Abstract
Background
Large outbreaks of hand, foot and mouth disease (HFMD) were observed in both 2008 and 2009 in China.
Methods
Using the national surveillance data since May 2, 2008, epidemiological characteristics of the outbreaks are summarized, and the transmissibility of the disease and the effects of potential risk factors were evaluated via a susceptible-infectious-recovered transmission model.
Results
Children of 1.0–2.9 years were the most susceptible group to HFMD (odds ratios [OR] > 2.3 as compared to other age groups). Infant cases had the highest incidences of severe disease (ORs > 1.4) and death (ORs > 2.4), as well as the longest delay from symptom onset to diagnosis (2.3 days). Males were more susceptible to HFMD than females (OR=1.56 [95% confidence interval=1.56, 1.57]). An one day delay in diagnosis was associated with increases in the odds of severe disease by 40.3% [38.7%, 41.9%] and in the odds of death by 53.7% [43.6%, 64.5%]. Compared to Coxsackie A16, enterovirus (EV) 71 is more strongly associated with severe disease (OR=15.6 [13.4, 18.1]) and death (OR=40.7 [13.0, 127.3]). The estimated local effective reproductive numbers among prefectures ranged from 1.4 to 1.6 (median=1.4) in spring and stayed below 1.2 in other seasons. A higher risk of transmission was associated with temperatures in the range of 70-80F, higher relative humidity, wind speed, precipitation, population density, and the periods in which schools were open.
Conclusion
HFMD is a moderately transmittable infectious disease, mainly among pre-school children. EV71 was responsible for most severe cases and fatalities. Mixing of asymptomatically infected children in schools might have contributed to the spread of HFMD. Timely diagnosis may be a key to reducing the high mortality rate in infants.
Hand, foot and mouth disease (HFMD) is a common illness mostly seen in children and caused by a spectrum of pathogens in the enterovirus (EV) family. Most cases involve mild/moderate symptoms such as fever, oral ulcer, extremity rash, pharyngitis and herpangina, with recovery in about three to six days without medication.1 Although relatively rare, severe neurological complication including aseptic meningitis, encephalitis and acute flaccid paralysis can develop in children under 5 years of age with HFMD, particularly among those infected with EV71.2
Historically, outbreaks of HFMD were sporadic and local, but this pattern has changed with 2628 cases reported in Malaysia in 1997, nearly 130,000 cases in Taiwan in 1998, and thousands in Singapore and Australia thereafter. Since then, small to medium epidemics have been continuously observed in the Asia-Pacific region.2,3 The number of HFMD cases has been increasing rapidly in mainland China since 2007. The cumulative reported number of cases in China reached 489,540 in 2008 and 1,155,575 in 2009, marking an era of unprecedented large-scale outbreaks in the Asia-Pacific area. The occurrence of consecutive annual outbreaks also departs from the previously observed pattern in which a significant outbreak was usually followed by a quiescent period of one to two years.4
The transmissibility of recent circulating enteroviruses was assessed in the household setting in a prospective family cohort study in Taiwan.5 However, households are unlikely to be the sole transmission environment, and transmission in other settings such as schools and communities is likely to be of equal or greater importance for control. HFMD outbreaks exhibit a significant seasonal pattern, with a rapid onset in the Spring or early Summer, a gradual decline after the peak, and a light second wave in the Fall. This pattern has been observed on different continents and during multiple years.2,6 The extensive surveillance data from China, combined with publicly available demographic and environmental data, provide a unique opportunity to study the temporal and spatial patterns of disease spread at the aggregate level and to assess associated risk factors. This type of analysis, although highly exploratory due to the aggregate nature of the data, can be used to identify valuable hypotheses to be tested in epidemiological field studies with individual-level data.
Method
The Surveillance System
The Chinese Information System for Disease Control and Prevention is an internet-based real-time surveillance system established by the Chinese Center for Disease Control and Prevention (CDC) for monitoring and reporting public health emergency events and 39 notifiable infectious diseases. As of 2009, the surveillance system covers all Chinese CDC branches, most hospitals at the county-level and above (> 90%), clinics at the township level (> 80%), and other medical institutes. The median time from receiving disease information by a local authority to reporting it via the surveillance system was about seven hours in 2009.
Data Collection
On May 2, 2008, HFMD became a reportable disease, with reporting required within 24 hours via the surveillance system or by mail for facilities not covered by the system. Clinical diagnosis of HFMD was guided by a two-level case definition:
Ordinary cases: fever with skin papular/vesicular rash on hand, foot, mouth or buttock. A few cases may not have fever.
-
Severe cases: definition differs slightly between the two years.
2008: symptoms in ordinary cases plus complications such as myoclonia, acute flaccid paralysis, encephalitis, cardiopulmonary failure or pulmonary edema.
2009: ordinary symptoms with neurological, respiratory or circulatory complications, plus increased peripheral white blood cells, abnormal cerebrospinal fluid, increased blood sugar, or any abnormality in electroencephalogram, cerebrospinal MRI, chest X-ray, or ultrasound cardiogram. Some severe cases may not have rash, and laboratory confirmation is necessary.
Laboratory confirmation is defined as:
Isolation of EV or detection of EV-specific RNA.
A titer of EV-specific neutralizing antibody ≥ 256, or at least 4-fold increase in the titer between the acute phase and the recovering phase.
Upon diagnosis of HFMD, the following individual information was recorded: birth date, gender, current home address, symptom onset date, diagnosis date, hospitalization dates, severe case or not, clinical symptoms, clinical outcome (recovered, stabilized, death or other), whether specimen was collected, and viral type if specimen was collected and tested. Clinical specimens were collected from all severe and fatal cases and the lesser of all or five ordinary cases per county per month. At the provincial level, at least 10 cases were serotyped per month. Serotyping and sequencing were performed at prefecture or provincial surveillance laboratories with quality control and training from Chinese CDC.
The addresses of cases enable their aggregation over different levels of administrative divisions such as, from higher to lower geographic resolution, township, county, prefecture and province. Geographic information, including boundaries and areas for each administrative division, was obtained from the National Fundamental Geographic Information System of China. Demographic information was found in annals of statistics of the administrative divisions. We obtained daily climate indices including temperature, dew point, wind speed and precipitation for nearly 300 weather stations in China from the National Climate Data Center of the Department of Commerce of the United States.
Epidemiological description
We define the illness attack rate as the proportion of reported cases among the general population, case severity ratio as the proportion of severe disease among all reported cases, case fatality ratio as the proportion of deaths among all reported cases, and severe case fatality ratio as the proportion of fatal cases among severe cases. Based on the age distribution of the cases in the surveillance data, availability of population-level demographics, and previous studies on age-specific immune response to enteroviruses7,8,9, we partitioned the population into five age groups: 0-0.9 years (infants), 1.0-2.9 years, 3.0-5.9 years, 6.0-9.9 years, and 10+ years. We partitioned all provinces of mainland China into seven geographical regions: Central North, Central South, South, Northeast, Southwest, Central West and West, shown in Figure S1. The division is determined by geographic location, population density, socioeconomic development, ethnicity, climate, etc. For instance, the South West and the West regions have relatively high proportions of minority ethnic groups. HFMD-related attack rates, case severity ratios, case fatality ratios and severe case fatality ratios were calculated by age group, gender and geographic region for 2008 and 2009 separately. The distribution of the time from symptom onset to diagnosis among cases and the distribution of the time from onset to death among fatal cases were reported by age group, gender and geographic region for the two years combined.
Transmissibility and effects of risk factors
We used a Poisson regression embedded within an aggregate Susceptible-Infected-Recovered statistical model to evaluate the transmissibility of the disease and the effects of potential risk factors on transmission. Specifically, we aggregated cases into weekly counts by g = 10 age-gender strata in each of the 342 prefectures in mainland China. We use prefectures as the geographic transmission units because (1) the resolution of the data at the prefecture level provides sufficient information for the risk factors that we collected, and (2) our method requires a sufficiently large susceptible population in each transmission unit which may not be available at the county level. Let be the number of reported cases in stratum k of prefecture i who were infected during week t. Assuming a one-week incubation period,10 is the number of reported cases with symptom onsets in week t + 1. We also assume that the infectious period starts from the symptom onset week and lasts for d weeks.
The reported cases were assumed to be infected as a result of exposure to infectious cases or an unknown local reservoir including but not limited to environmental persistence (e.g., sewage water) or long-term asymptomatic enterovirus carriers. Transmissions from the two types of sources are referred to as human-to-human and reservoir-to-human respectively. We consider two levels of human-to-human contact: within prefecture and between prefectures, the latter referring to between neighboring prefectures or between an ordinary prefecture and the capital prefecture of the province. Let λ0, λ1 and λ2 be the baseline reservoir-to-human, human-to-human within-prefecture, and human-to-human between-prefecture transmission rates (weekly mean numbers of new infections a source can generate). Let Ai be the collection of prefectures that are adjacent to prefecture i. Let be the covariates associated with the subpopulation in stratum k of prefecture i in week t. For the climate indices, the prefecture-specific weekly averages are used as covariates. Let be the transmission rate from an infectious person in stratum l of prefecture j, and the rate from the reservoir, to the susceptible population in stratum k of prefecture i during week t. The transmission rates are adjusted for covariates in regression models
where 1condition indicates whether the condition is true (1) or not (0) , and βS, βI and αS are the covariate effects on susceptibility (with subscript S) and infectiousness (with subscript I). For a covariate X and its effect β, exp(βx) is the risk ratio at X = x versus at X = 0. The overall transmission rate in stratum k of prefecture i during week t is
where {i} ∪ Ai is the set of prefecture i and all its adjacent prefectures.pt−τ is the probability a case infected in week τ is infectious in week t. The values of pl, l = 1, …, d, together with d, are assumed known and subject to sensitivity analysis. We assume p1 = 1 and pl < 1 for l > 1 to account for the waning of infectiousness since symptom onset. It is reasonable to assume d = 2 or 3 according to the range of the convalescent period in historic epidemics.11,12 Conditioning on , we assume that . Statistical inference is based on the maximum likelihood estimation. A similar model was used for influenza surveillance data in which the transmission rates were interpreted as autoregressive parameters.13 More discussions on the choice of the statistical model and regression covariates are given in the online supplementary materials. Data before the third week in May of 2008 were ignored to avoid potential bias due to incomplete reporting. The last week in 2009 has only three days and was also excluded from the analysis.
The local effective reproductive number (R) is defined as the average number of secondary infections a randomly selected case can generate in a fully susceptible population during his or her infectious period. In our setting, this quantity depends on the spatial and temporal location. The average number of secondary infections a case in stratum l of prefecture j with symptom onset in week t can generate during his/her infectious period is
The local effective R for a randomly selected case in prefecture with symptom onset in week t is the average weighted by the susceptibility levels across all strata:
where measures the susceptibility level of stratum l. is the probability that a randomly selected person is from stratum l, conditioning on that this person is a case. The infectiousness levels of infectious sources are identical for all susceptibles in the prefecture and thus cancel out in the conditional probability.
Results
Epidemiological characteristics
The epicurves of HFMD for the nation and its seven geographic regions during 2008 and 2009 in mainland China are shown in Figure 1. Most regions displayed a common trend of steady increase beginning in early February, rapid increase during March or April, a peak from April to June, a quick decline in summer, a slower declining rate or even a small second rise in early September, and finally, steady decrease until January. The picture of 2008 is incomplete before May, but the timing of peaks was similar.
Figure 1.
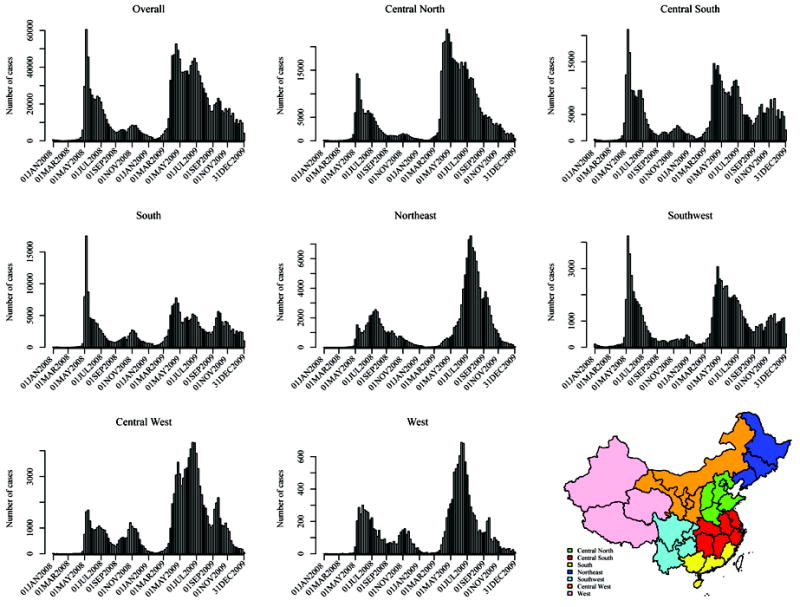
Epicurves of the 2008 and 2009 HFMD epidemics in China for the whole nation and seven geographic regions. Cases are aggregated by week, i.e., each unit of the horizontal axis is a week. Locations of the displayed dates are only approximate with a resolution up to the weeks they belong to.
Numbers of cases, severe cases and fatal cases are presented in Table 1 by age group, gender, region and year. Corresponding attack rates, case severity ratios, case fatality ratios and severe case fatality ratios are given in Table 2. Children under six years constituted > 93% of the cases, with the highest annual cumulative incidence, over 18 per thousand, in the 1.0–2.9 years group in 2009. The case severity ratio was 11.98 per thousand in 2009, a dramatic jump from 2.48 per thousand in 2008, partially due to the difference in the definition of severe cases. A slight increase in the case fatality ratio was also observed. The peaks of weekly case severity ratio mostly occurred in July and August, later than the peaks of the epicurves (Figure S2). The geographic distributions of prefecture-specific annual attack rates, case severity ratios, case fatality ratios and severe case fatality ratios across the nation are shown in Figures S3-S6. The dynamic changes of prefecture-specific weekly attack rates (online animation) show the movement of the disease from the southern and central regions to the northern and western ones in spring and summer and the remaining activity in the South during winter.
Table 1.
Numbers (percents) of reported hand, foot and mouth disease cases, severe cases and deaths by age group, gender, region and year.
| Grouping Factor |
Level | Population | 2008
|
2009
|
||||
|---|---|---|---|---|---|---|---|---|
| Cases | Severe Cases | Deaths | Cases | Severe Cases | Deaths | |||
| Age Group |
0–0.9 | 16878805 | 46939 (9.6) | 220 (18.2) | 38 (29.9) | 131873 (11.4) | 2681 (19.4) | 109 (30.9) |
| 1–2.9 | 32131101 | 244444 (49.6) | 701 (57.9) | 77 (60.6) | 586363 (50.7) | 8627 (62.4) | 207 (58.6) | |
| 3–5.9 | 47286361 | 161596 (33.0) | 244 (20.2) | 12 (9.5) | 368392 (31.9) | 2290 (16.6) | 35 (9.9) | |
| 6–9.9 | 57167634 | 26679 (5.5) | 35 (2.9) | 0 (0) | 51005 (4.4) | 201 (1.4) | 2 (0.6) | |
| ≥10 | 1146579188 | 9882 (2.0) | 10 (0.8) | 0 (0) | 17942 (1.6) | 36 (0.3) | 0 | |
|
| ||||||||
| Gender | Female | 632842767 | 178182 (36.4) | 400 (33.1) | 51 (40.2) | 429307 (37.2) | 4846 (35.0) | 126 (35.7) |
| Male | 667200322 | 311358 (63.6) | 810 (66.9) | 76 (59.8) | 726267 (62.8) | 8989 (65.0) | 227 (64.3) | |
|
| ||||||||
| Region1 | CN | 318022505 | 120349 (24.6) | 332 (27.4) | 22 (17.3) | 419006 (36.3) | 11090 (80.2) | 142 (40.2) |
| CS | 370840498 | 170180 (34.8) | 456 (37.7) | 47 (37.0) | 320284 (27.7) | 1008 (7.3) | 68 (19.3) | |
| S | 186769460 | 96335 (19.7) | 329 (27.2) | 46 (36.2) | 169943 (14.7) | 620 (4.5) | 43 (12.2) | |
| NE | 108510963 | 38294 (7.8) | 56 (4.6) | 7 (5.5) | 98080 (8.5) | 367 (2.6) | 33 (9.4) | |
| SW | 192608272 | 32702 (6.7) | 23 (1.9) | 2 (1.6) | 59103 (5.1) | 582 (4.2) | 41 (11.6) | |
| CW | 93941677 | 27007 (5.5) | 12 (1.0) | 2 (1.6) | 79900 (6.9) | 155 (1.1) | 25 (7.1) | |
| W | 29349714 | 4673 (0.9) | 2 (0.2) | 1 (0.8) | 9259 (0.8) | 13 (0.1) | 1 (0.3) | |
|
| ||||||||
| Overall | 1300043089 | 489540 | 1210 | 127 | 1155575 | 13835 | 353 | |
CN= Central North, CS=Central South, S=South, NE=Northeast, SW=Southwest, CW= Central West, W=West,
Table 2.
Attack Rates (AR), Case Severity Ratios (CSR), Case Fatality Ratio (CFR) and Severe Case Fatality Ratios (SCFR) by Age Group, Gender, Region and Year for the HFMD Epidemics in China during 2008–2009.
| Grouping Factor |
Level | 2008
|
2009
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| AR (/1000) |
CSR (/1000) |
CFR (/1000) |
SCFR | AR (/1000) |
CSR (/1000) |
CFR (/1000) |
SCFR | ||
| Age Group |
0–0.9 | 2.78 | 4.73 | 0.81 | 0.17 | 7.81 | 20.33 | 0.83 | 0.041 |
| 1–2.9 | 7.61 | 2.88 | 0.32 | 0.11 | 18.25 | 14.72 | 0.35 | 0.024 | |
| 3–5.9 | 3.42 | 1.52 | 0.074 | 0.049 | 7.79 | 6.22 | 0.095 | 0.015 | |
| 6–9.9 | 0.47 | 1.32 | 0 | 0 | 0.89 | 3.94 | 0.039 | 0.01 | |
| ≥10 | 0.0086 | 1.01 | 0 | 0 | 0.016 | 2.01 | 0 | 0 | |
|
| |||||||||
| Gender | Fenale | 0.28 | 2.25 | 0.29 | 0.13 | 0.68 | 11.29 | 0.29 | 0.026 |
| Male | 0.47 | 2.61 | 0.24 | 0.094 | 1.09 | 12.38 | 0.31 | 0.025 | |
|
| |||||||||
| Region1 | CN | 0.38 | 2.76 | 0.18 | 0.066 | 1.32 | 26.47 | 0.34 | 0.013 |
| CS | 0.46 | 2.71 | 0.28 | 0.10 | 0.86 | 3.15 | 0.21 | 0.067 | |
| S | 0.52 | 3.42 | 0.48 | 0.14 | 0.91 | 3.65 | 0.25 | 0.069 | |
| NE | 0.35 | 1.46 | 0.18 | 0.13 | 0.90 | 3.74 | 0.34 | 0.090 | |
| SW | 0.17 | 0.70 | 0.061 | 0.087 | 0.31 | 9.85 | 0.69 | 0.070 | |
| CW | 0.29 | 0.44 | 0.074 | 0.17 | 0.85 | 1.94 | 0.31 | 0.16 | |
| W | 0.16 | 0.43 | 0.21 | 0.5 | 0.32 | 1.40 | 0.11 | 0.077 | |
|
| |||||||||
| Overall | 0.38 | 2.48 | 0.26 | 0.10 | 0.89 | 11.98 | 0.31 | 0.026 | |
CN= Central North, CS=Central South, S=South, NE=Northeast, SW=Southwest, CW= Central West, W=West,
Effects of age group, gender and region on attack rate, case severity ratio, case fatality ratio and severe case fatality ratio were estimated for the two years separately in Table 3. The estimated odds ratios approximate relative risks as the numerators are much smaller than the denominators. The effects of age and gender are similar between the years, but the region effects appear temporally heterogeneous. We focus on the 2009 epidemic for its completeness in the data. Relative to the 1.0-2.9 years group, the risk of disease was about 40% in the 0–0.9 years and the 3.0–5.9 years groups, and dropped to 5% and 1% in the two oldest age groups. The youngest group had more than 20% higher risk, whereas the older age groups all had 50% or lower risk, of severe disease than the 1.0–2.9 years group. The pattern of the case fatality ratios and severe case fatality ratios across age groups matches that of the case severity ratios, but with more dramatic differences in the case fatality ratios. Males had 56% higher risk of disease and 10% higher risk of severe disease than females. Substantial geographic heterogeneity was observed for all four measures. The attack rate and case severity ratio were the highest in the Central North region, with more than 70% of the nationally reported severe cases in three provinces in this region: Shandong, Henan and Hebei. The Southwest region had the second highest case severity ratio and the highest case fatality ratio in 2009. Compared to the Central North region, all other regions, in particular the Central West region, had much higher severe case fatality ratios, ORs > 5.
Table 3.
Effects of Age Group, Gender and Region on the Annual Attack Rate (AR), Case Severity Ratio (CSR), Case Fatality Ratio (CFR), and Severe Case Fatality Ratio (SCFR). Presented are Odds Ratios and 95% Confidence Intervals from Logistic Regression Models.
| Year | Grouping Factor1 |
Level | AR
|
CSR
|
CFR
|
SCFR
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |||
| 2008 | Age (1–2.9) |
0–0.9 | 0.36 | 0.36, 0.37 | 1.59 | 1.36, 1.85 | 2.48 | 1.68, 3.66 | 1.72 | 1.12, 2.64 |
| 3–5.9 | 0.45 | 0.45, 0.45 | 0.55 | 0.48, 0.64 | 0.24 | 0.13, 0.45 | 0.41 | 0.22, 0.77 | ||
| 6–9.9 | 0.061 | 0.060, 0.062 | 0.49 | 0.35, 0.68 | 0 | 0 | ||||
| ≥10 | 0.0011 | 0.0011, 0.0011 | 0.38 | 0.20, 0.70 | 0 | 0 | ||||
| Gender (Female) |
Male | 1.61 | 1.60, 1.62 | 1.13 | 1.0, 1.27 | 0.82 | 0.57, 1.17 | 0.68 | 0.46, 1.0 | |
| Region2 (CN) |
CS | 1.26 | 1.25, 1.27 | 0.96 | 0.83, 1.10 | 1.43 | 0.86, 2.37 | 1.57 | 0.92, 2.68 | |
| S | 1.43 | 1.42, 1.45 | 1.20 | 1.03, 1.40 | 2.47 | 1.48, 4.10 | 2.26 | 1.32, 3.88 | ||
| NE | 1.02 | 1.01, 1.04 | 0.60 | 0.45, 0.79 | 1.29 | 0.55, 3.03 | 2.10 | 0.84, 5.26 | ||
| SW | 0.46 | 0.45, 0.47 | 0.26 | 0.17, 0.39 | 0.35 | 0.08, 1.47 | 1.78 | 0.38, 8.29 | ||
| CW | 0.77 | 0.76, 0.78 | 0.18 | 0.10, 0.32 | 0.50 | 0.12, 2.13 | 3.32 | 0.65,16.88 | ||
| W | 0.37 | 0.36, 0.38 | 0.18 | 0.04, 0.70 | 1.53 | 0.21, 11.40 | 1.98 | 1.03,381.23 | ||
|
| ||||||||||
| 2009 | Age (1–2.9) |
0–0.9 | 0.42 | 0.42, 0.42 | 1.42 | 1.35, 1.48 | 2.42 | 1.92, 3.06 | 1.85 | 1.45, 2.36 |
| 3–5.9 | 0.43 | 0.42, 0.43 | 0.47 | 0.45, 0.49 | 0.26 | 0.18, 0.37 | 0.54 | 0.37, 0.77 | ||
| 6–9.9 | 0.049 | 0.048, 0.049 | 0.30 | 0.26, 0.34 | 0.10 | 0.025, 0.41 | 0.33 | 0.080, 1.34 | ||
| ≥10 | 0.0008 | 0.0008, 0.0009 | 0.15 | 0.11, 0.21 | 0 | 0 | ||||
| Gender (Female) |
Male | 1.56 | 1.56, 1.57 | 1.10 | 1.06, 1.14 | 1.05 | 0.84, 1.30 | 0.96 | 0.77, 1.21 | |
| Region2 (CN) |
CS | 0.67 | 0.67, 0.68 | 0.12 | 0.11, 0.12 | 0.63 | 0.47, 0.84 | 5.80 | 4.30, 7.81 | |
| S | 0.72 | 0.72, 0.73 | 0.13 | 0.12, 0.14 | 0.72 | 0.51, 1.02 | 5.88 | 4.13, 8.37 | ||
| NE | 0.75 | 0.75, 0.76 | 0.17 | 0.15, 0.19 | 1.43 | 0.97, 2.09 | 8.61 | 5.78, 12.82 | ||
| SW | 0.24 | 0.24, 0.24 | 0.38 | 0.35, 0.42 | 2.20 | 1.55, 3.11 | 6.15 | 4.29, 8.81 | ||
| CW | 0.65 | 0.65, 0.66 | 0.083 | 0.071, 0.097 | 1.19 | 0.78, 1.83 | 16.67 | 10.47,26.53 | ||
| W | 0.21 | 0.20, 0.21 | 0.061 | 0.036, 0.11 | 0.43 | 0.06, 3.06 | 7.64 | 0.98, 59.77 | ||
Values in paretheses are reference levels for the ORs.
CN= Central North, CS=Central South, S=South, NE=Northeast, SW=Southwest, CW= Central West, W=West.
As shown in Table 4, the mean (median) duration from symptom onset to diagnosis among all reported cases of both years combined is 1.58 (1.0) days, shorter than that in severe cases, 2.24 (2.0) days. Infant cases had longer times from onset to diagnosis, no matter whether severe or not, and to death than older groups. The longer delay in diagnosis may partially account for the higher CSR and CFR among infants. When the logistic models for year 2009 in Table 3 are further adjusted for the onset-to-diagnosis time (durations > 5 days are set to 5 days), the odds of severe disease among infants relative to the 1–2.9 years group drops from 1.42 to 1.14 [95% confidence interval (CI): 1.09, 1.19], and the OR regarding death drops from 2.42 to 1.88 [1.48, 2.38]. In addition, one day delay in diagnosis raised the risk of severe disease by 40% [38.7%, 41.9%] and the risk of death by 53.7% [43.6%, 64.5%]. The south region has the longest mean delay in severe cases, about 3 days, followed by the northeast and central west regions. Histograms of the delay times in Figure S7 suggest that the long delay in the South region was due to an excessive number of severe cases with ≥ 5 days of delay in the Guangdong province.
Table 4.
Mean and Median Time (in Days) from Symptom Onset to Diagnosis among All Cases and Severe Cases, and Time from Symptom Onset to Death among Fatal Cases, by Age Group, Gender and Region for the 2008–2009 HFMD Epidemics in China. Intervals < 0 Days (n=27) are Excluded, and Intervals > 30 Days (n=1546) are Truncated at 30 Days. 95% Confidence Intervals are Not Given Where the Number of Observations is < 10.
| Grouping Factor |
Number of Days from
|
||||||
|---|---|---|---|---|---|---|---|
| Onset to Diagnosis
|
|||||||
| Cases
|
Severe Cases
|
Onset to Death
|
|||||
| Mean (Median) |
95% CI | Mean (Median) |
95% CI | Mean (Median) |
95% CI | ||
| Age Group |
0–0.9 | 2.31 (2) | 2.30, 2.32 | 2.69 (2) | 2.61, 2.78 | 4.62 (4) | 3.94, 5.41 |
| 1–2.9 | 1.59 (1) | 1.59, 1.59 | 2.18 (2) | 2.13, 2.22 | 3.69 (3) | 3.29, 4.14 | |
| 3–5.9 | 1.31 (1) | 1.30, 1.31 | 1.97 (1) | 1.89, 2.06 | 3.36 (3) | 2.71, 4.18 | |
| 6–9.9 | 1.46 (1) | 1.44, 1.47 | 2.29 (2) | 2.07, 2.53 | 5.0 (5) | - | |
| ≥10 | 1.89 (1) | 1.87, 1.92 | 2.30 (2) | 1.68, 3.16 | - | - | |
|
| |||||||
| Gender | Fenale | 1.59 (1) | 1.58, 1.59 | 2.23 (2) | 2.17, 2.30 | 4.06 (3) | 3.46, 4.77 |
| Male | 1.57 (1) | 1.57, 1.57 | 2.25 (2) | 2.21, 2.29 | 3.88 (3) | 3.51, 4.29 | |
|
| |||||||
| Region1 | CN | 1.47 (1) | 1.46, 1.47 | 2.11 (2) | 2.07, 2.14 | 3.85 (3) | 3.40, 4.37 |
| CS | 1.63 (1) | 1.63, 1.64 | 2.51 (2) | 2.36, 2.66 | 4.63 (3) | 3.69, 5.82 | |
| S | 1.76 (1) | 1.75, 1.77 | 3.05 (2) | 2.82, 3.30 | 3.44 (3) | 2.90, 4.08 | |
| NE | 1.32 (1) | 1.31, 1.33 | 2.88 (3) | 2.67, 3.11 | 3.73 (3) | 3.14, 4.41 | |
| SW | 1.89 (1) | 1.87, 1.90 | 2.40 (2) | 2.21, 2.59 | 4.37 (3) | 3.35, 5.70 | |
| CW | 1.47 (1) | 1.46, 1.48 | 2.74 (2) | 2.33, 3.22 | 3.22 (3) | 2.45, 4.24 | |
| W | 1.46 (1) | 1.43, 1.49 | 1.20 (0) | 0.59, 2.46 | 1.00 (1) | - | |
|
| |||||||
| Overall | 1.58 (1) | 1.57, 1.58 | 2.24 (0) | 2.21, 2.28 | 3.95 (1) | 3.62, 4.31 | |
CN= Central North, CS=Central South, S=South, NE=Northeast, SW=Southwest, CW= Central West, W=West,
A total of 33,576 (2%) HFMD cases were lab-confirmed for pathogens, of which 26% are Coxsackie A16 (CA16) and 48% are EV71 (Table 5). From 2008 to 2009, the relative proportion of CA16 among tested samples more than doubled, whereas those of EV71 and other enteroviruses (neither CA16 nor EV71, but exact types are unknown) decreased. In 2009, CA16 is mostly found in the Northeast region and the coastline of the Central South region (Figure S8), whereas EV71 cases clustered in the Central North region where most of the severe cases were reported (Figure S9). Among lab-confirmed severe cases, 81% were infected with EV71. With CA16 as the reference of the odds, the ORs between severe and mild cases during 2009 are 15.57 (95% CI: 13.40, 18.11) for EV71 and 4.20 (95% CI: 3.55, 4.97) for other enteroviruses, controlling for age group, gender and time from onset to diagnosis. With death as the outcome, the ORs are 40.69 (95% CI: 13.01, 127.31) for EV71 and 4.98 (95% CI: 1.41, 17.54) for other enteroviruses. The proportion of EV71 (72%) was the highest in the Southwest region in 2009, which may partially account for its high case severity ratio and case fatality ratio.
Table 5.
Numbers (Percentages) of Lab-Confirmed Cases, Severe Cases and Deaths by Pathogen Type and Year.
| Pathogen | 2008 | 2009 | Combined | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Severe Cases |
Deaths | Cases | Severe Cases |
Deaths | Cases | Severe Cases |
Deaths | |
| CA16 | 1276 (14.8) | 12 (3.0) | 1 (1.2) | 7584 (30.4) | 190 (4.6) | 3 (1.3) | 8860 (26.4) | 202 (4.4) | 4 (1.3) |
| EV71 | 4668 (54.0) | 333 (82.0) | 82 (95.3) | 11511 (46.2) | 3334 (80.6) | 205 (92.8) | 16179 (48.2) | 3667 (80.7) | 287 (93.5) |
| Other Enteroviruses | 2697 (31.2) | 61 (15.0) | 3 (3.5) | 5840 (23.4) | 613 (14.8) | 13 (5.9) | 8537 (25.4) | 674 (14.8) | 16 (5.2) |
|
| |||||||||
| Overall | 8641 | 406 | 86 | 24935 | 4137 | 221 | 33576 | 4543 | 307 |
Transmissibility and effects of risk factors
We use (p1, p2, p3) to represent a three-week infectious period with p2 × 100% and p3 × 100% infectiousness in the second and third weeks, respectively, relative to the first week, where p1 = 1 corresponds to the symptom onset week. We examine three settings: (1, 0.2, 0), (1, 0.5, 0) and (1, 0.6, 0.2), and report the results based on the setting of (1, 0.2, 0) as the primary findings because it provides a largest likelihood among the three settings.
The results of the aggregate Susceptible-Infected-Recovered transmission model are presented for transmission rates in Table 6. The effects of discrete risk factors are given as risk ratios in Table 7 for human-to-human transmission and in Table S1 for reservoir-to-human transmission. The effects of age group and gender are assumed common for the two types of transmission and therefore only presented in Table 7. The risk ratios for the continuous risk factors on human-to-human transmission are displayed in Figure 2.
Table 6.
Estimates and 95% Confidence Intervals for Reservoir-to-human (λ0), Within-Prefecture Human-to-Human (λ1) and Across-Prefecture Human-to-Human (λ2) Transmission Forces Based on an Aggregate Susceptible-Infected-Recovered Model for the 2008–2009 HFMD outbreaks in China Combined.
| Type of Infection Source | Parameter | Assumption about Infectious Period
|
|||||
|---|---|---|---|---|---|---|---|
| (1, 0. 2, 0)
|
(1, 0.5, 0)
|
(1, 0.6 , 0.2)
|
|||||
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | ||
| Local Reservoir | λ0(10−1) | 1.54 | 1.39, 1.70 | 1.56 | 1.41, 1.73 | 1.55 | 1.39, 1.72 |
| Human Cases | λ1(10−1) | 1.51 | 1.49, 1.53 | 1.19 | 1.18, 1.21 | 0.97 | 0.96, 0.98 |
| λ2(10−4) | 4.19 | 3.91, 4.50 | 3.17 | 2.94, 3.41 | 2.44 | 2.26, 2.64 | |
Table 7.
Effects of Discrete Risk Factors on Susceptibility to Transmission Risk from Human to Human in the 2008–2009 HFMD Epidemics in China. Presented are Risk Ratios (RR) and 95% Confidence Intervals from the Aggregate Susceptible-Infected-Recovered Model.
| Risk Factor | Assumption about Infectious Period
|
||||||
|---|---|---|---|---|---|---|---|
| (1 , 0.2, 0)
|
(1 , 0.5, 0)
|
(1, 0.6, 0.2)
|
|||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | ||
| Age Group |
0–0.9 | 0.22 | 0.21, 0.22 | 0.22 | 0.21, 0.22 | 0.22 | 0.21, 0.22 |
| 1–2.9 | |||||||
| 3–5.9 | 0.63 | 0.63, 0.64 | 0.63 | 0.63, 0.64 | 0.63 | 0.63, 0.64 | |
| 6–9.9 | 0.090 | 0.089, 0.090 | 0.090 | 0.089, 0.090 | 0.090 | 0.089, 0.090 | |
| ≥10 | 0.033 | 0.032, 0.033 | 0.033 | 0.032, 0.033 | 0.033 | 0.032, 0.033 | |
| Gender | Female | ||||||
| Male | 1.70 | 1.70, 1.71 | 1.70 | 1.70, 1.71 | 1.70 | 1.70, 1.71 | |
|
| |||||||
| School Open | No | ||||||
| Yes | 1.15 | 1.15, 1.16 | 1.18 | 1.17, 1.19 | 1.21 | 1.20, 1.22 | |
| Precipitation | Low | ||||||
| Medium | 1.05 | 1.05, 1.06 | 1.05 | 1.05, 1.06 | 1.05 | 1.05, 1.06 | |
| High | 1.04 | 1.03, 1.04 | 1.03 | 1.03, 1.04 | 1.02 | 1.02, 1.03 | |
| Region1 | CN | ||||||
| CS | 0.96 | 0.96, 0.97 | 0.97 | 0.96, 0.97 | 0.97 | 0.96, 0.97 | |
| S | 0.93 | 0.92, 0.94 | 0.93 | 0.93, 0.94 | 0.94 | 0.93, 0.95 | |
| NE | 1.08 | 1.08, 1.09 | 1.09 | 1.09, 1.10 | 1.11 | 1.10, 1.12 | |
| SW | 0.98 | 0.97, 0.98 | 0.98 | 0.97, 0.99 | 0.98 | 0.97, 0.99 | |
| CW | 1.04 | 1.03, 1.05 | 1.04 | 1.03, 1.05 | 1.04 | 1.03, 1.05 | |
| W | 1.07 | 1.05, 1.09 | 1.07 | 1.05, 1.09 | 1.07 | 1.05, 1.09 | |
CN= Central North, CS=Central South, S=South, NE=Northeast, SW=Southwest, CW= Central West, W=West
Figure 2.
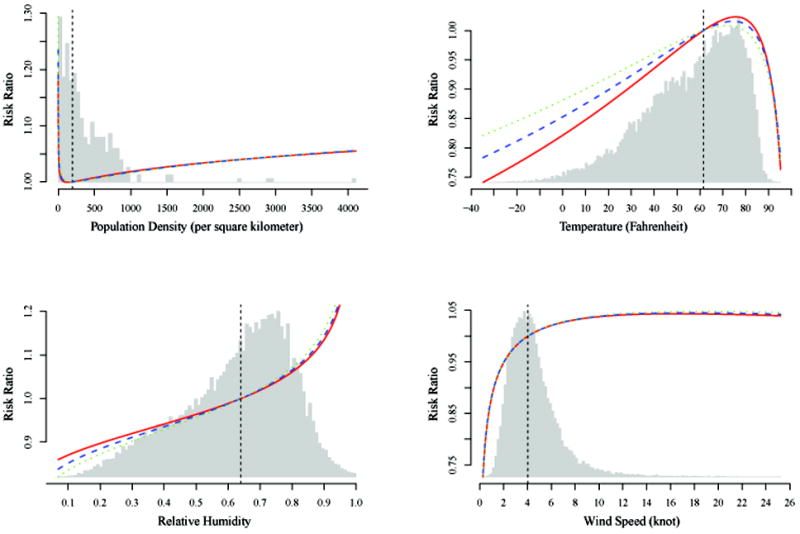
Effects of continuous risk factors, presented as risk ratios (RR), on aggregate human-to-human transmission rates. For each continuous risk factor X with a mean of X̄, the RR is calculated as exp (β̂1(X − X̄) + β̂2(X − X̄)2), where β̂1 and β̂2 are estimated regression coefficients. The red solid, blue dashed, and green dotted curves correspond to the assumptions about the infectious period, 1-0.2-0, 1-0.5-0 and 1-0.6-0.2, respectively. The black dashed line indicates where RR = 1. The grey area is the histogram for the distribution of the corresponding covariate.
Transmission of the disease was primarily driven by contacts within prefectures with a transmission rate more than 300 times of that between neighboring prefectures. Figure S14 shows the model-predicted weekly mean number of cases generated by the unobserved sources (grey area) and by the observed cases in neighbor prefectures. Between-prefecture transmissions dominated over the unobserved reservoir in spring and summer, suggesting that the movement of the disease across prefectures was likely driven by human-to-human transmission during that period. Reservoir-to-human transmission rates were relatively high in the South and the Central South regions (above 50 cases per week), which is also seen in Table S1.
The 10%, 50% and 90% percentiles of the model-based local effective R values among all prefectures are shown in Figure 3. Standard deviations range from 0.002 to 0.08 with a median of 0.004 and are not presented in the figure. The peak values appear around the beginning of March in most regions. The median local effective R reached 1.2 in the Central South and the South, and about 1.1 elsewhere, during the peak weeks. In these two regions, around 10% of the prefectures had local effective R values over 1.3 during the peak weeks. As the surveillance period does not cover the ascending phase of 2008, the model based on the overall surveillance data likely underestimates the local effective R. Using the data from only the ascending phase of the 2009 epidemic (January 20–April 20) and a model without adjusting for any risk factor, the estimated local R values among all prefectures range from 1.36 to 1.58 (standard deviations: 0.003–0.006) with a median of 1.41. Figure 3 also clearly demonstrates the effect of school closure in spring and summer on reducing human-to-human transmission.
Figure 3.
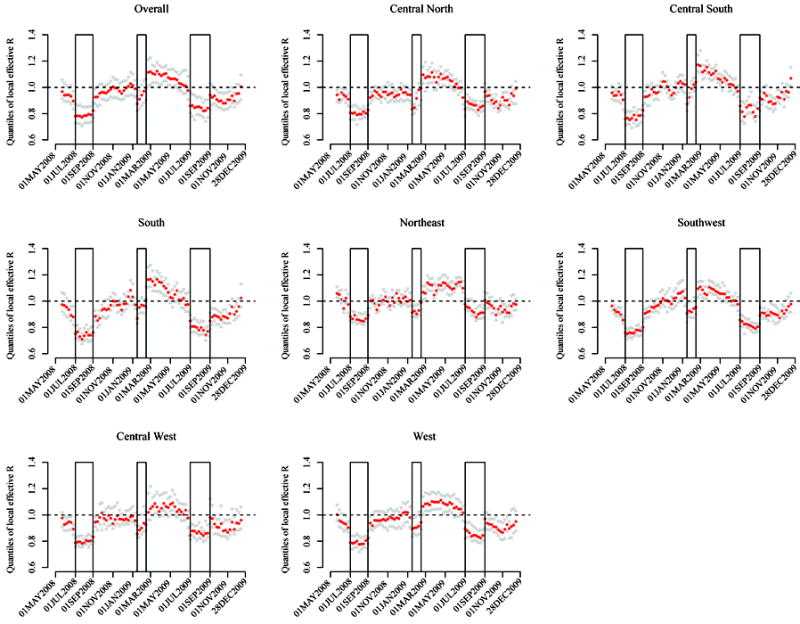
Time dependent prefecture-specific aggregate local effective reproductive numbers (adjusted for covariates) for the overall nation and seven geographic regions for the 2008-2009 HFMD epidemics in China. Medians are shown in red dots, and 10% and 90% quantiles are shown in grey triangles. The segments in boxes indicate school closure during the spring and summer breaks.
The age and gender effects on weekly risks based on the model differ in magnitudes from those for annual risks in Table 3, but the general trend is similar. Spatial heterogeneity was obvious for reservoir-to-human transmission, but not so for human-to-human transmission. Human-to-human transmission rates were 15% higher during school open periods than school closure periods. Higher population densities were associated with higher human-to-human transmission rates except at very low densities, but the differences are generally ≤ 5%. The disease favored warm temperature over either too cold or too hot, with 74°F as the most suitable for transmission. It also favored higher relative humidity levels, with changes ≤ 10%. The effect of wind speed was nearly monotonic increasing with much steeper slopes when the speed was ≤ 4 knots. Medium and high levels of precipitation raised the risk of disease by about 5%, suggesting that ground water be a potential medium for transmission.
Figure S15 contrasts the model-predicted (conditional on observation in previous weeks) with observed weekly counts of cases, and Figure S16 plots standardized residuals of prefecture-specific weekly case numbers over log-transformed case numbers (left) and over time (right). The left panel of Figure S16 indicates that the Poisson model is reasonable for the data, as most residuals lie in a strip of approximately equal width over the observed case numbers. Both Figure S15 and the right panel of Figure S16 suggest a reasonable goodness-of-fit. The model captured most of the temporal variation except for the rapid growth in a few weeks in April of 2009, in particular in week 65 (March 24-30) when the case number was more than doubled compared to the previous week.
Discussion
The HFMD epidemics in China during 2008-2009 share many similarities with previous large outbreaks in the Asia-Pacific area.14,15 Preschool children, particularly the 1.0–2.9 years old, had the highest risk of acquiring HFMD, whereas infants had the greatest risk of severe complication and death. Males were more susceptible to disease and complications. EV71 was responsible for the majority of severe complications and deaths, whereas CA16 usually caused only mild symptoms. We found that temperature and wind speed were important, and relative humidity and precipitation were mild, risk modifiers for aggregate human-to-human transmission. The favorable effect of high relative humidity for viral transmission has been previously documented for polioviruses.16
Despite the enormous numbers of cases, the reported annual attack rates, 0.38 and 0.89 per thousand in 2008 and 2009, are lower than 6.1 per thousand in Taiwan’s 1998 outbreak and 3.5+ per thousand in Singapore’s outbreaks during 2002 and 2005–2007.14,17 Ho et al. reported 0.083 per thousand for the proportion of severe cases and 19.3% for the severe case fatality ratio among people under 15 years of age in the 1998 epidemic of Taiwan,14 while the corresponding figures are 0.090 per thousand and 2.6% among children under 10 years of age in 2009 in mainland China. The gap in the severe case fatality ratio could result from better treatment options for Chinese patients ten years after the outbreak in Taiwan or from the difference in the definition of severe cases. The EV71 lineages differ between the two outbreaks, C2 in Taiwan and C4 in China, but we are not aware of any systematic comparison of the virulence between different lineages of EV71. The proportions of EV71 among lab-confirmed severe cases were similar (around 80%) in the 1998 outbreak of Taiwan and the 2008–2009 outbreaks of China.
We found that severe cases and infant cases are associated with longer delay in diagnosis. Extremity rash or oral ulcers appeared less frequently in severe cases than in mild cases, and neurological complications are not specific to enteroviruses, both of which might have contributed to the delay in diagnosis. It is possible that the delay in diagnosis had in turn contributed to further deterioration of the disease to cardiorespiratory complications or even death in China. Such deterioration, if occurred, often progressed fast, generally in three to five days.18 The delay in diagnosis in infants was likely related to the fact that infants have a higher baseline body temperature and are thus more tolerant with fever, and that they can not really communicate their discomfort to their parents. A timely diagnosis may be a key to lowering the high mortality rate in infants, which may be achieved by educational campaigns about monitoring signs of HFMD among parents of newborns. The reason for the longer delay in diagnosis in Guangdong province compared to the rest of China is much less obvious. Guangdong province has been traditionally more industrialized than other regions, with a large volume of migrant labor workers from rural areas of inland provinces. This subpopulation has a relatively low economic status and hygienic conditions, and medical services are likely to be less accessible to them. This conjecture may be verified by collecting socioeconomic, hygienic and behavioral data from cases in the future.
HFMD was moderately transmissible with the estimated local effective R between 1.4 and 1.6 during the peak season. The estimates for the basic reproductive number R0 for polioviruses, another family of enterovirus, range from 5 to 15,16 but these estimates were based on serosurveillance data and are not comparable to our estimates for the local effective R. The dominance of local transmission suggests that more information about risk determinants is hidden within prefectures and warrants future analysis at a finer spatial scale such as counties or townships. The lack of finer spatial resolution data for risk factors limited our analysis to the prefecture level.
While most cases were younger than six years of age, our analysis revealed a strong effect of school closure on the epidemic. A plausible explanation is that substantial asymptomatic infections occurred among young school children during school mixing, and these asymptomatic children further transmitted the disease to their younger siblings or neighbors at home. If this speculation is true, the re-opening of schools after the summer break may partially account for the second rise of the epicurve in September. This speculation is partially supported by a serosurvey study in Taiwan which found that nearly 70% of EV71 seropositive children under six years of age were asymptomatic and the New York virus watch program in the 1960s which found that > 40% children of ≥ 5 years infected with Coxsackie viruses had no illness.7,19 Adults were found to have high proportions of asymptomatic EV71 infection in Taiwan5 or of detectable EV71 antibodies in Germany.20,21 Future serosurvey studies are needed in China to confirm the role of asymptomatic infections among school children and adults in the epidemics. These people are possible targets for vaccine design as they generally have much better immune response than preschool children.
Neither asymptomatic infections nor under-reporting of symptomatic cases are explicitly considered in our model due to the lack of information. Under-reporting is possible if, for example, overwhelmed medical facilities tended to adopt more strict diagnosis criteria. Ignoring unobserved infections, asymptomatic or unreported, will not bias the estimates of aggregate human-to-human transmissibility under the assumptions that (1) unobserved cases are as infectious as observed symptomatic cases, and (2) the probability of not being observed given infection does not changes over time or space (online supplementary materials). However, the effect of a risk factor can be biased if the probability of not being observed differs across the levels of the risk factor. For example, infected adults may be more likely than children to be asymptomatic or less likely to seek medical care, which is a potential source of bias for the age effect on transmissibility.
It is more rational to model the transmission process of each type of enterovirus separately with appropriate consideration of inter-pathogen interaction. This may be pursued after the completion of the virological confirmation of local specimens in the national laboratories of Chinese CDC. On the other hand, the estimated transmission rates can be interpreted as the averages over the co-circulating pathogens, and the estimated risk ratios have the interpretation of average effects if the true risk ratios are similar across pathogens (online supplementary materials).
Our model fits the incidence of HFMD well for most of the year except for the peak weeks in April and May of 2009, suggesting that there are unknown risk factors that drove the epidemic to the peak in such a short time. To inform prevention and control strategies, it is crucial to identify these underlying risk factors through field studies. Some insights may be provided by conducting a case-control study that samples subjects over the duration of an outbreak and across spatial regions, traces exposure history of each subject, and performs environmental sampling of the viruses.
The aggregated nature of our data means that we are estimating average effects for risk factors collected at the population level, and so the results may not reflect the effects of these factors at the individual level, due to the “ecological fallacy”.22,23 For example, the estimated effects of the climate indices at the prefecture level may not reflect the effects at the township level. However, the age and gender effects based on our model can be interpreted as individual-level effects because each age-gender stratum of each prefecture was modeled as an transmission unit.
Following the large outbreaks in 2008 and 2009, the incidence of HFMD continued to rise during 2010 in the Western Pacific region, particularly in China, Japan, South Korea and Singapore.24 Despite the fact that HFMD is an ongoing threat to regional health and can potentially become an emerging threat to global health, there appears to be a lack of studies and plans for intervention strategies. Our quantification of the population-level transmissibility and the effects of risk factors using surveillance data provides a basis for the use of mathematical or statistical models to evaluate the effectiveness of possible intervention strategies. The epidemiological hypotheses generated by our analyses need to be addressed by both the collection of more information in surveillance questionaires and the implementation of appropriate field studies.
Supplementary Material
Acknowledgments
Supported by: 1. The National Institute of General Medical Sciences MIDAS grant U01-GM070749 (Y.Y., I.L.)
2. The National Institute of Health grant R01 CA095994 (J.W.)
3. Fred Hutchinson Cancer Research Center (FHCRC), China-FHCRC Initiative (S.S., L.Y., J.D.)
4. Chinese Center for Disease Control and Prevention (Y.W., Z.F., Y.G., J.Z., L.W., Z.W., W.Y.)
References
- 1.Hamaguchi T, Fujisawa H, Sakai KS, et al. Acute encephalitis caused by intrafamilial transmission of enterovirus 71 in adult. Emerging Infectious Diseases. 2008;14:828–830. doi: 10.3201/eid1405.071121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tseng FC, Huang HC, Chi CY, et al. Epidemiological survey of enterovirus infections occurring in Taiwan between 2000 and 2005: analysis of sentinel physician surveillance data. Journal of Medical Virology. 2007;79:1850–1860. doi: 10.1002/jmv.21006. [DOI] [PubMed] [Google Scholar]
- 3.McMinn PC. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiology Reviews. 2002;26:91–107. doi: 10.1111/j.1574-6976.2002.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 4.Solomon T, Lewthwaite P, Perera D, et al. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infectious Disease. 2010;10:778–790. doi: 10.1016/S1473-3099(10)70194-8. [DOI] [PubMed] [Google Scholar]
- 5.Chang LY, Tsao KC, Hsia SH, et al. Transmission and clinical features of enterovirus 71 infections in household contacts in Taiwan. JAMA. 2004;291:222–227. doi: 10.1001/jama.291.2.222. [DOI] [PubMed] [Google Scholar]
- 6.van der Sanden S, Koopmans M, Uslu G, et al. Epidemiology of enterovirus 71 in the Netherlands, 1963-2008. Journal of Clinical Microbiology. 2008 doi: 10.1128/JCM.00168-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang LY, King CC, Hsu KH, et al. Risk factors of enterovirus 71 infection and associated hand, foot, and mouth disease/herpangina in children during an epidemic in Taiwan. Pediatrics. 2002;109:e88–e93. doi: 10.1542/peds.109.6.e88. [DOI] [PubMed] [Google Scholar]
- 8.Luo ST, Chiang PS, Chao AS, et al. Enterovirus 71 maternal antibodies in infants, Taiwan. Emerging Infectious Disease. 2009;15:581–584. doi: 10.3201/1504.081550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ooi EE, Phoon MC, Ishak B, et al. Seroepidemiology of human enterovirus 71, Singapore. Emerging Infectious Disease. 2002;8:995–997. doi: 10.3201/eid0809.10.3201/eid0809.010397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goh KT, Doraisingham S, Tan JL, et al. An outbreak of hand, foot and mouth disease in Singapore. Bulletin of the World Health Organization. 1982;60:965–969. [PMC free article] [PubMed] [Google Scholar]
- 11.Sarma N, Sarkar A, Mukherjee A, et al. Epidemic of hand, foot and mouth disease in west Bengal, India in August, 2007: a multicentric study. Indian Journal of Dermatology. 2009;54:26–30. doi: 10.4103/0019-5154.48982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chong CY, Chan KP, Shah VA, et al. Hand, foot and mouth disease in Singapore: a comparison of fatal and non-fatal cases. Acta Paediatrica. 2003;92:1163–1169. [PubMed] [Google Scholar]
- 13.Paul M, Held L, Toschke AM. Multivariate modeling of infectious disease surveillance data. Statistics in Medcine. 2008;27:6250–6267. doi: 10.1002/sim.3440. [DOI] [PubMed] [Google Scholar]
- 14.Ho M, Chen ER, HSU KH, et al. An epidemic of enterovirus 71 infection in Taiwan. New England Journal of Medicine. 1999;341:929–935. doi: 10.1056/NEJM199909233411301. [DOI] [PubMed] [Google Scholar]
- 15.Tong CYW, Bible JM. Global epidemiology of enterovirus 71. Future Virology. 2009;4:501–510. [Google Scholar]
- 16.Fine PEM, Carneiro IAM. Transmissibility and persistence of oral polio vaccine viruses: implicationsfor the global poliomyelitis eradication initiative. American Journal of Epidemiology. 1999;150:1001–1021. doi: 10.1093/oxfordjournals.aje.a009924. [DOI] [PubMed] [Google Scholar]
- 17.Ang LW, Koh BKW, Chan KP, et al. Epidemiology and control of hand, foot and mouth disease in Singapore. Annals Academy of Medicine Singapore. 2009;38:106–112. [PubMed] [Google Scholar]
- 18.Ooi MH, Wong SC, Lewthwaite P, et al. Clinical features, diagnosis, and management of enterovirus 71. The Lancet. 2010;9:1097–1105. doi: 10.1016/S1474-4422(10)70209-X. [DOI] [PubMed] [Google Scholar]
- 19.Kogon A, Spigland I, Frothingham TE, et al. The virus watch program: a continuing surveillance of viral infections among metropolitan New York families. American Journal of Epidemiology. 1969;89:51–61. doi: 10.1093/oxfordjournals.aje.a120915. [DOI] [PubMed] [Google Scholar]
- 20.Diedrich S, Weinbrecht A, Schreier E. Seroprevalence and molecular epidemiology of enterovirus 71 in Germany. Arch Virol. 2009;154:1139–1142. doi: 10.1007/s00705-009-0413-x. [DOI] [PubMed] [Google Scholar]
- 21.Rabenau HF, Richter M, Doerr HW, et al. Hand, foot and mouth disease: seroprevalence of Coxsackie A16 and enterovirus 71 in Germany. Medical Microbiology and Immunology. 2010;199:45–51. doi: 10.1007/s00430-009-0133-6. [DOI] [PubMed] [Google Scholar]
- 22.Koopman JS, Longini IM. Ecological effects of individual exposures and non linear disease dynamics in populations. American Journal of Public Health. 1994;84:836–842. doi: 10.2105/ajph.84.5.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wakefield J. Ecologic studies revisited. Annual Review of Public Health. 2008;29:75–90. doi: 10.1146/annurev.publhealth.29.020907.090821. [DOI] [PubMed] [Google Scholar]
- 24. [May 11, 2011]; http://www.wpro.who.int/sites/csr/data/HFMD_TrendsNStatistics.htm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


