Abstract
Aims
To investigate the incidence and severity of the ‘head-turning sign’ (HTS), i.e. turning the head back to the caregiver(s) for help, in patients with various dementias and discuss its clinical specificity in Alzheimer's disease (AD).
Methods
We investigated the incidence and severity of HTS while administering a short cognitive test (the revised Hasegawa Dementia Rating Scale: HDSR) in outpatients with AD [125 patients, including 4 with AD + vascular dementia (VaD)], 8 with amnestic mild cognitive impairment (aMCI), 34 with dementia with Lewy bodies (DLB), 8 with progressive supranuclear palsy (PSP) and 6 with VaD.
Results
Significant differences were found among the 5 disease groups in the incidence and severity of HTS, and HDSR scores. Given the significant differences between AD and DLB in post hoc analyses, patients were dichotomized into AD-related (AD and aMCI) and AD-nonrelated (PSP, DLB and VaD) groups. Both incidence (41 vs. 17%, p = 0.002) and severity of HTS (0.80 ± 1.13 vs. 0.21 ± 0.60, p = 0.001) were significantly higher in the AD-related group, while average age and HDSR scores were comparable between both groups. AD-related disease, female gender and low HDSR score contributed significantly to the occurrence and severity of HTS.
Conclusions
HTS can be a clinical marker of AD and aMCI, and may represent a type of excuse behavior as well as a sign of dependency on and trust in the caregivers.
Key Words: Amnestic mild cognitive impairment, Behavioral symptoms, Caregivers, Dementia with Lewy bodies, Excuse behavior, Female preponderance, Head-turning sign, Neuropsychological signs, Progressive supranuclear palsy, Vascular dementia
Introduction
When facing questions that are beyond their cognitive capacity, patients with dementia may turn back to the caregiver(s) for help, as if they were asking silently for some cues or answers directly to the question. This behavior, simplistically designated as ‘head-turning sign’ (HTS), has often observed in our patients, especially in patients with Alzheimer's disease (AD), but has rarely been investigated or reported in the literature. A single description of HTS as a sign observed in AD is found in a paragraph of the book entitled Clinical Diagnosis and Management of Alzheimer's Disease[1]. A PubMed search using the key words ‘head-turning sign’ and ‘dementia’ revealed no related articles. Therefore, it is not yet known how frequently HTS is observed in patients with AD at different stages of disease, and whether or not incidence and severity of HTS differ from those in other types of dementia. Consequently, we have little knowledge as to whether HTS is rather specific for AD or is ubiquitously present in the general dementia population. Furthermore, whether or not HTS correlates with declining cognition remains to be determined. The purpose of this study is first to investigate the incidence (frequency) and severity of HTS in AD and make comparisons with other dementia types and amnestic mild cognitive impairment (aMCI), and secondly to discuss whether HTS represents a clinical marker of AD and its precursor stage (aMCI), and finally to study the pathophysiological aspects of HTS in more detail.
Patients and Methods
Patients
We studied consecutive outpatients with dementia who were treated regularly by one of the authors (T.F.) at the Neurology Department of the Showa University Northern Yokohama Hospital in the period from September 2010 to March 2011. The study patients included 125 with AD [including 4 with mixed vascular dementia (VaD)], 8 with aMCI, 34 with dementia with Lewy bodies (DLB), 8 with progressive supranuclear palsy (PSP) and 6 with VaD. The diagnosis of each disease was made based on the established diagnostic criteria: AD using NINCDS-ADRDA criteria [2], aMCI using Petersen's criteria [3], DLB using DLB Consortium criteria in 2005 [4], PSP by NINDS-SPSP (National Institute of Neurological Disorders and the Society for Progressive Supranuclear Palsy) criteria [5] and VaD using NINDS-AIREN (International Workshop of the National Institute of Neurological Disorders and Stroke and the Association Internationale pour la Recherche et l'Enseignement en Neurosciences) criteria [6]. The diagnosis of AD mixed with VaD was made when clinical features of AD as well as VaD, physical signs compatible with multiple vascular lesions and their radiological evidence all coexisted. Furthermore, in an attempt to enhance the specificity of the diagnosis of DLB, we included only patients who satisfied the diagnostic criteria and showed abnormalities on 123I-meta-iodobenzylguanidine (MIBG) myocardial scintigraphy, which is recommended as one of the supportive diagnostic measures. We adopted the cutoff values of an heart/mediastinum (H/M) uptake ratio <1.68 and/or a washout rate >23.6%, which are considered to be highly sensitive and specific for the diagnosis of DLB [7].
Methods
We carefully observed whether or not the patients demonstrated HTS while administering a short cognitive test (the revised Hasegawa Dementia Rating Scale: HDSR) that usually takes about 10 min. The caregiver(s) was asked to be seated quietly at 45° and approximately 1 m behind the patient. HTS was considered present only when patients turned back to their caregivers in the face of difficulties with the HDSR subitems that the patients could not deal with, and asked the caregivers to help them either explicitly or implicitly. Self-devoted head tilting when deep in thought and any head movements associated with agitation, attention deficits or denial were not considered as HTS. Then, we scored semiquantitatively the severity in HTS-positive patients: when patients turned back only once, a severity score of 1 was assigned, a score of 2 for twice, and 3 for three times or more during the HDSR session. Patients and caregivers gave informed consent for our administering of the HDSR and for observing patient behavior during the test. This study was approved by the Ethics Committee of the Showa University Northern Yokohama Hospital.
Statistics
We compared the incidence of HTS (percentage of patients who demonstrated HTS) in each disease group, and the severity scores of HTS as well as the demography data of the patients and average HDSR scores. First, we compared the 5 disease groups using the Kruskal-Wallis test for numerical data (age, HDSR and severity scores) and the χ2 test for categorical data (gender and incidence of HTS).
As the comparisons of the 5 groups and post hoc Mann-Whitney tests revealed significant differences in both incidence and severity of HTS between AD and DLB (details in Results), we secondly dichotomized the whole patients into AD-related (AD and aMCI) and AD-nonrelated (DLB, PSP and VaD) groups, and compared these two groups using the Mann-Whitney and χ2 tests.
Lastly, to determine independent factors possibly contributing to the occurrence and severity of HTS, age and gender of patients, HDSR score and disease type (AD related and nonrelated) were analyzed using logistic (presence or absence of HTS) and linear (severity scores) regression analyses. A value of p < 0.05 represented statistical significance.
Results
Comparisons among the 5 Disease Groups
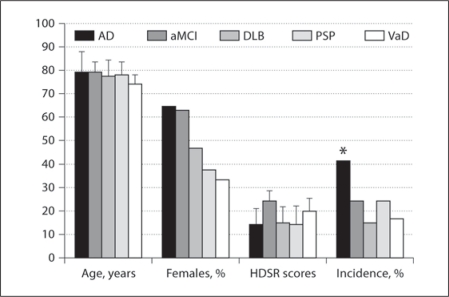
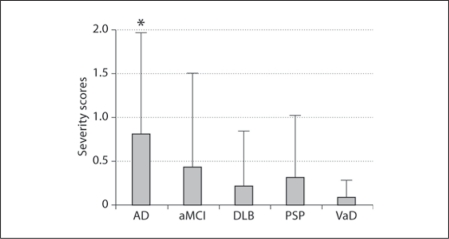
HTS incidence and/or severity scores in AD (incidence: 42%/severity score: 0.82 ± 1.13) and aMCI (25%/0.44 ± 1.05) were higher than in DLB (15%/0.21 ± 0.63), PSP (25%/0.31 ± 0.70) and VaD (17%/0.08 ± 0.20; table 1, fig. 1, 2). Group comparisons revealed that both the incidence (p = 0.029) and severity (p = 0.014) of HTS as well as HDSR scores (p = 0.0005) differed significantly among the 5 disease groups. Differences in the average age among the 5 groups, being apparently lower in VaD, showed a tendency toward significance (p = 0.06). The percentage of females was highest in AD and lowest in VaD patients, but group differences did not reach significance (p = 0.14). In post hoc analyses, significant differences in the incidence (p = 0.003) and severity (p = 0.003) of HTS were found between AD and DLB. The HDSR scores were significantly higher in aMCI than in AD, DLB and PSP (p = 0.0005–0.01), while they were comparable among AD, DLB and PSP. The HDSR score in VaD was significantly higher than in AD (p = 0.03) but lower than in aMCI, with a trend toward significance (p = 0.06).
Table 1.
Patient demography, average HDSR scores, and incidence and severity of HTS
| Groups | Age years | Females % | HDSR scores | HTS |
|
|---|---|---|---|---|---|
| incidence, % | severity scores | ||||
| AD | 79.1 ± 8.7 | 65 | 14.5 ± 6.6 | 42 | 0.82 ± 1.13 |
| aMCI | 79.3 ± 4.0 | 63 | 24.8 ± 3.9 | 25 | 0.44 ± 1.05 |
| DLB | 77.9 ± 6.3 | 47 | 15.1 ± 6.9 | 15 | 0.21 ± 0.63 |
| PSP | 78.4 ± 5.2 | 38 | 14.5 ± 8.0 | 25 | 0.31 ± 0.70 |
| VaD | 73.8 ± 3.2 | 33 | 20.5 ± 4.2 | 17 | 0.08 ± 0.20 |
| p value | 0.06 | 0.14 | 0.0005 | 0.029 | 0.014 |
Fig. 1.
Age, gender distribution, HDSR scores and incidence of HTS in AD, aMCI, DLB, PSP and VaD. * p = 0.03 vs. DLB.
Fig. 2.
HTS severity scores of the 5 disease groups. * p = 0.03 vs. DLB.
As partly mentioned in the Methods, these results, together with a general conception that in the majority of patients aMCI is pathophysiologically a precursor of AD [8], lead us to dichotomize the study patients into AD-related (AD and aMCI; n = 133) and AD-nonrelated (PSP, DLB and VaD; n = 48) groups.
Comparisons between AD-Related and AD-Nonrelated Groups
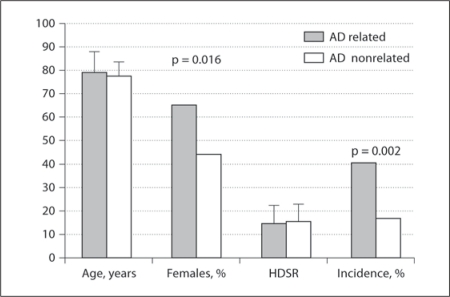
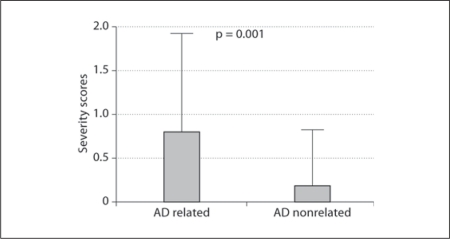
The percentage of females was significantly higher in the AD-related than in the AD-nonrelated group (65 vs. 44%, respectively; p = 0.016), while average age (p = 0.20) and HDSR scores (p = 0.54) were similar between both groups (table 2, fig. 3, 4). Both incidence (AD related 41% and AD nonrelated 17%; p = 0.002) and severity scores of HTS (AD related 0.80 ± 1.13 and AD nonrelated 0.21 ± 0.60; p = 0.001) were significantly higher in the AD-related group.
Table 2.
Patient demography, average HDSR scores, and incidence and severity of HTS between AD-related (AD and aMCI) and AD-nonrelated (DLB, PSP and VaD) groups
| Groups | Age years | Females % | HDSR scores | HTS |
|
|---|---|---|---|---|---|
| incidence, % | severity scores | ||||
| AD related | 79.1 ± 8.5 | 65 | 15.2 ± 6.8 | 41 | 0.80 ± 1.13 |
| AD nonrelated | 77.4 ± 5.9 | 44 | 15.7 ± 7.0 | 17 | 0.21 ± 0.60 |
| p value | 0.20 | 0.016 | 0.54 | 0.002 | 0.001 |
Fig. 3.
Age, gender distribution, HDSR scores and incidence of HTS in AD-related (AD and aMCI) and AD-nonrelated groups (DLB, PSP and VaD).
Fig. 4.
HTS severity scores of AD-related and AD-nonrelated groups.
Factors Contributing to HTS
Logistic regression showed that significant and independent contributors of HTS occurrence included AD-related disease (odds ratio [OR] 2.98, 95% confidence interval [CI] = 1.26–7.09, p = 0.013), female gender (OR 2.83, 95% CI = 1.39–5.75, p = 0.004) and HDSR scores (OR 0.94, 95% CI = 0.90–0.99, p = 0.016), explaining 18% of the variances. Similarly, AD-related diseases (β = 0.21, p = 0.004), female gender (β = 0.18, p = 0.015) and HDSR scores (β = −0.16, p = 0.018) independently predicted HTS severity scores, explaining 11% of the variances.
Discussion
Disease-specific signs and symptoms, despite their rather anecdotal character, are helpful and even essential for making accurate differential diagnoses especially when dealing with clinicopathologically overlapping diseases, such as AD, DLB and VaD, or PSP, corticobasal degeneration and frontotemporal dementia. Auxiliary diagnostic strategies represented by various imaging methods and biomarkers, such as MRI, SPECT, MIBG scintigraphy, amyloid imaging, β-amyloid and phosphorylated tau in cerebrospinal fluid and striatal 123I-FP-CIT SPECT are especially useful in differentiating AD from DLB or other diseases in the early phase; however, these sophisticated methods are not always available to all clinicians. This study addressed the importance of a single clinical sign (HTS) in the differentiation of AD from other dementia-causing diseases, namely DLB, PSP and VaD.
In the current study, diagnoses were made carefully at the initial clinical assessment and confirmed by a long-term follow-up. Furthermore, we paid specific attention to avoiding confusion between DLB and AD, PSP or VaD, because the initial symptoms of DLB may be highly variable [9]. The diagnosis of DLB was made only when patients satisfied the consensus criteria [4] and showed abnormalities on the MIBG myocardial scintigraphy that are considered specific for DLB [7]. Conversely, MIBG myocardial scintigraphy was utilized when the clinical differentiation between DLB and AD, PSP or VaD was difficult. When MIBG scintigraphy was introduced in the differential diagnosis, the diagnostic specificity for each disease may be higher than without it [7].
Having the clinical diagnoses made as correctly as possible, we compared the incidence and severity of HTS. As hypothesized at the beginning, results proved that HTS was most prevalent and severe in patients with AD, followed by aMCI regarding HTS severity. Both the frequency and the severity of HTS were significantly different among the 5 disease groups and post hoc analyses showed that significant differences existed between AD and DLB.
Based on these findings as well as the fact that aMCI is most likely a prodromal stage of AD [8], patients were grouped into AD related (AD and aMCI) and AD nonrelated (DLB, PSP and VaD). As expected, comparisons between these two groups showed that the incidence as well as the severity scores for HTS was significantly higher in the AD-related group. Thus, HTS can be regarded as one of the clinical features that characterize AD and aMCI, as previously described [1], and frequent HTS may suggest, although not conclusively, a diagnosis of AD or its prodromal stage (aMCI).
What are the pathophysiological factors underlying HTS? HTS was more prevalent and severer in AD-related diseases that highlight memory impairment with comparatively preserved frontal executive function. In contrast, HTS was rarer in AD-nonrelated diseases that may feature more apparent dysexecutive syndromes. HTS may represent rather intact executive strategies and tactics when facing memory-related difficulties that are beyond the cognitive abilities of patients. Conversely, patients with deficits in executive function, divided attention or set changing may not be able to come up on the spot with an idea of turning to their caregivers and asking for help as an alternative strategy for coping with troubles at hand. From this point of view, HTS may share some properties with excuse behaviors, often observed in AD patients, in an attempt to cover up memory deficits.
Concomitantly, HTS may also signify dependency on and trust in the caregivers/family members by shifting the responsibilities that patients are supposed to carry. Analysis of behaviors of patients with AD showed an extremely high incidence rate of dependency (87% of 106 patients with probable AD) together with denial of illness (63%) and motor agitation (55%) [10].
Independent of the epidemiological fact that AD is more prevalent in females, female gender was a statistically significant contributor to HTS. In general, women may feel easier at heart to become dependent on others when facing difficulties, while men tend to feel obligated to deal with adversities without help. In fact, a recent study compared the prevalence of behavioral and psychological symptoms of dementia in men and women with cognitive impairment and showed that ‘help seeking’ and depression were significantly more frequent in women, while aggressive and regressive behaviors were more prevalent in men [11]. HTS is essentially a behavior of help seeking and the finding from the current study that HTS is more frequent in women may strengthen a previous finding that help seeking was more prevalent in female patients.
HTS turned out to be negatively but weakly associated with HDSR scores: higher incidence and severity scores were associated with lower HDSR scores (OR < 1.0 for the presence of HTS and a negative β for the severity of HTS). Incidence and severity of HTS may correlate with the severity of dementia because memory impairment that triggers HTS may worsen as dementia progresses. In fact, a recent large-scale survey targeting 3,404 people with cognitive impairment suggested that behavioral and psychological symptoms of dementia in general correlated negatively with the level of cognition. However, some of these symptoms, e.g. attention-seeking behavior (help seeking), showed non-linear correlations, with the highest prevalence in middle-stage cognitive impairment [12]. Likewise, the incidence and severity of HTS correlated essentially with the levels of cognitive impairment measured by HDSR. However, HTS was unique in that its severity score was higher in aMCI in spite of significantly higher HDSR scores than in PSP, DLB and VaD. In patients with aMCI, although HDSR scores remain higher, the severity of memory deficits is far beyond that of other cognitive domains, including executive function, a situation that may elicit HTS. Given the same HDSR score, frontal executive dysfunction may be milder than memory deficits in an AD-related population, while the reverse may be true in AD-nonrelated diseases. As suggested above, HTS may be the consequence of imbalance between memory impairment and relatively preserved executive function.
Limitations
However, this study has some limitations. First, the numbers of patients in the 5 disease groups are not uniform because consecutive regularly visiting patients were studied. The numbers of patients may represent the prevalence of each disease in our dementia clinic. However, comparisons between AD-related (n = 133) and -nonrelated patients (n = 48) may be justified because it is generally understood that a sample-size difference smaller than 3:1 (2.8:1 in the present study) may be compatible with statistical procedures and would not reduce statistical robustness [13]. Thus, the results obtained from AD-related and -nonrelated groups may be considered statistically robust. Secondly, the incidence of HTS may vary depending on the persons who accompany the patients: husband, wife, children, siblings or professional caregivers. Because the majority of caregivers were spouses or children, the levels of familiarity between a patient and the caregiver(s) was supposedly similar from patient to patient. Regrettably, we could not take this factor into consideration since precise information was not available.
Conclusion
In spite of these shortcomings, the present study proposed that HTS, reflecting intact tactics as well as dependency and reliance on the caregivers when facing difficulties, may be one of the distinguishing clinical signs of AD-related cognitive impairment.
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Bouchard RW, Rossor MN. Typical clinical features. In: Gauthier S, editor. Clinical Diagnosis and Management of Alzheimer's Disease. London: Dunitz; 1996. pp. 35–50. [Google Scholar]
- 2.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 4.McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M, Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 5.Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH, Hallett M, Jankovic J, Quinn NP, Tolosa E, Zee DS. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 7.Yoshita M, Taki J, Yokoyama K, Noguchi-Shinohara M, Matsumoto Y, Nakajima K, Yamada M. Value of 123I-MIBG radioactivity in the differential diagnosis of DLB from AD. Neurology. 2006;66:1850–1854. doi: 10.1212/01.wnl.0000219640.59984.a7. [DOI] [PubMed] [Google Scholar]
- 8.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukui T, Hori K, Yoshimasu H. Onset patterns and initial symptoms of dementia with Lewy bodies: possible pathophysiological diversities deduced from a SPECT study. Dement Geriatr Cogn Disord Extra. 2011;1:237–248. doi: 10.1159/000330345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devanand DP, Brockington CD, Moody BJ, Brown RP, Mayeux R, Endicott J, Sackeim HA. Behavioral syndromes in Alzheimer's disease. Int Psychogeriatr. 1992;4(suppl 2):161–184. [PubMed] [Google Scholar]
- 11.Lövheim H, Sandman PO, Karlsson S, Gustafson Y. Sex differences in the prevalence of behavioral and psychological symptoms of dementia. Int Psychogeriatr. 2009;21:469–475. doi: 10.1017/S1041610209008497. [DOI] [PubMed] [Google Scholar]
- 12.Lövheim H, Sandman PO, Karlsson S, Gustafson Y. Behavioral and psychological symptoms of dementia in relation to level of cognitive impairment. Int Psychogeriatr. 2008;20:777–789. doi: 10.1017/S1041610208006777. [DOI] [PubMed] [Google Scholar]
- 13.Tsushima E: Analysis of Medical Data by the SPSS Program, ed 1. Tokyo, Tokyo Tosho, 2007.