Signaling from the chloroplast and mitochondria to the nucleus, termed retrograde signaling, regulates the expression of nuclear genes encoding organellar proteins and other cellular proteins to orchestrate organelle and cell development and metabolism. It is a critical component of plant response to abiotic stresses, such as high light and drought stress, which have a major impact on photosynthesis and primary metabolism that takes place within the chloroplast. However, the identity of signaling molecules active in retrograde signaling is clouded in uncertainty.
Indeed, there are likely multiple retrograde signals and signaling pathways to accommodate the different stimuli and transcriptional profiles of the several thousand proteins targeted to the organelles. Identifying bona fide signaling molecules from the soup of chloroplast metabolites is fraught with technical difficulty. One pathway, studied using the genomes uncoupled (gun) mutants, led to the identification of proteins that alter the expression of certain photosynthesis associated genes. Some of the GUN genes encode components of tetrapyrrole biosnthesis, and the tetrapyrrole magnesium protoporphyrin IX was considered a good candidate signaling molecule for some years, but recent evidence has cast doubt on this hypothesis. Mutations in proteins such as EXECUTOR1 and REGULATOR OF APX2 1 that regulate aspects of redox and reactive oxygen species have been shown to initiate retrograde signaling, but, to date, there is no solid evidence for a signaling molecule moving from the chloroplast to the nucleus (reviewed in Pfannschmidt, 2010).
Now, Estavillo et al. (pages 3992–4012) provide evidence that the phosphonucleotide 3′-phosphoadenosine 5′-phosphate (PAP) accumulates in response to high light or drought stress and moves from the chloroplast to the nucleus, where its activity leads to the activation of stress-responsive genes. They further show that the phosphatase SAL1 localizes in vivo to the chloroplast and mitochondria, where it functions as a negative regulator of PAP accumulation.
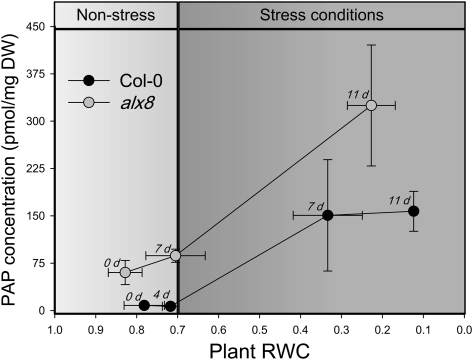
First, the authors show that PAP accumulates in sal1 mutants and during drought or high light stress (see figure) and that PAP is a substrate of SAL1 in vivo, based on the development of a fluorescence HPLC assay for accurate separation and monitoring of PAP and its precursors. SAL1 is then shown to be localized to chloroplasts and mitochondria, but not the cytosol, using three separate approaches: transient expression of SAL1:green fluorescent protein in Arabidopsis thaliana cells, isolation of protoplasts from stable transgenic lines expressing SAL1:green fluorescent protein, and the development of an improved plant extract fractionation protocol. The isolation of pure cytosolic and chloroplastic fractions from the same plant material constitutes a notable technical advance in plant tissue subcellular fractionation.
PAP accumulates under drought conditions in Arabidopsis. Correlation between relative water content (RWC) of plants and PAP concentration on a dry weight (DW) basis ± SD (n > 8) during drought. Days in drought is shown in italics. alx8 carries a loss-of-function SAL1 mutation. (Reprinted from Figure 3A of Estavillo et al. [2011].)
Next, the authors provide genetic evidence that PAP is a mobile signal that moves from the chloroplast to the nucleus and regulates nuclear gene expression by inhibiting nuclear exoribonucleases (XRNs) that target certain stress-responsive genes. This is supported by transcriptome analysis of xrn and sal1 mutants and the accumulation of XRN substrates in sal1 mutants. Thus, in the absence of SAL1 activity and in response to high light or drought stress, PAP accumulates in the chloroplast and then moves to the nucleus, where it inhibits XRNs, thereby triggering enhanced transcription of XRN target genes.
This work provides exciting evidence of a SAL1-PAP retrograde signaling pathway regulating the expression of various stress-related nuclear genes. Additional work is required to provide more direct evidence of the movement of PAP between organelles, how PAP levels are regulated during high light and drought stress, and how inhibition of XRNs regulates gene expression. Moreover, does SAL1 function directly as a stress sensor or are there additional upstream components?
References
- Estavillo G.M., et al. (2011). Evidence for a SAL1-PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis. Plant Cell 23: 3992–4012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfannschmidt T. (2010). Plastidial retrograde signalling—A true “plastid factor” or just metabolite signatures? Trends Plant Sci. 15: 427–435 [DOI] [PubMed] [Google Scholar]