Abstract
Purpose
To evaluate the dental and periodontal health of adolescents and young women with restrictive anorexia nervosa (AN), and relationship between bone mineral density (BMD) assessed by dual energy X-ray absorptiometry (DXA) and dental radiographs.
Study Design
Twenty-three young women, median age 17.6 years (range 14.4-27.2), were studied within three months of entering a clinical trial. DXA BMD measurements were obtained and subjects underwent a comprehensive dental examination, including panoramic and bitewing dental radiographs. Three observers assessed mandibular cortical width (MCW) in the mental foramen region on panoramic radiographs.
Results
Dental findings included very good to excellent oral hygiene. Gingival recession was evident in 10 participants (43%). Dental erosion was not seen and the mean decayed missing or filled teeth (DMFT) was 8.6. There was a weak positive correlation between BMD by DXA and MCW on radiographs.
Conclusions
We report dental manifestations associated with restrictive AN, and the association between bone assessments by DXA and dental radiographs in this patient group. Despite subnormal DXA measurements in most patients, essentially all adolescents had a normal dental examination. Dental providers should be cognizant of the fact that many patients with eating disorders may not display the “classic” findings reported in the literature.
Keywords: Adolescents, oral health, anorexia nervosa, body mass index, bone mineral density, DXA, MCW
INTRODUCTION
Anorexia nervosa (AN) is the third most common chronic disease among adolescent girls. According to the Diagnostic and Statistical Manual of Mental Disorders, 4th version (DSM-IV), AN is characterized by an intense fear of weight gain or obesity; refusal to maintain a healthy body weight; distorted body image; body mass index (BMI) < 18 kg/m2, or below 85% of ideal body weight for height; and amenorrhea ≥3 consecutive cycles (in postmenarchal females). The disease is classified as either restricting or binge-eating/purging (also known as bulimia) types. Both subtypes can have serious medical consequences, including effects on both bone and oral health.1-7 Previous studies suggest that a low bone mass is common in adolescent girls and young women with AN and that it may occur early in the course of their disease, compromising peak bone mass. As a result, AN may increase the risk for osteoporosis and fracture throughout life.2, 3
Unlike many healthcare practitioners, dentists and dental hygienists typically see patients on a regular basis, and often for the duration of a child or adolescent’s lifetime. Therefore, these providers have the unique opportunity to be the first to detect clinical evidence of an eating disorder. DeBate et al.4 found that a majority of dental health practitioners had little knowledge of oral clues associated with eating disorders. As dentists and dental hygienists are on the front lines of patient care, it is essential that they develop an understanding of the intra- and extra-oral effects of both binge-eating/purging and restricting AN. This knowledge could be critical to the early detection of eating disorders.5
Historically, studies describing the effects of eating disorders on oral health have focused on the consequences of the binge-eating/purging behavior subtype. These studies have shown a high prevalence of both tooth enamel erosion6 and dental caries7 within this patient group. However, the same attention towards dental health has not been given to patients with restrictive type AN and its potential relation to low bone mineral density (BMD).
Previous studies have shown that the malnutrition associated with AN puts these adolescent girls and young women at high risk for bone loss and early osteoporosis in later life. In conjunction, there have been studies in adult and elderly populations that provide evidence linking osteoporosis to decreased mandibular basal BMD,8 and gingival recession.9 However, a positive correlation between low BMD and variables associated with oral health in young women with AN has not previously been investigated.
The association between AN and bone loss has been intensely studied.2, 10-12 Carmo et al.3 found that while the severity of low BMD was dependent on the duration of amenorrhea, even with resumption of regular menstrual cycles and normalization of a healthy weight, some patients continue to have a low bone density. A major obstacle to combating bone loss is the failure to identify individuals with the condition until the clinical consequences have occurred.13 Early diagnosis is critical as several treatments have been shown to slow progressive bone loss, reduce the risk of fracture, and the subsequent morbidity and mortality associated with it. The serious implications of a low BMD and the associated risk of osteoporosis warrant a means to detect patients at risk without use of dual-energy X-ray absorptiometry (DXA). DXA is expensive, requires elaborate equipment, specialized operator training, has a large space requirement,14 and is not readily available to all communities. In contrast, a large majority of the population receives regular dental X-rays. Previous studies have shown the capability of dental radiographs to predict BMD successfully. Karayianni et al.13 found a correlation between mandibular cortical width (MCW) on dental panoramic radiographs and DXA T-scores. In another study, the coarseness of alveolar bone trabeculation was found to be an indicator of BMD in the forearm,15 while another study found that the maxillary alveolar bone density was an indicator of bone density at all other sites, including the anteroposterior lumbar spine and lateral lumbar spine.16 Therefore, dental panoramic radiographs could provide a convenient, affordable source of screening for osteoporosis with a lower radiation exposure for those patients who might not otherwise have access to conventional methods.
In this study, we examined a group of patients with primarily restrictive AN to evaluate for a correlation between BMD and oral health. The purpose of this study was to evaluate the dental and periodontal health of adolescents and young women with AN, and to examine the relationship between BMD as assessed by DXA and MCW obtained from dental panoramic radiographs.
METHODS
Subjects
Twenty-three young women participating in a clinical trial for bone health in AN were enrolled in this study within the first three months of entry into the clinical trial. All participants were recruited from the Children’s Hospital Boston Eating Disorder Program. Inclusion criteria included: criteria met for DSM-IV diagnosis of AN; secondary amenorrhea for at least 3 months; female; between the ages of 15-30 years; and participant was at least two years postmenarchal. Exclusion criteria included: participant had received previous hormonal replacement therapy (e.g., estrogen, oral contraceptives) within the past 3 months; diagnosis of confirmed Cushing syndrome, diabetes mellitus, skeletal dysplasia, or other disease known to affect bone metabolism; and/or receipt of glucocorticoid therapy or other medications known to have skeletal effects within previous 3 months.
Radiographic measurements
Dental panoramic radiographs using a Gendex Orthoralix 9200 (Kavo Dental Co., Lake Zurich, IL) and bitewing radiographs using a Gendex GX-770 (Kavo Dental Co., Lake Zurich, IL) X-ray unit were exposed on each subject at the dental clinic of Children’s Hospital Boston using standard exposure settings and radiation protection with a lead apron and thyroid collar. All films were digitized utilizing an Epson Expression 1680 (Epson America, Inc., Long Beach, CA) and transferred to Dolphin Imaging software (v10.0) (Dolphin Imaging and Management Solutions, Chatsworth, CA). Digital images were printed using a Canon iP 5200 printer (Canon U.S.A. Inc., Lake Success, NY). Measurements were made from either the films or prints of the digitized images. Three experienced healthcare professionals, one radiologist and two dentists independently made measurements using a Mitutoyo “Absolute Digimatic” digitometer (Mitutoyo Co., Aurora, IL) of MCW in the region of the mental foramen from the dental panoramic radiographs. The MCW of the body of the mandible was measured bilaterally on each film/print in the mental foramen region, from a perpendicular drawn equidistant between the apices of the premolars to a tangent to the lower border of the mandible (Figure 1). All measurements were made two times, on two separate occasions at least a week apart, and the observers were blinded to the reference osteoporotic diagnosis.
Figure 1.
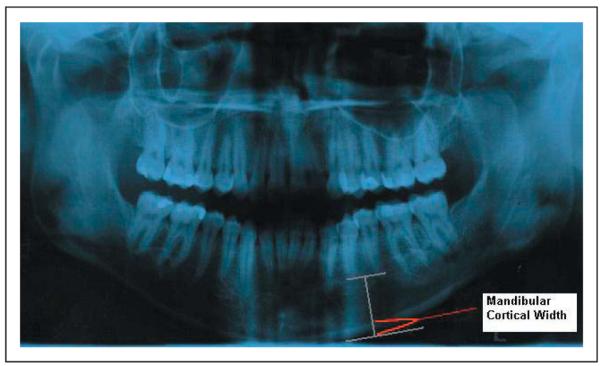
Measurement of mandibular cortical width (MCW) on panoramic radiograph. A line was drawn connecting the apices from premolar to premolar, from which a perpendicular was dropped to the external cortex of the mandible. A line tangent to the outer cortex was then drawn, to which a perpendicular was drawn and the MCW was measured bilaterally using a digitometer from the corticomedullary junction to the external cortex.
Data Collection
All study participants were seen pre-treatment in the pediatric dental clinic at Children’s Hospital Boston. Informed consent was obtained from all study participants after the nature of the procedures had been fully explained prior to the commencement of the dental examination and radiographs. Demographic data was obtained from a health history questionnaire which included information on age, gender, place of residence, and family demographics.
Height and weight were obtained at the Children’s Hospital Boston General Clinical Research Center using a calibrated stadiometer (Kalamazoo, MO) and scale. Bone mineral density data was acquired by DXA on a Hologic 4500 Delphi scanner (Hologic Inc, Bedford, MA), including total body, spine and hip BMD. All participants underwent a comprehensive visual-tactile clinical examination using an artificial light source, a #2 reflecting mirror, a #23 explorer, and a periodontal probe in a recumbent position in the Children’s Hospital Department of Dentistry evaluating: past medical history and dental history; decayed, missing, or filled teeth (DMFT); plaque score utilizing the Simplified Oral Hygiene Index (OHI-S, Table 1)17; periodontal disease utilizing the Modified Gingival Index (GI, Table 2)18 as well as probing depths (six surfaces around each tooth); and screening for oral cancer, dental erosion, and other oral pathology. For purposes of this study, dental caries was defined as an explorer stick with resistance to pull-back. Dental erosion was defined as a clinically, detectable change in the enamel smooth surface without evidence of dental caries. This study was approved by the Committee on Clinical Investigation (institutional review board) at Children’s Hospital Boston.
Table 1.
Simplified Oral Hygiene Index: Criteria for classifying debris (17)
| Scores | Criteria |
|---|---|
| 0 | No debris or stain present |
| 1 | Soft debris covering not more than one third of the tooth surface, or presence of extrinsic stains without other debris regardless of surface area covered |
| 2 | Soft debris covering more than one third, but not more than two thirds, of the exposed tooth surface. |
| 3 | Soft debris covering more than two thirds of the exposed tooth surface. |
Table 2.
Gingival Index System (18)
| Scores | Criteria |
|---|---|
| 0 | Absence of inflammation |
| 1 | Mild inflammation is present indicated by a slight change in color and texture |
| 2 | Moderate glazing, redness, edema, hypertrophy, and bleeding on pressure |
| 3 | Severe inflammation noted by marked redness, hyper- trophy, ulceration, and tendency to spontaneously bleed |
Statistical Analyses
DXA BMD Z-scores were compared to normal by one-sample Student t-test. Variability of MCW measurements between and within raters was assessed by mixed-effects analysis of variance. Correlation between MCW and DXA measures was assessed with Pearson coefficients. All computations were performed with SAS software version 9.1 (Cary, NC).
RESULTS
Twenty-three young women were enrolled into the study. Recruitment and examinations commenced in March 2004 and were completed in May 2008. A comprehensive dental examination and dental radiographs were obtained on all twenty-three participants (100%). The median age of the study sample was 17.6 years (range 14.4-27.2). The largest percentage of participants was Caucasian (83%) (Table 3).
Table 3.
Characteristics of Sample
| N (%) | Mean | SD | Median | Min | Max | |
|---|---|---|---|---|---|---|
| Age (yr) | 18.5 | 2.9 | 17.6 | 14.4 | 27.2 | |
| Race: Caucasian | 19 (83) | |||||
| African-American | 1 (4) | |||||
| Asian-American | 1 (4) | |||||
| Other | 2 (9) | |||||
| Weight (kg) | 48.0 | 6.3 | 49.2 | 39.6 | 67.9 | |
| BMI (kg/m2) | 17.5 | 1.7 | 17.5 | 14.8 | 22.9 | |
| Duration of anorexia (mo) | 2.5 | 2.9 | 13 | 1 | 96 | |
| Duration of amenorrhea (mo) | 18 | 32 | 7 | 1 | 144 |
Dental findings included very good to excellent oral hygiene according to the OHI-S (Simplified Oral Hygiene Index) (Table 4). Soft tissue pathology was limited to four (17%) patients with mild gingival inflammation localized to the maxillary and mandibular molar regions according to the GI. Gingival recession, determined as ≥3 surfaces 1 mm below the cementoenamel junction was evident in 10 partic-ipants (43%). There were no findings of oral cancer detected in any of the participants. Dental erosion was not seen in any participant. However, 26% of participants reported a history of binge-eating/purging activity. The mean DMFT was 8.6; nine participants (39%) had clinically detectable caries.
Table 4.
Dental findings.
| N (%) | Mean | SD | Median | Min | Max | |
|---|---|---|---|---|---|---|
| Mandibular Cortical Width (mm)* |
4.8 | 0.7 | 4.8 | 3.6 | 6.6 | |
| DMFT | 8.6 | 9.2 | 7.1 | 0 | 32 | |
| Binge-purge | 6 (26) | |||||
| Simplified Oral Hygiene Index | 18 (78)† | 0 | 0 | 4 | ||
| Gingival Index System | 19 (83)† | 0 | 0 | 3 |
Mean of 6 measurements by 3 raters.
Number and percentage of zero scores.
The mean group MCW measured by three examiners was 4.8 mm (range 3.4-7.4). Both inter-examiner and intra-examiner reliability were assessed. One examiner provided systematically higher values for MCW (Table 5). Of the total variance of MCW, 68% was true inter-subject variation with a standard deviation of 0.71 mm. The remaining variance was approximately equal parts variability among raters (19%, SD 0.37 mm) and residual measurement error within examiners (14%, SD 0.32 mm).
Table 5.
Mandibular cortical width (MCW): measurement variability.
| MCW, mm | |||||
|---|---|---|---|---|---|
| N | Mean | SD | Min | Max | |
| All | 138 | 4.80 | 0.83 | 3.4 | 7.4 |
| Rater 1 | 46 | 4.57 | 0.66 | 3.6 | 6.4 |
| Rater 2 | 46 | 4.59 | 0.76 | 3.4 | 6.5 |
| Rater 3 | 46 | 5.23* | 0.88 | 3.7 | 7.4 |
Different from Raters 1 and 2, p<0.001.
DXA revealed normal total body BMD measurements, but mean spinal and hip BMD were significantly diminished (Table 6). Total body BMD showed a slight, but statistically insignificant correlation with MCW (Figure 2). The correlation of MCW with other DXA measures was weak, but positive (Table 6).
Table 6.
DXA measurements.
| Site | Measure | Mean | SD | Median | Min | Max | Correlation with MCW* |
|
|---|---|---|---|---|---|---|---|---|
| r | p | |||||||
| Total Body | BMD (g/cm2) | 1.04 | 0.07 | 1.03 | 0.92 | 1.18 | 0.20 | 0.36 |
| Z-Score | 0.02 | 1.01 | −0.20 | −2.00 | 2.10 | −0.03 | 0.88 | |
| AP Spine | BMD (g/cm2) | 0.89 | 0.10 | 0.89 | 0.68 | 1.06 | 0.12 | 0.57 |
| Z-Score | −1.01† | 1.06 | −1.20 | −3.30 | 0.90 | 0.01 | 0.97 | |
| Hip | BMD (g/cm2) | 0.87 | 0.11 | 0.87 | 0.66 | 1.09 | 0.15 | 0.50 |
| Z-score | −0.49‡ | 0.91 | −0.50 | −2.20 | 1.30 | 0.09 | 0.69 | |
r is Pearson correlation coefficient between DXA measure and mean of 6 MCW measurements by 3 raters. p tests for significant difference from zero.
Significantly different from zero, p<0.001
p<0.02.
Figure 2.
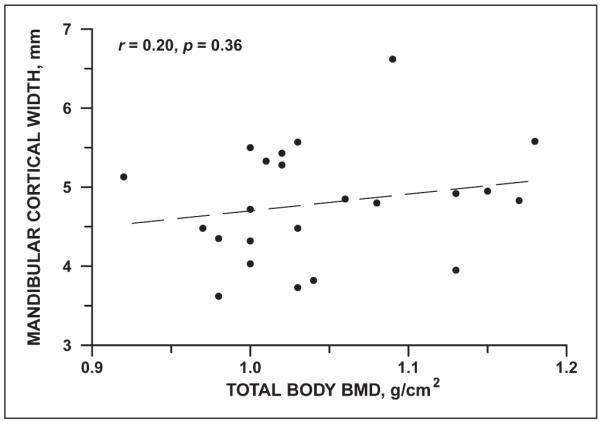
Correlation of mandibular cortical width with total body bone mineral density in 23 young women with anorexia nervosa.
DISCUSSION
Osteoporosis and its associated low-trauma fractures may have dramatic outcomes in terms of morbidity, mortality, and health care costs. Therefore, effective preventive strategies dependent on early diagnosis and treatment are essential to mitigate the long-term effects of this silent disease.19 This study examined the oral health of adolescent and young adult females suffering from restrictive AN, and in particular the correlation of BMD between DXA and dental panoramic radiographs. General trends in the dental findings included almost impeccable hygiene as determined through dental and periodontal exams, as well as soft tissue findings that suggest overzealous brushing. Our results are important ones for dental providers and hygienists to consider, given that they represent front-line providers for adolescent girls and young women, especially with regard to routine health screening.
Primary outcomes for this study included the MCW as detected on dental panoramic radiographs and bone density as determined by the standard BMD screening tool, DXA. Our choice of outcomes was based in part upon studies in the literature that identified the anterior region of the mandible, considering regions obtained on dental radiographs, as a favorable site for BMD detection.20-22 One study found that the cortical index for diagnosing osteoporosis was noted to have both low sensitivity and specificity.23 Use of MCW measurements, in lieu of mandibular cortical porosity in the mental foramen region, was influenced by the recent release of findings from the OSTEODENT project.24 That project concluded that MCW had better efficacy than the mandibular cortical index in detecting osteoporosis and that there was no benefit in combining the two measurements. Although there has been little consensus as to an appropriate mandibular cortical thickness in the literature, a measurement of ≤3 mm has been suggested as an optimal threshold for further investigation to rule out osteoporosis.22 Taguchi et al.25 claimed that 60% of their patients who presented with an MCW of less than 3 mm were osteoporotic. Horner et al.20 found that reduction of the mandibular cortex below 3 mm in the mental foramen region was also associated with low bone mass at the spine, femoral neck, or forearm. Lastly, Karayianni et al.13 stated that only those subjects with the thinnest mandibular cortices (≤3 mm) warrant referral for further investigation, as they are more likely to have the disease. Interestingly, none of the current participants had a MCW ≤3mm, and the range of measurements from all three observers on all subjects was 3.4 mm to 7.4 mm. These findings may be attributable to the differences in study populations, in that we studied adolescent and young adult females and previous studies have included primarily postmenopausal women. Our results might have differed had we utilized digital radiography and accounted for errors in magnification by including a mechanism in our methodology to control for it. In addition, we also might have improved the accuracy of our findings by utilizing an increased number of examiners for our MCW measurements. However, a previous study found that one important barrier to using cortical width measurements in primary dental care is the significant observer variability in measurement that does not improve with individualized instruction.26 Despite our minimally rigorous methodology, our study showed that there was a weak positive correlation between BMD detected by DXA and MCW as measured on dental panoramic radiographs.
Krall et al.27 found a relationship between calcium and vitamin D supplement intake and the risk of tooth loss in the elderly. Patients with AN have been shown previously to have a better vitamin D status compared to healthy control subjects.28 The benefit is related in part to more consistent compliance with vitamin D supplementation and potentially, to increased levels of bioavailable vitamin D in underweight patients. The positive effects of vitamin D on the oral health of the current patients with AN, in addition to not having the deleterious effects of the binging/purging behavior, may have improved the overall oral health of this current clinic-based sample.
Willumsen and Graugaard29 found that women with eating disorders, overall, have a higher level of dental fear than do women within the general population. Furthermore, they reported that almost half of the women with high dental fear restricted contact with dentists. Their study also found that women with higher dental fear reported more significant dental pathology. These findings suggest that the patients who volunteered to participate in the current study could have potentially had less dental fear, and therefore fewer dental problems as they may have been more compliant with regular cleanings and dental assessments. As a result of the direct association between poorer dental health and higher dental fear, it is likely that we may have enrolled a sample enriched with better dental health, potentially introducing selection bias.
Limitations should be considered and acknowledged. First, the small number of study participants limited our statistical power. Secondly, there was a lack of correction for magnification errors during the radiographic imaging. In addition, not “prestaining” the subjects’ dentition with Red Cote tablets prior to checking for plaque may have led to a less stringent detection of plaque. Most studies have utilized plaque staining per World Health Organization guidelines.30 Finally, some data were obtained via self-report questionnaires which has its own inherent limitations, especially in adolescents.
There is the concern that a dentist may see an under-weight patient who has normal dental health and may over-look other symptomatology associated with AN, especially the classic dental signs of an eating disorder (e.g., increased incidence of caries, tooth enamel erosion). In the absence of these findings, dental professionals (both dentists and hygienists) could be falsely reassured regarding the bone health of AN patients due to a lack of awareness regarding objective findings associated with eating disorders, especially those of the restrictive subtype. This study highlights dental and periodontal findings in young patients with restrictive AN.
CONCLUSIONS
The findings of this study will potentially help dentists and dental hygienists who perform examinations on their adolescent and young adult patients become more cognizant of the wide variability of objective findings in those afflicted with an eating disorder. Dentists are a valuable resource in that they have access to clinical and radiographic information that enables them to play a critical role in the initial screening of patients for eating disorders, osteoporosis, and other conditions that affect the oral soft and hard tissues.
While not statistically significant in this small study, we noted a positive trend in the relationship between BMD via DXA and MCW obtained through dental panoramic images. A larger study and development of a normative database will be necessary to evaluate definitively whether panoramic radiographs can be utilized as a screening test for proper referral and to predict the overall risk of osteoporosis and its associated sequelae in adolescents and young women with AN.
ACKNOWLEDGMENTS
We gratefully acknowledge our patients and their families for their participation, and Katie Clegg, Jamie Nydegger, Jessica Sexton, Dr. Cheri Cox and Julie Ringelheim for excellent technical assistance. Supported by grant RO1 HD043869 from the Eunice Kennedy Shriver NICHD; NIH grant MO1-RR-2172 to the Children’s Hospital Boston General Clinical Research Center; the Department of Defense, US Army Bone Health and Military Readiness Program; and Project 5-T71-MC-00009-14 from the Maternal and Child Health Bureau.
REFERENCES
- 1.http://www.niams.nih.gov/Health_Info/bone/default.asp
- 2.Wentz E, Mellstrom D, Gillberg IC, Gillberg C, Rastam M. Brief report: Decreased bone mineral density as a long-term complication of teenage-onset anorexia nervosa. Eur Eat Disord Rev. 2007;15:290–5. doi: 10.1002/erv.795. [DOI] [PubMed] [Google Scholar]
- 3.Carmo I, Mascarenhas M, Macedo A, et al. A study of bone density change in patients with anorexia nervosa. Eur Eat Disord Rev. 2007;15:457–462. doi: 10.1002/erv.812. [DOI] [PubMed] [Google Scholar]
- 4.DeBate RD, Tedesco LA, Kerschbaum WE. Knowledge of oral and physical manifestations of anorexia and bulimia nervosa among dentists and dental hygienists. J Dent Educ. 2005;69:346–354. [PubMed] [Google Scholar]
- 5.Robb ND, Smith BG. Anorexia and bulimia nervosa (the eating disorders): conditions of interest to the dental practitioner. J Dent. 1996;24:7–16. doi: 10.1016/0300-5712(95)00002-x. [DOI] [PubMed] [Google Scholar]
- 6.Montecchi PP, Custureri V, Polimeni A, et al. Oral manifestations in a group of young patients with anorexia nervosa. Eat Weight Disord. 2003;8:164–7. doi: 10.1007/BF03325007. [DOI] [PubMed] [Google Scholar]
- 7.Rytomaa I, Jarvinen V, Kanerva R, Heinonen OP. Bulimia and tooth erosion. Acta Odontol Scand. 1998;56:36–40. doi: 10.1080/000163598423045. [DOI] [PubMed] [Google Scholar]
- 8.Jeffcoat MK, Lewis CE, Reddy MS, Wang CY, Redford M. Postmenopausal bone loss and its relationship to oral bone loss. Periodontol. 2000;23:94–102. doi: 10.1034/j.1600-0757.2000.2230109.x. [DOI] [PubMed] [Google Scholar]
- 9.Mohammad AR, Brunsvold M, Bauer R. The strength of association between systemic postmenopausal osteoporosis and periodontal disease. Int J Prosthodont. 1996;9:479–483. [PubMed] [Google Scholar]
- 10.Gordon CM, Goodman E, Emans SJ, et al. Physiologic regulators of bone turnover in young women with anorexia nervosa. J Pediatr. 2002;141:64–70. doi: 10.1067/mpd.2002.125003. [DOI] [PubMed] [Google Scholar]
- 11.Legroux-Gerot I, Vignau J, D’Herbomez M, et al. Evaluation of bone loss and its mechanisms in anorexia nervosa. Calcif Tissue Int. 2007;81:174–182. doi: 10.1007/s00223-007-9038-9. [DOI] [PubMed] [Google Scholar]
- 12.Wentz E, Mellstrom D, Gillberg C, Sundh V, Gillberg IC, Rastam M. Bone density 11 years after anorexia nervosa onset in a controlled study of 39 cases. Int J Eat Disord. 2003;34:314–8. doi: 10.1002/eat.10192. [DOI] [PubMed] [Google Scholar]
- 13.Karayianni K, Horner K, Mitsea A, et al. Accuracy in osteoporosis diagnosis of a combination of mandibular cortical width measurement on dental panoramic radiographs and a clinical risk index (OSIRIS): the OSTEODENT project. Bone. 2007;40:223–9. doi: 10.1016/j.bone.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 14.Koch A, Landesberg R, Siris E, Tsay R, Eisig S, Michaeli D. Identification of individuals at high risk for osteoporosis using digital panoramic analysis: A pilot study. Journal of the William Jarvie Society. 2006;49:53–4. [Google Scholar]
- 15.Jonasson G, Bankvall G, Kiliaridis S. Estimation of skeletal bone mineral density by means of the trabecular pattern of the alveolar bone, its interdental thickness, and the bone mass of the mandible. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:346–352. doi: 10.1067/moe.2001.116494. [DOI] [PubMed] [Google Scholar]
- 16.Southard KA, Southard TE, Schlechte JA, Meis PA. The relationship between the density of the alveolar processes and that of post-cranial bone. J Dent Res. 2000;79:964–9. doi: 10.1177/00220345000790041201. [DOI] [PubMed] [Google Scholar]
- 17.Greene JC, Vermillion JR. The simplified oral hygiene index. JADA. 1964;68:7–13. doi: 10.14219/jada.archive.1964.0034. [DOI] [PubMed] [Google Scholar]
- 18.Loe H. The Gingival Index, the Plaque Index, and the Retention Index. J Periodontol. 1967;38:610–6. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 19.Dervis E. Oral implications of osteoporosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:349–356. doi: 10.1016/j.tripleo.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Horner K, Devlin H, Harvey L. Detecting patients with low skeletal bone mass. J Dent. 2002;30:171–5. doi: 10.1016/s0300-5712(02)00010-6. [DOI] [PubMed] [Google Scholar]
- 21.Devlin H, Allen PD, et al. Automated osteoporosis risk assessment by dentists: A new pathway to diagnosis. Bone. 2006;40:835–842. doi: 10.1016/j.bone.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 22.Klemetti E, Kolmakov S, Kroger H. Pantomography in assessment of the osteoporosis risk group. Scand J Dent Res. 1994;102:68. doi: 10.1111/j.1600-0722.1994.tb01156.x. [DOI] [PubMed] [Google Scholar]
- 23.Klemetti E, Kolmakow S. Morphology of the mandibular cortex on panoramic radiographs as an indicator of bone quality. Dentomaxillofac Radiol. 1997;26:22–5. doi: 10.1038/sj.dmfr.4600203. [DOI] [PubMed] [Google Scholar]
- 24.Devlin H, Karayianni K, et al. Diagnosing osteoporosis by using dental panoramic radiographs: The OSTEODENT project. Oral Surg Oral Med Oral Pathol Oral Radiol Endod Dec. 2007;104:821–828. doi: 10.1016/j.tripleo.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 25.Taguchi A, Ohtsuka M, Nakamoto T, Tanimoto K. Screening for osteoporosis by dental panoramic radiographs. Clin. Calcium. 2006;16:67–73. [PubMed] [Google Scholar]
- 26.Devlin CV, Horner K, Devlin H. Variability in measurement of radiomorphometric indices by general dental practitioners. Dentomaxillofac. Radiol. 2001;30:120–5. doi: 10.1038/sj/dmfr/4600594. [DOI] [PubMed] [Google Scholar]
- 27.Krall EA, Wehler C, Garcia RI, Harris SS, Dawson-Hughes B. Calcium and vitamin D supplements reduce tooth loss in the elderly. Am J Med. 2001;111:452–6. doi: 10.1016/s0002-9343(01)00899-3. [DOI] [PubMed] [Google Scholar]
- 28.Haagensen AL, Feldman HA, Ringelheim J, Gordon CM. Low prevalence of vitamin D deficiency among adolescents with anorexia nervosa. Osteoporos Int. 2008;19:289–94. doi: 10.1007/s00198-007-0476-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willumsen T, Graugaard PK. Dental fear, regularity of dental attendance and subjective evaluation of dental erosion in women with eating disorders. Eur J Oral Sci. 2005;113:297–302. doi: 10.1111/j.1600-0722.2005.00227.x. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization . Oral health surveys: basic methods. 4th edition WHO; Geneva: 1997. [Google Scholar]


