Abstract
FBW7 is a ubiquitin E3 ligase substrate adaptor that targets many important oncoproteins—such as Notch, c-Myc, cyclin E and c-Jun—for ubiquitin-dependent proteolysis. By doing so, it plays crucial roles in many cellular processes, including cell cycle progression, cell growth, cellular metabolism, differentiation and apoptosis. Loss of FBW7 has been observed in many types of human cancer, and its role as a tumour suppressor was confirmed by genetic ablation of FBW7 in mice, which leads to the induction of tumorigenesis. How FBW7 exerts its tumour suppression function, and whether loss of FBW7 leads to de-differentiation or acquisition of stemness—a process frequently seen in human carcinomas—remains unclear. Emerging evidence shows that FBW7 controls stem cell self-renewal, differentiation, survival and multipotency in various stem cells, including those of the haematopoietic and nervous systems, liver and intestine. Here, we focus on the function of FBW7 in stem cell differentiation, and its potential relevance to human disease and therapeutics.
Keywords: stem cell, FBW7, differentiation, Notch, c-Jun
See Glossary for abbreviations used in this article.
Glossary.
BLBP brain lipid-binding protein
DAPT N-[N-(3,5-difluorophenacetyl)- l-alanyl]-S-phenylglycine t-butyl
FBW7 F-box and WD-repeat-domain-containing 7
GSK-3 glycogen synthase kinase 3
HECT homologous to E6-associated protein carboxyl terminus
Hes hairy and enhancer of split 1
Hey hairy/enhancer-of-split related with YRPW motif protein 1
KLF Kruppel-like factor
mTOR mammalian target of rapamycin
RBR ring between ring fingers
SOX SRY-box
SREBP sterol regulatory element-binding protein
Wnt wingless-type MMTV integration site family
What is FBW7?
Ubiquitination is a post-translational modification that has many regulatory roles in the cell. An important one is the promotion of protein degradation by the 26S proteasome, a multi-subunit protease complex. This pathway controls diverse cellular processes, including cell proliferation, cell cycle progression, transcription, immune response, DNA damage repair and apoptotic cell death (Nalepa et al, 2006). Ubiquitination is mediated through a cascade of three enzymatic reactions catalysed by the ubiquitin-activating enzyme (E1), the ubiquitin-conjugating enzyme (E2) and the ubiquitin ligases (E3s), which can physically bind to and add ubiquitin chains to the target protein, often resulting in its recognition and degradation in an ATP-dependent manner by the proteasome (Nalepa et al, 2006).
On the basis of common structural motifs, E3s are grouped into several main classes: RING-finger, HECT, U-box, PHD-finger and RBR (Eisenhaber et al, 2007; Rotin & Kumar, 2009). RING-finger E3 ligases are grouped into subfamilies, one of which consists of the Cullin-based E3 ligases, including the Skp1–Cullin 1–F-box (SCF) complex (Frescas & Pagano, 2008). The core of the SCF complex consists of Skp1, Rbx1 and Cullin 1; it additionally contains variable F-box proteins (Frescas & Pagano, 2008). Each F-box protein has two main functional domains: the F-box, which interacts with the SCF core complex through binding to Skp1; and the carboxy-terminal domains—such as WD40 or the LRR domain—which bind to specific substrates (Nakayama & Nakayama, 2006). More than 70 putative F-box proteins have been found in the human genome (Winston et al, 1999), and their function and physiological substrates are mostly unknown. Among them, FBW7—also known as Fbxw7, Ago, CDC4 and Sel10—has been reported to target various oncogenic proteins for degradation (Welcker & Clurman, 2008). There are three mammalian FBW7 isoforms —FBW7α, FBW7β and FBW7γ—derived from alternative splicing, which encode unique amino-termini (Welcker & Clurman, 2008). This leads to differences in cellular localization and tissue distribution: FBW7α and FBW7γ localize in the nucleus and nucleolus, respectively, whereas FBW7β is mainly expressed in the cytoplasm (Welcker & Clurman, 2008). The exact physiological role of each isoform remains largely unknown.
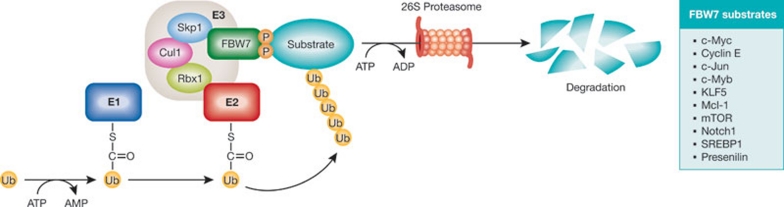
Only a handful of FBW7-specific substrates have been described, such as Aurora (Finkin et al, 2008), cyclin E (Koepp et al, 2001; Moberg et al, 2001; Strohmaier et al, 2001), c-Jun (Nateri et al, 2004; Wei et al, 2005), c-Myc (Welcker et al, 2004; Yada et al, 2004), c-Myb (Kanei-Ishii et al, 2008), KLF5 (Zhao et al, 2010), Mcl-1 (Inuzuka et al, 2011; Wertz et al, 2011), mTOR (Mao et al, 2008), Notch (Gupta-Rossi et al, 2001; Hubbard et al, 1997; Oberg et al, 2001; Wu et al, 2001), presenilin (Rocher-Ros et al, 2010) and SREBP (Sundqvist et al, 2005; Fig 1). All are oncogenic proteins often overexpressed in human cancers that have essential roles in signalling pathways involved in cell growth, division, differentiation and apoptosis. Therefore, FBW7 is believed to function as a tumour suppressor due to the negative regulation of these oncogenic proteins. Indeed, FBW7 is frequently inactivated by deletion, mutation or promoter hypermethylation in cancer (Crusio et al, 2010). FBW7 mutations occur in approximately 30% of T-cell acute lymphoblastic leukaemia (T-ALL), 16% of primary endometrial cancer and 11% of colorectal cancer. Overall, approximately 6% of all human primary tumours have mutations in FBW7 (Perry & Li, 2008).
Figure 1.
Pathway of FBW7-mediated degradation. Proteins are targeted for degradation by the ubiquitin proteasome system through an enzymatic cascade involving three enzymes: the ubiquitin-activating E1, the ubiquitin-conjugating E2 and a ubiquitin ligase, E3. The initial step is ATP-dependent and involves the linkage of ubiquitin to E1. Ubiquitin is then activated and transferred to E2. The ubiquitin-charged E2 then interacts with a specific E3 partner such as SCF(FBW7), which transfers the ubiquitin molecule to the substrate, resulting in its recognition and degradation in an ATP-dependent manner by the 26S proteasome. FBW7, F-box and WD-repeat-domain-containing 7; Ub, ubiquitin.
How does FBW7 exert its anti-tumoral activity? The exact molecular mechanisms by which FBW7 suppresses cancer development and progression are still not fully understood (Sidebar A). However, several studies have begun to shed some light on this question. For example, FBW7 deletion leads to activation of p53 through c-Myc accumulation in mature T lymphocytes (Onoyama et al, 2007). Although FBW7 deletion alone is not sufficient for tumorigenesis, the combination of FBW7 and p53 inactivation efficiently promotes lymphomagenesis (Onoyama et al, 2007). Furthermore, loss of FBW7 induces gut adenomas mainly due to overexpression of Notch and c-Jun (Babaei-Jadidi et al, 2011), and inhibition of FBW7 induces the development of thymic lymphoma through upregulation of c-Myc (Onoyama et al, 2007). Additionally, depletion of FBW7 delays c-Myb turnover and increases its abundance in a GSK3-dependent manner in myeloid leukaemia cells (Kitagawa et al, 2009). FBW7 targets mTOR for ubiquitination and degradation, and loss of FBW7 leads to activation of mTOR in human breast cancer (Mao et al, 2008). More recently, FBW7 was described to target Mcl-1 for ubiquitination and destruction (Inuzuka et al, 2011; Wertz et al, 2011). As a result, elevated expression of the pro-survival factor Mcl-1 in FBW7-deficient cells results in resistance to anti-tubulin chemotherapeutic agents and accelerated tumorigenesis (Wertz et al, 2011). Similarly, FBW7-deficient T-ALL cells with higher expression of Mcl-1 are more sensitive to the Mcl-1 antagonist sorafenib, while acquiring resistance to the Bcl-2 family inhibitor ABT-737 (Inuzuka et al, 2011). Although inhibition of FBW7 substrates might contribute to suppress tumour growth, many questions remain before we will fully understand the complex roles of FBW7 as a tumour suppressor (Sidebar A).
Sidebar A | In need of answers.
How does loss of FBW7 result in cancer?
How does FBW7 regulate abnormal cell division, resulting in cancer?
Why are numerous oncoproteins regulated by one ubiquitin E3 ligase, SCF(FBW7)?
How does FBW7 control stem cell self-renewal and differentiation?
Does loss of the FBW7 tumour suppressor lead to de-differentiation or acquisition of the cancer stem cell phenotype, thus contributing to tumour development?
Can one specific signalling pathway control stem cell differentiation to a specific cell type?
Does targeting FBW7 regulate stem cell differentiation? Can this be harnessed to design novel therapeutic strategies for human disease?
Stem cells at a glance
In addition to its known role in tumour suppression, FBW7 has recently been implicated in the control of stem cell biology, which is the focus of this review. Stem cells are classically defined as cells with unlimited capacity for self-renewal and a remarkable potential to differentiate into a full spectrum of cells, allowing the formation of tissues and/or full organisms (Reya et al, 2001). There are three main types of stem cell: embryonic, germinal and somatic. Embryonic stem cells (ESCs) are derived from the blastocyst—a 3–5-day-old embryo—and have the capacity to generate all the cell types of a mature organism, as well as the ability to replicate unlimitedly (Young, 2011). Germinal stem cells from the germinal layer of the embryo eventually undergo differentiation to generate either eggs or sperms (Barroca et al, 2009). Somatic stem cells (also known as adult stem cells, ASCs) differentiate into many characteristic cell types within a specific organ or tissue (Wagers & Weissman, 2004). Recently, a new type of stem cell, termed induced pluripotent stem cells (iPSCs), were generated by transduction of four defined transcription factors—OCT4, SOX2, KLF4 and c-Myc—thus allowing some specialized adult cells to be genetically reprogrammed into a stem-cell-like state (Takahashi & Yamanaka, 2006; Wu & Hochedlinger, 2011). However, further studies revealed that iPSCs differ significantly from ESCs in many aspects (Pappas & Yang, 2008). The concept of cancer stem cells (CSCs)—also known as cancer-stem-like cells—was introduced a few years ago and remains controversial (Clevers, 2011). CSCs with the capacity to self-renew and differentiate have been identified and isolated from a wide variety of human cancers including haematopoietic tumours, breast, lung, prostate, colon, brain, head and neck, and pancreatic cancer (Clevers, 2011). However, it remains unclear how CSCs contribute to tumorigenesis in vivo.
Stem cells are found in particular locations or microenvironments within tissues, which are known as stem cell niches (Lin, 2002). The niche consists of neighbouring proliferating and differentiating cells that provide the appropriate environment so that the stem cells can remain undifferentiated (Lin, 2002). In tissues such as the gut and bone marrow, stem cells function to repair damaged cells and/or replace those that were lost due to normal wear, tear or injury (Yen & Wright, 2006). However, in other organs—including the heart and pancreas—stem cells only divide under special conditions, such as in response to injury (Laflamme & Murry, 2011; Zaret & Grompe, 2008). Interestingly, unlike somatic cell division, which yields two identical daughter cells, stem cell division is asymmetrical. Each daughter cell has the potential to either remain a stem cell or give rise to a specialized cell, such as a red blood, muscle or brain cell (Cohen & Melton, 2011).
Although research on stem cells has generated several important discoveries over the past decades, we still do not understand the signals that trigger the process of stem cell differentiation and have only a limited picture of what maintains ‘stemness’. Therefore, research into these questions is urgently needed. Several factors and signals, such as components of Notch, Wnt and Sonic hedgehog, have been shown to have pivotal roles in stem cell differentiation (Reya et al, 2001) and have been comprehensively reviewed elsewhere (Takebe et al, 2011). In addition, a body of recent literature has shown that FBW7 is also involved in stem cell differentiation (Hoeck et al, 2010; Iriuchishima et al, 2011; Matsumoto et al, 2011; Matsuoka et al, 2008; Perry & Li, 2008; Reavie et al, 2010; Thompson et al, 2008). The following sections discuss the potential roles of FBW7 in stem cell differentiation.
FBW7 in stem cell differentiation
It is important to note that ESC-specific depletion of FBW7 does not induce phenotypic changes or alter the expression of germline markers, suggesting that FBW7 is dispensable for the self-renewal of ESCs (Reavie et al, 2010). Nevertheless, several studies have shown that FBW7 regulates several transcription factors—such as c-Myc, Notch and c-Jun—involved in the quiescence and self-renewal of haematopoietic stem cells (HSCs; Iriuchishima et al, 2011; Matsuoka et al, 2008; Thompson et al, 2008). Furthermore, two independent groups have shown that FBW7 is a key regulator of neural stem cell (NSC) maintenance and differentiation in the brain (Hoeck et al, 2010; Matsumoto et al, 2011). FBW7 also regulates the maintenance of intestinal progenitors, as progenitor cells accumulate in the crypts of mice lacking FBW7 in the intestine and their differentiation into goblet cells is impaired (Sancho et al, 2010). However, the expression of stem cell markers remained unchanged in the intestines of FBW7-deficient mice, arguing against an expansion of the stem cell compartment (Sancho et al, 2010). More recently, FBW7 deficiency has also been shown to lead to a shift in the differentiation of liver stem cells from the hepatocyte lineage to cholangiocytes, as well as to an increase in cell proliferation (Onoyama et al, 2011). In the following subsections, we discuss the contributions of FBW7 to the maintenance and differentiation of some stem cell types.
Neural stem cells. The adult brain contains NSCs, which are able to generate three main cell types: neurons, astrocytes and oligodendrocytes (Sahni & Kessler, 2010). NSCs are known to change their competency during the development process. They initially give rise to neurons and later to other cell types that are also known as later-born cell types and include astrocytes, oligodendrocytes and ependymal cells. Both the cell number and the later-born cell types are decreased if NSCs are prematurely depleted, suggesting that NSC maintenance is necessary to generate the proper cell quantity and full cellular diversity before the final stage of development (Hatakeyama et al, 2004). In fact, the size and shape of the nervous system primarily depends on the number of times an NSC periodically re-enters the cell cycle (Ohnuma & Harris, 2003). In support of this idea, mutation of cyclin-dependent kinase inhibitor p27 blocks cell cycle progression and delays cell differentiation, leading to an enlarged brain (Nakayama et al, 1996). Furthermore, cell differentiation delayed by expression of stabilized β-catenin in turn increased the size of NSCs and neurons, also resulting in an enlarged brain (Chenn & Walsh, 2002). In addition, the inactivation of the Notch targets Hes1, Hes3 and Hes5 accelerates cell differentiation and causes a wide range of brain malformations (Hatakeyama et al, 2004).
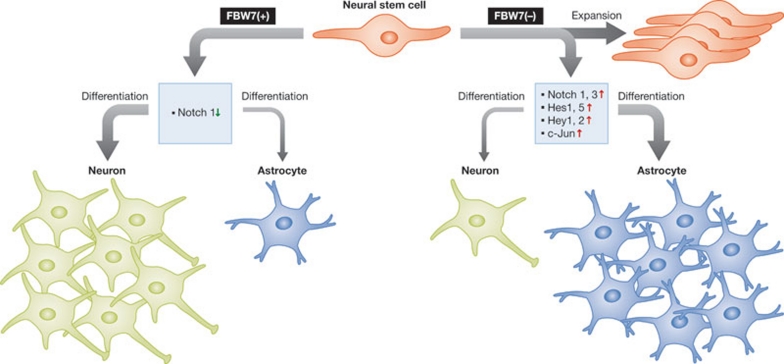
FBW7 has been recently shown to have a crucial role in NSCs to regulate the abundance of Notch proteins (Matsumoto et al, 2011). Mice with conditional ablation of FBW7 in the brain die shortly after birth and lack suckling behaviour (Matsumoto et al, 2011). In these mice, the differentiation of neural progenitor cells was skewed towards astrocytes rather than neurons. This phenotype is consistent with the observed accumulation of Notch 1 and Notch 3, as well as overexpression of Notch downstream target genes, including Hes1, Hes5, Hey1, Hey2 and BLBP, most of which are associated with the maintenance of NSCs (Matsumoto et al, 2011). Inhibition of the Notch pathway by the pharmacological inhibitor DAPT increased the number of neurons and reduced the number of astrocytes (Matsumoto et al, 2011), indicating that excessive and persistent Notch signalling impairs the differentiation of NSCs to neurons, favouring astrocytes instead.
In agreement with an important role of FBW7 in NSCs, another study identified that FBW7 is a key regulator of neural progenitor viability and NSC differentiation (Hoeck et al, 2010). This group also used conditional knockout mice to inactivate FBW7 in the nervous system and found that mice lacking FBW7 died during the perinatal period. The absence of FBW7 resulted in decreased neurogenesis and an accumulation of cells expressing radial glia markers in cultured neurospheres, indicating that FBW7 inactivation might lead to increased generation of radial glia neural stem cells (Hoeck et al, 2010). In addition, the loss of FBW7 led to impaired NSC differentiation and markedly increased apoptotic death of neural progenitors, due to the upregulation of Notch 1 and c-Jun, respectively. Inhibition of the Notch pathway with DAPT alleviated the blocking of stem cell differentiation (Hoeck et al, 2010). Together, these two independent studies demonstrate that FBW7 might be required for NSC differentiation (Fig 2; Hoeck et al, 2010; Matsumoto et al, 2011).
Figure 2.
FBW7 is required for neural stem cell differentiation. FBW7 controls NSC differentiation through downregulation of its ubiquitin substrates, such as Notch 1. Loss of FBW7 leads to impaired NSC differentiation due to the upregulation of c-Jun, Notch 1, Notch 3 and the Notch target genes Hes1, Hes5, Hey1 and Hey2. After deletion of FBW7, differentiation of NSCs is skewed towards astrocytes at the expense of neurons, suggesting that excessive and persistent Notch signalling impairs the differentiation of NSCs to neurons. FBW7, F-box and WD-repeat-domain-containing 7; Hes, hairy and enhancer of split 1; Hey, hairy/enhancer-of-split related with YRPW motif protein 1; NSC, neural stem cell.
Haematopoietic stem cells. These cells reside in the bone marrow and give rise to all mature blood cell types: red blood cells, B and T lymphocytes, natural killer cells, neutrophils, basophils, eosinophils, monocytes, macrophages and platelets (Schroeder, 2010). HSCs remain quiescent or dormant during homeostasis, although they are the adult stem cell with the highest potential to generate a large number of progenitor cells (Schroeder, 2010). To maintain homeostasis and respond rapidly to haematopoietic stresses—such as bleeding, toxic insults and chemotherapeutic agents—HSCs self-renew and differentiate to produce new blood cells (Schroeder, 2010). Several signalling pathways and molecules have been found to control the fate of HSCs, including Notch (Clements et al, 2011; Loeffler et al, 2011), Sonic hedgehog (Trowbridge et al, 2006), Smad (Blank et al, 2008; Larsson & Karlsson, 2005), Wnt (Duncan et al, 2005) and c-Myc (Hoffman et al, 2002; Laurenti et al, 2008), indicating that the self-renewal and quiescence of HSCs are controlled by a highly orchestrated integration of intrinsic and extrinsic signals. Several independent groups have recently shown that FBW7 regulates HSC quiescence and differentiation (Matsuoka et al, 2008; Reavie et al, 2010; Thompson et al, 2008).
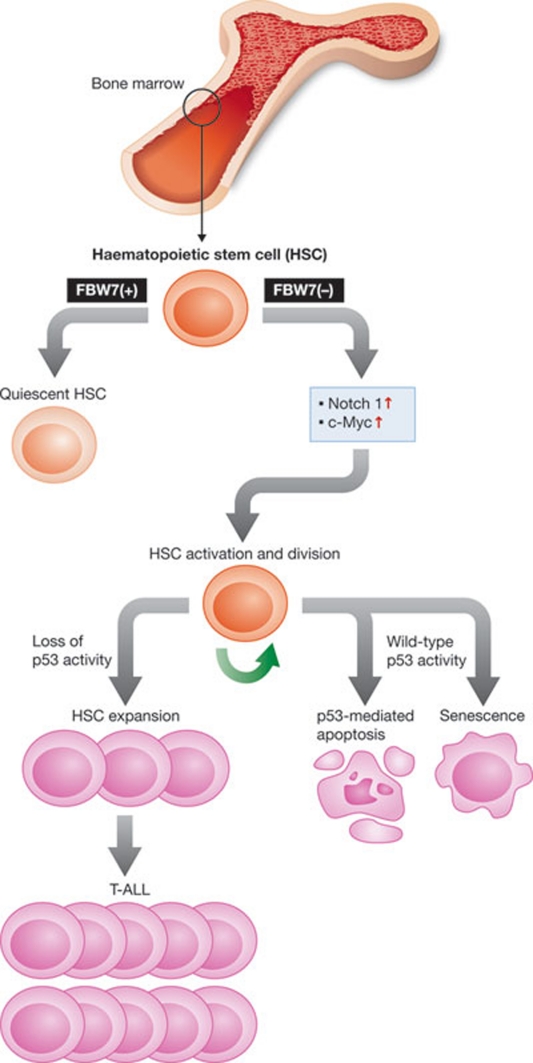
FBW7-deficient mice die at embryonic day 10.5 due to defects in haematopoiesis and vascular development (Tetzlaff et al, 2004; Tsunematsu et al, 2004). The inactivation of FBW7 in bone marrow HSCs causes premature HSC death through p53-dependent apoptosis (Matsuoka et al, 2008). Furthermore, FBW7-deficient HSCs upregulate c-Myc and Notch 1 expression, and downregulate Mdm2 expression, which suppresses p53 function (Matsuoka et al, 2008). Interestingly, loss of FBW7 confers a selective advantage to cells in which p53 function is inhibited, resulting in the development of T-ALL; this suggests that FBW7 acts as a fail-safe mechanism against both premature HSC loss and leukaemia (Matsuoka et al, 2008). In addition, FBW7 controls HSC quiescence and self-renewal, as its deletion leads to defective stem cell quiescence, which results in impaired self-renewal and loss of repopulating capacity (Thompson et al, 2008). Deletion of FBW7, which is highly expressed in non-cycling HSCs, specifically affects the expression of several important regulators of cell cycle entry, such as p57 and E2F2, inducing exit from quiescence and entry into the cell cycle (Thompson et al, 2008). As in the previous study, deletion of FBW7 in bone marrow stem cells and progenitor cells also induced c-Myc stabilization, suggesting that c-Myc overexpression could be an important regulator of cell cycle entry in HSCs (Thompson et al, 2008). Slight changes in the stability and abundance of c-Myc—which is controlled by FBW7—can profoundly modify the transcriptional programme of HSC and therefore trigger their quiescence or self-renewal (Reavie et al, 2010).
FBW7α is the FBW7 isoform preferentially expressed in primitive HSCs (Iriuchishima et al, 2011). Overexpression of FBW7α causes cell-cycle dormancy, mainly through downregulation of FBW7 substrates such as c-Myc, Notch 1 and the mTOR target S6 (Iriuchishima et al, 2011). In addition, c-Myc expression is inhibited in an FBW7α-dependent manner during hypoxia, which is an essential factor for HSC quiescence (Iriuchishima et al, 2011). Thus, FBW7α sustains HSC quiescence through the inhibition of the c-Myc, Notch 1 and mTOR pathways. Interestingly, only attenuation of c-Myc rescues the proliferative abnormality of FBW7-null CD4+CD8+ cells (Onoyama et al, 2007; Welcker & Clurman, 2008). All of these studies point to c-Myc as a crucial FBW7 substrate in the context of HSC self-renewal and maintenance of the haematopoietic system. On the basis of these data, we propose a pathway through which inactivation of FBW7 leads to exhaustion of quiescent HSCs, mediated in part through activation of the c-Myc and Notch signalling pathways (Fig 3).
Figure 3.
Hypothesis of FBW7 function in haematopoietic stem cells. FBW7 is required for maintenance of HSC quiescence. Loss of FBW7 results in the accumulation of Notch 1 and c-Myc, leading to an exit from quiescence and entry into the cell cycle, which results in HSC expansion. HSC expansion is inhibited by either p53-dependent apoptosis or senescence. Loss of p53 activity leads to uncontrolled HSC expansion and eventually results in leukaemia. FBW7, F-box and WD-repeat-domain-containing 7.
Cancer stem cells. Although the existence of CSCs was first proposed more than a century ago, they have recently regained considerable attention due to advances in stem cell research (Visvader & Lindeman, 2008). Nevertheless, the concept of CSCs remains controversial and the role they play in tumour biology probably depends on the type of tumour. They are defined as a population of cells that have the capacity to self-renew and, thus, maintain tumours. Specific markers that identify CSCs in a variety of human cancers have been described (Visvader & Lindeman, 2008). Emerging evidence suggests that cancers could arise from CSCs, as they can self-renew, differentiate and regenerate the phenotypic cells of the original tumour when implanted into severely combined immunodeficient mice (Visvader & Lindeman, 2008). The concept of CSCs will undoubtedly help us to understand tumour biology and hopefully design novel therapeutic strategies for the complete eradication of tumour growth by targeting CSCs.
Notch—an FBW7 substrate—is deregulated in CSCs, leading to their uncontrolled self-renewal and a predisposition to tumour development, indicating that FBW7 could have a role in CSC biology through the Notch pathway (Ranganathan et al, 2011). Indeed, the Notch signalling network is frequently overexpressed in human malignancies including cervical, lung, colon, head and neck, and pancreatic cancers, renal carcinoma, acute myeloid leukaemia, and Hodgkin and large-cell lymphomas (Wang et al, 2010). The fate of CSCs has been shown recently to be controlled in part by the Notch pathway. For example, breast CSCs upregulate Notch, and its inhibition decreases the number of stem cells and their self-renewal capacity (Harrison et al, 2010; Kondratyev et al, 2011). Furthermore, breast CSCs contribute to the development of brain metastases due in part to increased Notch activity (Harrison et al, 2010; McGowan et al, 2011). Therefore, inhibition of Notch 1 reduces the number of CSCs and the formation of brain metastases from breast cancer (McGowan et al, 2011). Similar trends were observed in other CSCs: inhibition of Notch 1 decreased glioma CSC proliferation and glioma growth (Wang et al, 2011), as well as the growth of hepatocellular carcinoma (Nishina et al, 2011) and leukaemia (Alcalay et al, 2003). Notably, FBW7 deletion leads to stem cell activation and leukaemogenesis through the Notch target c-Myc (Matsuoka et al, 2008). Taken together, FBW7 targets Notch for degradation, which is a key regulatory process that controls CSC self-renewal. Furthermore, c-Myc is one of the crucial factors in the generation of iPSCs (Takahashi & Yamanaka, 2006). It is therefore possible that the accumulation of c-Myc (Takahashi & Yamanaka, 2006) or KLF5 (Liu et al, 2010; Zhao et al, 2010) contributes to the partial induction of stem-cell-like phenotypes in FBW7−/− cancers.
Clinical implications for therapy
Given the potential of stem cells to undergo self-renewal and differentiation, their therapeutic use in the context of transplantation, regenerative medicine and cancer treatment is under intense investigation. In fact, adult bone-marrow HSCs have been used in transplants for more than 40 years (Blade et al, 2010). As stem cells exist in many organs, including the brain and heart, these cells could become the basis of transplantation-based therapies if we can harness the conditions to control stem cell differentiation. Stem cell research also opens a new therapeutic window for regenerative or reparative medicine for diseases such as diabetes and heart disease (Wagers & Weissman, 2004). For example, it is now possible to regenerate bone by using cells derived from bone marrow stroma, to repair damaged heart muscle with cardiac muscle cells and to develop insulin-producing cells for the possible treatment of diabetes (Wagers & Weissman, 2004). To reach these goals, we need to be able to generate large quantities of adult stem cells and to differentiate them into specific, fully functional cell types.
As mentioned above, FBW7 plays a pivotal role in stem cell self-renewal and differentiation. It is also required for the maintenance of HSC quiescence; consequently, the loss of FBW7 promotes transient HSC proliferation, eventually leading to their exhaustion (Matsuoka et al, 2008; Thompson et al, 2008). On the other hand, loss of FBW7 leads to impaired NSC differentiation and increased apoptotic death of neural progenitors (Hoeck et al, 2010). Therefore, regulation of FBW7 could theoretically be used to control stem cell differentiation for potential clinical therapies. In this regard, NSC manipulation could potentially be used in the treatment of neurological diseases such as stroke, multiple sclerosis and Parkinson disease. Furthermore, due to the role of FBW7 in CSCs, it might be possible to target and eliminate them through upregulation of FBW7 activity, which would consitute a novel strategy for cancer treatment. Overexpression of FBW7, or induction of FBW7 expression, is possible by manipulating its upstream regulators, including p53 (Yokobori et al, 2009) and microRNAs (Lerner et al, 2011; Mavrakis et al, 2011). This would subsequently reduce Notch activity, eventually inhibiting the ability of CSCs to repopulate the cells forming the tumour mass. For example, gliomas—which are the most common types of tumour of the central nervous system—have inactivation of FBW7 (Hagedorn et al, 2007) and higher expression of Notch (Stockhausen et al, 2010), and might arise from neural CSCs. Given its ability to promote degradation of Notch and subsequently enhance NSC differentiation, FBW7 is expected to be a novel target to treat gliomas. Interestingly, several types of CSC were shown to share many properties—including self-renewal, pluripotency and quiescence—with normal HSCs (Clevers, 2011; Rasheed et al, 2011), in which FBW7 is required for survival by suppression of c-Myc activity. Therefore, inhibition of FBW7 might also be effective for eradication of CSCs. However, it should be noted that considerable additional research is required to understand fully how to use stem cells for cell-based or tissue-based therapies to treat human diseases.
Conclusions and future perspectives
We have discussed recent studies that identify FBW7 as a key player in controlling the balance between stem cell dormancy and self-renewal. It is noteworthy that the function of FBW7 in stem cells is dependent on the organ system of interest. For example, FBW7 is required for neural differentiation, while loss of FBW7 in HSCs leads to defective maintenance of quiescence and premature depletion of HSCs (Hoeck et al, 2010; Thompson et al, 2008). These observations suggest that the lack of FBW7 leads to opposite functional consequences in HSCs and NSCs. Given its ability to control stem cell differentiation, regulation of FBW7 by novel approaches is likely to have a significant impact in designing new disease-specific therapies, including cancer treatment.
However, at the current stage, it is imperative to elucidate further our molecular understanding of how FBW7 controls the self-renewal and survival capacity of stem cells. Although some progress has been made in this regard, we have only touched the tip of the iceberg. Future studies should address many important outstanding questions (Sidebar A). For example, how are stem cells maintained in the adult tissues? In addition to the well-characterized lateral inhibition by the Notch signalling pathway, what are other molecular mechanisms that allow stem cells to remain undifferentiated after the cells around them have undergone differentiation? Can one specific signalling pathway control stem cell differentiation to a specific cell type? How does FBW7 regulate abnormal cell division, resulting in cancer? The answers to these questions will help us to understand how cancer cells are regulated, and the involvement of FBW7 in this process. Furthermore, addressing these questions might enable us to control stem cell differentiation and thereby grow cells or tissues for medical purposes, such as cell-based therapies, as well as develop novel strategies to target cancer and other complex diseases.
Acknowledgments
We sincerely apologize to all the colleagues whose important work was not cited in this review due to space limitations. We thank Alan Lau, Pengda Liu and Tao Huang for critical reading of the manuscript. The authors' work cited in this review was funded by grants from the National Institute of General Medicines, National Institutes of Health (GM089763), the Massachusetts Life Science Center New Investigator award and the Department of Defense Prostate New Investigator award, all to W.W. Z.W. is supported by an NRSA Fellowship.
Footnotes
The authors declare that they have no conflict of interest.
References
- Alcalay M et al. (2003) Acute myeloid leukemia fusion proteins deregulate genes involved in stem cell maintenance and DNA repair. J Clin Invest 112: 1751–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaei-Jadidi R et al. (2011) FBXW7 influences murine intestinal homeostasis and cancer, targeting Notch, Jun, and DEK for degradation. J Exp Med 208: 295–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroca V, Lassalle B, Coureuil M, Louis JP, Le Page F, Testart J, Allemand I, Riou L, Fouchet P (2009) Mouse differentiating spermatogonia can generate germinal stem cells in vivo. Nat Cell Biol 11: 190–196 [DOI] [PubMed] [Google Scholar]
- Blade J, Rosinol L, Cibeira MT, Rovira M, Carreras E (2010) Hematopoietic stem cell transplantation for multiple myeloma beyond 2010. Blood 115: 3655–3663 [DOI] [PubMed] [Google Scholar]
- Blank U, Karlsson G, Karlsson S (2008) Signaling pathways governing stem-cell fate. Blood 111: 492–503 [DOI] [PubMed] [Google Scholar]
- Chenn A, Walsh CA (2002) Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 297: 365–369 [DOI] [PubMed] [Google Scholar]
- Clements WK, Kim AD, Ong KG, Moore JC, Lawson ND, Traver D (2011) A somitic Wnt16/Notch pathway specifies haematopoietic stem cells. Nature 474: 220–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H (2011) The cancer stem cell: premises, promises and challenges. Nat Med 17: 313–319 [DOI] [PubMed] [Google Scholar]
- Cohen DE, Melton D (2011) Turning straw into gold: directing cell fate for regenerative medicine. Nat Rev Genet 12: 243–252 [DOI] [PubMed] [Google Scholar]
- Crusio KM, King B, Reavie LB, Aifantis I (2010) The ubiquitous nature of cancer: the role of the SCF(Fbw7) complex in development and transformation. Oncogene 29: 4865–4873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AW et al. (2005) Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol 6: 314–322 [DOI] [PubMed] [Google Scholar]
- Eisenhaber B, Chumak N, Eisenhaber F, Hauser MT (2007) The ring between ring fingers (RBR) protein family. Genome Biol 8: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkin S, Aylon Y, Anzi S, Oren M, Shaulian E (2008) Fbw7 regulates the activity of endoreduplication mediators and the p53 pathway to prevent drug-induced polyploidy. Oncogene 27: 4411–4421 [DOI] [PubMed] [Google Scholar]
- Frescas D, Pagano M (2008) Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer 8: 438–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta-Rossi N, Le Bail O, Gonen H, Brou C, Logeat F, Six E, Ciechanover A, Israel A (2001) Functional interaction between SEL-10, an F-box protein, and the nuclear form of activated Notch1 receptor. J Biol Chem 276: 34371–34378 [DOI] [PubMed] [Google Scholar]
- Hagedorn M, Delugin M, Abraldes I, Allain N, Belaud-Rotureau MA, Turmo M, Prigent C, Loiseau H, Bikfalvi A, Javerzat S (2007) FBXW7/hCDC4 controls glioma cell proliferation in vitro and is a prognostic marker for survival in glioblastoma patients. Cell Div 2: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison H, Farnie G, Brennan KR, Clarke RB (2010) Breast cancer stem cells: something out of notching? Cancer Res 70: 8973–8976 [DOI] [PubMed] [Google Scholar]
- Hatakeyama J, Bessho Y, Katoh K, Ookawara S, Fujioka M, Guillemot F, Kageyama R (2004) Hes genes regulate size, shape and histogenesis of the nervous system by control of the timing of neural stem cell differentiation. Development 131: 5539–5550 [DOI] [PubMed] [Google Scholar]
- Hoeck JD, Jandke A, Blake SM, Nye E, Spencer-Dene B, Brandner S, Behrens A (2010) Fbw7 controls neural stem cell differentiation and progenitor apoptosis via Notch and c-Jun. Nat Neurosci 13: 1365–1372 [DOI] [PubMed] [Google Scholar]
- Hoffman B, Amanullah A, Shafarenko M, Liebermann DA (2002) The proto-oncogene c-myc in hematopoietic development and leukemogenesis. Oncogene 21: 3414–3421 [DOI] [PubMed] [Google Scholar]
- Hubbard EJ, Wu G, Kitajewski J, Greenwald I (1997) sel-10, a negative regulator of lin-12 activity in Caenorhabditis elegans, encodes a member of the CDC4 family of proteins. Genes Dev 11: 3182–3193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inuzuka H et al. (2011) SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature 471: 104–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriuchishima H, Takubo K, Matsuoka S, Onoyama I, Nakayama KI, Nojima Y, Suda T (2011) Ex vivo maintenance of hematopoietic stem cells by quiescence induction through Fbxw7α overexpression. Blood 117: 2373–2377 [DOI] [PubMed] [Google Scholar]
- Kanei-Ishii C, Nomura T, Takagi T, Watanabe N, Nakayama KI, Ishii S (2008) Fbxw7 acts as an E3 ubiquitin ligase that targets c-Myb for nemo-like kinase (NLK)-induced degradation. J Biol Chem 283: 30540–30548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa K, Hiramatsu Y, Uchida C, Isobe T, Hattori T, Oda T, Shibata K, Nakamura S, Kikuchi A, Kitagawa M (2009) Fbw7 promotes ubiquitin-dependent degradation of c-Myb: involvement of GSK3-mediated phosphorylation of Thr-572 in mouse c-Myb. Oncogene 28: 2393–2405 [DOI] [PubMed] [Google Scholar]
- Koepp DM, Schaefer LK, Ye X, Keyomarsi K, Chu C, Harper JW, Elledge SJ (2001) Phosphorylation-dependent ubiquitination of cyclin E by the SCFFbw7 ubiquitin ligase. Science 294: 173–177 [DOI] [PubMed] [Google Scholar]
- Kondratyev M, Kreso A, Hallett RM, Girgis-Gabardo A, Barcelon ME, Ilieva D, Ware C, Majumder PK, Hassell JA (2011) Gamma-secretase inhibitors target tumor-initiating cells in a mouse model of ERBB2 breast cancer. Oncogene [in the press] [DOI] [PubMed] [Google Scholar]
- Laflamme MA, Murry CE (2011) Heart regeneration. Nature 473: 326–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson J, Karlsson S (2005) The role of Smad signaling in hematopoiesis. Oncogene 24: 5676–5692 [DOI] [PubMed] [Google Scholar]
- Laurenti E et al. (2008) Hematopoietic stem cell function and survival depend on c-Myc and N-Myc activity. Cell Stem Cell 3: 611–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M et al. (2011) miRNA-27a controls FBW7/hCDC4-dependent cyclin E degradation and cell cycle progression. Cell Cycle 10: 2172–2183 [DOI] [PubMed] [Google Scholar]
- Lin H (2002) The stem-cell niche theory: lessons from flies. Nat Rev Genet 3: 931–940 [DOI] [PubMed] [Google Scholar]
- Liu N et al. (2010) The Fbw7/human CDC4 tumor suppressor targets proproliferative factor KLF5 for ubiquitination and degradation through multiple phosphodegron motifs. J Biol Chem 285: 18858–18867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler D, Kokkaliaris KD, Schroeder T (2011) Wnt to notch relay signaling induces definitive hematopoiesis. Cell Stem Cell 9: 2–4 [DOI] [PubMed] [Google Scholar]
- Mao JH, Kim IJ, Wu D, Climent J, Kang HC, DelRosario R, Balmain A (2008) FBXW7 targets mTOR for degradation and cooperates with PTEN in tumor suppression. Science 321: 1499–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A, Onoyama I, Sunabori T, Kageyama R, Okano H, Nakayama KI (2011) Fbxw7-dependent degradation of Notch is required for control of “stemness” and neuronal–glial differentiation in neural stem cells. J Biol Chem 286: 13754–13764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S et al. (2008) Fbxw7 acts as a critical fail-safe against premature loss of hematopoietic stem cells and development of T-ALL. Genes Dev 22: 986–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrakis KJ et al. (2011) A cooperative microRNA-tumor suppressor gene network in acute T-cell lymphoblastic leukemia (T-ALL). Nat Genet 43: 673–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PM, Simedrea C, Ribot EJ, Foster PJ, Palmieri D, Steeg PS, Allan AL, Chambers AF (2011) Notch1 inhibition alters the CD44hi/CD24lo population and reduces the formation of brain metastases from breast cancer. Mol Cancer Res 9: 834–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg KH, Bell DW, Wahrer DC, Haber DA, Hariharan IK (2001) Archipelago regulates cyclin E levels in Drosophila and is mutated in human cancer cell lines. Nature 413: 311–316 [DOI] [PubMed] [Google Scholar]
- Nakayama KI, Nakayama K (2006) Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer 6: 369–381 [DOI] [PubMed] [Google Scholar]
- Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY (1996) Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 85: 707–720 [DOI] [PubMed] [Google Scholar]
- Nalepa G, Rolfe M, Harper JW (2006) Drug discovery in the ubiquitin–proteasome system. Nat Rev Drug Discov 5: 596–613 [DOI] [PubMed] [Google Scholar]
- Nateri AS, Riera-Sans L, Da Costa C, Behrens A (2004) The ubiquitin ligase SCFFbw7 antagonizes apoptotic JNK signaling. Science 303: 1374–1378 [DOI] [PubMed] [Google Scholar]
- Nishina S et al. (2011) Restored expression of the tumor suppressor gene RUNX3 reduces cancer stem cells in hepatocellular carcinoma by suppressing Jagged1–Notch signaling. Oncol Rep 26: 523–531 [DOI] [PubMed] [Google Scholar]
- Oberg C, Li J, Pauley A, Wolf E, Gurney M, Lendahl U (2001) The Notch intracellular domain is ubiquitinated and negatively regulated by the mammalian Sel-10 homolog. J Biol Chem 276: 35847–35853 [DOI] [PubMed] [Google Scholar]
- Ohnuma S, Harris WA (2003) Neurogenesis and the cell cycle. Neuron 40: 199–208 [DOI] [PubMed] [Google Scholar]
- Onoyama I, Tsunematsu R, Matsumoto A, Kimura T, de Alboran IM, Nakayama K, Nakayama KI (2007) Conditional inactivation of Fbxw7 impairs cell-cycle exit during T cell differentiation and results in lymphomatogenesis. J Exp Med 204: 2875–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoyama I, Suzuki A, Matsumoto A, Tomita K, Katagiri H, Oike Y, Nakayama K, Nakayama KI (2011) Fbxw7 regulates lipid metabolism and cell fate decisions in the mouse liver. J Clin Invest 121: 342–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas JJ, Yang PC (2008) Human ESC vs. iPSC—pros and cons. J Cardiovasc Transl Res 1: 96–99 [DOI] [PubMed] [Google Scholar]
- Perry JM, Li L (2008) Self-renewal versus transformation: Fbxw7 deletion leads to stem cell activation and leukemogenesis. Genes Dev 22: 1107–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan P, Weaver KL, Capobianco AJ (2011) Notch signalling in solid tumours: a little bit of everything but not all the time. Nat Rev Cancer 11: 338–351 [DOI] [PubMed] [Google Scholar]
- Rasheed ZA, Kowalski J, Smith BD, Matsui W (2011) Concise review: emerging concepts in clinical targeting of cancer stem cells. Stem Cells 29: 883–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reavie L et al. (2010) Regulation of hematopoietic stem cell differentiation by a single ubiquitin ligase–substrate complex. Nat Immunol 11: 207–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL (2001) Stem cells, cancer, and cancer stem cells. Nature 414: 105–111 [DOI] [PubMed] [Google Scholar]
- Rocher-Ros V, Marco S, Mao JH, Gines S, Metzger D, Chambon P, Balmain A, Saura CA (2010) Presenilin modulates EGFR signaling and cell transformation by regulating the ubiquitin ligase Fbw7. Oncogene 29: 2950–2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotin D, Kumar S (2009) Physiological functions of the HECT family of ubiquitin ligases. Nat Rev Mol Cell Biol 10: 398–409 [DOI] [PubMed] [Google Scholar]
- Sahni V, Kessler JA (2010) Stem cell therapies for spinal cord injury. Nat Rev Neurol 6: 363–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho R, Jandke A, Davis H, Diefenbacher ME, Tomlinson I, Behrens A (2010) F-box and WD repeat domain-containing 7 regulates intestinal cell lineage commitment and is a haploinsufficient tumor suppressor. Gastroenterology 139: 929–941 [DOI] [PubMed] [Google Scholar]
- Schroeder T (2010) Hematopoietic stem cell heterogeneity: subtypes, not unpredictable behavior. Cell Stem Cell 6: 203–207 [DOI] [PubMed] [Google Scholar]
- Stockhausen MT, Kristoffersen K, Poulsen HS (2010) The functional role of Notch signaling in human gliomas. Neuro Oncol 12: 199–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohmaier H, Spruck CH, Kaiser P, Won KA, Sangfelt O, Reed SI (2001) Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature 413: 316–322 [DOI] [PubMed] [Google Scholar]
- Sundqvist A, Bengoechea-Alonso MT, Ye X, Lukiyanchuk V, Jin J, Harper JW, Ericsson J (2005) Control of lipid metabolism by phosphorylation-dependent degradation of the SREBP family of transcription factors by SCF(Fbw7). Cell Metab 1: 379–391 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676 [DOI] [PubMed] [Google Scholar]
- Takebe N, Harris PJ, Warren RQ, Ivy SP (2011) Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol 8: 97–106 [DOI] [PubMed] [Google Scholar]
- Tetzlaff MT, Yu W, Li M, Zhang P, Finegold M, Mahon K, Harper JW, Schwartz RJ, Elledge SJ (2004) Defective cardiovascular development and elevated cyclin E and Notch proteins in mice lacking the Fbw7 F-box protein. Proc Natl Acad Sci USA 101: 3338–3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BJ, Jankovic V, Gao J, Buonamici S, Vest A, Lee JM, Zavadil J, Nimer SD, Aifantis I (2008) Control of hematopoietic stem cell quiescence by the E3 ubiquitin ligase Fbw7. J Exp Med 205: 1395–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge JJ, Scott MP, Bhatia M (2006) Hedgehog modulates cell cycle regulators in stem cells to control hematopoietic regeneration. Proc Natl Acad Sci USA 103: 14134–14139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunematsu R, Nakayama K, Oike Y, Nishiyama M, Ishida N, Hatakeyama S, Bessho Y, Kageyama R, Suda T, Nakayama KI (2004) Mouse Fbw7/Sel-10/Cdc4 is required for notch degradation during vascular development. J Biol Chem 279: 9417–9423 [DOI] [PubMed] [Google Scholar]
- Visvader JE, Lindeman GJ (2008) Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 8: 755–768 [DOI] [PubMed] [Google Scholar]
- Wagers AJ, Weissman IL (2004) Plasticity of adult stem cells. Cell 116: 639–648 [DOI] [PubMed] [Google Scholar]
- Wang J, Wang C, Meng Q, Li S, Sun X, Bo Y, Yao W (2011) siRNA targeting Notch-1 decreases glioma stem cell proliferation and tumor growth. Mol Biol Rep [in the press] [DOI] [PubMed] [Google Scholar]
- Wang Z, Li Y, Ahmad A, Azmi AS, Banerjee S, Kong D, Sarkar FH (2010) Targeting Notch signaling pathway to overcome drug resistance for cancer therapy. Biochim Biophys Acta 1806: 258–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Jin J, Schlisio S, Harper JW, Kaelin WG Jr (2005) The v-Jun point mutation allows c-Jun to escape GSK3-dependent recognition and destruction by the Fbw7 ubiquitin ligase. Cancer Cell 8: 25–33 [DOI] [PubMed] [Google Scholar]
- Welcker M, Clurman BE (2008) FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer 8: 83–93 [DOI] [PubMed] [Google Scholar]
- Welcker M, Orian A, Jin J, Grim JE, Harper JW, Eisenman RN, Clurman BE (2004) The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc Natl Acad Sci USA 101: 9085–9090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz IE et al. (2011) Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature 471: 110–114 [DOI] [PubMed] [Google Scholar]
- Winston JT, Koepp DM, Zhu C, Elledge SJ, Harper JW (1999) A family of mammalian F-box proteins. Curr Biol 9: 1180–1182 [DOI] [PubMed] [Google Scholar]
- Wu G, Lyapina S, Das I, Li J, Gurney M, Pauley A, Chui I, Deshaies RJ, Kitajewski J (2001) SEL-10 is an inhibitor of notch signaling that targets notch for ubiquitin-mediated protein degradation. Mol Cell Biol 21: 7403–7415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SM, Hochedlinger K (2011) Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol 13: 497–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yada M, Hatakeyama S, Kamura T, Nishiyama M, Tsunematsu R, Imaki H, Ishida N, Okumura F, Nakayama K, Nakayama KI (2004) Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7. EMBO J 23: 2116–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen TH, Wright NA (2006) The gastrointestinal tract stem cell niche. Stem Cell Rev 2: 203–212 [DOI] [PubMed] [Google Scholar]
- Yokobori T, Mimori K, Iwatsuki M, Ishii H, Onoyama I, Fukagawa T, Kuwano H, Nakayama KI, Mori M (2009) p53-Altered FBXW7 expression determines poor prognosis in gastric cancer cases. Cancer Res 69: 3788–3794 [DOI] [PubMed] [Google Scholar]
- Young RA (2011) Control of the embryonic stem cell state. Cell 144: 940–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret KS, Grompe M (2008) Generation and regeneration of cells of the liver and pancreas. Science 322: 1490–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Zheng HQ, Zhou Z, Chen C (2010) The Fbw7 tumor suppressor targets KLF5 for ubiquitin-mediated degradation and suppresses breast cell proliferation. Cancer Res 70: 4728–4738 [DOI] [PubMed] [Google Scholar]