EMBO Rep (2011) advance online publication. doi:; DOI: 10.1038/embor.2011.223
Conventional wisdom states that reactive oxygen species (ROS) are deleterious to living organisms. Indeed, neutrophils and macrophages use a respiratory burst to kill ingested microbes. Nevertheless, ROS at low doses can convey important biological information to regulate plant cell growth, for example, or as a prerequisite for the differentiation of some haematopoietic progenitor cells in insects (Owusu-Ansah & Banerjee, 2009). These effects had been thought to be largely cell-autonomous. However, in this issue of EMBO reports, the Banerjee group shows that parasitization of Drosophila larvae by wasp eggs leads to increased ROS levels in haematopoietic niche cells, which then emit a diffusible signal required for the transdifferentiation of haemocytes into a cell type adapted to kill parasites (Sinenko et al, 2011).
Drosophila has become an interesting model to study haematopoiesis, as many signalling pathways and transcription factors that regulate this process are conserved between humans and Drosophila. The Notch, Hedgehog, JAK–STAT and Wingless pathways, as well as Collier/EBF, have been shown to control blood cell homeostasis in the lymph glands (Crozatier & Vincent, 2011). However, in contrast to the plethora of cytokines that are known to participate in the control of mammalian haematopoiesis, almost no such molecules have been identified in Drosophila, with the notable exception of a ligand for the receptor of the sole JAK–STAT pathway that exists in flies.
Drosophila—which lacks a lymphoid lineage and, hence, an adaptive immune system—provides a simple, genetically tractable system for the study of haematopoiesis. It starts during embryogenesis, generating a population of circulating and sessile haemocytes in larvae, which are mostly plasmatocytes—macrophage-like cells—and crystal cells (Lanot et al, 2001). We refer to them as haemocoelic haemocytes. A haematopoietic organ, the lymph gland, develops during larval stages, and at the onset of pupation releases the haemocytes required to control infections during metamorphosis and involved in tissue remodelling. In vertebrates, haematopoietic stem cells can either self-renew or differentiate, decisions that are controlled by signals provided by the haematopoietic ‘niche’. The niche consists of a structural microenvironment that sustains the long-term renewal of stem cells. A similar structure, called the posterior signalling centre (PSC), exists in the Drosophila lymph gland and, under normal conditions, maintains a pool of neighbouring precursor cells (prohaemocytes) in an undifferentiated state in the medullary zone (Crozatier & Vincent, 2011). This is achieved in a non-cell-autonomous manner, by delivering cues—including a Hedgehog signal—and thus keeping prohaemocytes in a quiescent precursor state, a process that also requires autocrine JAK–STAT signalling in the medullary zone prohaemocytes. Enhanced ROS expression in progenitor cells of the medullary zone is required for their subsequent differentiation into haemocytes (Owusu-Ansah & Banerjee, 2009).
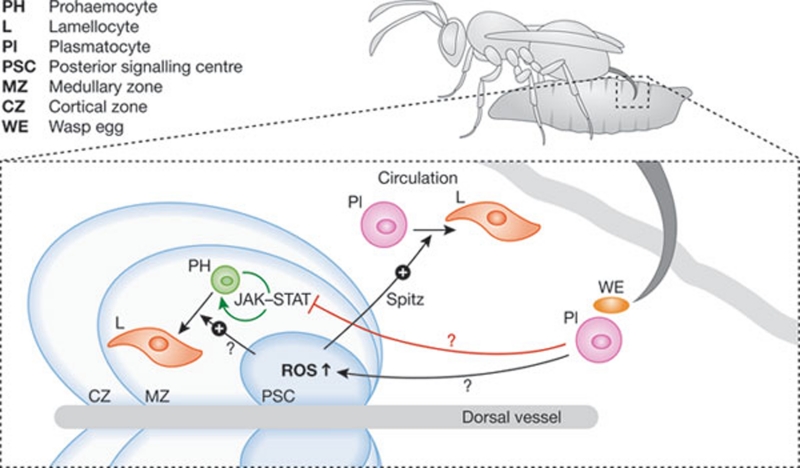
A major threat to any insect larva in its natural environment is parasitoid attacks by wasps. These hymenopterans also lay their eggs in Drosophila larvae, where they develop at the expense of the host. Once the Drosophila larva pupates, the parasitoid larva undergoes metamorphosis, consumes the host, and a wasp adult emerges from the fly pupal case. To fight such infestation, Drosophila larvae have developed a spectacular cellular response: on detection of the parasitoid egg, their immune system triggers the massive differentiation of prohaemocytes in the lymph gland and the transdifferentiation of circulating and/or sessile plasmatocytes into a specialized blood cell type—the lamellocyte (Fig 1; Lanot et al, 2001; Markus et al, 2009). The abundant newly formed lamellocytes then eliminate the invader by encapsulating the wasp egg, which is eventually killed within the melanized capsule. Although wasps have evolved strategies to escape this vigorous defence mechanism, the fly can thereby limit parasitoid developmental success.
…parasitization of Drosophila larvae by wasp eggs [increases] ROS levels in haematopoietic niche cells [inducing] the transdifferentiation of haemocytes…
Figure 1.
Haematopoietic reaction to wasp infection in Drosophila larvae. When a parasitoid wasp lays an egg in a larva, it is detected by circulating blood cells (plasmatocytes), which probably signal to the haematopoietic tissues, including the lymph gland. This inhibits the JAK–STAT pathway in the precursor cells (prohaemocytes) of the medullary zone of the lymph gland, licencing them to exit the undifferentiated state. It also increases ROS levels in the PSC, inducing the transdifferentiation of haemocoelic plasmatocytes into lamellocytes and the differentiation of prohaemocytes to lamellocytes. A cue emitted by the PSC, proposed to be the EGFR ligand Spitz, is needed for circulating cells to adopt the lamellocyte fate.
The PSC has a dual function, as a niche for haemocyte progenitors and also as a master coordinator in the host immune response against wasp eggs. Indeed, although wasp eggs are mainly encapsulated by lamellocytes that originate from circulating, haemocoelic plasmatocytes, the lymph gland—and more specifically the PSC—is required to drive their transdifferentiation. Indeed, collier mutant larvae, which lack a PSC, fail to mount an immune response against the parasitoid (Crozatier et al, 2004). Hence, a signal is thought to originate from the egg after its detection by plasmatocytes, allowing the PSC to drive the lamellocyte programme in the lymph gland and in haemocoelic haemocytes non-cell-autonomously (Fig 1).
Sinenko and colleagues now show that wasp parasitization leads to increased ROS levels in PSC cells (Sinenko et al, 2011). Furthermore, artificially raising ROS levels in PSC cells of nonparasitized larvae by genetically interrupting complex I of the electron transport chain (ETC) leads to the differentiation of lamellocytes in the lymph gland and in the larval body. This phenomenon can be blocked by increasing either the expression of superoxide dismutase 2 or that of its transcriptional regulator Forkhead box O (FoxO), which is under the control of the PI3 kinase–Akt pathway and not the JNK pathway. Unexpectedly, blocking complex IV of the ETC, which decreases ATP levels but does not generate ROS, also causes the ectopic differentiation of (pro)haemocytes into lamellocytes. Having excluded the usual suspects (JAK–STAT, eiger/JNK) as mediators of the signal originating from PSC cells that drives transdifferentiation of haemocoelic plasmatocytes, the authors show that the genetic inactivation of the EGF receptor ligand Spitz in the PSC prevents the formation of circulating lamellocytes either after wasp infestation or after artificial generation of ROS in PSC cells. Moreover, ectopic expression of Spitz in the PSC of nonparasitized larvae also drives transdifferentiation of haemocytes in the larval body. These results were confirmed by inactivating or overactivating the EGF receptor in haemocytes. Lineage tracing experiments with a plasmatocyte-specific marker showed that the specific expression of a dominant-negative EGF receptor in these cells prevented their recovery in the population of transdifferentiated lamellocytes after wasp infestation. The authors therefore propose that Spitz acts as a cytokine driving the transdifferentiation of haemocoelic plasmatocytes that is elicited by ROS signalling in PSC cells. Because wasps attempt to thwart the immune response, in some cases by lysing lamellocytes (Rizki & Rizki, 2004), one might also consider the alternative hypothesis that Spitz provides a survival signal that protects haemocytes from the wasp attack and that the differentiation signal is actually provided by another source. An easy way to disprove this possibility would be to check that lineage-traced haemocytes remain as plasmatocytes and do not disappear from circulation in the experiment described above, an observation that the authors might have already made. It is nevertheless intriguing that the number of PSC cells seem to be strongly reduced when Spitz signalling is impaired in parasitized larvae.
Some key questions remain, such as whether Spitz also drives the differentiation of prohaemocytes in the lymph gland; the identification of the signal conveyed to the PSC after the detection of the wasp egg by the immune system; and whether the same signal is also directly responsible for blocking JAK–STAT signalling in medullary zone prohemocytes (Makki et al, 2010). Answering these questions would help decipher how ROS are generated in the receiving PSC cells. This is unlikely to be associated to the proximity of an oxygen source as in the ‘vascular niche’ of the mammalian bone (Crozatier & Vincent, 2011), as the fly is too small to have hypoxic zones. One mechanism might be related to enhanced mitochondrial activity. Alternatively, the ROS might be generated by one of the two Drosophila oxidases, NOX (NADPH oxidase) or DUOX (dual oxidase), the latter of which has a key role in host defence against intestinal infections (Ha et al, 2005). The overarching evolutionary mystery that remains to be solved is why such a complex systemic crosstalk between haemocoelic haemocytes and the lymph gland exists, as a local response could in principle be sufficient to produce an adapted cellular response, in as much as the lymph gland does not exist in many species.
References
- Crozatier M, Vincent A (2011) Dis Model Mech 4: 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozatier M et al. (2004) PLoS Biol 2: E196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha EM et al. (2005) Science 310: 847. [DOI] [PubMed] [Google Scholar]
- Lanot R et al. (2001) Dev Biol 230: 243. [DOI] [PubMed] [Google Scholar]
- Makki R et al. (2010) PLoS Biol 8: e1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus R et al. (2009) Proc Natl Acad Sci USA 106: 480519261847 [Google Scholar]
- Owusu-Ansah E, Banerjee U (2009) Nature 461: 537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizki RM, Rizki TM (1991) J Exp Zool 257: 236. [DOI] [PubMed] [Google Scholar]
- Sinenko SA et al. (2011) EMBO Rep [Epub ahead of print] doi:; DOI: 10.1038/embor.2011.223 [DOI] [PMC free article] [PubMed] [Google Scholar]