1, which is indirectly associated with RAD51 (supplementary Fig S4C–F online). These observations indicate that SYCP3 affects the molecules in close proximity to RAD51.
Knockdown of SYCP3 recovers the function of RAD51
Next, we evaluated the IR-induced foci formation of recombination molecules by reducing SYCP3 expression in HepG2 cells, which endogenously express SYCP3. Western blot analysis showed the successful knockdown of SYCP3 after transfection of small interfering RNA (siRNA) targeting SYCP3, with expression levels about half of those in wild-type cells and cells transfected with control non-targeting siRNA (supplementary Fig S5A,B online). We also performed rescue experiments by expressing the siRNA-resistant FLAG–SYCP3 construct, to confirm the selectivity of the siRNA for the SYCP3 gene and the specificity of the phenotype. Expression of the exogenous FLAG–SYCP3 protein was not abolished by SYCP3 siRNA, but the endogenous SYCP3 protein was still successfully knocked down (supplementary Fig S5B online), indicating that the siRNA was selective for the endogenous SYCP3 mRNA.
The frequency of IR-induced RAD51 foci-positive cells increased from 12.3±0.6% (mean±s.d.) to 31.3±1.5% after knockdown of SYCP3, but was rescued to 16.7±2.3% when co-transfected with the siRNA-resistant FLAG–SYCP3 construct (Fig 2E,F). Focusing on cells in S and G2 phases, a robust increase in the frequency of IR-induced RAD51 foci-positive cells from 53.7±2.3% to 94.3±2.5% was observed after silencing SYCP3, which was rescued to 53.3±3.1% in rescue experiments with the siRNA-resistant FLAG–SYCP3 construct (Fig 2G), confirming the phenotype specificity for the SYCP3 gene. In contrast, no changes were observed in foci formation of either NBS1 or BRCA1 after knockdown of SYCP3 (supplementary Fig S5C–F online). These observations in siRNA experiments support the results from stable expression of SYCP3 in RPE cells.
Expression of SYCP3 reduces HR efficiency
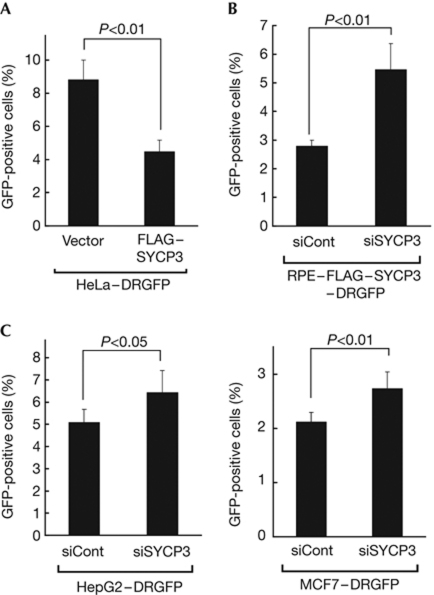
Because the results described above indicate that SYCP3 impairs the HR pathway, we next measured the effect of SYCP3 expression on HR efficiency with the DR–green fluorescent protein (GFP) assay (Pierce & Jasin, 2005). First, we stably expressed the exogenous FLAG–SYCP3 protein in HeLa–DRGFP cells (Sakamoto et al, 2007; supplementary Fig S6A online), in which GFP-positive cells can be induced on expression of the I-SceI restriction enzyme. Results indicated a significant reduction in the proportion of GFP-positive cells from 8.8±1.2% in mock cells to 4.5±0.7% in FLAG–SYCP3-expressing HeLa–DRGFP cells (P<0.01, two-tailed t-test; Fig 3A). Second, we established RPE–FLAG–SYCP3 cells in which the DR–GFP reporter system is stably integrated, and found that knockdown of exogenous FLAG–SYCP3 in these cells recovered the proportion of GFP-positive cells from 2.8±0.2% to 5.5±0.9% (P<0.01; Fig 3B; supplementary Fig S6B online). Finally, we established HepG2–DRGFP cells and MCF7–DRGFP cells and assessed the effect of knockdown of endogenous SYCP3 in these cells. Silencing endogenous SYCP3 in these cells significantly recovered the proportion of GFP-positive cells from 5.1±0.6% to 6.5±1.0% (P<0.05) and from 2.1±0.2% to 2.7±0.3% (P<0.01), respectively (Fig 3C; supplementary Fig S6B online). These findings provide direct evidence that SYCP3 reduces the intrinsic HR activity of mitotic cells.
Figure 3.
SYCP3 inhibits the homologous recombination repair activity measured by the DR–GFP assay. (A) The proportions of the green fluorescent protein (GFP)-positive cells in HeLa–DRGFP–FLAG–SYCP3 cells and mock cells stably transfected with an empty vector. (B,C) The proportions of the GFP-positive cells on knockdown of exogenous FLAG–SYCP3 in RPE–FLAG–SYCP3–DRGFP cells (B) or on knockdown of endogenous SYCP3 in HepG2–DRGFP cells and MCF7–DRGFP cells (C). In A, B and C, columns and bars represent the mean and s.d. of three independent experiments (A,B) or five independent experiments (C), respectively.
SYCP3 reduces sister chromatid exchanges
A reduction of sister chromatid exchange (SCE) levels is the hallmark of a defect in sister-chromatid-based recombination. A significant reduction in the levels of IR-induced SCE was observed in SYCP3-expressing RPE cells. The frequencies of IR-induced SCEs per cell were 7.7±2.1 in mock cells (mean±s.d., n=50), and 5.8±1.8 and 6.5±2.1 in the two RPE clones expressing SYCP3 (n=50) (P<0.001, Mann–Whitney U-test). These observations support the findings that SYCP3 impairs the intrinsic HR pathway.
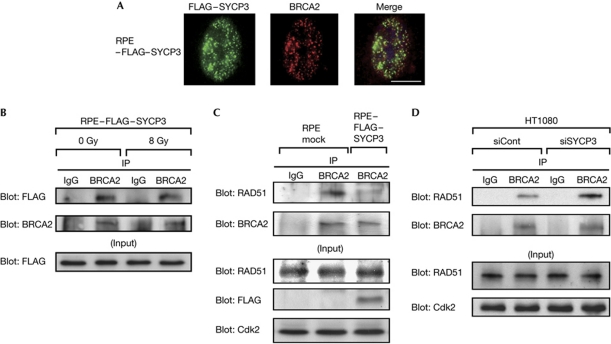
The mitotic SYCP3 protein interferes with BRCA2
To identify the molecule that is targeted by SYCP3, we assessed the localization of the SYCP3 protein and key molecules involved in HR in RPE cells stably expressing FLAG–SYCP3 by immunofluorescence. We found the partial colocalization of FLAG–SYCP3 and BRCA2 in the nucleus (Fig 4A). Moreover, the anti-BRCA2 antibody pulled down FLAG–SYCP3 in these cells (Fig 4B), indicating that SYCP3 forms a complex with BRCA2. The interaction between SYCP3 and BRCA2 was not modified in response to IR-induced DNA damage (Fig 4B).
Figure 4.
The mitotic SYCP3 protein interacts with BRCA2 and inhibits interaction between BRCA2 and RAD51 in mitotic cells. (A) Immunofluorescence visualization of FLAG–SYCP3 (left panel) and BRCA2 (middle panel). Cells were stained with anti-FLAG (green) and anti-BRCA2 (red) antibodies and 4,6-diamidino-2-phenylindole (blue), and merged (right panel). Scale bar, 10 μm. (B) Association of FLAG–SYCP3 with BRCA2 in FLAG–SYCP3-expressing RPE cells unirradiated or irradiated with 8 Gy X-ray 2 h before collecting the cell lysates. (C) Coimmunoprecipitation of BRCA2 with RAD51 from lysates of mock cells and RPE cells stably expressing FLAG–SYCP3 collected 3 h after inducing DNA damage by treatment with 50 μM cisplatin for 1 h. (D) Coimmunoprecipitation of BRCA2 with RAD51 from lysates of HT1080 cells transfected with a non-targeting small interfering RNA (siRNA) control or siRNA for SYCP3. In B, C and D, 500 μg of total cell lysates was precipitated using the anti-BRCA2 antibody or normal mouse immunoglobulin G (IgG) as a negative control and visualized by western blotting using the anti-FLAG antibody (B, upper panel), the anti-RAD51 antibody (C,D, upper panel) or the anti-BRCA2 antibody (lower panels). IP, immunoprecipitation.
We next determined whether the DNA damage-induced interaction between BRCA2 and RAD51 is affected by SYCP3 expression. Compared with mock cells, the FLAG–SYCP3-expressing RPE clones showed a reduction in the binding of BRCA2 with RAD51, whereas the protein levels of BRCA2 were not affected (Fig 4C). Conversely, the interaction of these two proteins was recovered by silencing SYCP3 in HT1080 cells, which express SYCP3 endogenously (Fig 4D; supplementary Fig S6C online), confirming that expression of SYCP3 inhibits the interaction between BRCA2 and RAD51 in response to DNA damage.
SYCP3 confers hypersensitivity to a PARP inhibitor
Poly(ADP-ribose) polymerase 1 (PARP1) is a DNA-binding enzyme that is activated by DNA breaks and facilitates the access of base excision/single-strand break repair proteins to the site of damage. Tumours deficient in BRCA1 or BRCA2, which are characterized by defective HR, have been shown to be sensitive to therapies that use PARP inhibitors to achieve synthetic lethality (Bryant et al, 2005; Farmer et al, 2005).
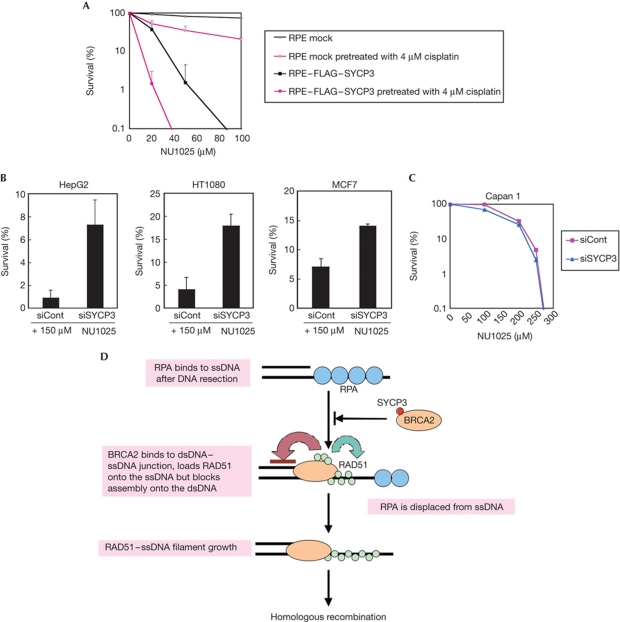
As our findings showed that SYCP3 expression inhibits the BRCA2 function, we assumed that the mitotic cells expressing SYCP3 would be sensitive to PARP inhibitors. Indeed, RPE cells expressing FLAG–SYCP3 showed extreme hypersensitivity to the PARP inhibitor NU1025, and addition of 4 μM cisplatin markedly enhanced this hypersensitivity (Fig 5A). Conversely, reduced SYCP3 expression levels in HepG2, HT1080 and the breast cancer cell line MCF7 increased the colony survival after treatment with 150 μM NU1025 from 0.9±0.77% (mean±s.d.) to 7.2±2.2% in HepG2 cells, from 4.0±2.5% to 17.8±2.7% in HT1080 cells and from 7.0±1.5% to 14.0±0.4% in MCF7 cells, respectively (Fig 5B; supplementary Fig S6C online).
Figure 5.
SYCP3 impairs the BRCA2 recombination mediator activity in mitotic cells and hypersensitizes the cells to the inhibitor of PARP. (A) Colony outgrowth of the RPE cells expressing or not expressing FLAG–SYCP3 either untreated or treated with 4 μM cisplatin for 1 h before continuous exposure to the poly(ADP-ribose) polymerase (PARP) inhibitor NU1025. The representative result of three independent experiments is shown. The mean and s.d. of the triplicate dishes are indicated. (B,C) Colony survival of HepG2 cells (B), HT1080 cells (B), MCF7 cells (B) and Capan1 cells (C) transfected with SYCP3-targeting small interfering RNA (siRNA) and a non-targeting siRNA control after continuous treatment with NU1025. Columns and bars represent the mean of three independent experiments and s.d., respectively. (D) A proposed model for inhibition of RAD51-dependent homologous recombination by SYCP3. BRCA2 binds to the double-stranded DNA (dsDNA)–single-stranded DNA (ssDNA) junction and loads RAD51 onto ssDNA while suppressing RAD51–dsDNA binding. SYCP3 impairs the recruitment of RAD51 to resected DSBs by forming a complex with BRCA2.
The sensitivity to PARP inhibitors in cells that are naturally deficient in BRCA2 should therefore not be affected by SYCP3 expression. The pancreatic cancer cell line Capan1, which expresses endogenous SYCP3 (supplementary Fig S1A online), carries a 6174delT mutation in one BRCA2 allele accompanied by loss of the wild-type allele and is reported to be sensitive to PARP inhibitors (McCabe et al, 2005). Supporting this prediction, silencing of SYCP3 in Capan1 cells did not affect the sensitivity to the PARP inhibitor (Fig 5C; supplementary Fig S6C online).
Discussion
Our findings indicate that, in mitotic cells, SYCP3 forms a complex with BRCA2, inhibits the interaction between BRCA2 and RAD51, and impairs the recruitment of RAD51 to resected DSBs, which is a crucial step in the early stages of HR (Fig 5D). This provides a new underlying mechanism for chromosomal instability, in which the function of the BRCA2 tumour suppressor is impaired by the synaptonemal complex protein SYCP3. Importantly, this inhibition of HR might be common in tumours of various tissue origins, as well as in breast and ovarian cancers with no detected BRCA2 mutations, because SYCP3 is expressed in various tumours.
The BRCA2-interacting proteins have a crucial role in modulating the HR pathway. In contrast to the previously identified proteins such as PALB2, BCCIP and DSS1, which promote the BRCA2–RAD51 repair machinery (Gudmundsdottir et al, 2004; Lu et al, 2005; Oliver et al, 2009), SYCP3 is a new type because it inhibits the repair pathway. Although our preliminary data showed a direct association between SYCP3 and full-length BRCA2 (data not shown), further characterization of the interaction between SYCP3 and BRCA2 would be needed to address how SYCP3 interacts with BRCA2 and how it inhibits the binding of RAD51 to BRCA2.
Finally, our findings provide a new insight in therapeutic strategies for cancer. Tumours with aberrant SYCP3 expression should show a satisfactory response to radiotherapy, cisplatin-based chemotherapy and especially PARP inhibitors. Although clinical studies indicated that PARP inhibitors are highly effective for cancers with BRCA1 or BRCA2 mutation (Fong et al, 2009), our finding that SYCP3 expression confers extreme hypersensitivity to PARP inhibitors would be applied to a broader range of tumours expressing SYCP3, including cancers with no detected BRCA1 or BRCA2 mutations. Establishment of this new therapeutic strategy would require further analyses in large patient cohorts and tumour models.
Methods
Cell lines, samples, antibodies, expression analysis and vector constructions. Detailed descriptions are provided in the supplementary information online.
Stable expression of SYCP3 cDNA in mitotic cells. The expression vectors for SYCP3 or FLAG–SYCP3 were transfected at 1,200 V and 10 μF into RPE cells and at 250 V and 950 μF into HeLa–DRGFP cells using the Bio-Rad Gene Pulser II. Stable clones were selected with 900 μg ml−1 Zeocin (Invitrogen).
siRNA. The siRNA targeting the 3′ untranslated region of SYCP3 mRNA (5′-GCUUUCAGCUCUUUAGUAAUGAUAG-3′) was synthesized by Invitrogen and used to knock down endogenous SYCP3. To knock down exogenous FLAG–SYCP3, the predesigned siRNA (AM16704) from Ambion was used. An siCONTROL non-targeting siRNA from Dharmacon was used as a control. For transfection of the siRNA, see supplementary information online.
Immunoprecipitation, immunofluorescence, fluorescence in situ hybridization analysis, cell survival assays, analysis of SCEs and cell cycle analysis. Detailed protocols are provided in the supplementary information online.
The DR–GFP assay. Stable integrants were obtained by transfecting the RPE–FLAG–SYCP3 cells, MCF7 cells and HepG2 cells with the linearized phprtDR–GFP construct and selecting with puromycin as described previously (Sakamoto et al, 2007). For more details, see supplementary information online.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We are grateful to Maria Jasin for the DR–GFP construct and the I-SceI expression plasmid, and Kenshi Komatsu and Junya Kobayashi for providing us with HeLa–DRGFP cells. We also thank Eri Nagata and Hidekazu Suzuki for their technical assistance. This work was supported in part by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science to K.M. and to N.H., by SHISEIDO Grants for Women Scientists to N.H. and by the Itoe Okamoto Scientific Award, SHISEIKAI, to N.H.
Author contributions: N.H. performed most of the experiments. M.O., A.K., Y.F., T.H. and J.S. assisted with the experiments. N.H., S.T. and K.M. analysed data. N.H. and K.M. wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T (2005) Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434: 913–917 [DOI] [PubMed] [Google Scholar]
- Farmer H et al. (2005) Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434: 917–921 [DOI] [PubMed] [Google Scholar]
- Fong PC et al. (2009) Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 361: 123–134 [DOI] [PubMed] [Google Scholar]
- Gudmundsdottir K, Ashworth A (2006) The roles of BRCA1 and BRCA2 and associated proteins in the maintenance of genomic stability. Oncogene 25: 5864–5874 [DOI] [PubMed] [Google Scholar]
- Gudmundsdottir K, Lord CJ, Witt E, Tutt AN, Ashworth A (2004) DSS1 is required for RAD51 focus formation and genomic stability in mammalian cells. EMBO Rep 5: 989–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartlerode AJ, Scully R (2009) Mechanisms of double-strand break repair in somatic mammalian cells. Biochem J 423: 157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang TH, Noh KH, Kim JH, Bae HC, Lin KY, Monie A, Pai SI, Hung CF, Wu TC, Kim TW (2010) Ectopic expression of X-linked lymphocyte-regulated protein pM1 renders tumor cells resistant to antitumor immunity. Cancer Res 70: 3062–3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Guo X, Meng X, Liu J, Allen C, Wray J, Nickoloff JA, Shen Z (2005) The BRCA2-interacting protein BCCIP functions in RAD51 and BRCA2 focus formation and homologous recombinational repair. Mol Cell Biol 25: 1949–1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maatouk DM, Kellam LD, Mann MR, Lei H, Li E, Bartolomei MS, Resnick JL (2006) DNA methylation is a primary mechanism for silencing postmigratory primordial germ cell genes in both germ cell and somatic cell lineages. Development 133: 3411–3418 [DOI] [PubMed] [Google Scholar]
- McCabe N, Lord CJ, Tutt AN, Martin NM, Smith GC, Ashworth A (2005) BRCA2-deficient CAPAN-1 cells are extremely sensitive to the inhibition of poly (ADP-ribose) polymerase: an issue of potency. Cancer Biol Ther 4: 934–936 [DOI] [PubMed] [Google Scholar]
- Niemeyer P, Tureci O, Eberle T, Graf N, Pfreundschuh M, Sahin U (2003) Expression of serologically identified tumor antigens in acute leukemias. Leuk Res 27: 655–660 [DOI] [PubMed] [Google Scholar]
- Nojima K et al. (2005) Multiple repair pathways mediate tolerance to chemotherapeutic cross-linking agents in vertebrate cells. Cancer Res 65: 11704–11711 [DOI] [PubMed] [Google Scholar]
- Oliver AW, Swift S, Lord CJ, Ashworth A, Pearl LH (2009) Structural basis for recruitment of BRCA2 by PALB2. EMBO Rep 10: 990–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page SL, Hawley RS (2004) The genetics and molecular biology of the synaptonemal complex. Annu Rev Cell Dev Biol 20: 525–558 [DOI] [PubMed] [Google Scholar]
- Pierce AJ, Jasin M (2005) Measuring recombination proficiency in mouse embryonic stem cells. Methods Mol Biol 291: 373–384 [DOI] [PubMed] [Google Scholar]
- Sakamoto S, Iijima K, Mochizuki D, Nakamura K, Teshigawara K, Kobayashi J, Matsuura S, Tauchi H, Komatsu K (2007) Homologous recombination repair is regulated by domains at the N- and C-terminus of NBS1 and is dissociated with ATM functions. Oncogene 26: 6002–6009 [DOI] [PubMed] [Google Scholar]
- Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ (2005) Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer 5: 615–625 [DOI] [PubMed] [Google Scholar]
- Tashiro S, Walter J, Shinohara A, Kamada N, Cremer T (2000) Rad51 accumulation at sites of DNA damage and in postreplicative chromatin. J Cell Biol 150: 283–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.