Abstract
Non-random positioning of chromosomal domains relative to each other and to nuclear landmarks is a common feature of eukaryotic genomes. In particular, the distribution of DNA loci relative to the nuclear periphery has been linked to both transcriptional activation and repression. Nuclear pores and other integral membrane protein complexes are key players in the dynamic organization of the genome in the nucleus, and recent advances in our understanding of the molecular networks that organize genomes at the nuclear periphery point to a further role for non-random locus positioning in DNA repair, recombination and stability.
In eukaryotes, several processes mediate the packaging of a large linear genome into a relatively small nucleus. The association of DNA with architectural proteins creates an entity known as chromatin. The fundamental unit of chromatin, the nucleosome, is formed by the wrapping of 147 base pairs of DNA around core histone proteins1. Higher-order nucleosome packing and chromatin compaction create a chromosome. It has long been appreciated that chromosome folding and compaction are crucial for faithful mitotic chromosome segregation and cell division. It is now clear that in interphase the positioning of chromosome domains relative to each other and to nuclear landmarks is not random and has important roles in genome function2,3.
Chromosomal regions harbouring ribosomal DNA (rDNA) repeats, which provide the foundation for the ribosome manufacturing compartment known as the nucleolus, are preferentially located at the nuclear periphery in yeast and closer to the centre of the nucleus in mammals4,5. The main silent chromatin domains can localize to the nuclear periphery in different organisms. For example, all yeast telomeres localize at the periphery, and perinuclear telomeres are present in flies, plants and mammals3,6–10. Centromeres also localize to the nuclear periphery in yeast, flies and plants8,11. However, strictly linking the nuclear periphery to transcriptional repression would be an oversimplification. Whether perinuclear association of DNA favours, represses or does not affect transcription varies depending on the locus itself, how it is recruited to the periphery and how it is positioned relative to various nuclear neighbourhoods2.
The nucleus is spatially defined by two membrane bilayers, the inner nuclear membrane (INM) and outer nuclear membrane (ONM), which are perforated by nuclear pores that control traffic in and out of the nucleus (FIG. 1a). Some INM and ONM proteins interact in the nuclear lumen and form trans-envelope linkages that physically connect nuclear chromatin to cytoplasmic filaments in several organisms, including yeast, worms and mammals12–14. Thus, the nuclear envelope can be viewed both as a divider and physical linker between the nucleus and cytoplasm. In animal cells, intermediate filament proteins called lamins form a web between the INM and DNA and connect nuclear pores to each other (FIG. 1a). Lamins help maintain the spherical geometry of nuclei in organisms with large genomes. Mutations in lamins, nuclear membrane proteins and their binding partners are associated with several human diseases including muscular dystrophies and laminopathies, highlighting the importance of perinuclear molecular interactions15,16. Although lamins are not present in plants and fungi, many proteins of the INM, ONM and nuclear pores are conserved on a structural and functional level, suggesting that the mechanisms involved in organizing nuclear contents are at least partially conserved. Whereas proteins that are integral to the INM are generally linked to transcriptional silencing, nuclear pore complexes (NPCs) or their components have been linked to both transcriptionally inactive and active loci in different organisms3,17–21 (FIG. 1a). In addition to its impact on gene expression, perinuclear DNA positioning has been linked to the protection of the DNA sequence itself in several recent studies conducted mainly in yeast4,22–27.
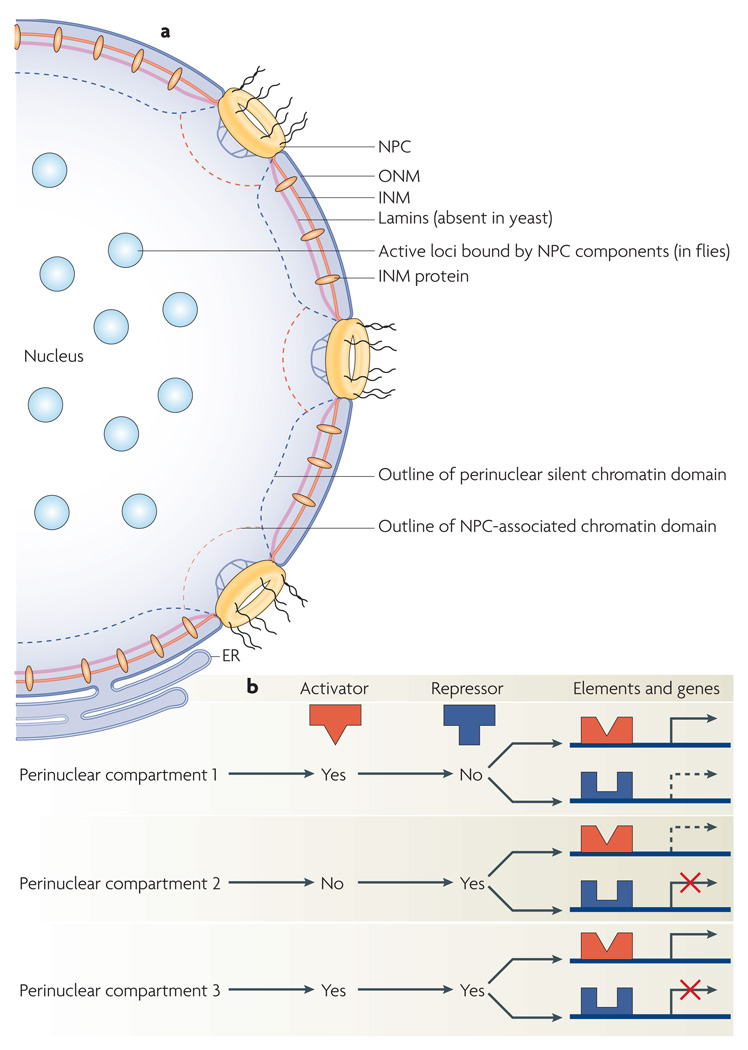
Figure 1. The nuclear periphery and gene expression.
a | An overview of perinuclear components and compartments. The nuclear envelope encompasses an outer nuclear membrane (ONM) and an inner nuclear membrane (INM), which are perforated with nuclear pore complexes (NPCs). In animal cells, filamentous proteins called lamins form a web between the INM and DNA, connect nuclear pores to each other and help maintain the spherical geometry of the nucleus. INM proteins and lamins are frequently implicated in transcriptional gene silencing. Perinuclear NPCs can be preferentially associated with active loci (as in yeast) or silent loci (as in Drosophila melanogaster and humans), depending on the species. At least in fly cells, nucleoplasmic NPC components are localized to active loci in the nuclear interior. The membrane of the endoplasmic reticulum (ER) is continuous with the ONM. b | Although active or silent chromatin compartments exist at the nuclear periphery, the effect of gene targeting to a perinuclear compartment depends on the presence or absence (yes or no, respectively) of transcriptional regulators (activators or repressors) at that compartment and the regulatory sequence elements controlling transcription at the targeted locus. Transcriptional effects related to the relocation of two genes to different perinuclear compartments are shown. Scenarios with increased transcription (black arrows), decreased transcription (red X marks) or unaltered transcription (dashed arrows) are shown.
Here, we review how the non-random organization of DNA relative to the nuclear periphery affects genome expression and stability. First, we discuss the effect of perinuclear DNA association on chromatin and gene expression. Second, we discuss the role of emerging perinuclear networks of protein–protein and protein–DNA interactions in regulating DNA recombination and repair, and highlight the relationships of these networks to gene expression. We conclude by discussing how these processes are integrated at the nuclear periphery, highlighting common themes and pending questions.
Perinuclear gene expression
The study of DNA spatial organization was revolutionized by the introduction of the concept of radial subnuclear positioning, which measures the location of a genetic locus along an axis extending from the centre of the nucleus to its periphery28. By suggesting that a gene-dense chromosome is internal and a gene-light chromosome is perinuclear, early studies relying on radial DNA positioning launched the quest to determine whether the position of a gene affects its function28. Since then, many questions have been answered but even more have emerged.
Perinuclear proteins in silencing
DNA-tagging methods have been used to assess the extent of perinuclear DNA positioning on a genome-wide scale. One such method is DNA adenine methyltransferase identification (DamID), which can detect loci located near the nuclear periphery by identifying DNA that becomes methylated following the expression of the Escherichia coli Dam methylase fused to a protein located at the nuclear periphery, such as an INM or lamin protein29. These studies revealed that transcriptionally silent chromosomal domains are more likely to interact with perinuclear landmarks such as lamins in both flies and humans20,21. These domains are poor in active chromatin marks, are flanked by regions enriched in insulator protein-binding sites and often contain co-regulated genes. Interestingly, lamin-associated genes can be released from lamins on transcriptional activation following treatment with histone deacetylase inhibitors, suggesting that silencing promotes perinuclear association20. These findings support a long-standing and classical view of heterochromatin as dense chromosomal regions residing primarily next to the nuclear membrane30–32.
Several recent studies looking at specific loci or reporter genes have also physically linked silent chromatin to the nuclear periphery4,22,26,33–37. One of the earliest of these studies revealed that the artificial weakening of a silencer sequence adjacent to the budding yeast Saccharomyces cerevisiae HMR mating-type locus (BOX 1) caused the locus to be released from the nuclear periphery and become transcribed33. More importantly, artificially returning this crippled locus to the periphery by marking it with sequence tags and expressing a sequence tag-binding protein fused to a perinuclear protein restored silencing at the HMR locus. However, complete removal of the silencer element rendered the HMR locus transcriptionally active whether or not it was targeted to the nuclear periphery. This was an early indication that perinuclear targeting and other factors are important for silencing in natural settings and that anchoring alone may not be sufficient for silencing. In fact, a peripheral gene can remain silent even when excised from its location in the yeast genome, providing that heterochromatin factors such as the silent information regulator (SIR) complex (composed of Sir2, Sir3 and Sir4) maintain interactions with the wandering gene38,39.
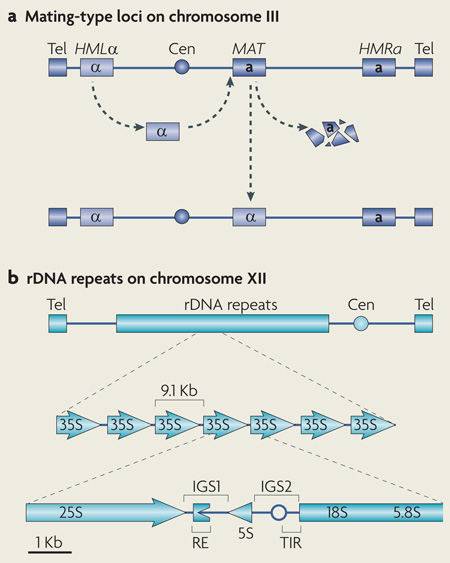
Box 1 | Organization of yeast mating-type loci and rDNA repeats.
The budding yeast Saccharomyces cerevisiae has mating types or sexes known as α and a (see the figure, part a). Three separate copies of mating-type information are located on chromosome III. The HMLα and HMRa loci contain copies of the α and a genes, respectively. Both of these loci are silenced by silent information regulator (SIR) proteins. The allele present at the MAT locus is expressed and determines the mating type. Mating-type switching involves the replacement of the allele present at the MAT locus with a copy of the α or a allele present at HMLα or HMRa. The S. cerevisiae ribosomal DNA (rDNA) locus is located on chromosome XII and contains a tandem array of ~190 repeated units (see the figure, part b). Each unit yields an RNA polymerase I (Pol I)-transcribed 35S precursor rRNA, which is processed into 25S, 18S and 5.8S rRNAs, and a Pol III-transcribed 5S rRNA. Each unit also contains intergenic spacers (IGS1 and IGS2) that maintain repeat integrity by mediating silent chromatin assembly and promoting perinuclear rDNA-repeat anchoring. IGS1 contains recombination-enhancing (RE) sequences that harbour replication fork-blocking sequences, which can stall replication forks and induce double strand breaks. The locations of the centromere (Cen), telomeres (Tel), Pol I transcription initiation region (TIR) and DNA replication origin (empty circle) are shown in the figure.
In mammalian cells, the effect of targeting reporter genes to the nuclear periphery ranged from very weak to strong with regard to transcriptional inhibition34,35,40. Whereas perinuclear tethering based on INM proteins (human lamina-associated polypeptide 2β (LAP2β; also known as TMPO) or mouse emerin)34,35 repressed transcription of several reporter genes, targeting methods that relied on lamins (human lamin B1, which is a B-type lamin)40 only had negligible effects on gene expression (TABLE 1). Varying effects may be linked to different promoters and methodologies used. Nonetheless, the silencing of testis-specific gene clusters in fly somatic cells relies on recruitment of the clusters to the nuclear periphery by B-type lamins, which thus seem to control gene expression in natural settings41. Together, these findings suggest that the interaction of DNA with lamins or INM proteins is more closely associated with the silencing of gene expression.
Table 1.
Functions of INM and NPC proteins that link chromatin to the nuclear periphery*
| Protein | Organism | Locus | Function at genetic loci | Refs |
|---|---|---|---|---|
| INM proteins: LEM domain proteins and others | ||||
| Emerin | Mus musculus | Reporter gene and immunoglobulin loci | Perinuclear recruitment by truncated emerin lowers histone acetylation and gene expression; inactive immunoglobin loci at the periphery are in contact with emerin | 34 |
| LAP2β | Homo sapiens | Chromosomal domains or chromatin | Perinuclear recruitment of chromosomal domains by LAP2β reduces the expression of several but not all genes | 35 |
| Heh1 | Saccharomyces cerevisiae | rDNA | Forms CLIP with Nur1 and binds cohibin; maintains rDNA repeat stability but not silencing | 4, 68 |
| Telomeres | Role in subtelomeric gene silencing | 36 | ||
| Centromeres | Unknown | 36 | ||
| Nur1 | S. cerevisiae | rDNA | Forms CLIP with Heh1 and binds cohibin; maintains rDNA repeat stability but not silencing | 4, 68 |
| INM proteins: SUN domain proteins, KASH domain proteins and others | ||||
| Mps3 | S. cerevisiae | Telomeres and loci with DSBs | Mitotic tethering, silencing and suppression of aberrant recombination at telomeres; mitotic DSBs are targeted to the nuclear periphery to maintain genome stability; and meiotic telomere bouquet formation | 23, 24, 37, 83, 98, 102 |
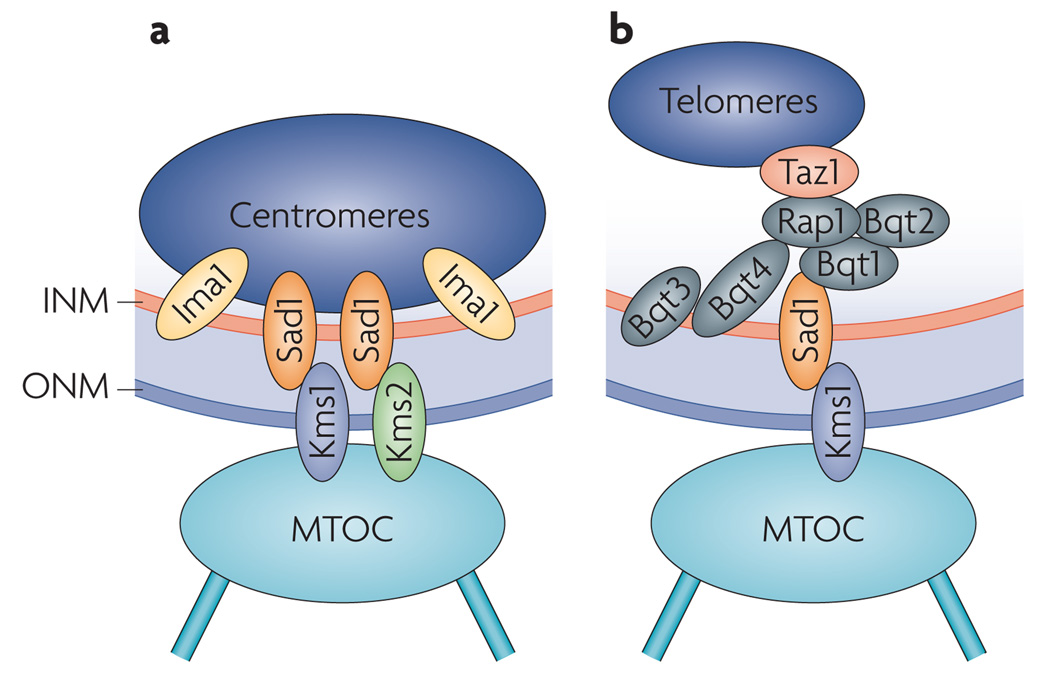
| Sad1, Kms1, Kms2 and Ima1 | Schizosaccharomyces pombe | Centromeres | Buffer cytoskeletal forces to maintain nuclear integrity; the Ndc80 complex helps stabilize this perinuclear anchoring system | 14 |
| Sad1 and Kms1 | S. pombe | Telomeres | Mediate meiotic telomere clustering at the nuclear envelope and bouquet formation by cooperating with Bqt1, Bqt2, Bqt3 and Bqt4. | 92, 94 |
| SUN-1 and ZYG-12 | Caenorhabditis elegans | Chromosomal domains or chromatin | Meiotic chromosome reorganization and homologue recognition | 96 |
| SUN1 | M. musculus | Telomeres | Perinuclear meiotic telomere anchoring, homologue pairing, synapsis and gametogenesis | 99 |
| SUN2 | M. musculus and Rattus rattus | Telomeres | Perinuclear meiotic telomere anchoring | 100 |
| NPC proteins | ||||
| Nup84 complex | S. cerevisiae | Telomeres | Induced DNA breaks require Nup84 complex proteins for repair of lesions by NHEJ | 26 |
| Mating-type locus‡ | Collapsed replication forks are recruited to the Nup84–Slx5–Slx8 complex for repair | 22 | ||
| NPC proteins | S. cerevisiae | Telomeres | Eroded telomeres remain perinuclear but move to nuclear pores through interactions with NPC proteins | 27 |
Bqt, bouquet formation protein; CLIP, chromosome linkage INM proteins; DSB, double strand break; Heh1, helix extension helix 1; INM, inner nuclear membrane; KASH, klarsicht–Anc1–SYNE homology; Kms, karyogamy meiotic segregation protein; LAP2β, lamina-associated polypeptide 2β; LEM, LAP2β–emerin–MAN1; Mps3, monopolar spindle 3; NHEJ, non-homologous end joining; NPC, nuclear pore complex; Nur1, nuclear rim 1; rDNA, ribosomal DNA; Slx, synthetic lethal of unknown function; SUN, Sad1–Unc84 homology.
Additional factors involved in perinuclear targeting of DNA such as the proteasome and specific histone deacetylase complexes are not presented here but are reviewed elsewhere47,116.
A modified MAT locus was used.
Activation and silencing by NPCs
Is the nuclear periphery of eukaryotes strictly linked to the repression of transcription? The answer is certainly not. In fact, several studies using genome-wide or targeted approaches have reported that the association of genes with NPCs promotes transcription in S. cerevisiae42–47 (FIG. 1a). This association seems to be at least partly dependent on DNA zip codes called gene recruitment sequences (GRSs), which target DNA to the NPCs48. Furthermore, GRSs are functional in the fission yeast Schizosaccharomyces pombe, suggesting mechanistic conservation over a billion years of evolution48. Such sequences may help reveal why some loci are preferentially transcribed at or away from the nuclear periphery. Whether similar functions for NPCs exist in other organisms is unclear. Although NPCs have been primarily linked to transcriptionally active regions in Drosophila melanogaster, recent studies in this organism add another layer of complexity to the study of the role of NPCs in gene expression control17–19. Whereas the functional architecture of the NPC at the nuclear envelope mediates subcellular transport, various NPC components are dynamic and continuously exchange between the nucleoplasm and the nuclear pores, at least in larger eukaryotes such as rats and flies18,49. This suggests that pools of NPC proteins may have different functions depending on their subnuclear localization. Indeed, it was recently proposed that nucleoplasmic NPC components can activate the expression of internally localized genes, whereas DNA–NPC interactions at the periphery may be linked to inactive genes in D. melanogaster17,18 (FIG. 1a). A genome-wide study revealed that the treatment of human cells with histone deacetylase inhibitors can exchange silent chromatin for active domains at the NPC50. Together, these findings suggest that association of DNA with NPCs may have differential effects on transcription depending on the organism, cell type, signalling cues and NPC component localization.
Perinuclear heterogeneity
It has long been known that yeast perinuclear compartments such as regions near the nucleolus, telomeric cluster regions and spindle pole body regions differ in protein and DNA composition4,51. In addition, advances in high-resolution microscopy recently revealed that the nuclear periphery is not a monotone area but, instead, harbours different types of domains in mouse cells52. Although the exact protein and DNA composition of perinuclear domains is unclear, how such a differential organization may affect gene expression can be imagined. Whereas one domain might be enriched in a given transcriptional repressor, another can have a high concentration of a specific activator. Thus, effects on the transcription of a gene positioned in any of these perinuclear domains can depend on the nature of regulatory elements controlling the locus (FIG. 1b). For example, a gene lacking regulatory elements required to recruit a specific activator is unlikely to be activated when recruited to a perinuclear domain where the activator is enriched. By contrast, localization of a repressive DNA element to a perinuclear region enriched in silencing factors boosts silencing. Consistent with this, artificial targeting of a chromosomal domain to the nuclear periphery can reversibly suppress the expression of some endogenous human genes located near the tethering sites and even genes further away35. However, the expression of many other genes is not detectably reduced and location at the nuclear periphery is not incompatible with active transcription. Furthermore, the observed reduction of gene expression at the periphery was dependent on histone deacetylases, suggesting that only some genes were sensitive to histone deacetylation at the periphery. Taken together, these findings reveal a complex heterogeneity at the nuclear periphery and highlight crosstalk between genes, genetic elements, perinuclear compartments and the nuclear envelope.
Chromosome organization at the nuclear periphery and the enrichment of specific components in perinuclear domains seem to involve positive feedback mechanisms. The clustering of yeast telomeres at the periphery is thought to increase the local concentration of silencing factors and this higher concentration may in turn promote further clustering3,51. However, we note some exceptions to this interdependency. For example, as mentioned above, silent chromatin domains in yeast can persist after their dissociation from the nuclear periphery38,53. The activation of genes targeted to NPCs may also be a consequence of faster processing and export of transcripts rather than a targeted activation of transcription or alleviation of repressive mechanisms. The full extent of the compartmentalization of transcription at the nuclear periphery is only starting to emerge and needs further characterization.
Perinuclear links to genome stability
Does association with the nuclear periphery affect processes other than silent chromatin assembly and transcription? Can perinuclear DNA positioning regulate genome stability by regulating processes such as DNA repair, recombination or replication fork rescue? Recent studies conducted primarily in yeast suggest that the answer to both of these questions is yes4,22–27,54–56. These findings suggest that the radial positioning of DNA loci and the connections they establish at the nuclear periphery are crucial to genome replication and stability.
LEM domain proteins linked to rDNA stability
If not properly regulated, repetitive DNA sequences that are abundant in eukaryotes can initiate the gain or loss of chromosomal regions by engaging in homologous recombination, leading to genomic instability and cancer57,58. S. cerevisiae is often used as a model system to study how eukaryotes stabilize chromosomes harbouring repetitive sequences. In this yeast, the rDNA locus, which is located on chromosome XII, contains a tandem array of ~190 repeating units encoding RNA polymerase I- and RNA polymerase III-transcribed rRNAs59 (FIG. 2; BOX 1). rDNA repeats are spatially separated from the bulk of nuclear DNA and provide the foundation for the nucleolus (FIG. 2). Yeast has one crescent-shaped nucleolus that occupies up to one-third of the nuclear volume and abuts the INM. In addition to harbouring rRNA-coding DNA sequences, each unit contains intergenic spacers (IGS1 and IGS2), which regulate rRNA transcription and repeat integrity (BOX 1). IGS1 contains recombination-enhancing sequences that can be activated by fork-blocking protein 1 (Fob1)60–62. Interestingly, Fob1 mediates its own suppression by recruiting several nucleolar factors to IGS1. These factors are the protein complexes RENT (regulator of nucleolar silencing and telophase exit; composed of Net1 (also known as Cfi1), Cdc14 and the NAD+-dependent histone deacetylase Sir2) and cohibin (composed of the monopolin sub units Lrs4 and Csm1), as well as a protein with sequence homology to Net1 called topoisomerase 1-associated factor 2 (Tof2)63–68 (FIG. 2; BOX 1). Suppression of Fob1-dependent recombination is linked to the ability of the RENT and cohibin complexes to induce rDNA silencing, which involves the establishment of less accessible chromatin structures, thereby preventing transcription by RNA polymerase II from endogenous or foreign promoters positioned in IGS regions. This differs from RNA polymerase I silencing, which limits rRNA synthesis4,68–73. Chromatin-dependent silencing mechanisms similar to rDNA silencing are observed at the two other main loci in S. cerevisiae, the mating-type loci and telomeres, but the complexes that mediate silencing at these loci are different74 (BOX 1).
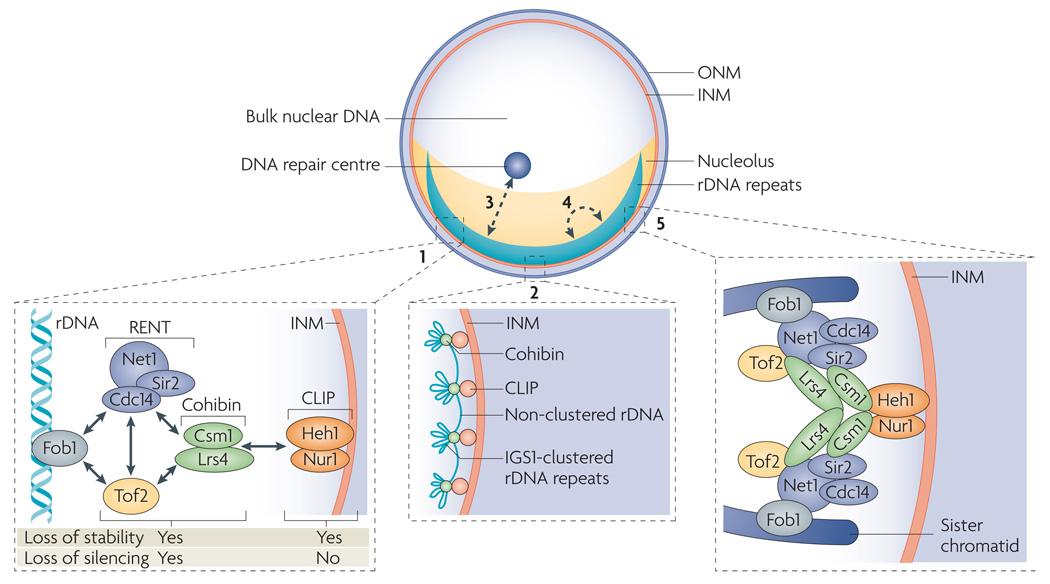
Figure 2. The nuclear envelope in gene expression and protection of repetitive DNA sequences.
In Saccharomyces cerevisiae, ribosomal DNA (rDNA) repeats sit next to the inner nuclear membrane (INM), are separated from the bulk of nuclear DNA and are located in the ribosome-manufacturing compartment, the nucleolus. Fork-blocking protein 1 (Fob1) binds and activates recombination-enhancing sequences in the first intergenic spacer (IGS1) of rDNA, thereby promoting recombination (1). This function of Fob1 is repressed by several nucleolar factors, which include the regulator of nucleolar silencing and telophase exit (RENT) complex (composed of Net1 (also known as Cfi1), Cdc14 and silent information regulator 2 (Sir2)), the cohibin complex (composed of the monopolin subunits Lrs4 and Csm1) and topoisomerase-related factor 2 (Tof2). Suppression of Fob1 activity is linked to the ability of these factors to promote silent chromatin assembly in IGS1. Cohibin also interacts with the CLIP (chromosome linkage INM proteins) complex (composed of helix extension helix 1 (Heh1; also known as Src1) and nuclear rim 1 (Nur1; also known as YDL089W)), linking rDNA repeats to the nuclear envelope4,68 (2). CLIP is required for the maintenance of rDNA repeat stability but not for silencing at IGS1 (REF. 4). The effects of deleting different members of the network on chromosome stability and silencing at rDNA repeats are indicated (1). Cohibin has a role in silencing that is independent of its association with CLIP and rDNA perinuclear localization and may involve clustering IGS1 sites from different rDNA units, which are then attached to the INM through CLIP (2). Perinuclear anchoring may also stabilize rDNA repeats by preventing their exit from the nucleolus and limiting their exposure to nucleoplasmic DNA repair centres (3), spreading the repeats along the INM to limit recombination-promoting long-range interactions between different rDNA units (4), and by promoting the alignment of sister chromatids during replication to limit aberrant recombination events4,66,68 (5).
A combination of affinity purifications, proteomic analyses and chromosome and cell biology techniques have uncovered a network of protein–protein interactions extending from rDNA repeats to the nuclear envelope of S. cerevisiae4 (FIG. 2). Connection to the nuclear periphery relies on two integral INM proteins, helix extension helix 1 (Heh1; also known as Src1) and nuclear rim 1 (Nur1; also known as YDL089W), forming a complex called CLIP (chromosome linkage INM proteins) (FIG. 2). Heh1, the orthologue of human MAN1, is a member of a family of INM proteins containing a highly conserved LAP2β–emerin–MAN1 (LEM) domain. LEM domain proteins are linked to multiple clinical conditions, such as Emery–Dreifuss muscular dystrophy, through emerging roles in several key processes including gene expression and chromatin organization4,15,36,75–78. CLIP physically links the rDNA-associated cohibin complex to the nuclear envelope. Interestingly, releasing rDNA repeats from the nuclear membrane by the disruption of CLIP compromises chromosome stability by promoting aberrant recombination events in the repeats without affecting rDNA silencing4 (FIG. 2). rDNA repeats unleashed from the nuclear envelope seem to accumulate lesions that can move from the nucleolus to the nucleoplasm, in which high concentrations of functional recombination proteins promote recombination events compromising chromosome stability4,79 (FIG. 2). In addition, repeat instability can be partially repressed in mutant cells when rDNA is artificially tethered back to the INM4. General roles for LEM domain proteins in yeast genome stability are likely, as Heh1 also localizes to telomeres and contributes to their silencing36. Importantly, some functions of LEM domain proteins have emerged only after considering redundancies between members of this protein family and so future studies may benefit from similar considerations80.
Taken together, these findings suggest that INM-mediated perinuclear chromosome tethering has an important role in stabilizing the highly repetitive rDNA locus in S. cerevisiae, even though tethering is not required for silent chromatin assembly (TABLE 1; FIG. 2). Thus, silencing and tethering are at least partly independent processes that stabilize rDNA repeats. However, cohibin, which links rDNA to the INM CLIP complex, is required for rDNA silencing68. This requirement indicates a role for cohibin in silencing that is independent of its role as an attachment point for CLIP. Although the function of cohibin in silencing is not understood, rDNA IGS regions cluster in the nucleolus63 and the silencing function of cohibin may be linked to its possible role in this clustering (FIG. 2).
Esc1 and SUN domain proteins: telomere protection
In addition to LEM domain proteins, other classes of perinuclear proteins have been linked to various chromosomal domains, including telomeres. If not properly protected, telomeres can be recognized as DNA breaks and cause genomic havoc81. Cells have therefore evolved a number of mechanisms to protect telomeres and maintain genome stability.
In S. cerevisiae, 32 telomeres cluster in 4–8 foci at the nuclear periphery of interphase cells3,82 (FIG. 3a). The INM protein monopolar spindle 3 (Mps3) helps cluster telomeres at the nuclear envelope of yeast cells83. Mps3 belongs to a family of INM proteins that contain a highly conserved domain called SUN (Sad1–Unc84 homology) and are physically linked to several genetic loci13. This prompted others to closely examine the role of Mps3 in perinuclear telomere tethering and silent chromatin domains in interphase.
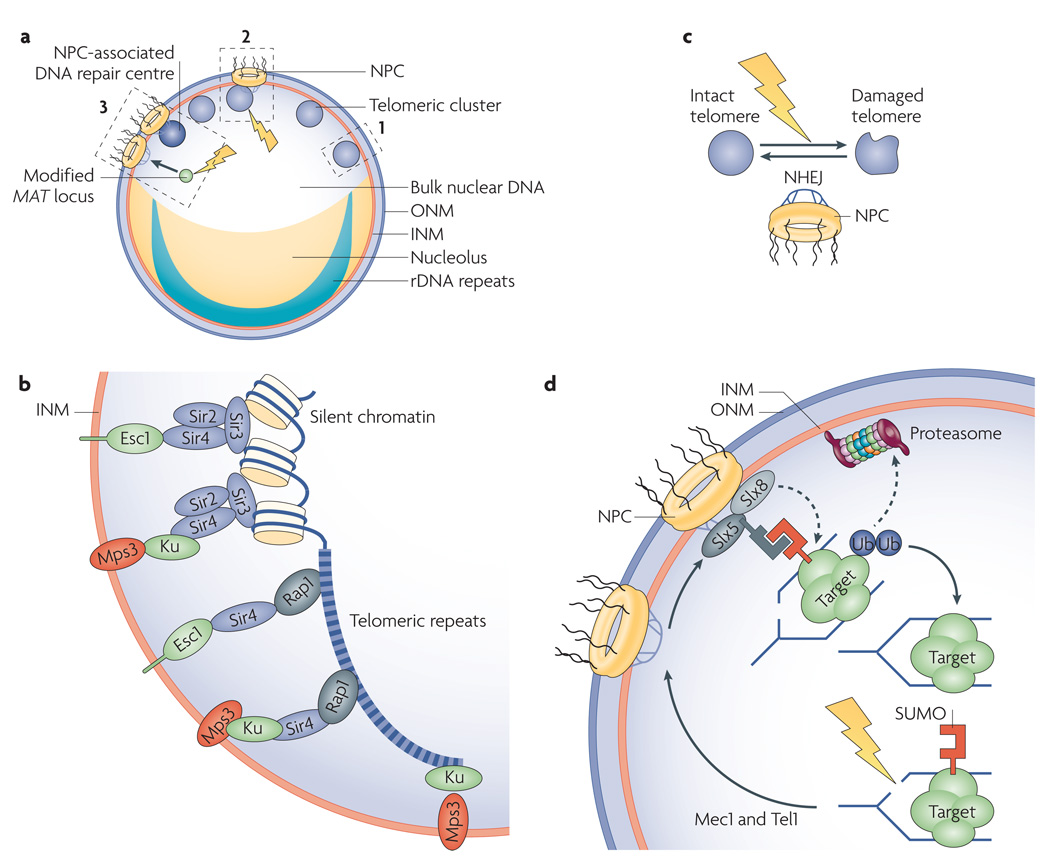
Figure 3. Perinuclear interactions stabilize a genome.
a | Saccharomyces cerevisiae genome organization. Nucleolar ribosomal DNA (rDNA) repeats are separated from the bulk of nuclear DNA and stabilized by tethering to the inner nuclear membrane (INM). Silencing and suppression of recombination near telomeres is promoted by their perinuclear clustering. Induction of double strand breaks (DSBs) at a modified mating-type MAT locus triggers the relocation of MAT (solid arrow) from a random position to nuclear pore complexes (NPCs). Dashed boxes 1–3 are highlighted in panels b–d, respectively. b | S. cerevisiae telomere anchoring. Telomere repeat-bound repressor and activator site-binding protein 1 (Rap1) recruits the silent information regulator (SIR) complex (composed of Sir2, Sir3 and Sir4), which promotes its own spreading to nearby chromatin. Rap1 can also interact with Sir4 alone. Enhancer of silent chromatin 1 (Esc1), Ku proteins and the INM protein monopolar spindle 3 (Mps3) anchor telomeres. In S phase, Sir4–Ku or Sir4–Esc1 anchors are present. Mps3 is linked to Sir4–Ku anchors, but recently identified Ku–Mps3 connections can contribute to telomere anchoring when Sir4–Ku interactions are compromised. Ku proteins are the main anchors in G1 phase24,37,82–86. c | NPCs are required for perinuclear telomere anchoring and non-homologous end joining (NHEJ)-dependent repair of subtelomeric DSBs26. d | Following DSB induction, two kinases (mitosis entry checkpoint 1 (Mec1) and telomere maintenance 1 (Tel1)) help target damaged replication forks to small ubiquitin-related modifier (SUMO)-targeted ubiquitin ligase (STUbL) complexes (composed of synthetic lethal of unknown function 5 (Slx5) and Slx8), which are enriched at NPCs. This may fix damage through SUMO-dependent substrate recognition, ubiquitylation and proteasomal degradation22. ONM, outer nuclear membrane; Ub, ubiquitin.
In S. cerevisiae, chromatin silencing at telomeres requires the SIR complex, Ku (composed of yeast Ku70 and Ku80) and other DNA-associated factors74,82–86. Telomeres are tethered to the nuclear periphery by several mechanisms. A poorly understood mechanism relying primarily on Ku proteins is thought to tether telomeres to the nuclear envelope in G1 phase82,85. We know more about S phase perinuclear telomere anchoring, which is only partially dependent on Ku proteins and relies on additional factors. In S phase, conventional telomere tethering relies on cooperation between the telomere-associated Sir4 and either Ku proteins or a perinuclear protein called Esc1 (establishes silent chromatin 1). The SUN domain protein Mps3 was found to cooperate with Sir4 during S phase to promote perinuclear targeting and efficient silencing of telomeres in an Esc1-independent manner37 (FIG. 3b). Therefore, Mps3 may be linked to telomeres by Ku or other Sir4-interacting factors to mediate perinuclear anchoring and clustering of telomeres and increase the local concentration of chromatin silencing factors. Esc1 does not have a transmembrane domain and may rely on its known post-translational lipid modifications or another bona fide membrane protein to associate with the INM86. Future work should clarify the complex interactions linking S. cerevisiae telomeres to the nuclear envelope. Mps3 may also have broad effects on genome function, as loss of telomere clustering and subsequent dispersion of silencing factors across the genome results in widespread changes in gene expression, including the erroneous silencing of non-subtelomeric genes87.
If Mps3 is crucial to telomere stability, then it might cooperate with other key factors that protect telomeres such as the enzyme telomerase. By templating DNA synthesis from its own RNA subunit, telomerase counteracts telomere shortening, which can accumulate over DNA replication and cell division cycles and induce senescence. Disruption of telomerase results in chromosome end joining and genomic instability88. In S. cerevisiae, telomerase is composed of a telomerase reverse transcriptase catalytic core particle called ever shorter telomeres protein 2 (Est2), its binding factors Est1 and Est3, as well as the RNA subunit Tlc1, which interacts with Ku proteins. A recent study tested the ability of LexA fusion proteins to mediate perinuclear anchoring of a chromosomal locus tagged with LexA-binding sites24. Among the proteins tested was a Sir4 binding-deficient Ku80 protein fused to LexA. Interestingly, the ability of this modified Ku80 fusion protein to mediate perinuclear anchoring in S phase cells required Est1 and interactions between the fusion protein and Tlc1. An Est2–LexA fusion protein was also capable of mediating perinuclear anchoring in S phase cells in an Est1-dependent manner. This S phase anchoring was only weakly affected by disruption of Esc1. Perinuclear anchoring in G1 phase is less dependent on telomerase subunits and points to different requirements or additional factors implicated in G1 phase anchoring. These findings suggest that, at least in the absence of Sir4–Ku80 interactions, Ku80 may cooperate with telomerase in an alternative telomere tethering (ATT) process during S phase.
This telomerase-mediated recruitment of a tagged chromatin locus to the nuclear envelope does not depend on NPCs but seems to rely on Mps3 (REF. 24), which is also required for perinuclear telomere anchoring in S phase cells24,37. Interestingly, the proper function of the SIR complex on chromatin seems to be required for ATT, but it is unclear why this is the case. Importantly, expression of the N terminus of Mps3 can act in a dominant-negative manner and disrupt ATT. Cells lacking the ataxia telangiectasia mutated (ATM) kinase homologue telomere length regulation 1 (Tel1) have reduced SIR complex localization to telomeres and were thus predicted to be more dependent on ATT, especially as a fairly robust telomeric silencing is maintained in these cells24,89. Indeed, introduction of the dominant-negative Mps3 fragment in Tel1-negative cells leads to unprotected hyper-recombinant telomeres and a senescence-like phenotype24. These findings suggest that Mps3 may cooperate with telomerase to mediate perinuclear telomere anchoring and to suppress aberrant recombination events at telomeres, especially in cells with deficiencies in Sir4-based telomere anchoring pathways. Consistent with a broad role for Mps3 in yeast genome stability, double strand breaks (DSBs) are sequestered to the nuclear periphery through a process requiring Mps3 and the transient action of sumoylated histone variant H2A.Z to guide efficient DNA repair23,56. Together, these studies highlight the key role of perinuclear proteins such as Esc1 and Mps3 in DNA organization, chromatin silencing and genome stability (TABLE 1; FIG. 3b). The existence of several partially overlapping telomere tethering pathways highlights the importance of this process.
How does perinuclear localization promote genome stability? The simplest mechanism may be that the nuclear membrane acts as a large solid support, allowing cells to control the radial distribution of many genetic loci relative to each other and to other nuclear components. For example, perinuclear tethering can stabilize DNA repeats by limiting their exposure to nucleoplasmic recombination factors, by spreading the repeats along the INM to limit recombination-promoting long-range interactions between different DNA units, and by keeping sister chromatids properly aligned during replication to limit unequal recombination events (FIG. 2; FIG. 3). It is important to note that the tandem arrangement of DNA repeats on the same chromosome or the clustering of different chromosomal domains such as telomeres allows for the co-regulation of genes or chromosomal domains. In addition, such arrangements can promote beneficial recombination events by promoting genetic diversity in the cell population under stress. Such beneficial recombination can occur at telomeres following telomerase deletion and can alter the size of rDNA repeats under stress90,91. Thus, repetitive loci are arranged in a manner that promotes co-regulation and limits aberrant recombination while being conducive to beneficial recombination.
Cytoskeleton–DNA bridges
Recently, a series of interactions that reach across the nuclear envelope and link the cytoskeleton to chromatin have been identified. A group of integral membrane proteins located in the ONM harbour a highly conserved nuclear lumenal domain called KASH (klarsicht–Anc1–SYNE homology) and associate with the cytoskeleton. In S. pombe, worms, flies and mammals, lumenal KASH domains also interact with the INM-localized SUN domain proteins, which interact with chromatin as described above13.
INM–ONM connections: nuclear integrity
Given that SUN domain proteins can interact with chromatin, SUN–KASH protein interactions constitute a link that stretches across the nuclear envelope and connects the cytoskeleton to DNA. In S. pombe, the KASH domain proteins karyogamy meiotic segregation protein 1 (Kms1) and Kms2 and the SUN domain protein Sad1 physically link the cytoskeleton to heterochromatin-associated peri-centromeric chromosome domains14 (TABLE 1; FIG. 4a). Importantly, disruption of centromeric heterochromatin weakens the ability of these trans-membrane linkages to withstand cytoskeletal forces exerted on the nucleus, leading to nuclear deformation and sometimes even to the generation of nuclear membrane foci in the cytoplasm. These findings reveal that INM–ONM bridges linking the cytoskeleton to key chromosomal sites are crucial to the balancing of sub-cellular forces as well as to the maintenance of nuclear structure and overall genome integrity.
Figure 4. Transmembrane coupling of chromatin to the cytoskeleton during the mitotic and meiotic cell cycle.
a | In interphase of Schizosaccharomyces pombe cells engaged in a mitotic (also called vegetative) cell cycle, centromeres are connected to the primary microtubule organizing centre (MTOC; or spindle pole body (SPB), the centrosome equivalent in fungi) through interactions between the SUN (Sad1–Unc84 homology) domain protein Sad1 and the KASH (klarsicht–Anc1–SYNE homology) domain proteins karyogamy meiotic segregation protein 1 (Kms1) and Kms2. Connections are stabilized by the inner nuclear membrane (INM) protein Ima1 and are required for the maintenance of nuclear integrity14. b | In S. pombe cells in meiotic prophase, meiosis-specific bouquet formation protein 1 (Bqt1) and Bqt2 connect telomere-associated proteins (repressor activator protein 1 (Rap1) and Taz1) to Sad1, promoting telomere clustering and formation of the telomere bouquet chromosomal arrangement, which is crucial for homologous chromosome pairing, meiotic recombination and progression through meiosis. Sad1–Kms1 interactions are thought to translate cytoskeletal forces to chromosome movements in the nucleus. These connections are stabilized by Bqt3 and Bqt4 (REFS 92–94). ONM, outer nuclear membrane.
INM–ONM connections: meiotic chromosome pairing
Haploid yeast cells can bud or fission by progressing through the mitotic cell cycle, which is also called the vegetative cell cycle, to generate two haploid cells. Following the mating of two haploid cells of the opposite mating type, the emerging diploid yeast cell can follow a meiotic cell cycle to generate four haploid daughter cells. In the meiotic cell cycle, DNA replication is followed by two rounds of cell division. In addition to the above described roles in the mitotic cell cycle of yeast, SUN–KASH domain interactions also have important roles in cells engaged in the meiotic cell cycle. The bundling of meiotic chromosomes through their telomeres results in the formation of a ‘bouquet’ arrangement, which pairs homologous chromosomes, thereby ensuring recombination and progression through meiosis. In S. pombe, activation of meiosis results in the induction of genes encoding two proteins, bouquet formation protein 1 (Bqt1) and Bqt2 (REFS 92,93). Together with Bqt3 and Bqt4, these ubiquitously expressed Bqt proteins connect telomere-associated factors (repressor and activator site-binding protein 1 (Rap1) and Taz1) to the SUN domain protein Sad1 (REFS 92–94) (FIG. 4b). These connections are crucial for the localization of telomeres to the nuclear periphery, bouquet formation and progression through meiosis, and Sad1–Kms1 interactions are thought to translate cytoskeletal forces to chromosome movements in the nucleus92 (TABLE 1; FIG. 4b). Consistent with this, meiotic chromosome movements were recently found to be driven by forces transduced through the nuclear envelope of S. cerevisiae95. In addition, similar cytoskeletal forces are relayed across the nuclear envelope by SUN–KASH interactions to coordinate meiotic chromosome pairing and synapsis in worms96,97 (TABLE 1). Thus, these forces are likely to be widely conserved, especially as the clustering of meiotic telomeres and other chromosomal regions at the nuclear periphery requires SUN domain proteins, such as Mps3 in yeast, worms, mice and rats98–102 (TABLE 1).
Interestingly, if the roles of SUN domain proteins in the meiotic and vegetative cell cycle are similar, Mps3 might also cooperate with ONM proteins to translate cytoskeletal forces into a dynamic reorganization of telomeres or other loci during vegetative growth. Similar to meiosis, this reorganization might promote or prevent the occurrence of recombination events or chromosome maintenance steps depending on genome status. Taken together, these studies reveal that interactions between INM and ONM proteins provide a direct mode of communication between the cytoskeleton and the nucleus. This suggests that the nucleus and cytoplasm can exchange information without transporting factors through NPCs.
NPCs, lamins and DNA stability
In addition to the LEM, SUN and KASH domain proteins (TABLE 1), the involvement of NPCs — the most conspicuous nuclear envelope structures — and lamins in genome function are under intense investigation.
NPCs and subtelomeric DNA repair
A role for NPCs in telomere anchoring, silencing and stability has been the focus of several studies. The initial proposal that NPCs anchor chromosome ends to the periphery was met with skepticism as telomeric clusters do not significantly localize to nuclear pores under normal conditions. In addition, data from different studies did not agree on the role of the NPC proteins myosin-like protein 1 (Mlp1) and Mlp2 in perinuclear anchoring and transcriptional silencing of telomeres in yeast103–105. Although these proteins are now thought to be more important for telomere length control than general telomere anchoring or silencing, a recent study suggested that a core NPC subcomplex called Nup84 might at least contribute to the anchoring of some telomeres at the nuclear periphery26,104. Nup84 is a conserved seven-subunit subcomplex, and several Nup84 subunits behave like Sir4, Esc1 and Ku proteins in that they are required for the perinuclear localization of the left arm of chromosome XI of S. cerevisiae. Interestingly, deletion of Nup84 components also abrogates the ability of cells to repair DSBs by non-homologous end joining (NHEJ) only when artificially generated in the subtelomeric region of the chromosome (TABLE 1; FIG. 3a,c). In addition, the severity of telomeric silencing and repair defects did not always correlate in cells lacking Nup84 subunits or Esc1, suggesting that perinuclear subtelomeric silencing and repair may be separable4,26.
DNA damage targeted to NPCs
A study conducted in S. cerevisiae reported that the induction of a DSB at a modified MAT locus, which is somewhat randomly located in the nucleus, can result in the relocation of the damaged locus to the NPC. Importantly, NPC association occurs only when donor sequences, which can be used as a template for repair by homologous recombination, are not available22. The constraint of the tagged locus increased over time after DSB induction. When synchronized cells were subjected to conditions promoting DNA polymerase stalling, early firing replication origins remained randomly distributed in the nucleus unless replication fork breakage was enhanced by treating cells with additional toxic agents. This led the authors to propose that replication fork-associated breaks, rather than DNA polymerase stalling alone, induce perinuclear targeting to prevent genomic havoc. In addition, the damaged locus is recruited to Nup84, which itself interacts in a damage-independent manner with the small ubiquitin-related modifier (SUMO)-targeted ubiquitin ligase (STUbL) complex (composed of synthetic lethal of unknown function 5 (Slx5) and Slx8). The STUbL complex is stimulated by SUMO-modified substrates, harbours SUMO-interacting motifs and forms SUMO-dependent nuclear foci, including DNA repair centres. Recruitment to the NPC was dependent on mitosis entry checkpoint 1 (Mec1) and Tel1, which are kinases typically recruited to DSBs to trigger repair and delay progression through the cell cycle in S. cerevisiae. Disruption of Nup84–STUbL complexes results in an increased formation of spontaneous DNA repair foci and gross chromosomal rearrangements, suggesting that this complex resolves DNA damage at collapsed replication forks (FIG. 3a,d). This led to a model in which collapsed replication forks may be localized to the nuclear periphery to allow the Nup84-anchored STUbL to target key substrates for ubiquitin-dependent proteolysis22 (TABLE 1; FIG. 3d). This is thought to promote a specialized form of DNA repair at the nuclear periphery in order to rescue damaged replication forks. Future work should help reveal the exact role or substrate of STUbL at the nuclear periphery and the nature of this specialized form of DNA repair. Interestingly, the damaged MAT locus relocated to the nuclear periphery at sites other than telomere clusters22. Consistent with this, the artificial damaging of telomeres does not disrupt their perinuclear localization but, instead, causes the damaged domains to move closer to and interact with NPCs22,27 (TABLE 1). These findings suggest that damaged loci, whether attached to the nuclear envelope or not, may be targeted to NPCs for repair in S. cerevisiae.
Taken together, these studies suggest that NPCs are crucial components of DNA repair processes at the nuclear periphery. How does the NPC carry out this function? It may be that key forms of certain repair factors, such as STUbL, are specifically enriched at NPCs. Another possibility is that the NPC acts as a solid support, allowing the formation of chromatin structures that are conducive to DNA repair or replication fork rescue. Such functions would not be mutually exclusive and, irrespective of the precise mechanism, these findings suggest that NPCs define subnuclear locations with important roles in DNA damage repair in S. cerevisiae.
Lamins and genome integrity
The nuclear periphery has also been linked to the maintenance of genome stability in mammalian cells. Although most mammalian telomeres localize to the nuclear interior, lamin-associated DNA domains are present at several subtelomeres, and A-type lamins were found to target subtelomeres containing repetitive DNA sequences called D4Z4 repeats to the nuclear periphery in human cells6,21. Interestingly, the number of D4Z4 repeats seems to be lower in patients affected with autosomal dominant facio-scapulo-humeral muscular dystrophy, suggesting that perinuclear telomere targeting defects may contribute to this disease6. Another study reported that 20% of telomeres in mouse embryonic fibroblasts localize to the nuclear periphery7. The loss of A-type lamins results in defects in telomere length, telomeric heterochromatin structures and telomere processing by NHEJ, but it is unclear if these effects are related to direct lamin–telomere interactions at the nuclear periphery7. Thus, similar to LEM, SUN and KASH domain proteins, lamins and NPCs are key perinuclear factors that interact with various chromosomal domains to regulate transcription and protect the genome.
A general role for lamins and NPCs in genome stability is also supported by several other studies. Human lamins are preferentially linked to chromosomal regions that are characterized by low transcriptional activity and thought to constitute architectural units of interphase chromosomes21. Lamin mutations linked to laminopathies are associated with severe losses in the structural integrity of the nucleus and with subnuclear chromatin organization defects15. In addition, lamins have been linked to various transcription regulatory programmes, including the sequestration of transcription factors in inactive complexes at the nuclear envelope106. In yeast, mutations in NPC subcomplexes are synthetic lethal, with mutations impairing homologous recombination, rendering cells hypersensitive to DNA damaging agents and inducing spontaneous DNA damage107,111. Taken together, the above studies suggest that lamins and NPCs are crucial for eukaryotic genome integrity.
Concluding remarks
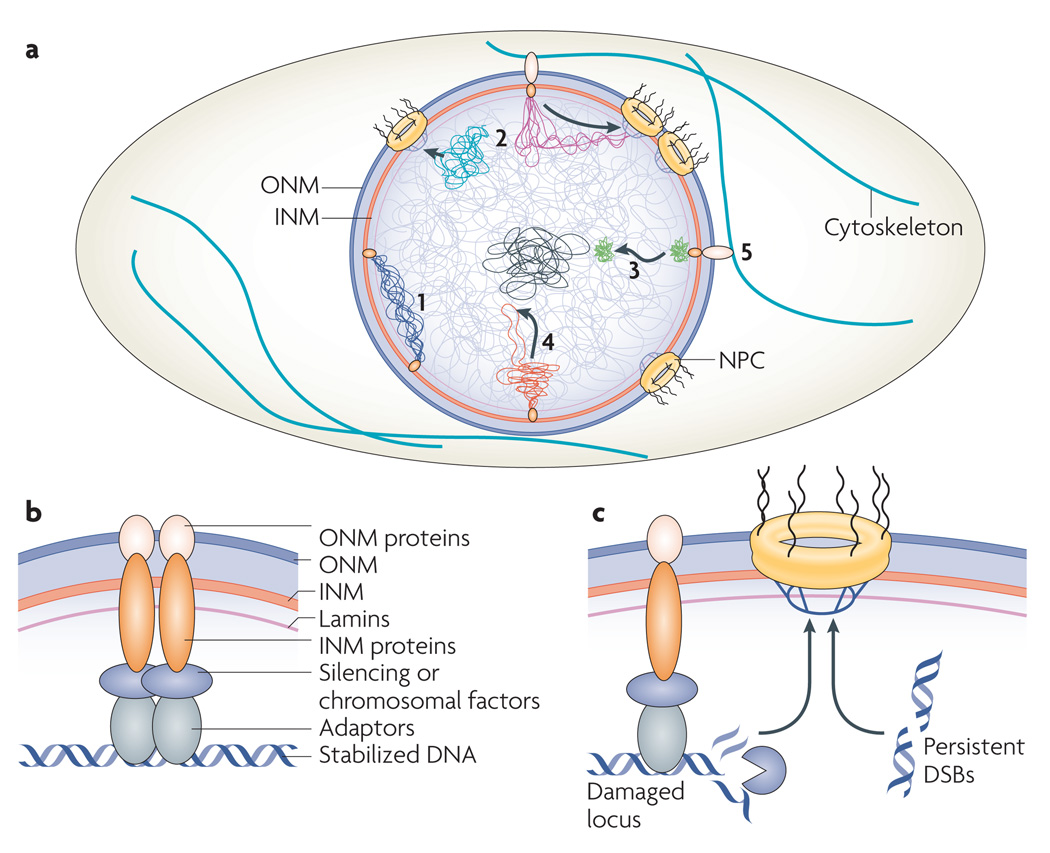
Genome spatial organization is controlled by processes that involve interactions with the nuclear periphery (FIG. 5a). The impact of the nuclear periphery on the genome is exerted at least partially by INM proteins, NPCs and cytoskeletal components. In particular, INM proteins and lamins are emerging as tethers for chromosomal domains and can prevent or minimize damage to DNA4,24,37–39 (FIG. 5b). However, NPCs seem to take over once a genomic locus accumulates significant damage22,23,26,27 (FIG. 5c).
Figure 5. Perinuclear tethering and global genome organization.
a | General view of genome organization at the nuclear periphery. Perinuclear networks relying on inner nuclear membrane (INM) proteins tether ‘at risk’ chromosome domains to the nuclear envelope, separating them from bulk nuclear DNA and maintaining genome stability (1). Whether membrane-bound (pink) or not (blue), loci in crisis are relocated to nuclear pore complex (NPC)-associated repair centres (2). The subnuclear relocation of a locus located in a dense chromosome domain may influence the subnuclear relocation of many loci in the same chromosomal region or in surrounding nuclear neighborhoods (3 and 4; chromosome domain in (3) is denser than that in (4)). The cytoskeleton modulates chromosome dynamics through INM–outer nuclear membrane (ONM) protein connections (5). b | Molecular interactions linking DNA to the nuclear envelope. DNA-binding adaptors recruit chromatin to the nuclear periphery by relying on various complexes including silencing complexes and INM proteins. Whereas the loss of silencing proteins disrupts tethering, deletion or disruption of INM proteins abrogates anchoring and promotes aberrant recombination events, but does not always affect silencing. INM proteins may not always rely on interactions with ONM proteins to move DNA in the nucleus. In yeast, these connections are maintained in the absence of lamins. c | Damaged loci or genomic regions with persistent double strand breaks (DSBs) are relocated along the nuclear periphery or from an internal nuclear location to NPCs, which mediate specific forms of DNA repair and protection.
The findings discussed here highlight the role of the nuclear periphery in chromatin silencing, genome stability and progression through the cell cycle. How these processes are coordinated in the nucleus, integrated with nucleoplasmic genome organization and affected during cell division are only some of the key questions that remain unanswered. Is all DNA movement random or is it guided? Although several studies point to a role for actin and myosin in the movement of genomic regions over long distances, random DNA movement has also been observed112–114. Moreover, various non-coding RNA molecules are transcribed from many of the genomic regions with stability that is maintained by perinuclear localization115. In addition to modulating chromatin structure115, these RNA molecules may have roles in locus clustering and perinuclear localization. Further characterization of perinuclear networks will probably uncover many of the secrets of non-random genome organization and its role in gene regulation, genome stability and disease.
Acknowledgements
We thank J.N.Y. Chan and other members of our laboratories for discussions and comments. K.M. is supported by the Canadian Institutes of Health Research (CIHR) Institute of Aging and the CIHR Institute of Genetics Lap-Chee Tsui Award. D.M. is supported by the National Institutes of Health (NIH) and is an investigator of the Howard Hughes Medical Institute (HHMI).
Glossary
- Nucleolus
A nuclear compartment that is home to genes encoding rRNA and is the site of most ribosome manufacturing steps.
- Telomere
A segment at the end of each chromosome arm that consists of repetitive sequences and prevents the normal chromosome ends from being recognized as DSBs.
- Centromere
A specialized region on a chromosome that is composed of highly condensed heterochromatin and is the platform for the assembly of kinetochores.
- Intermediate filament protein
A member of a large family of cytoplasmic and nuclear proteins that polymerize into stable filaments of ~10 nm in diameter.
- Muscular dystrophies
A group of diseases characterized by the gradual loss of muscle structure and function. These diseases can be caused by mutations affecting various proteins, including lamins and INM proteins.
- Laminopathies
A group of diseases that include premature ageing syndromes and certain types of muscular dystrophies and are associated with mutations in genes encoding lamins.
- DamID
A technology that identifies the DNA sites with which a protein interacts by fusing the protein to an adenine methyltransferase. This allows for site identification by detection of the foreign adenosine methylation mark across the genome.
- Insulator protein
A protein that binds an insulator element, which is a genetic boundary element that can block enhancers.
- Histone deacetylase
An enzyme that removes post-translational acetylation marks from amino acids on the tails of histone proteins.
- Heterochromatin
A genomic region characterized by a compact form of chromatin that makes the DNA less accessible to the protein factors that usually bind.
- B-type lamin
One of a group of proteins that include the ubiquitously expressed lamin B1 and lamin B2, which are encoded by the LMNB1 and LMNB2 genes, respectively.
- RENT
A protein complex that ensures silent chromatin assembly at the intergenic spacers of ribosomal DNA, maintains the stability of ribosomal DNA repeats and prevents premature exit from the cell cycle.
- Cohibin
A protein complex that maintains the stability of rDNA repeats by ensuring silent chromatin assembly and attachment to the nuclear periphery.
- CLIP
A complex of INM proteins that maintains the stability of ribosomal DNA repeats by anchoring them to the nuclear envelope.
- Emery–Dreifuss muscular dystrophy
A disease that is characterized by muscle wasting and cardiac defects and is associated with mutations in genes encoding lamins or the LEM domain protein emerin.
- Telomerase
An enzyme that uses its own RNA subunit as a template to catalyse the lengthening of telomeres following replication.
- Non-homologous end joining
A pathway that repairs DNA DSBs by directly ligating the broken ends, without the need for a homologous template.
- A-type lamin
One of a group of lamins that are primarily expressed after the gastrulation stage of development. This class includes the splice variants lamin A and lamin C, which are encoded by the LMNA gene.
- Facio-scapulo-humeral muscular dystrophy
A common form of muscular dystrophy that is characterized by the progressive weakening or loss of skeletal muscles. Common locations of these weaknesses at disease onset are the face (facio), shoulder (scapulo) and upper arms (humeral).
- Synthetic lethal
A genetic interaction in which the deletion of two genes at the same time results in lethality. An organism in which one gene is deleted and the other gene is present will still be viable.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
DATABASES
UniProtKB: http://www.uniprot.org
Bqt1 | Bqt2 | Cdc14 | Csm1 | emerin | Esc1 | Est1 | Est2 | Est3 | Fob1 | Heh1 | Kms1 | Kms2 | Ku70 | Ku80 | lamin B1 | LAP2β | Lrs4 | Mec1 | Mlp1 | Mlp2 | Mps3 | Net1 | Nur1 | Rap1 | Sad1 | Sir2 | Sir3 | Sir4 | Slx5 | Slx8 | Taz1 | Tel1 | Tof2
FURTHER INFORMATION
Karim Mekhail’s homepage: http://individual.utoronto.ca/mekhailgenome/
Danesh Moazed’s homepage: https://moazed.med.harvard.edu
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
Contributor Information
Karim Mekhail, Email: karim.mekhail@utoronto.ca.
Danesh Moazed, Email: danesh@hms.harvard.edu.
References
- 1.Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 2.Takizawa T, Meaburn KJ, Misteli T. The meaning of gene positioning. Cell. 2008;135:9–13. doi: 10.1016/j.cell.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Towbin BD, Meister P, Gasser SM. The nuclear envelope — a scaffold for silencing? Curr. Opin. Genet. Dev. 2009;19:180–186. doi: 10.1016/j.gde.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 4. Mekhail K, Seebacher J, Gygi SP, Moazed D. Role for perinuclear chromosome tethering in maintenance of genome stability. Nature. 2008;456:667–670. doi: 10.1038/nature07460. Yeast rDNA repeats are stabilized by perinuclear membrane tethers.
- 5.Mekhail K, et al. Regulation of ubiquitin ligase dynamics by the nucleolus. J. Cell Biol. 2005;170:733–744. doi: 10.1083/jcb.200506030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ottaviani A, et al. Identification of a perinuclear positioning element in human subtelomeres that requires A-type lamins and CTCF. EMBO J. 2009;28:2428–2436. doi: 10.1038/emboj.2009.201. A-type lamins recruit subtelomeric regions to the nuclear periphery in human cells.
- 7.Gonzalez-Suarez I, et al. Novel roles for A-type lamins in telomere biology and the DNA damage response pathway. EMBO J. 2009;28:2414–2427. doi: 10.1038/emboj.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Funabiki H, Hagan I, Uzawa S, Yanagida M. Cell cycle-dependent specific positioning and clustering of centromeres and telomeres in fission yeast. J. Cell Biol. 1993;121:961–976. doi: 10.1083/jcb.121.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agard DA, Sedat JW. Three-dimensional architecture of a polytene nucleus. Nature. 1983;302:676–681. doi: 10.1038/302676a0. [DOI] [PubMed] [Google Scholar]
- 10.Hochstrasser M, Mathog D, Gruenbaum Y, Saumweber H, Sedat JW. Spatial organization of chromosomes in the salivary gland nuclei of Drosophila melanogaster. J. Cell Biol. 1986;102:112–123. doi: 10.1083/jcb.102.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang Y, Spector DL. Centromere positioning and dynamics in living Arabidopsis plants. Mol. Biol. Cell. 2005;16:5710–5718. doi: 10.1091/mbc.E05-08-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starr DA, Han M. Role of ANC-1 in tethering nuclei to the actin cytoskeleton. Science. 2002;298:406–409. doi: 10.1126/science.1075119. [DOI] [PubMed] [Google Scholar]
- 13.Starr DA. A nuclear-envelope bridge positions nuclei and moves chromosomes. J. Cell Sci. 2009;122:577–586. doi: 10.1242/jcs.037622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. King MC, Drivas TG, Blobel G. A network of nuclear envelope membrane proteins linking centromeres to microtubules. Cell. 2008;134:427–438. doi: 10.1016/j.cell.2008.06.022. Cooperation between SUN–KASH domain interactions and silent chromatin at centromeres in the buffering of subcellular forces and the maintenance of nuclear integrity in yeast.
- 15.Capell BC, Collins FS. Human laminopathies: nuclei gone genetically awry. Nature Rev. Genet. 2006;7:940–952. doi: 10.1038/nrg1906. [DOI] [PubMed] [Google Scholar]
- 16.Schirmer EC, Florens L, Guan T, Yates JR, 3rd, Gerace L. Nuclear membrane proteins with potential disease links found by subtractive proteomics. Science. 2003;301:1380–1382. doi: 10.1126/science.1088176. [DOI] [PubMed] [Google Scholar]
- 17.Kalverda B, Pickersgill H, Shloma VV, Fornerod M. Nucleoporins directly stimulate expression of developmental and cell-cycle genes inside the nucleoplasm. Cell. 2010;140:360–371. doi: 10.1016/j.cell.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 18. Capelson M, et al. Chromatin-bound nuclear pore components regulate gene expression in higher eukaryotes. Cell. 2010;140:372–383. doi: 10.1016/j.cell.2009.12.054. References 17 and 18 show that, in D. melanogaster, nucleoplasmic NPC components interact with active loci and perinuclear NPCs interact with a smaller group of silent genes.
- 19.Vaquerizas JM, et al. Nuclear pore proteins Nup153 and Megator define transcriptionally active regions in the Drosophila genome. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1000846. e1000846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pickersgill H, et al. Characterization of the Drosophila melanogaster genome at the nuclear lamina. Nature Genet. 2006;38:1005–1014. doi: 10.1038/ng1852. [DOI] [PubMed] [Google Scholar]
- 21.Guelen L, et al. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature. 2008;453:948–951. doi: 10.1038/nature06947. [DOI] [PubMed] [Google Scholar]
- 22. Nagai S, et al. Functional targeting of DNA damage to a nuclear pore-associated SUMO-dependent ubiquitin ligase. Science. 2008;322:597–602. doi: 10.1126/science.1162790. A locus with persistent damage relocates to NPCs at the nuclear periphery.
- 23.Oza P, Jaspersen SL, Miele A, Dekker J, Peterson CL. Mechanisms that regulate localization of a DNA double-strand break to the nuclear periphery. Genes Dev. 2009;23:912–927. doi: 10.1101/gad.1782209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schober H, Ferreira H, Kalck V, Gehlen LR, Gasser SM. Yeast telomerase and the SUN domain protein Mps3 anchor telomeres and repress subtelomeric recombination. Genes Dev. 2009;23:928–938. doi: 10.1101/gad.1787509. A SUN domain protein and telomerase cooperate to maintain yeast telomere stability.
- 25.Gartenberg MR. Life on the edge: telomeres and persistent DNA breaks converge at the nuclear periphery. Genes Dev. 2009;23:1027–1031. doi: 10.1101/gad.1805309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Therizols P, et al. Telomere tethering at the nuclear periphery is essential for efficient DNA double strand break repair in subtelomeric region. J. Cell Biol. 2006;172:189–199. doi: 10.1083/jcb.200505159. DSBs require NPC components for repair by NHEJ in yeast.
- 27.Khadaroo B, et al. The DNA damage response at eroded telomeres and tethering to the nuclear pore complex. Nature Cell Biol. 2009;11:980–987. doi: 10.1038/ncb1910. [DOI] [PubMed] [Google Scholar]
- 28. Croft JA, et al. Differences in the localization and morphology of chromosomes in the human nucleus. J. Cell Biol. 1999;145:1119–1131. doi: 10.1083/jcb.145.6.1119. Introduction of the concept of radial locus positioning and association of gene-light chromosomes with the nuclear periphery.
- 29.van Steensel B, Henikoff S. Identification of in vivo DNA targets of chromatin proteins using tethered Dam methyltransferase. Nature Biotechnol. 2000;18:424–428. doi: 10.1038/74487. [DOI] [PubMed] [Google Scholar]
- 30.Comings DE. Arrangement of chromatin in the nucleus. Hum. Genet. 1980;53:131–143. doi: 10.1007/BF00273484. [DOI] [PubMed] [Google Scholar]
- 31.Palladino F, et al. Sir3 and Sir4 proteins are required for the positioning and integrity of yeast telomeres. Cell. 1993;75:543–555. doi: 10.1016/0092-8674(93)90388-7. [DOI] [PubMed] [Google Scholar]
- 32.Csink AK, Henikoff S. Genetic modification of heterochromatic association and nuclear organization in Drosophila. Nature. 1996;381:529–531. doi: 10.1038/381529a0. [DOI] [PubMed] [Google Scholar]
- 33. Andrulis ED, Neiman AM, Zappulla DC, Sternglanz R. Perinuclear localization of chromatin facilitates transcriptional silencing. Nature. 1998;394:592–595. doi: 10.1038/29100. An early study linking perinuclear localization to silent chromatin in yeast.
- 34. Reddy KL, Zullo JM, Bertolino E, Singh H. Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature. 2008;452:243–247. doi: 10.1038/nature06727. INM protein-dependent localization of genes to the nuclear envelope promotes heterochromatin-dependent transcriptional silencing in metazoans.
- 35.Finlan LE, et al. Recruitment to the nuclear periphery can alter expression of genes in human cells. PLoS Genet. 2008;4 doi: 10.1371/journal.pgen.1000039. e1000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grund SE, et al. The inner nuclear membrane protein Src1 associates with subtelomeric genes and alters their regulated gene expression. J. Cell Biol. 2008;182:897–910. doi: 10.1083/jcb.200803098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bupp JM, Martin AE, Stensrud ES, Jaspersen SL. Telomere anchoring at the nuclear periphery requires the budding yeast Sad1-UNC-84 domain protein Mps3. J. Cell Biol. 2007;179:845–854. doi: 10.1083/jcb.200706040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gartenberg MR, Neumann FR, Laroche T, Blaszczyk M, Gasser SM. Sir-mediated repression can occur independently of chromosomal and subnuclear contexts. Cell. 2004;119:955–967. doi: 10.1016/j.cell.2004.11.008. A gene excised from its genomic location at the nuclear periphery remains silenced owing to the maintenance of silencing protein interactions at the wandering locus.
- 39.Ansari A, Gartenberg MR. Persistence of an alternate chromatin structure at silenced loci in vitro. Proc. Natl Acad. Sci. USA. 1999;96:343–348. doi: 10.1073/pnas.96.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumaran RI, Spector DL. A genetic locus targeted to the nuclear periphery in living cells maintains its transcriptional competence. J. Cell Biol. 2008;180:51–65. doi: 10.1083/jcb.200706060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shevelyov YY, et al. The B-type lamin is required for somatic repression of testis-specific gene clusters. Proc. Natl Acad. Sci. USA. 2009;106:3282–3287. doi: 10.1073/pnas.0811933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Casolari JM, et al. Genome-wide localization of the nuclear transport machinery couples transcriptional status and nuclear organization. Cell. 2004;117:427–439. doi: 10.1016/s0092-8674(04)00448-9. [DOI] [PubMed] [Google Scholar]
- 43.Taddei A, et al. Nuclear pore association confers optimal expression levels for an inducible yeast gene. Nature. 2006;441:774–778. doi: 10.1038/nature04845. [DOI] [PubMed] [Google Scholar]
- 44.Brickner JH, Walter P. Gene recruitment of the activated INO1 locus to the nuclear membrane. PLoS Biol. 2004;2:e342. doi: 10.1371/journal.pbio.0020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cabal GG, et al. SAGA interacting factors confine sub-diffusion of transcribed genes to the nuclear envelope. Nature. 2006;441:770–773. doi: 10.1038/nature04752. [DOI] [PubMed] [Google Scholar]
- 46.Dieppois G, Iglesias N, Stutz F. Cotranscriptional recruitment to the mRNA export receptor Mex67p contributes to nuclear pore anchoring of activated genes. Mol. Cell. Biol. 2006;26:7858–7870. doi: 10.1128/MCB.00870-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akhtar A, Gasser SM. The nuclear envelope and transcriptional control. Nature Rev. Genet. 2007;8:507–517. doi: 10.1038/nrg2122. [DOI] [PubMed] [Google Scholar]
- 48. Ahmed S, et al. DNA zip codes control an ancient mechanism for gene targeting to the nuclear periphery. Nature Cell Biol. 2010;12:111–118. doi: 10.1038/ncb2011. Ancient DNA zip codes target active genes to the nuclear periphery in S. cerevisiae and S. pombe.
- 49.Rabut G, Doye V, Ellenberg J. Mapping the dynamic organization of the nuclear pore complex inside single living cells. Nature Cell Biol. 2004;6:1114–1121. doi: 10.1038/ncb1184. [DOI] [PubMed] [Google Scholar]
- 50.Brown CR, Kennedy CJ, Delmar VA, Forbes DJ, Silver PA. Global histone acetylation induces functional genomic reorganization at mammalian nuclear pore complexes. Genes Dev. 2008;22:627–639. doi: 10.1101/gad.1632708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maillet L, et al. Evidence for silencing compartments within the yeast nucleus: a role for telomere proximity and Sir protein concentration in silencer-mediated repression. Genes Dev. 1996;10:1796–1811. doi: 10.1101/gad.10.14.1796. [DOI] [PubMed] [Google Scholar]
- 52.Schermelleh L, et al. Subdiffraction multicolor imaging of the nuclear periphery with 3D structured illumination microscopy. Science. 2008;320:1332–1336. doi: 10.1126/science.1156947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gasser SM, Hediger F, Taddei A, Neumann FR, Gartenberg MR. The function of telomere clustering in yeast: the circe effect. Cold Spring Harb. Symp. Quant. Biol. 2004;69:327–337. doi: 10.1101/sqb.2004.69.327. [DOI] [PubMed] [Google Scholar]
- 54.Ishii K, Arib G, Lin C, Van Houwe G, Laemmli UK. Chromatin boundaries in budding yeast: the nuclear pore connection. Cell. 2002;109:551–562. doi: 10.1016/s0092-8674(02)00756-0. [DOI] [PubMed] [Google Scholar]
- 55.Pai CY, Corces VG. The nuclear pore complex and chromatin boundaries. Trends Cell Biol. 2002;12:452–455. doi: 10.1016/s0962-8924(02)02367-x. [DOI] [PubMed] [Google Scholar]
- 56.Kalocsay M, Hiller NJ, Jentsch S. Chromosome-wide Rad51 spreading and SUMO-H2A.Z-dependent chromosome fixation in response to a persistent DNA double-strand break. Mol. Cell. 2009;33:335–343. doi: 10.1016/j.molcel.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 57.Sung P, Klein H. Mechanism of homologous recombination: mediators and helicases take on regulatory functions. Nature Rev. Mol. Cell Biol. 2006;7:739–750. doi: 10.1038/nrm2008. [DOI] [PubMed] [Google Scholar]
- 58.Franco S, Alt FW, Manis JP. Pathways that suppress programmed DNA breaks from progressing to chromosomal breaks and translocations. DNA Repair (Amst) 2006;5:1030–1041. doi: 10.1016/j.dnarep.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 59.Nomura M. Ribosomal RNA genes, RNA polymerases, nucleolar structures, and synthesis of rRNA in the yeast Saccharomyces cerevisiae. Cold Spring Harb. Symp. Quant. Biol. 2001;66:555–565. doi: 10.1101/sqb.2001.66.555. [DOI] [PubMed] [Google Scholar]
- 60.Keil RL, Roeder GS. Cis-acting, recombination-stimulating activity in a fragment of the ribosomal DNA of S. cerevisiae. Cell. 1984;39:377–386. doi: 10.1016/0092-8674(84)90016-3. [DOI] [PubMed] [Google Scholar]
- 61.Brewer BJ, Fangman WL. A replication fork barrier at the 3′ end of yeast ribosomal RNA genes. Cell. 1988;55:637–643. doi: 10.1016/0092-8674(88)90222-x. [DOI] [PubMed] [Google Scholar]
- 62.Kobayashi T, Horiuchi T. A yeast gene product, Fob1 protein, required for both replication fork blocking and recombinational hotspot activities. Genes Cells. 1996;1:465–474. doi: 10.1046/j.1365-2443.1996.d01-256.x. [DOI] [PubMed] [Google Scholar]
- 63.Straight AF, et al. Net1, a Sir2-associated nucleolar protein required for rDNA silencing and nucleolar integrity. Cell. 1999;97:245–256. doi: 10.1016/s0092-8674(00)80734-5. [DOI] [PubMed] [Google Scholar]
- 64.Visintin R, Hwang ES, Amon A. Cfi1 prevents premature exit from mitosis by anchoring Cdc14 phosphatase in the nucleolus. Nature. 1999;398:818–823. doi: 10.1038/19775. [DOI] [PubMed] [Google Scholar]
- 65.Shou W, et al. Exit from mitosis is triggered by Tem1-dependent release of the protein phosphatase Cdc14 from nucleolar RENT complex. Cell. 1999;97:233–244. doi: 10.1016/s0092-8674(00)80733-3. [DOI] [PubMed] [Google Scholar]
- 66.Huang J, Moazed D. Association of the RENT complex with nontranscribed and coding regions of rDNA and a regional requirement for the replication fork block protein Fob1 in rDNA silencing. Genes Dev. 2003;17:2162–2176. doi: 10.1101/gad.1108403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rabitsch KP, et al. Kinetochore recruitment of two nucleolar proteins is required for homolog segregation in meiosis I. Dev. Cell. 2003;4:535–548. doi: 10.1016/s1534-5807(03)00086-8. [DOI] [PubMed] [Google Scholar]
- 68.Huang J, et al. Inhibition of homologous recombination by a cohesin-associated clamp complex recruited to the rDNA recombination enhancer. Genes Dev. 2006;20:2887–2901. doi: 10.1101/gad.1472706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bryk M, et al. Transcriptional silencing of Ty1 elements in the RDN1 locus of yeast. Genes Dev. 1997;11:255–269. doi: 10.1101/gad.11.2.255. [DOI] [PubMed] [Google Scholar]
- 70.Smith JS, Boeke JD. An unusual form of transcriptional silencing in yeast ribosomal DNA. Genes Dev. 1997;11:241–254. doi: 10.1101/gad.11.2.241. [DOI] [PubMed] [Google Scholar]
- 71.Gottlieb S, Esposito RE. A new role for a yeast transcriptional silencer gene, SIR2, in regulation of recombination in ribosomal DNA. Cell. 1989;56:771–776. doi: 10.1016/0092-8674(89)90681-8. [DOI] [PubMed] [Google Scholar]
- 72.Fritze CE, Verschueren K, Strich R, Easton Esposito R. Direct evidence for SIR2 modulation of chromatin structure in yeast rDNA. EMBO J. 1997;16:6495–6509. doi: 10.1093/emboj/16.21.6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith JS, Caputo E, Boeke JD. A genetic screen for ribosomal DNA silencing defects identifies multiple DNA replication and chromatin-modulating factors. Mol. Cell. Biol. 1999;19:3184–3197. doi: 10.1128/mcb.19.4.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moazed D. Common themes in mechanisms of gene silencing. Mol. Cell. 2001;8:489–498. doi: 10.1016/s1097-2765(01)00340-9. [DOI] [PubMed] [Google Scholar]
- 75.Hellemans J, et al. Loss-of-function mutations in LEMD3 result in osteopoikilosis, Buschke-Ollendorff syndrome and melorheostosis. Nature Genet. 2004;36:1213–1218. doi: 10.1038/ng1453. [DOI] [PubMed] [Google Scholar]
- 76.Brachner A, Reipert S, Foisner R, Gotzmann J. LEM2 is a novel MAN1-related inner nuclear membrane protein associated with A-type lamins. J. Cell Sci. 2005;118:5797–5810. doi: 10.1242/jcs.02701. [DOI] [PubMed] [Google Scholar]
- 77.Rodriguez-Navarro S, Igual JC, Perez-Ortin JE. SRC1: an intron-containing yeast gene involved in sister chromatid segregation. Yeast. 2002;19:43–54. doi: 10.1002/yea.803. [DOI] [PubMed] [Google Scholar]
- 78.Bione S, et al. Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nature Genet. 1994;8:323–327. doi: 10.1038/ng1294-323. [DOI] [PubMed] [Google Scholar]
- 79.Torres-Rosell J, et al. The Smc5–Smc6 complex and SUMO modification of Rad52 regulates recombinational repair at the ribosomal gene locus. Nature Cell Biol. 2007;9:923–931. doi: 10.1038/ncb1619. [DOI] [PubMed] [Google Scholar]
- 80.Liu J, et al. MAN1 and emerin have overlapping function(s) essential for chromosome segregation and cell division in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA. 2003;100:4598–4603. doi: 10.1073/pnas.0730821100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blasco MA. The epigenetic regulation of mammalian telomeres. Nature Rev. Genet. 2007;8:299–309. doi: 10.1038/nrg2047. [DOI] [PubMed] [Google Scholar]
- 82.Taddei A, Gasser SM. Repairing subtelomeric DSBs at the nuclear periphery. Trends Cell Biol. 2006;16:225–228. doi: 10.1016/j.tcb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 83.Antoniacci LM, Kenna MA, Skibbens RV. The nuclear envelope and spindle pole body-associated Mps3 protein bind telomere regulators and function in telomere clustering. Cell Cycle. 2007;6:75–79. doi: 10.4161/cc.6.1.3647. [DOI] [PubMed] [Google Scholar]
- 84.Andrulis ED, et al. Esc1, a nuclear periphery protein required for Sir4-based plasmid anchoring and partitioning. Mol. Cell. Biol. 2002;22:8292–8301. doi: 10.1128/MCB.22.23.8292-8301.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hediger F, Neumann FR, Van Houwe G, Dubrana K, Gasser SM. Live imaging of telomeres: yKu and Sir proteins define redundant telomere-anchoring pathways in yeast. Curr. Biol. 2002;12:2076–2089. doi: 10.1016/s0960-9822(02)01338-6. [DOI] [PubMed] [Google Scholar]
- 86.Taddei A, Hediger F, Neumann FR, Bauer C, Gasser SM. Separation of silencing from perinuclear anchoring functions in yeast Ku80, Sir4 and Esc1 proteins. EMBO J. 2004;23:1301–1312. doi: 10.1038/sj.emboj.7600144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Taddei A, et al. The functional importance of telomere clustering: global changes in gene expression result from SIR factor dispersion. Genome Res. 2009;19:611–625. doi: 10.1101/gr.083881.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lingner J, Cooper JP, Cech TR. Telomerase and DNA end replication: no longer a lagging strand problem? Science. 1995;269:1533–1534. doi: 10.1126/science.7545310. [DOI] [PubMed] [Google Scholar]
- 89.Hediger F, Berthiau AS, van Houwe G, Gilson E, Gasser SM. Subtelomeric factors antagonize telomere anchoring and Tel1-independent telomere length regulation. EMBO J. 2006;25:857–867. doi: 10.1038/sj.emboj.7600976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kobayashi T, Ganley AR. Recombination regulation by transcription-induced cohesin dissociation in rDNA repeats. Science. 2005;309:1581–1584. doi: 10.1126/science.1116102. [DOI] [PubMed] [Google Scholar]
- 91.Muntoni A, Reddel RR. The first molecular details of ALT in human tumor cells. Hum. Mol. Genet. 2005;14:R191–R196. doi: 10.1093/hmg/ddi266. [DOI] [PubMed] [Google Scholar]
- 92.Chikashige Y, et al. Meiotic proteins Bqt1 and Bqt2 tether telomeres to form the bouquet arrangement of chromosomes. Cell. 2006;125:59–69. doi: 10.1016/j.cell.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 93.Tang X, Jin Y, Cande WZ. Bqt2p is essential for initiating telomere clustering upon pheromone sensing in fission yeast. J. Cell Biol. 2006;173:845–851. doi: 10.1083/jcb.200602152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chikashige Y, et al. Membrane proteins Bqt3 and -4 anchor telomeres to the nuclear envelope to ensure chromosomal bouquet formation. J. Cell Biol. 2009;187:413–427. doi: 10.1083/jcb.200902122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Koszul R, Kim KP, Prentiss M, Kleckner N, Kameoka S. Meiotic chromosomes move by linkage to dynamic actin cables with transduction of force through the nuclear envelope. Cell. 2008;133:1188–1201. doi: 10.1016/j.cell.2008.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Penkner AM, et al. Meiotic chromosome homology search involves modifications of the nuclear envelope protein Matefin/SUN-1. Cell. 2009;139:920–933. doi: 10.1016/j.cell.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 97.Sato A, et al. Cytoskeletal forces span the nuclear envelope to coordinate meiotic chromosome pairing and synapsis. Cell. 2009;139:907–919. doi: 10.1016/j.cell.2009.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Conrad MN, Lee CY, Wilkerson JL, Dresser ME. MPS3 mediates meiotic bouquet formation in Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA. 2007;104:8863–8868. doi: 10.1073/pnas.0606165104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ding X, et al. SUN1 is required for telomere attachment to nuclear envelope and gametogenesis in mice. Dev. Cell. 2007;12:863–872. doi: 10.1016/j.devcel.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 100.Schmitt J, et al. Transmembrane protein SUN2 is involved in tethering mammalian meiotic telomeres to the nuclear envelope. Proc. Natl Acad. Sci. USA. 2007;104:7426–7431. doi: 10.1073/pnas.0609198104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Penkner A, et al. The nuclear envelope protein Matefin/SUN-1 is required for homologous pairing in C. elegans meiosis. Dev. Cell. 2007;12:873–885. doi: 10.1016/j.devcel.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 102.Conrad MN, et al. Rapid telomere movement in meiotic prophase is promoted by NDJ1, MPS3, and CSM4 and is modulated by recombination. Cell. 2008;133:1175–1187. doi: 10.1016/j.cell.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 103.Feuerbach F, et al. Nuclear architecture and spatial positioning help establish transcriptional states of telomeres in yeast. Nature Cell Biol. 2002;4:214–221. doi: 10.1038/ncb756. [DOI] [PubMed] [Google Scholar]
- 104.Hediger F, Dubrana K, Gasser SM. Myosin-like proteins 1 and 2 are not required for silencing or telomere anchoring, but act in the Tel1 pathway of telomere length control. J. Struct. Biol. 2002;140:79–91. doi: 10.1016/s1047-8477(02)00533-6. [DOI] [PubMed] [Google Scholar]
- 105.Galy V, et al. Nuclear pore complexes in the organization of silent telomeric chromatin. Nature. 2000;403:108–112. doi: 10.1038/47528. [DOI] [PubMed] [Google Scholar]
- 106.Andres V, Gonzalez JM. Role of A-type lamins in signaling, transcription, and chromatin organization. J. Cell Biol. 2009;187:945–957. doi: 10.1083/jcb.200904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bennett CB, et al. Genes required for ionizing radiation resistance in yeast. Nature Genet. 2001;29:426–434. doi: 10.1038/ng778. [DOI] [PubMed] [Google Scholar]
- 108.Loeillet S, et al. Genetic network interactions among replication, repair and nuclear pore deficiencies in yeast. DNA Repair (Amst) 2005;4:459–468. doi: 10.1016/j.dnarep.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 109.Pan X, et al. A DNA integrity network in the yeast Saccharomyces cerevisiae. Cell. 2006;124:1069–1081. doi: 10.1016/j.cell.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 110.Palancade B, et al. Nucleoporins prevent DNA damage accumulation by modulating Ulp1-dependent sumoylation processes. Mol. Biol. Cell. 2007;18:2912–2923. doi: 10.1091/mbc.E07-02-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao X, Wu CY, Blobel G. Mlp-dependent anchorage and stabilization of a desumoylating enzyme is required to prevent clonal lethality. J. Cell Biol. 2004;167:605–611. doi: 10.1083/jcb.200405168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chuang CH, et al. Long-range directional movement of an interphase chromosome site. Curr. Biol. 2006;16:825–831. doi: 10.1016/j.cub.2006.03.059. [DOI] [PubMed] [Google Scholar]
- 113. Dundr M, et al. Actin-dependent intranuclear repositioning of an active gene locus in vivo. J. Cell Biol. 2007;179:1095–1103. doi: 10.1083/jcb.200710058. References 112 and 113 show evidence for actin–myosin-guided movement of DNA loci.
- 114.Holaska JM, Kowalski AK, Wilson KL. Emerin caps the pointed end of actin filaments: evidence for an actin cortical network at the nuclear inner membrane. PLoS Biol. 2004;2:E231. doi: 10.1371/journal.pbio.0020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moazed D. Small RNAs in transcriptional gene silencing and genome defence. Nature. 2009;457:413–420. doi: 10.1038/nature07756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sexton T, Schober H, Fraser P, Gasser SM. Gene regulation through nuclear organization. Nature Struct. Mol. Biol. 2007;14:1049–1055. doi: 10.1038/nsmb1324. [DOI] [PubMed] [Google Scholar]