Abstract
Positron emission tomography (PET) imaging with radiolabeled monoclonal antibodies has always been a dynamic area in molecular imaging. With decay half-life (3.3 d) well matched to the circulation half-lives of antibodies (usually on the order of days), 89Zr has been extensively studied over the last decade. This review article will give a brief overview on 89Zr isotope production, the radiochemistry generally used for 89Zr-labeling, and the PET tracers that have been developed using 89Zr. To date, 89Zr-based PET imaging has been investigated for a wide variety of cancer-related targets, which include human epidermal growth factor receptor 2, epidermal growth factor receptor, prostate-specific membrane antigen, splice variant v6 of CD44, vascular endothelial growth factor, carbonic anhydrase IX, insulin-like growth factor 1 receptor, among others. With well-developed radiochemistry, commercial availability of chelating agents for 89Zr labeling, increasingly widely available isotope supply, as well as successful proof-of-principle in pilot human studies, it is expected that PET imaging with 89Zr-based tracers will be a constantly evolving and highly vibrant field in the near future.
Keywords: Zirconium-89 (89Zr), positron emission tomography (PET), molecular imaging, radioimmunoPET, monoclonal antibody (mAb), cancer
INTRODUCTION
Radiolabeled antibodies have been used in the clinic for diagnostic and therapeutic purposes for over 40 years [1-3]. The use of radiolabeled antibodies as imaging probes to visualize tumors has always been a vibrant area in molecular imaging. In particular, positron emission tomography (PET) with radiolabeled monoclonal antibodies (mAbs), sometimes termed as “immunoPET”, is an attractive method for non-invasive tumor detection since this strategy combines the high sensitivity of PET with the high antigen specificity of mAbs. If the mAb is used for systemic therapy of cancer (either as a single agent or in combination with other anti-cancer drugs), immunoPET with the radiolabeled mAb can be used for tumor detection as well as treatment planning.
Among the more than 100 PET isotopes, the majority are not suitable for PET imaging applications because of undesirable decay half-life, poor availability, high production cost, underdeveloped radiochemistry, among others. Within the commonly used PET isotopes, only a few of them are suitable for antibody labeling since immunoPET requires that the PET isotope can be attached to the mAb with good in vivo stability and the decay half-life of the isotope should match the pharmacokinetics of the mAb. Currently, some of the commonly used PET isotopes for antibody labeling include 89Zr (t1/2 = 3.3 d), 124I (t1/2 = 4.2 d), 64Cu (t1/2 = 12.7 h), 86Y (t1/2 = 14.7 h), among others. This review article will summarize the current status of PET tracers based on 89Zr.
Zirconium is a transition metal in Group IVB of the periodic table. 89Zr, which decays by positron emission (23%) and electron capture (77%) to the stable isotope 89Y, has attractive characteristics for immunoPET applications. The physical decay half-life of 89Zr is 3.3 days, which is compatible with the time needed to achieve optimal tumor-to-background ratios for intact mAbs (typically a few days). The Emax of 897 keV and Eave of 396.9 keV for its positron emission, lower than that of 124I (which has a similar decay half-life), could result in PET images with good spatial resolution. Ideally, a positron emitter should not have prompt photons with energy near 511 keV in order to achieve better quantitative accuracy. The spontaneous gamma decay of 89Zr gives rise to 908.97 keV photons (99% abundance), which can be easily gated off by setting the energy window of a PET scanner.
There are several advantages of using 89Zr over other PET isotopes with comparable decay half-lives such as 124I. For example, there is no need to use highly enriched target and the energy required for 89Zr production is lower [4]. 124I linked directly to mAbs via tyrosine residues can be subjected to dehalogenation in vivo, which can lead to significant radioactivity uptake in non-targeted organs unrelated to mAb-antigen binding [5]. In addition, if the mAb undergoes internalization after binding to its target, lysosomal proteolysis of the mAb generally results in rapid loss of 124I from the cell but accumulation of radiometals such as 89Zr [6]. The major disadvantages of 89Zr include limited availability and the high energy gamma emission at 908.97 keV, which may limit the radioactive dose that can be administered into patients.
PRODUCTION OF 89Zr
89Zr can be produced via either 89Y(p,n)89Zr or 89Y(d,2n)89Zr reaction [7-12]. The chemistry for separating 89Zr from the target is the same independent of the production method. In a typical 89Y(p,n)89Zr reaction, proton beam with 14-14.5 MeV energy is used to bombard inexpensive natural yttrium foil mounted onto an aluminum/copper disc for 2-3 h (65-80 μA) and isotope separation is done via ion exchange chromatography or solvent extraction after bombardment. With optimal irradiation time, this method can lead to < 0.2% impurity level of 88Zr [4]. Alternatively, the 89Y(d,2n)89Zr reaction can be used to minimize the level of 88Zr. Yttrium pellet is irradiated with a 16 MeV deuteron beam and 89Zr can be separated from the target by ion exchange chromatography with an overall yield of ~80% [12]. The impurity level of 88Zr can be kept as low as 0.008% at the end of bombardment. Although the yield of the 89Y(d,2n)89Zr reaction can be improved by using higher energy deuteron beam, the impurity level of 88Zr may also be higher. The radionuclidic purity of the product can be determined by gamma spectroscopy, where a germanium detector is coupled to a multichannel analyzer. Currently, high specific activity 89Zr (typically in the form of 89Zr-chloride) can be produced by several facilities around the world, which can be shipped to remote sites [13].
RADIOLABELING OF 89Zr
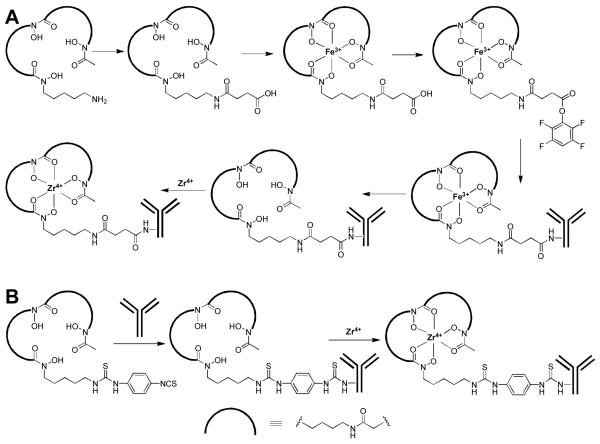
89Zr labeling of antibodies can be achieved through various types of chelators, primarily desferrioxamine B (Df) which can form a stable chelate with 89Zr through the 3 hydroxamate groups [14]. A representative route for 89Zr-labeling is shown in Fig. (1A). Generally, mAbs are conjugated with a bifunctional derivative of Df via an amide linkage for subsequent labeling with 89Zr [15]. The hydroxamate groups within Df need to be temporarily blocked with Fe(III) before mAb conjugation. Subsequently, Fe(III) is removed by transchelation to ethylenediaminetetraacetic acid (EDTA) before the conjugate is exposed to 89Zr. The choice of Df as the chelator for 89Zr is attractive because it has been safely used in the clinic for many years. In the past and ongoing clinical studies, neither adverse reactions nor significant changes in blood and urine values were observed after injection of Df-containing conjugates. Moreover, no antibody responses directed against the Df chelate were observed, indicating that its immunogenicity is quite low [16].
Fig. (1).
Representative radiochemistry for 89Zr-labeling. (A) The 6-step route. (B) The 2-step method.
Despite the success of this strategy, the multi-step procedure is quite complicated and time-consuming which makes it challenging to produce 89Zr-labeled mAbs in compliance to the current Good Manufacturing Practice (cGMP) for clinical investigations. Recently, a new bifunctional chelate was reported for 89Zr labeling: p-isothiocyanatobenzyl-desferrioxamine B (Df-Bz-NCS) [17]. As shown in Fig. (1B), labeling of 89Zr has been significantly simplified into a 2-step procedure (the initial strategy has 6 steps) [18]. Coupling of Df-Bz-NCS to mAbs was very efficient and it has been reported that a reproducible chelate:mAb ratio of 1.5:1 could be obtained using only a three-fold molar excess of Df-Bz-NCS [17]. Such a low chelate:mAb ratio can avoid alteration of the pharmacokinetics or immunoreactivity of the mAb. Comparing the 2 strategies, the rate of 89Zr complexation was very similar, indicating that different chemical linkages (e.g. S instead of O in the side chain which might be involved in 89Zr4+ coordination) have little influence on the radiochemistry. At the optimal pH (7.0), almost quantitative complexation was achieved after 30 min incubation at room temperature, with no impairment of the immunoreactivity of the mAbs.
The need for protection of radioimmunoconjugates against radiation damage during storage has been demonstrated in various studies [9,19]. The presence of the antioxidant ascorbic acid during storage of high-dose 90Y/131I-labeled mAbs has been proven to be beneficial. However, ascorbic acid cannot be used for storage of 89Zr-labeled mAbs since it can cause detachment of 89Zr from Df by reducing Zr4+ to Zr2+ [9]. It was suggested that 89Zr-Df-Bz-NCS-mAb can be best stored at 4 °C in sodium acetate buffer in presence of the antioxidant gentisic acid. It is worth noting that under certain storage conditions, the 89Zr-Df-Bz-NCS-mAb conjugate is slightly less stable than the conjugate obtained from the 6-step strategy. In particular, the presence of Cl− in the storage buffer can impair the integrity of the radioimmunoconjugates. This is likely due to radiation-induced formation of OCl− which can react with the thiol group of the enolised thiourea unit, which in turn can lead to a series of events such as coupling reactions and cleavage of methionyl peptide bonds.
Besides the two abovementioned methods, the reaction between N-(S-acetyl)thioacetyl-Df (SATA-Df) and maleimide-conjugated mAb was also investigated [20,21]. However, the resulting conjugates were found to be unstable in human serum at 37°C [9]. Recently, several thiol-reactive Df ligands were tested for labeling mAbs in a site-specific manner [22], using engineered mAbs containing selectively positioned cysteine residues. In this study, the amino group of Df was acylated by various chemicals to obtain thiol-reactive reagents bromoacetyl-desferrioxamine (Df-Bac), iodoacetyl-desferrioxamine (Df-Iac) and maleimidocyclohexyl-desferrioxamine (Df-Chx-Mal), respectively. Df-Bac and Df-Iac alkylated the thiol groups of thio-trastuzumab by nucleophilic substitution, while Df-Chx-Mal led to the conjugate Df-Chx-Mal-thio-trastuzumab. Each Df-modified thio-trastuzumab conjugate was labeled with 89Zr in high yield and the resulting tracers exhibited good tumor-to-blood ratio in a breast cancer model.
PET IMAGING WITH 89Zr
To date, a wide variety of mAbs have been labeled with 89Zr and several of these 89Zr-labeled mAbs have entered clinical investigation with promising results. With wider availability of 89Zr and simpler chemistry for radiolabeling, it is expected that more and more PET tracers based on 89Zr will be developed in the coming years.
Imaging HER2
Human epidermal growth factor receptor 2 (HER2), a member of the ErbB tyrosine kinase receptor family, is involved in cell survival, differentiation, proliferation, metastasis, and angiogenesis [23-25]. Overexpression of HER2 has been found in a wide variety of human cancers. Currently, HER2 overexpression is determined using immunohistochemistry and fluorescence in situ hybridization (FISH) at the time of diagnosis of the primary tumor. Non-invasive imaging of HER2 expression and localization of HER2-overexpressing tumor lesions using immunoPET could be a practical method in the clinic to guide HER2-targeted therapy.
Trastuzumab (i.e. Herceptin), an anti-HER2 mAb that was approved by the Food and Drug Administration (FDA) to treat HER2-positive breast cancer, has been extensively investigated for imaging applications over the last decade [23,24]. In one study, clinical-grade 89Zr-trastuzumab was developed for potential clinical immunoPET imaging applications [26]. In nude mice bearing HER2-positive tumors, 89Zr-trastuzumab exhibited excellent tumor uptake (~30%ID/g) with high tumor-to-nontumor ratios. The biodistribution pattern of 89Zr-trastuzumab was similar to that of 111In-trastuzumab, which was reported in a previous clinical study [27]. In addition, 89Zr-trastuzumab was very stable in both buffer solutions and human serum.
The ability of 89Zr-trastuzumab PET to quantify the alternations in HER2 expression level after treatment with a heat shock protein 90 (Hsp90) inhibitor has been investigated [28]. PET imaging revealed significant decrease of tracer uptake in the tumor, indicating that 89Zr-trastuzumab PET can be employed for non-invasive quantification of HER2 down-regulation after treatment. Similar findings were also observed in a separate study using a differently labeled 89Zr-trastuzumab and a different Hsp90 inhibitor [29]. Recently, site-specific labeling of engineered trastuzumab (through cysteine residues) with 89Zr was reported [22]. The resulting tracers were stable in serum and showed PET imaging properties comparable to conventionally prepared 89Zr-trastuzumab, where labeling was achieved through the lysine residues.
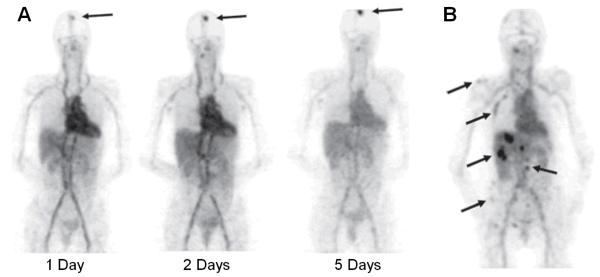
First-in-human study of 89Zr-labeled trastuzumab for PET imaging of HER2-positive lesions has been reported [30]. The tracer showed excellent tumor uptake which allowed detection of most of the known lesions and, more importantly, some lesions that had not been detected earlier (Fig. (2)). Interestingly, the dose of trastuzumab for optimal PET imaging performance was chosen to be 37 MBq of 89Zr-trastuzumab in a total of 50 mg protein for trastuzumab-naive patients or 10 mg protein for patients that are already on trastuzumab treatment. The exact mechanisms underlying such dose-dependent pharmacokinetics of 89Zr-trastuzumab are not known. One possible explanation is that it involves rapid but saturable elimination of low doses from the circulation during the first elimination phase, which has a half-life of ~4 days. Another mechanism that may play a role in increasing 89Zr-trastuzumab clearance in trastuzumab-naive patients is the presence of high plasma levels of extracellular domains shed by HER2. After trastuzumab (or 89Zr-trastuzumab) binds to these extracellular domains, the resulting complex is cleared by the liver and excreted into the intestines.
Fig. (2).
PET imaging of HER2 expression in patients with 89Zr-trastuzumab. (A) 89Zr-trastuzumab PET scans of a patient already on trastuzumab treatment at different time points post-injection revealed an increase over time in the tumor-to-nontumor ratio of tracer uptake. Arrow indicates 89Zr-trastuzumab uptake in the only lesion. (B) 89Zr-trastuzumab PET of a patient with liver and bone metastases at 5 days post-injection. A number of lesions are indicated by arrows. Adapted from [30].
Recently, another study indicated that trastuzumab pharmacokinetics and organ distribution can also be heavily affected by an extensive tumor load [31]. Therefore, a study with a more patient-tailored trastuzumab dosing schedule on the basis of tumor volume in addition to bodyweight should be considered in the future. However, one needs to bear in mind that such dose-dependent pharmacokinetics may not be applicable to 89Zr-labeled antibodies which bind to other cancer-related targets.
Imaging EGFR
The epidermal growth factor receptor (EGFR), another member of the ErbB family, is a 170 kDa protein which plays a critical role in tumor cell proliferation, differentiation, and survival [23,32]. EGFR overexpression has been associated with a number of cancers such as breast carcinoma, lung cancer, bladder cancer, and colon carcinoma [25,33]. In addition, EGFR expression is often associated with more aggressive tumors, poor prognosis, and resistance to treatment with cytotoxic agents. Therefore, EGFR is one of the most extensively studied targets in oncology and many mAbs have been developed against EGFR for cancer therapy [34].
Cetuximab (Erbitux, ImClone System Inc.) is a chimeric IgG1 mAb that can block EGFR activation by binding to the ligand-binding domain, which induces internalization of EGFR thereby preventing downstream signaling [35]. 89Zr-labeled cetuximab has been investigated in several preclinical studies [36,37]. In one early report, 89Zr-cetuximab PET was used as a scouting procedure before radioimmunotherapy (RIT) to confirm tumor targeting and allow estimation of radiation dose delivery to tumors and normal tissues [36]. It was concluded that 89Zr-cetuximab could serve as a surrogate for scouting the biodistribution of 90Y-cetuximab and 177Lu-cetuximab in tumor-bearing mice.
In another study, disparity between in vivo EGFR expression level and 89Zr-cetuximab uptake in the tumors was observed in mouse models, suggesting that additional pharmacokinetic or pharmacodynamic mechanisms may influence the tumor uptake of cetuximab as well as the therapeutic efficacy of this agent [37]. It was suggested that these additional mechanisms may explain why receptor expression levels alone are not sufficient to predict patient response to anti-EGFR therapies. Various studies have revealed that a majority of tumors responding to EGFR kinase inhibitors harbor activating mutations in the EGFR kinase domain [38]. Therefore, imaging of EGFR mutant expression might be more useful in selecting the right patient population for personalized treatment as well as predicting the therapeutic response [39].
Imaging PSMA
The prostate-specific membrane antigen (PSMA), a 100 kDa type II transmembrane glycoprotein, is one of the best characterized targets in oncology [40]. PSMA expression levels have been shown to exhibit a positive correlation with increased tumor progression, development of castration resistance, and/or resistance to hormone-based therapies. Although PSMA expression has also been detected in a limited range of normal tissues including benign prostatic epithelium, renal proximal tubule, small bowel, and the brain, these normal tissue sites only express PSMA at levels 2-3 orders of magnitude lower than that observed in more than 95% of clinical prostate cancer specimens [41]. In addition, expression of PSMA in normal tissues is highly polarized to the apical or luminal sides of various tissues which are not readily accessible to circulating mAbs, thus making anti-PSMA mAbs functionally tumor-specific.
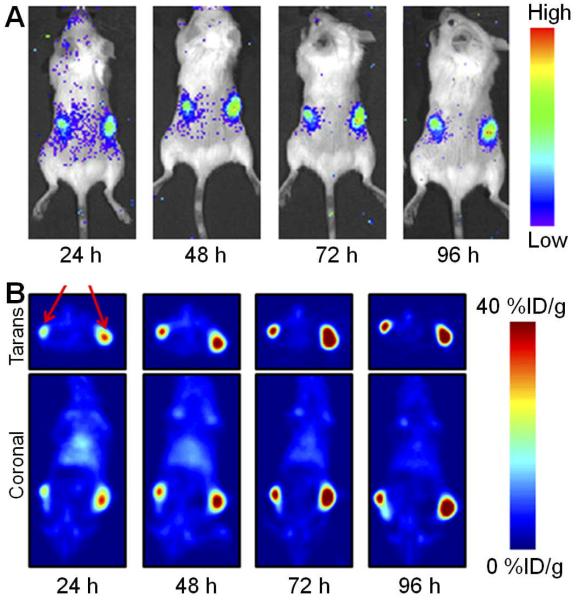
In a recent study, a 89Zr-labeled anti-PSMA mAb, J591, was reported for immunoPET and quantification of PSMA expression in vivo [42]. PET imaging of male athymic nude mice bearing subcutaneous LNCaP (PSMA-positive) or PC-3 (PSMA-negative) tumors was conducted and 89Zr-J591 provided excellent image contrast for delineating the LNCaP xenografts at a few days after tracer administration, which may potentially be used in the clinic to non-invasively identify and quantify PSMA-positive prostate tumors in patients. Interestingly, a “multimodality” approach to image PSMA expression has also been carried out using 89Zr-J591 [43]. Taken advantage of the phenomenon known as the Cerenkov effect (the emission of light from a transparent substance when a charged particle, such as an electron, travels through the material with a speed faster than the speed of light in that material), Cerenkov luminescence imaging (CLI) of tumors in vivo, using a small animal optical scanner, was carried out. The results obtained from optical scans correlated well with those obtained from immunoPET studies in a quantitative manner (Fig. (3)). Since CLI can be used to image radionuclides that do not emit either positrons or γ-rays and are thereby unsuitable for use with current nuclear imaging modalities, it may serve as a potential new imaging modality for rapid and high-throughput screening of radiopharmaceuticals.
Fig. (3).
Multimodality imaging of PSMA with 89Zr-J591. (A) Cerenkov luminescence imaging of tumor-bearing mice injected with 89Zr-J591 in a small animal optical scanner. (B) Corresponding coronal and transverse immunoPET images of the mouse. The signal originates from the LNCaP tumors (PSMA-positive). Mouse bearing two different sized tumors of the same origin was used to demonstrate that differential uptake between the small and large tumors can be discerned by Cerenkov luminescence imaging. Adapted from [43].
Imaging CD44v6
CD44 is a cell-surface glycoprotein involved in a wide variety of biological processes such as lymphocyte-endothelial cell interactions, adhesion of cells to extracellular matrix proteins, lymphohematopoiesis, homotypic adhesion, T cell activation/adherence, cytokine release, metastasis formation, and lateral movement of cells [44,45]. The CD44 protein family is composed of several isoforms, encoded by standard exons and many alternatively spliced variant exons, which are expressed in a tissue-specific manner. The splice variant v6 of CD44 (denoted as “CD44v6”) has been implicated in tumorigenesis, tumor cell invasion, and metastasis [46,47]. Although CD44v6 is expressed only in a subset of normal epithelial tissues (e.g. thyroid and prostate glands), homogeneous expression of CD44v6 has been found in various solid tumor types such as squamous cell carcinoma of the head and neck (HNSCC), lung, skin, esophageal, and cervical cancer [48]. In addition, heterogeneous expression of CD44v6 has been found in adenocarcinomas of the breast, lung, colon, pancreas, and stomach.
In order to avoid human antimouse immune responses, the chimeric IgG1 derivative of a murine mAb (U36 which binds to CD44v6) was constructed. 186Re-labeled chimeric mAb (cmAb), termed as “cU36”, was then tested in phase I trials which showed promising anti-tumor effects in patients with HNSCC [49,50]. Subsequently, 89Zr-labeled cU36 was investigated in xenograft-bearing mice [9]. Further, 89Zr-cU36 was also tested for the first time in HNSCC patients who were at high risk of having neck lymph node metastases [16]. Serial PET scans after injection of 2.0 mCi of 89Zr-cU36 successfully detected the primary head and neck tumors as well as metastases in the neck, with sensitivity at least as good as computed tomography (CT) or magnetic resonance imaging (MRI). However, a few patients developed an antibody response directed against cU36 although no evidence was found for antibody reactions against the chelate. In addition, 89Zr-cU36 was not able to detect micrometastases in patients, similar to the findings of a previous biodistribution study with the mAb U36 [51]. In another study of cU36, it was reported that 89Zr-labeled mAbs are more suitable for scouting of therapeutic doses of 90Y-labeled mAbs while 124I-labeled mAbs are better for scouting of 131I- and 186Re-labeled mAbs [52].
Radiation dosimetry estimation in patients injected with 89Zr-cU36 revealed that 89Zr-cU36 was safe and well-tolerated in all patients [53]. Although the mean radiation dose for patients in this study was estimated to be around 40 mSv, which would limit repeated application of 89Zr immunoPET, the constant improvement of new clinical PET/CT scanners may allow the acquisition of better-quality PET images with a lower injected radioactivity dose. In a recent study, different 89Zr-labeling chemistry (i.e. the 2-step and 6-step methods; Fig. (1)) was compared for cU36 in a mouse model [17]. High level accumulation of 89Zr-labeled cU36 in the tumors and low level of tracer uptake in normal tissues were observed for both tracers.
Imaging VEGF
Vascular endothelial growth factor (VEGF), a potent mitogen in embryonic and somatic angiogenesis, plays a pivotal role in both normal vascular tissue development and many disease processes such as tumor development and metastasis [54]. Overexpression of VEGF and/or VEGF receptors (VEGFRs) has been implicated as a marker of poor prognosis in various clinical studies [55]. The humanized anti-VEGF mAb, bevacizumab (i.e. Avastin), can block VEGF-induced tumor angiogenesis and was approved by the FDA to treat multiple metastatic cancers [56].
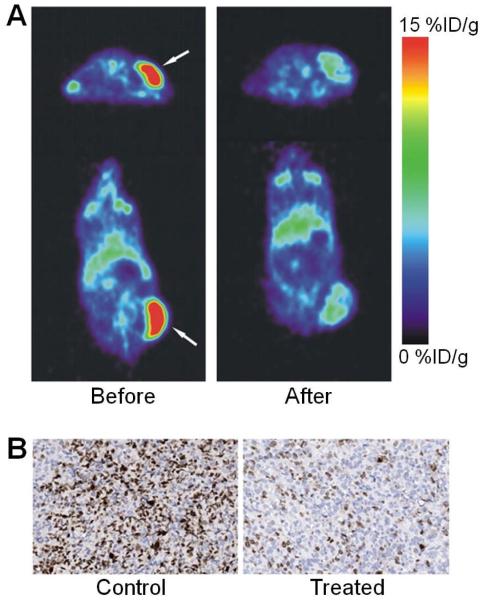
In 2007, 89Zr-labeled bevacizumab was investigated in nude mice bearing human ovary cancer SKOV-3 xenograft tumors [57]. Comparing 89Zr-bevacizumab and 89Zr-IgG, which served as a control, PET showed uptake of 89Zr-bevacizumab in well-perfused organs at 24 h post-injection and clear tumor localization after 72 h. The uptake of 89Zr-bevacizumab was significantly higher than that of 89Zr-IgG, suggesting target specificity of the tracer. Recently, 89Zr-bevacizumab was also successfully used to detect the early anti-angiogenic tumor response to treatment with a Hsp90 inhibitor (Fig. (4)), indicating that 89Zr-bevacizumab PET can be a sensitive and non-invasive technique for monitoring the anti-tumor effect [58].
Fig. (4).
PET imaging of VEGF expression. (A) Transversal (top) and coronal (bottom) PET images of 89Zr-bevacizumab in xenograft mice before (left) and after (right) treatment with a Hsp90 inhibitor. Arrows indicate the tumor. (B) Ki67 staining of the tumor tissue corroborated the PET results. Adapted from [58].
Imaging Other Targets
Besides the abovementioned targets, several other proteins have also been investigated using 89Zr-based PET. Hypoxic tumor cells are resistant to radiotherapy and various chemotherapeutic agents. Carbonic anhydrase IX (CAIX), an endogenous hypoxia-related protein, is upregulated in many tumor types [59]. Therefore, pre-therapeutic assessment of intratumoral hypoxia may allow selection of patients for intensified treatment regimens. Recently, 89Zr-labeled cG250-F(ab’)2, an anti-CAIX antibody fragment, was shown to allow visualization of tumor hypoxia with PET in a xenograft tumor model, which correlated spatially to the microscopic distribution of CAIX-expressing cells [60]. This study suggested a potential role of 89Zr-cG250-F(ab’)2 for non-invasive imaging of hypoxia in head and neck carcinomas, which deserves further exploration in the future.
In one report, expression of the insulin-like growth factor 1 receptor (IGF1R) was measured by PET with 89Zr-labeled R1507, a mAb against IGF1R [61]. Excellent contrast and prominent tracer uptake in the tumor was observed in mouse bearing triple-negative breast cancer tumors, which may enable future patient selection for IGF1R-targeted therapy in the clinic since currently there is no effective therapeutic regimes for this sub-population of breast cancer patients.
Single-walled carbon nanotubes (SWCNTs) have unique properties which make them suitable for applications in a wide variety of imaging modalities, such as MRI, near-infrared fluorescence, Raman spectroscopy, photoacoustic tomography, and radionuclide-based imaging [62,63]. Recently, SWCNTs were conjugated to E4G10, an antibody specifically binds to the monomeric vascular endothelial cadherin (VE-cad) epitope expressed in angiogenic tumor vessels, and labeled with 89Zr [64]. Dynamic and longitudinal PET imaging of this agent in LS174T human colorectal tumor-bearing mice demonstrated rapid blood clearance and target-specific tumor accumulation. Due to the large surface areas of SWCNTs, a single construct could be designed to incorporate both imaging and therapeutic agents onto the same platform. In addition, since VE-cad is expressed by the tumor vasculature, it was suggested that a single agent may potentially be employed to image or treat a variety of different tumors types.
CONCLUSIONS
Over the last several years, PET imaging with 89Zr-based agents has been a highly dynamic research area. To date, the majority of agents that have been labeled with 89Zr are mAbs, because the decay half-life of 89Zr matches very well with the biological half-life of antibodies. While this manuscript was in preparation, PET imaging of integrin αvβ3 expression using 89Zr-labeled arginine-glycine-aspartic acid (RGD) peptides were reported [65]. Although efficient radiolabeling of peptides with 89Zr was achieved and the tracer gave good PET images in xenograft models, it was concluded that labeling peptides with 89Zr is not optimal due to the relatively rapid clearance of peptide tracers from the tumor region, as well as the increased bone uptake of transchelated 89Zr over time. Therefore, it appears that the most promising applications of 89Zr are for the labeling and PET imaging of long-circulating agents such as mAbs and certain nanoparticles.
Currently, labeling of 89Zr is primarily through desferrioxamine B which serves as the chelator. Based on the available literature data, the 89Zr-Df conjugate is quite stable for in vivo applications. Therefore, the key to in vivo stability of 89Zr-based tracers lies in the linkage between Df and the antibody or the nanoparticle. Although site-specific labeling of 89Zr will not compromise the immunoreactivity of the resulting antibody conjugate, while labeling through the lysine side chains of mAbs may (even though the number of Df groups per antibody is quite low), the requirement of protein engineering expertise will limit the wide-spread adoption of such site-specific labeling strategy.
With significantly simplified radiochemistry (i.e. the 2-step method), commercially available chelating agents for 89Zr labeling, increasingly widely available isotope supply, as well as successful proof-of-principle in pilot human studies, it is expected that PET imaging with 89Zr-based tracers will be a constantly evolving and highly vibrant field for the next decade. Much further efforts are needed to accelerate the translation of more promising 89Zr-based tracers into clinical use.
ACKNOWLEDGEMENTS
This work is supported, in part, by the Wisconsin Partnership Program, the University of Wisconsin Carbone Cancer Center, a Susan G. Komen Postdoctoral Fellowship, and a DOD PCRP IDEA Award.
REFERENCES
- [1].Goldenberg DM, Sharkey RM. Advances in cancer therapy with radiolabeled monoclonal antibodies. Q. J. Nucl. Med. Mol. Imaging. 2006;50:248–264. [PubMed] [Google Scholar]
- [2].Goldenberg DM, Sharkey RM. Novel radiolabeled antibody conjugates. Oncogene. 2007;26:3734–3744. doi: 10.1038/sj.onc.1210373. [DOI] [PubMed] [Google Scholar]
- [3].McCardle RJ, Harper PV, Spar IL, Bale WF, Andros G, Jiminez F. Studies with iodine-131-labeled antibody to human fibrinogen for diagnosis and therapy of tumors. J. Nucl. Med. 1966;7:837–847. [PubMed] [Google Scholar]
- [4].Nayak TK, Brechbiel MW. Radioimmunoimaging with longer-lived positron-emitting radionuclides: potentials and challenges. Bioconjug. Chem. 2009;20:825–841. doi: 10.1021/bc800299f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Koehler L, Gagnon K, McQuarrie S, Wuest F. Iodine-124: a promising positron emitter for organic PET chemistry. Molecules. 2010;15:2686–2718. doi: 10.3390/molecules15042686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lee FT, Scott AM. Immuno-PET for tumor targeting. J. Nucl. Med. 2003;44:1282–1283. [PubMed] [Google Scholar]
- [7].Tang L. Radionuclide production and yields at Washington University School of Medicine. Q. J. Nucl. Med. Mol. Imaging. 2008;52:121–133. [PubMed] [Google Scholar]
- [8].Lewis JS, Welch MJ, Tang L. Workshop on the production, application and clinical translation of “non-standard” PET nuclides: a meeting report. Q. J. Nucl. Med. Mol. Imaging. 2008;52:101–106. [PMC free article] [PubMed] [Google Scholar]
- [9].Verel I, Visser GW, Boellaard R, Stigter-van Walsum M, Snow GB, van Dongen GA. 89Zr immuno-PET: comprehensive procedures for the production of 89Zr-labeled monoclonal antibodies. J. Nucl. Med. 2003;44:1271–1281. [PubMed] [Google Scholar]
- [10].Pagani M, Stone-Elander S, Larsson SA. Alternative positron emission tomography with non-conventional positron emitters: effects of their physical properties on image quality and potential clinical applications. Eur. J. Nucl. Med. 1997;24:1301–1327. doi: 10.1007/s002590050156. [DOI] [PubMed] [Google Scholar]
- [11].Dejesus OT, Nickles RJ. Production and purification of 89Zr, a potential PET antibody label. Int. J. Radiat. Appl. Instrum., A, Appl. Radiat. Isot. 1990;41:789–790. [Google Scholar]
- [12].Zweit J, Downey S, Sharma HL. Production of no-carrier-added zirconium-89 for positron emission tomography. Int. J. Radiat. Appl. Instrum., A, Appl. Radiat. Isot. 1991;42:199–201. [Google Scholar]
- [13].Holland JP, Sheh Y, Lewis JS. Standardized methods for the production of high specific-activity zirconium-89. Nucl. Med. Biol. 2009;36:729–739. doi: 10.1016/j.nucmedbio.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Meijs WE, Herscheid JDM, Haisma HJ, Pinedo HM. Evaluation of desferal as a bifunctional chelating agent for labeling antibodies with Zr-89. Int. J. Radiat. Appl. Instrum., A, Appl. Radiat. Isot. 1992;43:1443–1447. doi: 10.1016/0883-2889(92)90170-j. [DOI] [PubMed] [Google Scholar]
- [15].Herscheid JD, Hoekstra A, Vos CM. N-Succinyldesferrioxamine B: a potential radiopharmaceutical for assessing renal function. Eur. J. Nucl. Med. 1984;9:508–510. doi: 10.1007/BF00263255. [DOI] [PubMed] [Google Scholar]
- [16].Borjesson PK, Jauw YW, Boellaard R, de Bree R, Comans EF, Roos JC, Castelijns JA, Vosjan MJ, Kummer JA, Leemans CR, Lammertsma AA, van Dongen GA. Performance of immuno-positron emission tomography with zirconium-89-labeled chimeric monoclonal antibody U36 in the detection of lymph node metastases in head and neck cancer patients. Clin. Cancer Res. 2006;12:2133–2140. doi: 10.1158/1078-0432.CCR-05-2137. [DOI] [PubMed] [Google Scholar]
- [17].Perk LR, Vosjan MJ, Visser GW, Budde M, Jurek P, Kiefer GE, van Dongen GA. p-Isothiocyanatobenzyl-desferrioxamine: a new bifunctional chelate for facile radiolabeling of monoclonal antibodies with zirconium-89 for immuno-PET imaging. Eur. J. Nucl. Med. Mol. Imaging. 2010;37:250–259. doi: 10.1007/s00259-009-1263-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Vosjan MJ, Perk LR, Visser GW, Budde M, Jurek P, Kiefer GE, van Dongen GA. Conjugation and radiolabeling of monoclonal antibodies with zirconium-89 for PET imaging using the bifunctional chelate p-isothiocyanatobenzyl-desferrioxamine. Nat. Protoc. 2010;5:739–743. doi: 10.1038/nprot.2010.13. [DOI] [PubMed] [Google Scholar]
- [19].Chakrabarti MC, Le N, Paik CH, De Graff WG, Carrasquillo JA. Prevention of radiolysis of monoclonal antibody during labeling. J Nucl Med. 1996;37:1384–1388. [PubMed] [Google Scholar]
- [20].Meijs WE, Haisma HJ, Klok RP, van Gog FB, Kievit E, Pinedo HM, Herscheid JD. Zirconium-labeled monoclonal antibodies and their distribution in tumor-bearing nude mice. J. Nucl. Med. 1997;38:112–118. [PubMed] [Google Scholar]
- [21].Meijs WE, Haisma HJ, Van der Schors R, Wijbrandts R, Van den Oever K, Klok RP, Pinedo HM, Herscheid JD. A facile method for the labeling of proteins with zirconium isotopes. Nucl. Med. Biol. 1996;23:439–448. doi: 10.1016/0969-8051(96)00020-0. [DOI] [PubMed] [Google Scholar]
- [22].Tinianow JN, Gill HS, Ogasawara A, Flores JE, Vanderbilt AN, Luis E, Vandlen R, Darwish M, Junutula JR, Williams SP, Marik J. Site-specifically 89Zr-labeled monoclonal antibodies for ImmunoPET. Nucl. Med. Biol. 2010;37:289–297. doi: 10.1016/j.nucmedbio.2009.11.010. [DOI] [PubMed] [Google Scholar]
- [23].Cai W, Niu G, Chen X. Multimodality imaging of the HER-kinase axis in cancer. Eur. J. Nucl. Med. Mol. Imaging. 2008;35:186–208. doi: 10.1007/s00259-007-0560-9. [DOI] [PubMed] [Google Scholar]
- [24].Niu G, Cai W, Chen X. Molecular imaging of human epidermal growth factor receptor 2 (HER-2) expression. Front. Biosci. 2008;13:790–805. doi: 10.2741/2720. [DOI] [PubMed] [Google Scholar]
- [25].Gross ME, Shazer RL, Agus DB. Targeting the HER-kinase axis in cancer. Semin. Oncol. 2004;31:9–20. doi: 10.1053/j.seminoncol.2004.01.005. [DOI] [PubMed] [Google Scholar]
- [26].Dijkers EC, Kosterink JG, Rademaker AP, Perk LR, van Dongen GA, Bart J, de Jong JR, de Vries EG, Lub-de Hooge MN. Development and characterization of clinical-grade 89Zr-trastuzumab for HER2/neu immunoPET imaging. J Nucl Med. 2009;50:974–981. doi: 10.2967/jnumed.108.060392. [DOI] [PubMed] [Google Scholar]
- [27].Perik PJ, Lub-De Hooge MN, Gietema JA, van der Graaf WT, de Korte MA, Jonkman S, Kosterink JG, van Veldhuisen DJ, Sleijfer DT, Jager PL, de Vries EG. Indium-111-labeled trastuzumab scintigraphy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J. Clin. Oncol. 2006;24:2276–2282. doi: 10.1200/JCO.2005.03.8448. [DOI] [PubMed] [Google Scholar]
- [28].Oude Munnink TH, Korte MA, Nagengast WB, Timmer-Bosscha H, Schroder CP, Jong JR, Dongen GA, Jensen MR, Quadt C, Hooge MN, Vries EG. 89Zr-trastuzumab PET visualises HER2 downregulation by the HSP90 inhibitor NVP-AUY922 in a human tumour xenograft. Eur. J. Cancer. 2010;46:678–684. doi: 10.1016/j.ejca.2009.12.009. [DOI] [PubMed] [Google Scholar]
- [29].Holland JP, Caldas-Lopes E, Divilov V, Longo VA, Taldone T, Zatorska D, Chiosis G, Lewis JS. Measuring the pharmacodynamic effects of a novel Hsp90 inhibitor on HER2/neu expression in mice using Zr-DFO-trastuzumab. PLoS One. 2010;5:e8859. doi: 10.1371/journal.pone.0008859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dijkers EC, Oude Munnink TH, Kosterink JG, Brouwers AH, Jager PL, de Jong JR, van Dongen GA, Schroder CP, Lub-de Hooge MN, de Vries EG. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin. Pharmacol. Ther. 2010;87:586–592. doi: 10.1038/clpt.2010.12. [DOI] [PubMed] [Google Scholar]
- [31].Oude Munnink TH, Dijkers EC, Netters SJ, Lub-de Hooge MN, Brouwers AH, Haasjes JG, Schroder CP, de Vries EG. Trastuzumab pharmacokinetics influenced by extent human epidermal growth factor receptor 2-positive tumor load. J. Clin. Oncol. 2010;28:e355–356. doi: 10.1200/JCO.2010.28.4604. [DOI] [PubMed] [Google Scholar]
- [32].Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon DS. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- [33].Mitsudomi T, Yatabe Y. Epidermal growth factor receptor in relation to tumor development: EGFR gene and cancer. FEBS J. 2010;277:301–308. doi: 10.1111/j.1742-4658.2009.07448.x. [DOI] [PubMed] [Google Scholar]
- [34].Herbst RS, Shin DM. Monoclonal antibodies to target epidermal growth factor receptor-positive tumors: a new paradigm for cancer therapy. Cancer. 2002;94:1593–1611. doi: 10.1002/cncr.10372. [DOI] [PubMed] [Google Scholar]
- [35].Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin. Cancer Res. 1995;1:1311–1318. [PubMed] [Google Scholar]
- [36].Perk LR, Visser GW, Vosjan MJ, Stigter-van Walsum M, Tijink BM, Leemans CR, van Dongen GA. 89Zr as a PET surrogate radioisotope for scouting biodistribution of the therapeutic radiometals 90Y and 177Lu in tumor-bearing nude mice after coupling to the internalizing antibody cetuximab. J. Nucl. Med. 2005;46:1898–1906. [PubMed] [Google Scholar]
- [37].Aerts HJ, Dubois L, Perk L, Vermaelen P, van Dongen GA, Wouters BG, Lambin P. Disparity between in vivo EGFR expression and 89Zr-labeled cetuximab uptake assessed with PET. J. Nucl. Med. 2009;50:123–131. doi: 10.2967/jnumed.108.054312. [DOI] [PubMed] [Google Scholar]
- [38].Pines G, Kostler WJ, Yarden Y. Oncogenic mutant forms of EGFR: lessons in signal transduction and targets for cancer therapy. FEBS Lett. 2010;584:2699–2706. doi: 10.1016/j.febslet.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yeh HH, Ogawa K, Balatoni J, Mukhapadhyay U, Pal A, Gonzalez-Lepera C, Shavrin A, Soghomonyan S, Flores L, 2nd, Young D, Volgin AY, Najjar AM, Krasnykh V, Tong W, Alauddin MM, Gelovani JG. Molecular imaging of active mutant L858R EGF receptor (EGFR) kinase-expressing nonsmall cell lung carcinomas using PET/CT. Proc. Natl. Acad. Sci. USA. 2011 doi: 10.1073/pnas.1010744108. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Olson WC, Heston WD, Rajasekaran AK. Clinical trials of cancer therapies targeting prostate-specific membrane antigen. Rev. Recent Clin. Trials. 2007;2:182–190. doi: 10.2174/157488707781662724. [DOI] [PubMed] [Google Scholar]
- [41].Manyak MJ. Indium-111 capromab pendetide in the management of recurrent prostate cancer. Expert Rev. Anticancer Ther. 2008;8:175–181. doi: 10.1586/14737140.8.2.175. [DOI] [PubMed] [Google Scholar]
- [42].Holland JP, Divilov V, Bander NH, Smith-Jones PM, Larson SM, Lewis JS. 89Zr-DFO-J591 for immunoPET of prostate-specific membrane antigen expression in vivo. J. Nucl. Med. 2010;51:1293–1300. doi: 10.2967/jnumed.110.076174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ruggiero A, Holland JP, Lewis JS, Grimm J. Cerenkov luminescence imaging of medical isotopes. J. Nucl. Med. 2010;51:1123–1130. doi: 10.2967/jnumed.110.076521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Webb DS, Shimizu Y, Van Seventer GA, Shaw S, Gerrard TL. LFA-3, CD44, and CD45: physiologic triggers of human monocyte TNF and IL-1 release. Science. 1990;249:1295–1297. doi: 10.1126/science.1697984. [DOI] [PubMed] [Google Scholar]
- [45].Jacobson K, O’Dell D, Holifield B, Murphy TL, August JT. Redistribution of a major cell surface glycoprotein during cell movement. J. Cell Biol. 1984;99:1613–1623. doi: 10.1083/jcb.99.5.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Nestor M, Andersson K, Lundqvist H. Characterization of 111In and 177Lu-labeled antibodies binding to CD44v6 using a novel automated radioimmunoassay. J. Mol. Recognit. 2008;21:179–183. doi: 10.1002/jmr.883. [DOI] [PubMed] [Google Scholar]
- [47].Mulder JW, Kruyt PM, Sewnath M, Oosting J, Seldenrijk CA, Weidema WF, Offerhaus GJ, Pals ST. Colorectal cancer prognosis and expression of exon-v6-containing CD44 proteins. Lancet. 1994;344:1470–1472. doi: 10.1016/s0140-6736(94)90290-9. [DOI] [PubMed] [Google Scholar]
- [48].Heider KH, Kuthan H, Stehle G, Munzert G. CD44v6: a target for antibody-based cancer therapy. Cancer Immunol. Immunother. 2004;53:567–579. doi: 10.1007/s00262-003-0494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Colnot DR, Quak JJ, Roos JC, van Lingen A, Wilhelm AJ, van Kamp GJ, Huijgens PC, Snow GB, van Dongen GA. Phase I therapy study of 186Re-labeled chimeric monoclonal antibody U36 in patients with squamous cell carcinoma of the head and neck. J. Nucl. Med. 2000;41:1999–2010. [PubMed] [Google Scholar]
- [50].Colnot DR, Ossenkoppele GJ, Roos JC, Quak JJ, de Bree R, Borjesson PK, Huijgens PC, Snow GB, van Dongen GA. Reinfusion of unprocessed, granulocyte colony-stimulating factor-stimulated whole blood allows dose escalation of 186Relabeled chimeric monoclonal antibody U36 radioimmunotherapy in a phase I dose escalation study. Clin. Cancer Res. 2002;8:3401–3406. [PubMed] [Google Scholar]
- [51].de Bree R, Roos JC, Plaizier MA, Quak JJ, van Kamp GJ, den Hollander W, Snow GB, van Dongen GA. Selection of monoclonal antibody E48 IgG or U36 IgG for adjuvant radioimmunotherapy in head and neck cancer patients. Br. J. Cancer. 1997;75:1049–1060. doi: 10.1038/bjc.1997.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Verel I, Visser GW, Boerman OC, van Eerd JE, Finn R, Boellaard R, Vosjan MJ, Stigter-van Walsum M, Snow GB, van Dongen GA. Long-lived positron emitters zirconium-89 and iodine-124 for scouting of therapeutic radioimmunoconjugates with PET. Cancer Biother. Radiopharm. 2003;18:655–661. doi: 10.1089/108497803322287745. [DOI] [PubMed] [Google Scholar]
- [53].Borjesson PK, Jauw YW, de Bree R, Roos JC, Castelijns JA, Leemans CR, van Dongen GA, Boellaard R. Radiation dosimetry of 89Zr-labeled chimeric monoclonal antibody U36 as used for immuno-PET in head and neck cancer patients. J. Nucl. Med. 2009;50:1828–1836. doi: 10.2967/jnumed.109.065862. [DOI] [PubMed] [Google Scholar]
- [54].Cai W, Chen X. Multimodality imaging of vascular endothelial growth factor and vascular endothelial growth factor receptor expression. Front. Biosci. 2007;12:4267–4279. doi: 10.2741/2386. [DOI] [PubMed] [Google Scholar]
- [55].Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat. Rev. Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- [56].Middleton G, Lapka DV. Bevacizumab (Avastin) Clin. J. Oncol. Nurs. 2004;8:666–669. doi: 10.1188/04.CJON.663-669. [DOI] [PubMed] [Google Scholar]
- [57].Nagengast WB, de Vries EG, Hospers GA, Mulder NH, de Jong JR, Hollema H, Brouwers AH, van Dongen GA, Perk LR, Lub-de Hooge MN. In vivo VEGF imaging with radiolabeled bevacizumab in a human ovarian tumor xenograft. J. Nucl. Med. 2007;48:1313–1319. doi: 10.2967/jnumed.107.041301. [DOI] [PubMed] [Google Scholar]
- [58].Nagengast WB, de Korte MA, Oude Munnink TH, Timmer-Bosscha H, den Dunnen WF, Hollema H, de Jong JR, Jensen MR, Quadt C, Garcia-Echeverria C, van Dongen GA, Lub-de Hooge MN, Schroder CP, de Vries EG. 89Zr-bevacizumab PET of early antiangiogenic tumor response to treatment with HSP90 inhibitor NVP-AUY922. J. Nucl. Med. 2010;51:761–767. doi: 10.2967/jnumed.109.071043. [DOI] [PubMed] [Google Scholar]
- [59].Swietach P, Hulikova A, Vaughan-Jones RD, Harris AL. New insights into the physiological role of carbonic anhydrase IX in tumour pH regulation. Oncogene. 2010;29:6509–6521. doi: 10.1038/onc.2010.455. [DOI] [PubMed] [Google Scholar]
- [60].Hoeben BA, Kaanders JH, Franssen GM, Troost EG, Rijken PF, Oosterwijk E, van Dongen GA, Oyen WJ, Boerman OC, Bussink J. PET of hypoxia with 89Zr-labeled cG250-F(ab’)2 in head and neck tumors. J. Nucl. Med. 2010;51:1076–1083. doi: 10.2967/jnumed.109.073189. [DOI] [PubMed] [Google Scholar]
- [61].Heskamp S, van Laarhoven HW, Molkenboer-Kuenen JD, Franssen GM, Versleijen-Jonkers YM, Oyen WJ, van der Graaf WT, Boerman OC. ImmunoSPECT and immunoPET of IGF-1R expression with the radiolabeled antibody R1507 in a triple-negative breast cancer model. J. Nucl. Med. 2010;51:1565–1572. doi: 10.2967/jnumed.110.075648. [DOI] [PubMed] [Google Scholar]
- [62].Cai W, Chen X. Nanoplatforms for targeted molecular imaging in living subjects. Small. 2007;3:1840–1854. doi: 10.1002/smll.200700351. [DOI] [PubMed] [Google Scholar]
- [63].Hong H, Gao T, Cai W. Molecular imaging with single-walled carbon nanotubes. Nano Today. 2009;4:252–261. doi: 10.1016/j.nantod.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ruggiero A, Villa CH, Holland JP, Sprinkle SR, May C, Lewis JS, Scheinberg DA, McDevitt MR. Imaging and treating tumor vasculature with targeted radiolabeled carbon nanotubes. Int. J. Nanomedicine. 2010;5:783–802. doi: 10.2147/IJN.S13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Jacobson O, Zhu L, Niu G, Weiss ID, Szajek LP, Ma Y, Sun X, Yan Y, Kiesewetter DO, Liu S, Chen X. MicroPET imaging of integrin αvβ3 expressing tumors using 89Zr-RGD peptides. Mol. Imaging Biol. 2011 doi: 10.1007/s11307-010-0458-y. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]