Abstract
Oxidative stress induced by high levels of reactive oxygen species (ROS) is associated with the development of different pathological conditions, including cancers and autoimmune diseases. We analysed whether oxidatively challenged tissue can have systemic effects on the development of cellular immune responses using Drosophila as a model system. Indeed, the haematopoietic niche that normally maintains blood progenitors can sense oxidative stress and regulate the cellular immune response. Pathogen infection induces ROS in the niche cells, resulting in the secretion of an epidermal growth factor-like cytokine signal that leads to the differentiation of specialized cells involved in innate immune responses.
Keywords: haemocyte, haematopoietic niche, FoxO, ROS, Spitz
Introduction
Studies during the past decade have shown an important role of oxidative stress sensing in the development and function of different tissues in metazoans (Finkel & Holbrook, 2000; Ghaffari, 2008; Owusu-Ansah et al, 2008; Johannsen & Ravussin, 2009). While most investigations were primarily centred on the cell autonomous effects of oxidative stress, the question of whether the stressed cells can generate signals that mediate cell non-autonomous effects on neighbouring or distant tissues has not been properly addressed. This question is of particular importance for the regulation of innate and adaptive immune responses and has possible involvement in the aetiology of immunological and haematopoietic diseases. In this paper we have investigated cell-non-autonomous effects caused by oxidative stress that alters the differentiation status of the stress-sensing myeloid-like Drosophila blood system. A haematopoietic organ of Drosophila known as the lymph gland consists of three developmentally distinct cellular zones (Jung et al, 2005). Haematopoietic progenitor cells reside in a medullary zone (MZ) of the organ. During development, these progenitors differentiate to give rise to mature blood cells that populate the cortical zone. A cluster of cells located in the posterior end of the gland represents its third compartment, called the posterior signalling centre (PSC) that primarily functions as a haematopoietic niche or microenvironment, maintaining the stem-like progenitor cells within the MZ (Krzemien et al, 2007; Mandal et al, 2007). The cellular component of the Drosophila innate immune system is constituted of three types of effector blood cell: plasmatocytes, crystal cells and lamellocytes. Plasmatocytes are responsible for phagocytosis/scavenging and crystal cells are responsible for blood clotting; they are maintained in relatively constant numbers during development (Lanot et al, 2001). In contrast, lamellocytes are barely detectable under normal conditions but are rapidly generated on infestation by parasitic intruders such as wasp eggs, and these cells are responsible for the inactivation and removal of the invaders (Rizki & Rizki, 1992; Sorrentino et al, 2002; Markus et al, 2005). Lamellocyte differentiation induced by immune challenge fails to occur in the absence of collier expression in the PSC (Crozatier et al, 2004), suggesting the possibility that a signal from an intruder event mediates changes in the PSC, which in turn sends a signal to initiate differentiation of lamellocytes. In this study we have addressed the mechanism by which this systemic response is generated.
Results And Discussion
High ROS in the PSC induces cellular immune response
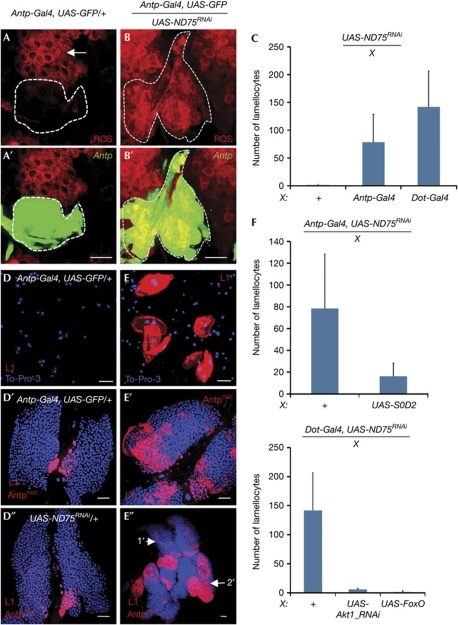
Abnormal metabolism is often associated with oxidative stress that results in increased production of ROS by mitochondria (Finkel & Holbrook, 2000; Owusu-Ansah et al, 2008; Starkov, 2008). Different concentrations of ROS and their derivatives are required for proper maintenance, proliferation, differentiation and apoptosis of stem cells and their committed progenitors (Kops et al, 2002; Giorgio et al, 2005; Tothova et al, 2007; Owusu-Ansah & Banerjee, 2009; Sinenko et al, 2010). In Drosophila, developmentally regulated levels of ROS are critical for maintenance of haematopoietic progenitors within the MZ of the lymph gland (Owusu-Ansah & Banerjee, 2009). In contrast, under normal growth conditions, PSC cells in wild-type larvae had very low levels of ROS expression (Fig 1A,A′) compared with that in the progenitor population of cells within the MZ (Fig 1A). To induce oxidative stress in the PSC we inactivated ND75, a component of complex I of the electron transport chain (ETC; Owusu-Ansah & Banerjee, 2009), with double-stranded RNA (dsRNA) using the Gal4/UAS misexpression system (Brand & Perrimon, 1993) and the PSC-specific Antp-Gal4 driver. As previously observed in other tissues (Owusu-Ansah et al, 2008), ND75 inactivation caused a readily detectable increase in ROS in the PSC cells (Fig 1B,B′), rising to levels similar to those seen in the progenitor cells of the MZ. The phenotypic consequence of inducing oxidative stress in the cells of the PSC was a remarkably robust increase in numbers of circulating lamellocytes (Fig 1C–E). Such an elevated number of lamellocytes was usually observed in wild-type larvae only if they were infested by parasitic wasps (Fig 2E,H). Although Antp-Gal4 is not expressed anywhere in the blood system, except the PSC, this driver is also expressed in other larval tissues. To exclude the possibility that the effect was due to a non-PSC expression of Antp-GaI4, the function of ND75 was also eliminated using the Dot-Gal4 driver normally expressed at high levels in the PSC, and this resulted in an identical lamellocyte response (Fig 1C). In contrast, oxidative challenge to various other larval tissues, including the fat body (LSP2-GaI4), the epidermis (A58-GaI4), the neurons (C127-GaI4), the dorsal vessel (Hand-GaI4), the ring gland (5015-GaI4), the wing imaginal disc (ap-Gal4) or the trachea (btl-GaI4), did not have a significant effect on lamellocyte differentiation (supplementary Fig S1A online). Furthermore, high ROS levels generated within the progenitor cells (dome-GaI4) of the lymph gland, which causes autonomous differentiation of this population (Owusu-Ansah and Banerjee, 2009), also did not have any significant effect on the non-autonomous differentiation of lamellocytes in the circulation (supplementary Fig S1A online). In contrast, oxidative challenge of the PSC caused non-autonomous lamellocyte response in circulation as well as within the lymph gland (Fig 1D′–E″). The PSC-mediated effect was due to mitochondrial dysfunction and not specifically linked to the product of the ND75 gene, because attenuation of PDSW (another complex I component), cytochrome-c oxidase, subunit Va (CoVa, a component of ETC complex IV) or Marf (mitochondrial assembly regulatory factor) function in the PSC, all induced increases in lamellocyte differentiation (supplementary Fig S1B online). The strength of the lamellocyte response to complex I inactivation depended on the strength of the dsRNA construct used in the experiment. Temporally, induction of the mutation in the second-larval instar caused the lamellocyte response to be seen in the third instar (not shown). This correlates well with the timescale of response to parasitic wasp infection (Crozatier et al, 2004). Finally, this oxidative stress elicited a cell-specific response; for example, no significant effect was seen on the differentiation of crystal cells and plasmatocytes in circulation (supplementary Fig S1C,D online). These results establish that the oxidative status of the PSC has a specific and non-autonomous role in lamellocyte differentiation as an immune response to parasitic invasion.
Figure 1.
ETC-mediated oxidative stress in the posterior signalling centre induces differentiation of lamellocytes. Cell markers are colour coded and indicated on each panel; genotypes are indicated at the top of each panel. All error bars represent standard deviation from the mean. (A,A′) Posterior signalling centre (PSC) cells (marked by Antp-Gal4, UAS-GFP, green, outlined) have very low levels of reactive oxygen species (ROS) compared with neighbouring progenitor population (arrow, red). (B,B′) Inactivation of ND75 in the PSC (green) causes significant upregulation of ROS expression in these cells (red). Scale bar, 10 μm. (C) Lamellocytes are rarely, if at all, seen in circulation of the wild-type larvae (+); n=14. Oxidative stress induced by inactivation of ND75 (UAS-ND75RNAi) in the PSC cells with the use of Antp-Gal4 (n=14) or Dot-Gal4 (n=16) causes a significant increase in the number of circulating lamellocytes. Additional drivers are included as Gal4 lines shown along the X axis. (D–D″) In wild-type larvae, lamellocytes (L1, red membrane stain) do not develop in circulation (D) or within the lymph gland (D′,D″; genotypes indicated; scale bar, 25 μm). The red staining in the PSC region is nuclear, marks Antp and is distinguishable from the extensive L1 membrane staining (as in E–E″). (E–E″) Inactivation of ND75 in PSC causes extensive lamellocyte differentiation in larval circulation (E), the primary (E′) and secondary (arrow, E″) lobes of the linkage group. (F,G) Superoxide dismutase-2 (SOD2) and Forkhead box O (FoxO) are crucial in controlling lamellocyte response in the PSC. The controls (+), show that inactivation of ND75 (UAS-ND75RNAi) in the PSC with Antp-Gal4 (F, n=14) or Dot-Gal4 (G, n=14) causes significant increase in lamellocyte numbers. Overexpression of SOD2 (F, P<0.001, n=18) or Foxo (G, n=14) or inactivation of Akt1 (G, n=14) in the ND75-deficient PSC suppresses this lamellocyte response. Misexpression constructs are indicated along the X axis. ETC, Electron transport chain; GFP, green fluorescent protein; RNAi, RNA-mediated interference; UAS, upstream activating sequence.
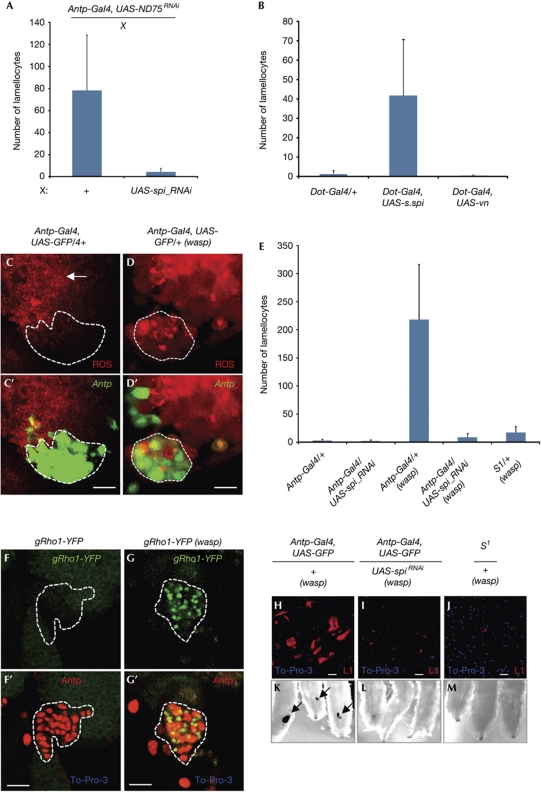
Figure 2.
Spitz signal from the oxidatively challenged PSC is required for the development of the lamellocyte response. (A) Inactivation of ND75 in PSC causes a significant increase in numbers of circulating lamellocytes (+); n=14. Inactivation of spitz (spiRNAi) in the ND75-deficient PSC significantly suppresses the lamellocyte numbers; n=16. (B) Overexpression of secreted Spitz (Dot-Gal4, UAS-s.spi, n=11) but not the alternative epidermal growth factor receptor (EGFR) ligand vein (Dot-Gal4, UAS-vn, n=10) in the PSC causes a significant increase in the lamellocyte numbers, compared with the wild-type background (Dot-Gal4/+, n=14). (C,C′) Under normal growth conditions, PSC cells (marked by Antp-Gal4, UAS-GFP, green outlined) are largely negative for reactive oxygen species (ROS; red); scale bar, 10 μm. (D,D′) ROS levels (red) are significantly increased in the PSC cells after 12 h of wasp infestation. (E) Lamellocytes do not develop in wild-type (Antp/+, n=12) background or when spitz (spi) is inactivated in the PSC with the use of Antp-Gal4 (Antp-Gal4, UAS-spiRNAi, n=15). Large numbers of lamellocytes develop in wasp-infested wild-type larvae (Antp/+ (wasp), n=10). Loss of spi function in the PSC (Antp-Gal4, UAS-spiRNAi(wasp), n=15) or loss of a single copy of Star (S1/+, n=15) results in a suppression of lamellocyte production in the infested mutant larvae. (F,F′) Under normal growth conditions PSC cells (marked by Antp, red outlined) do not express Rho (gRho1-YFP, green); scale bar, 10 μm. (G,G′) Rho (gRho1-YFP, green) is expressed as punctate dots in the cells of the PSC after 12–18 h of wasp infestation. (H) Large numbers of circulating lamellocytes develop in larvae infested with wasp eggs; scale bar, 50 μm. (I) Differentiated lamellocytes do not develop in the wasp-infested larvae that lack spi function in the PSC. (J) Differentiated lamellocytes do not develop in wasp-infested larvae that lack of single dose of S protein. (K) Melanotic capsules (arrows) are seen in wasp-infested larvae. (L) Melanotic capsules do not develop in the wasp-infested larvae that lack spi function in the PSC. (M) Melanotic capsules do not develop in the wasp-infested larvae of mutants lacking a single copy of Star. Cell markers are colour coded and indicated on each panel; genotypes are indicated at the top of each panel. The error bars shown in A, B and E represent standard deviation from the mean. GFP, green fluorescent protein; PSC, posterior signalling centre; RNAi, RNA-mediated interference; UAS, upstream activating sequence; vn, Vein; YFP, yellow fluorescent protein.
Akt1/FoxO controls oxidative sensing of the PSC niche
The status of the PSC cells on oxidative stress conditions was further analysed in some detail. ND75 dysfunction does not affect proliferation or maintenance of the PSC, because the number of PSC cells, which maintain expression of Antp, remains intact in this mutant background (Fig 1A–B′). In addition, no apoptosis is detected in ND75-deficient PSC cells (not shown; Owusu-Ansah et al, 2008; Owusu-Ansah & Banerjee, 2009), and also, apoptosis in the PSC alone, specifically induced by overexpression of Hid/Rpr, has no effect on lamellocyte differentiation (supplementary Fig S1E online).
Overexpression of superoxide dismutase-2 (SOD2) as a scavenger for ROS (Finkel & Holbrook, 2000) in ND75-deficient PSC is able to suppress the lamellocyte response significantly (Fig 1F). Furthermore, activation of the Forkhead box O (FoxO) transcription factor that positively regulates expression of antioxidant enzymes, including SOD2 (Nemoto & Finkel, 2002), completely suppresses the dsND75-induced lamellocyte response (Fig 1G). Inactivation of the Akt1 protein kinase in PSC also results in a near-complete suppression of the dsND75-induced lamellocyte response (Fig 1G), suggesting a role for the PI3K/Akt pathway in the regulation of FoxO. This is an important issue because FoxO activity can also be controlled by the Jun N-terminal kinase (JNK) pathway (Essers et al, 2004; Owusu-Ansah & Banerjee, 2009), but in the PSC the AKT pathway mediates this effect. The JNK reporter (puc69-lacZ) is not expressed in the PSC, and inactivation of JNK (encoded by the basket gene) using the dominant-negative form (bskDN) does not suppress dsND75-induced lamellocyte response (supplementary Fig S2A online). The FoxO reporter (4E-BP-lacZ) is robustly activated in the ND75-deficient PSC (supplementary Fig S2B–C′ online); however, loss of translational inhibition mediated by 4E-BP does not mimic this effect (not shown). It is important to point out that under wild-type non-stressed conditions, the PSC has relatively low levels of ROS, and therefore inactivation of either Foxo or SOD2 has no phenotypic consequence (supplementary Fig S2D online). We interpret these data to indicate that metabolic dysfunction induces an oxidatively stressed PSC that causes the activation of this pathway and the lamellocyte response.
Spitz from the PSC activates cellular immune response
Differentiation of lamellocytes has been associated with the JAK/STAT, JNK and Ras/Erk signalling pathways (Harrison et al, 1995; Sorrentino et al, 2004; Zettervall et al, 2004; Williams et al, 2006; Makki et al, 2010). We genetically altered these pathways in an ND75-deficient PSC background to identify which, if any, is involved in the lamellocyte response. Inactivation of the unpaired ligands (upd3, upd2 or upd) that activate the JAK/STAT pathway or of eiger (egr), which activates JNK signalling, did not suppress the lamellocyte phenotype (supplementary Fig S3A online). This strongly suggests that these pathways are not involved in the process downstream of ROS in the PSC and is consistent with previous studies showing that components of the JAK/STAT pathway (upd3, dome and Tep4; Krzemien et al, 2007; Makki et al, 2010) and JNK (puc69-lacZ reporter) are not involved in the functioning of the PSC. However, these pathways are likely to be involved in direct regulation of lamellocyte differentiation independently of the PSC function (Zettervall et al, 2004; Makki et al, 2010). In contrast, inactivation of spitz (spi), encoding the ligand for epidermal growth factor receptor (EGFR), in the context of ND75-deficient PSC significantly suppresses the lamellocyte response (Fig 2A). Furthermore, overexpression of the secreted form of Spi (s.Spi), but not the alternative EGFR ligand, Vein (Vn; Golembo et al, 1996; Schnepp et al, 1996) in the PSC, causes increased differentiation of circulating lamellocytes (Fig 2B) in an otherwise wild-type larva. EGFR mutant EgfrTS/Egfr18 lymph glands develop normally (supplementary Fig S3B,C online), suggesting that EGFR signalling is not required for normal lymph gland development but rather is involved in the regulation of a cellular immune response as a signalling event from the PSC only when the latter is oxidatively stressed.
The PSC-dependent parasitic challenge induced by wasp egg infestation and the mechanism described above both give rise to the same cellular response. We therefore decided to determine whether parasitization causes oxidative stress to the PSC. We found that immune challenge caused by wasp infestation induces high levels of ROS in the PSC cells as seen 12 h after invasion (Fig 2C–D′). The most prominent effect is on superoxide radicals detected with dihydroethidium staining (Fig 2D,D′); a smaller but detectable elevation of peroxide radicals revealed by RedoxSensor staining (supplementary Fig S4A–B′ online) is also apparent in PSC cells on this immune challenge. Scavenging these ROS types in the PSC by overexpressing SOD2 or catalase (Cat; Finkel & Holbrook, 2000) but not glutathione peroxidase (GPx), which reduces thioredoxin-mediated effects (Missirlis et al, 2003), significantly suppresses the lamellocyte response caused by wasp infestation (supplementary Fig S4C online). These genetic results are consistent with a model in which parasitic infection by wasp eggs raises ROS levels in the PSC, which then causes lamellocyte induction by expressing Spitz (Fig 3E). To test this model, we inactivated spi within the PSC in larvae infected by parasitic wasps. This caused a strong suppression of the lamellocyte response (Fig 2E,H,I,K,L); the few remaining L1 marker–positive cells are immature, as indicated by their relatively small cell size and their morphology (Fig 2H,I). In addition, melanotic capsules that are indicative of extensive cellular immune response to parasitic infection do not develop in a spi mutant background during wasp infestation (Fig 2K,L). Inactivation of spitz in the PSC did not affect the increase in ROS triggered by wasp infeststion (supplementary Fig S4D–F′ online). Thus spi does not regulate the ROS levels in the PSC; rather, wasp infection raises ROS levels, which leads to release of the s.Spi. Previous studies have shown that s.Spi production requires the function of the trafficking protein Star (S), and the protease Rhomboid (Rho1; Kolodkin et al, 1994; Urban et al, 2001; Tsruya et al, 2002). We found that the wasp-induced lamellocyte response and melanotic capsule formation are robustly suppressed on the loss of a single copy of Star (Fig 2E,J,M). More importantly, parasite-induced immune challenge specifically upregulates Rho1 in the PSC (Fig 2F,G′) by an as yet unidentified mechanism. These data establish that S and Rho1 are canonically required for processing and releasing the Spitz from the PSC.
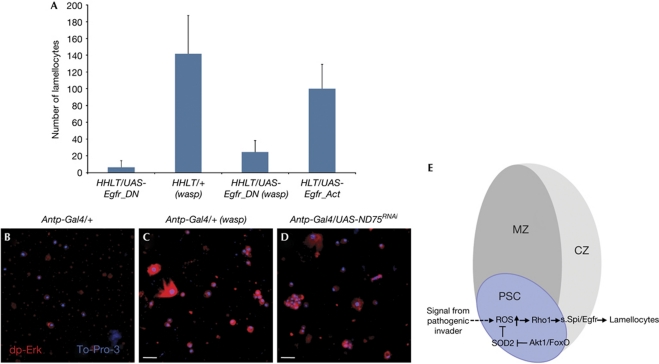
Figure 3.
Spi/EGFR signalling mediates lamellocyte cellular immune response. (A) As controls, lamellocytes are barely detected in wild-type circulation or when Egfr is inactivated in all haemocytes (HHLT-Gal4, UAS-EgfrDN, n=10). All error bars shown represent standard deviation from the mean. Large numbers of lamellocytes develop in wasp-infested wild-type larvae (HHLT-Gal4/+ (wasp), n=10). Loss of Egfr function in all haemocyte populations (HHLT-Gal4, UAS-EgfrDN(wasp), P=0.0001, n=10) results in a suppression of lamellocyte production in the infested mutant larvae. Overexpression of EgfrAct in the haemocyte precursors (HLT-Gal4, UAS-EgfrAct) induces the generation of large number of lamellocytes. (B–D) Under normal growth conditions dpErk expression is extremely low (B, red). Oxidative stress in the PSC induced by wasp infestation (C, red) or by inactivation of ND75 in the PSC (D, red) causes a significant increase in dpErk-positive cells in larval circulation. Genotypes are indicated at the top of the panels. Scale bar, 25 μm. (E) Proposed model. Signal from parasitic invader induces changes in the oxidative status of the PSC that in turn causes the production of secreted Spitz (s.Spi) protein, which signals via EGFR receptor, inducing lamellocyte differentiation. The Akt/FoxO pathway is involved in the regulation of the oxidative status of the PSC. CZ, cortical zone; DN, dominant-negative; EGFR, epidermal growth factor receptor; FoxO, Forkhead box O; HHLT, Hand-Hml lineage traced; HLT, Hml lineage traced; MZ, medullary zone; PSC, posterior signalling centre; ROS, reactive oxygen species; SOD, Superoxide dismutase-2; Spi, Spitz.
Secreted Spitz is known to bind to EGFR and activate the Ras/Erk pathway (Seger & Krebs, 1995). A dominant-negative form of EGFR (EgfrDN) strongly suppresses the lamellocyte response induced by wasp infestation when it is expressed in the lymph gland and the circulating haemocytes using the pan-haemocyte HHLT Gal4 driver (Fig 3A). This phenotype is virtually identical to that seen when spiRNAi is expressed in the PSC using Antp-Gal4. In addition, we used compartment-specific drivers and found that inactivation of the receptor in the cortical zone of the lymph gland and in circulating haemocytes (using lineage-traced HmlΔ-Gal4 line) prevents Hml-positive cells from becoming lamellocytes on wasp infestation (supplementary Fig S4G online). Importantly, we also found that a small subset of lamellocytes does not express Hml in the wild-type background and consequently EgfrDN is not expressed in these cells when HmlΔ-Gal4 is used as a driver. These Hml−,L1+ lamellocytes are easily detectable in this genetic background and act as an internal control. Expression of an activated form of EGFR (EgfrAct) in Hml+ haemocytes causes a robust increase in lamellocyte differentiaion (Fig 3A). This is also consistent with previous work, which showed that activated Ras induces an increase in the total number of haemocytes, including lamellocytes (Asha et al, 2003). Finally, both loss of ND75 in the PSC and wasp infestation cause robust activation of Erk as evident by an increase in dpErk staining in circulating haemocytes including lamellocytes (Fig 3B–D). This indicates that lamellocytes in circulation differentiate from precursor cells on activation of Spi/EGFR/Erk signalling. Taken together, these data provide strong support for the model shown in Fig 3E.
PSC cells have two independent functions: they serve as a haematopoietic niche in the lymph gland, where they orchestrate the maintenance and proper differentiation of haematopoietic progenitors (Krzemien et al, 2007; Mandal et al, 2007), and they regulate the cellular immune response by controlling lamellocyte differentiation in response to infection (Crozatier et al, 2004; Makki et al, 2010). The results presented here establish the mechanism for this latter function. Changes in oxidative status, caused by events of parasite invasion or ETC dysfunction, initiates a signal within this immunocompetent compartment causing the secretion of a cytokine ligand, Spitz, that induces differentiation of lamellocyte precursors in the circulatory system of the larva (Fig 3E). The identified mechanism is consistent with previously reported studies in mammals, which have shown that mitochondrial ROS can trigger systemic signals that reinforce the innate immune response (Arsenijevic et al, 2000). Our studies raise the possibility that specific populations of cells also exist in mammalian systems that sense oxidative stress due to infection and non-autonomously signal myeloid progenitors to initiate differentiation and enhance the immune response. Whether such populations are to be found within the haematopoietic niche as in Drosophila remains a speculation that can be tested in future studies.
Methods
For sources of stocks and antibodies used, please see supplementary information.
Genetics. Hand-Hml lineage-traced Gal4 (HHLT Gal4) and Hml lineage-traced Gal4 (HLT Gal4) lines were used to express transgenes in all haemocyte populations and Hml-positive haemocytes, respectively (Evans et al, 2009); Evans and Banerjee, unpublished). Two independent insertions of each UAS–dsRNA constructs were used. For controls, larvae from crosses of particular Gal4 line and w1118 were used. EgfrTS/Egfr18 mutants were shifted to restrictive temperature (29 °C) during the late embryonic stage—early first-larval instar and lymph glands analysed were dissected from third-instar larvae. Crosses were maintained on standard Drosophila cornmeal/sucrose/yeast medium at 25 or 29 °C.
Wasp infestation experiment. Late second or early third-instar larvae were treated with wasps (Leptopilina boulardi) for 12 h at room temperature. At 12 or 48 h (25 °C) after infestation (Crozatier et al, 2004), haemocyte samples were collected from third-instar larvae on glass slides and immunostained with L1 antibodies as described (Sinenko et al, 2004). Statistical analyses were performed using Student's t-test. Data in the figures represent mean±s.d.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank R. Nagaraj, C.J. Evans and other members of the Banerjee Laboratory for valuable comments during these studies. We also thank I. Ando, M. Freeman, B.-Z. Shilo, A. Simcox and P.H. Taghert for sharing reagents. We are grateful to the Bloomington Drosophila Stock Center, Mishima Genetic Strain Research Center, Vienna Drosophila RNAi Center and Developmental Studies Hybridoma Bank at the University of Iowa. J.S. is supported by a Broad Stem Cell Research Center Training Grant. This work was supported by a National Institutes of Health grant (5R01 HL067395) to U.B.
Author contributions: S.A.S. and U.B. developed the project. S.A.S designed and performed experiments. J.S. performed experiments. S.A.S. and U.B. discussed results and wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Arsenijevic D et al. (2000) Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet 26: 435–439 [DOI] [PubMed] [Google Scholar]
- Asha H, Nagy I, Kovacs G, Stetson D, Ando I, Dearolf CR (2003) Analysis of Ras-induced overproliferation in Drosophila hemocytes. Genetics 163: 203–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415 [DOI] [PubMed] [Google Scholar]
- Crozatier M, Ubeda JM, Vincent A, Meister M (2004) Cellular immune response to parasitization in Drosophila requires the EBF orthologue collier. PLoS Biol 2: E196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essers MA, Weijzen S, de Vries-Smits AM, Saarloos I, de Ruiter ND, Bos JL, Burgering BM (2004) FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J 23: 4802–4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CJ et al. (2009) G-TRACE: rapid Gal4-based cell lineage analysis in Drosophila. Nat Methods 6: 603–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408: 239–247 [DOI] [PubMed] [Google Scholar]
- Ghaffari S (2008) Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid Redox Signal 10: 1923–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio M et al. (2005) Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 122: 221–233 [DOI] [PubMed] [Google Scholar]
- Golembo M, Raz E, Shilo BZ (1996) The Drosophila embryonic midline is the site of Spitz processing, and induces activation of the EGF receptor in the ventral ectoderm. Development 122: 3363–3370 [DOI] [PubMed] [Google Scholar]
- Harrison DA, Binari R, Nahreini TS, Gilman M, Perrimon N (1995) Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. EMBO J 14: 2857–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsen DL, Ravussin E (2009) The role of mitochondria in health and disease. Curr Opin Pharmacol 9: 780–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SH, Evans CJ, Uemura C, Banerjee U (2005) The Drosophila lymph gland as a developmental model of hematopoiesis. Development 132: 2521–2533 [DOI] [PubMed] [Google Scholar]
- Kolodkin AL, Pickup AT, Lin DM, Goodman CS, Banerjee U (1994) Characterization of Star and its interactions with sevenless and EGF receptor during photoreceptor cell development in Drosophila. Development 120: 1731–1745 [DOI] [PubMed] [Google Scholar]
- Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM (2002) Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 419: 316–321 [DOI] [PubMed] [Google Scholar]
- Krzemien J, Dubois L, Makki R, Meister M, Vincent A, Crozatier M (2007) Control of blood cell homeostasis in Drosophila larvae by the posterior signalling centre. Nature 446: 325–328 [DOI] [PubMed] [Google Scholar]
- Lanot R, Zachary D, Holder F, Meister M (2001) Postembryonic hematopoiesis in Drosophila. Dev Biol 230: 243–257 [DOI] [PubMed] [Google Scholar]
- Makki R et al. (2010) A short receptor downregulates JAK/STAT signalling to control the Drosophila cellular immune response. PLoS Biol 8: e1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal L, Martinez-Agosto JA, Evans CJ, Hartenstein V, Banerjee U (2007) A Hedgehog- and Antennapedia-dependent niche maintains Drosophila haematopoietic precursors. Nature 446: 320–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus R, Kurucz E, Rus F, Ando I (2005) Sterile wounding is a minimal and sufficient trigger for a cellular immune response in Drosophila melanogaster. Immunol Lett 101: 108–111 [DOI] [PubMed] [Google Scholar]
- Missirlis F, Rahlfs S, Dimopoulos N, Bauer H, Becker K, Hilliker A, Phillips JP, Jackle H (2003) A putative glutathione peroxidase of Drosophila encodes a thioredoxin peroxidase that provides resistance against oxidative stress but fails to complement a lack of catalase activity. Biol Chem 384: 463–472 [DOI] [PubMed] [Google Scholar]
- Nemoto S, Finkel T (2002) Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science 295: 2450–2452 [DOI] [PubMed] [Google Scholar]
- Owusu-Ansah E, Banerjee U (2009) Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature 461: 537–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu-Ansah E, Yavari A, Mandal S, Banerjee U (2008) Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nat Genet 40: 356–361 [DOI] [PubMed] [Google Scholar]
- Rizki TM, Rizki RM (1992) Lamellocyte differentiation in Drosophila larvae parasitized by Leptopilina. Dev Comp Immunol 16: 103–110 [DOI] [PubMed] [Google Scholar]
- Schnepp B, Grumbling G, Donaldson T, Simcox A (1996) Vein is a novel component in the Drosophila epidermal growth factor receptor pathway with similarity to the neuregulins. Genes Dev 10: 2302–2313 [DOI] [PubMed] [Google Scholar]
- Seger R, Krebs EG (1995) The MAPK signaling cascade. FASEB J 9: 726–735 [PubMed] [Google Scholar]
- Sinenko SA, Hung T, Moroz T, Tran QM, Sidhu S, Cheney MD, Speck NA, Banerjee U (2010) Genetic manipulation of AML1-ETO-induced expansion of hematopoietic precursors in a Drosophila model. Blood 116: 4612–4620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinenko SA, Kim EK, Wynn R, Manfruelli P, Ando I, Wharton KA, Perrimon N, Mathey-Prevot B (2004) Yantar, a conserved arginine-rich protein is involved in Drosophila hemocyte development. Dev Biol 273: 48–62 [DOI] [PubMed] [Google Scholar]
- Sorrentino RP, Carton Y, Govind S (2002) Cellular immune response to parasite infection in the Drosophila lymph gland is developmentally regulated. Dev Biol 243: 65–80 [DOI] [PubMed] [Google Scholar]
- Sorrentino RP, Melk JP, Govind S (2004) Genetic analysis of contributions of dorsal group and JAK-Stat92E pathway genes to larval hemocyte concentration and the egg encapsulation response in Drosophila. Genetics 166: 1343–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkov AA (2008) The role of mitochondria in reactive oxygen species metabolism and signaling. Ann N Y Acad Sci 1147: 37–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tothova Z et al. (2007) FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128: 325–339 [DOI] [PubMed] [Google Scholar]
- Tsruya R, Schlesinger A, Reich A, Gabay L, Sapir A, Shilo BZ (2002) Intracellular trafficking by Star regulates cleavage of the Drosophila EGF receptor ligand Spitz. Genes Dev 16: 222–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban S, Lee JR, Freeman M (2001) Drosophila rhomboid-1 defines a family of putative intramembrane serine proteases. Cell 107: 173–182 [DOI] [PubMed] [Google Scholar]
- Williams MJ, Wiklund ML, Wikman S, Hultmark D (2006) Rac1 signalling in the Drosophila larval cellular immune response. J Cell Sci 119: 2015–2024 [DOI] [PubMed] [Google Scholar]
- Zettervall CJ, Anderl I, Williams MJ, Palmer R, Kurucz E, Ando I, Hultmark D (2004) A directed screen for genes involved in Drosophila blood cell activation. Proc Natl Acad Sci USA 101: 14192–14197 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.