Abstract
A synthetic approach is presented for the synthesis of galacturonic acid and d-fucosyl modified KRN7000. The approach allows for late-stage functionalisation of both the sugar 6”-OH and the sphingosine amino groups, which enables convenient synthesis of promising 6”-modified KRN7000 analogues.
Introduction
Similar to major histocompatibility complex (MHC) class I or class II molecules that present peptide antigens to the classic CD8+ and CD4+ T cells of the immune system, the evolutionary related CD1 molecules are specialized in presenting lipid and glycolipid antigens to non-MHC-restricted T lymphocytes.1 To accommodate these glycolipids, CD1 proteins have evolved a unique hydrophobic pocket in which the lipid chains are buried.2 From the five CD1 proteins present in humans, only a single isoform is present in mice, namely mCD1d, which was found homologous to the human isoform CD1d. CD1d presents glycolipids to invariant natural killer T (NKT) cells that express a semi-invariant T cell receptor (TCR) with a conserved TCR α-chain. Like natural killer (NK) cells, NKT cells are activated in the first line of immune response leading to secretion of proinflammatory T helper 1 (Th1) and immunomodulatory Th2 cytokines that initiate the proliferation of lymphocytes for inflammation and immunoregulation activities.3 NKT cell activation propagates rapidly to other cell types, such as NK cells, dendritic cells and subsets of B and conventional T cells.
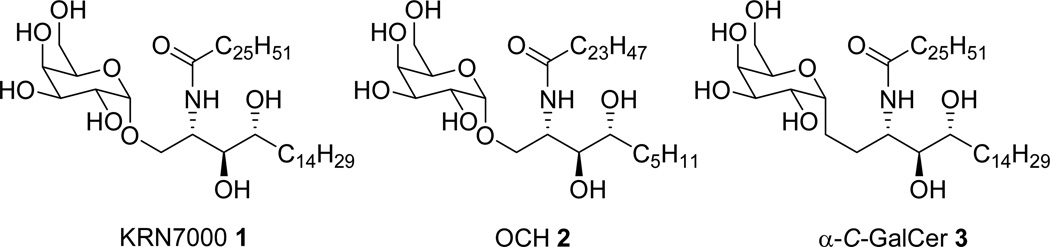
The archetypal CD1d ligand is α-galactosylceramide (α-GalCer, aka KRN7000, 1, Figure 1). KRN7000 was derived from the Agelasphins, a group of galactosylceramides isolated from the marine sponge Agelas mauritianus that showed potent activity in prolonging the life span of mice intraperitoneally inoculated with mouse melanoma cells.4 An unusual structural feature of KRN7000, which is uncommon in mammalian glycolipids but critical for NKT-cell activation, is the α-linkage between the sugar and the ceramide.
Figure 1.
Structures of KRN7000, OCH and α-C-GalCer
Although KRN7000 appears promising for a broad range of therapeutic applications, the concomitant stimulation of both Th1-type (INF-γ) and Th2-type (IL-4) cytokines, which counteract each other’s effects, is believed to be responsible for the limited therapeutic outcome in the clinic.5 It is suggested that α-GalCer analogues capable of inducing a biased Th1 or Th2 response are required for superior clinical effectiveness.6 Hence, several attempts have been undertaken to modify α-GalCer to alter its stimulatory profile (recently reviewed in ref. 7). The best documented examples are OCH 2, an α-GalCer analogue with a shortened sphingosine moiety resulting in a Th2-biased NKT cell activation profile,8 and α-C-GalCer 3, which shifts the profile towards Th1 responses.9
Several studies have been performed to assess the S.A.R. of the galactose part of α-GalCer. The 2”-OH group of the galactose was found critical for binding to CD1d. Upon removal of this hydroxyl group10 or its replacement by a methoxy,11 or acetamide group,12 the cytokine response was dramatically decreased. Howell and coworkers recently demonstrated that 3”- or 4”-deoxy or -fluoro analogues of KRN7000 retained antigenic activity,13 in contrast to similar modification at the 2”-position.14 Inversion of the 4”-OH (resulting in a GluCer analog of KRN7000) also affected the activity,15 while 3”-O-sulfo-α-GalCer gave comparable activity to the parent compound.16
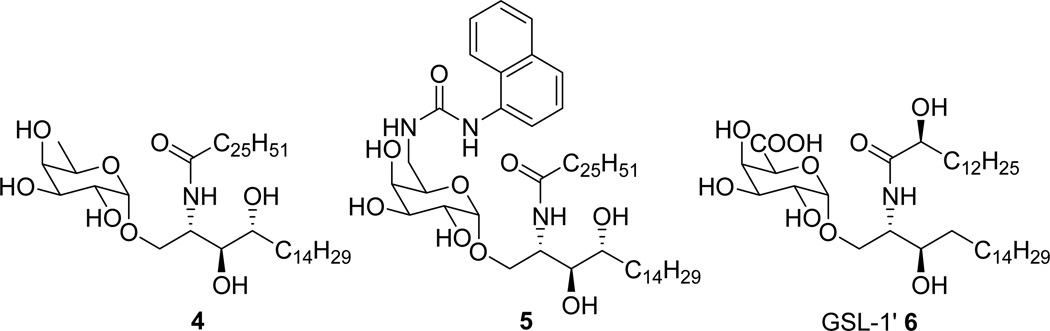
In contrast to the galactose secondary alcohol groups, modifications at the primary 6”-OH group are generally well-tolerated. Savage et al. demonstrated that attachment of small fluorophores at that position resulted in modified α-GalCers that retained the capacity to stimulate NKT cells.17 The Gal(α1→6)GalCer was able to stimulate NKT cells without processing to the parent monoglycosylceramide, in contrast to the corresponding diglycosylceramides at the other OH-groups (i.e., Gal (α1→2)GalCer, Gal (β1→3)GalCer and Gal(α1→4)GalCer), which lost their activity if the additional sugar cap was not enzymatically processed.18 Guzman and coworkers used a 6”-amide group to construct a water soluble pegylated α-GalCer analogue with specificity for CD1d and stimulatory properties on immune cells (e.g., DC and NK cells).19 Mori and coworkers reported a series of 6”-modified analogues of α-GalCer. Among others, the α-d-fucopyranosyl analogue (RCAI-58, 4, Figure 2) was found superior to α-GalCer in inducing IFN-γ in mice.20 Finally, we recently reported a series of 6”-amide and 6”-urea derivatives (e.g., 5) that retained or even surpassed the antigenic potency of KRN7000, and as an additional benefit, proved capable of producing a significant Th1-skewed cytokine response leading to superior tumor protection in vivo.21,22
Figure 2.
Structures of key 6”-modified glycosylceramides.
The tolerance for 6”-OH modifications could be explained by the crystal structure23 of the human NKT TCR in complex with CD1d bound to α-GalCer, which indicates that the Gal 6”-OH is the only sugar hydroxyl group not involved in H-bond formation.
Recently isolated immunoresponsive glycolipids from Sphingomonas species, which were capable of specifically stimulating human and mouse NKT cells in a CD1d-dependent manner, contained another 6”-OH modification.24 Structurally these mainly differ from α-GalCer in that they contain an α-linked glucuronic (GSL-1) or galacturonic acid glycone (GSL-1’, 6).
In view of the interesting NKT-cell mediated responses induced by the 6”-derivatised α-GalCer analogues, synthetic methodology that allows late-stage introduction of a sugar 6”-substituent and sphingosine amine functionalisation is desired for subsequent S.A.R. studies. Apart from limiting sugar protecting group manipulations, the situation for galacturonic acid and fucosyl analogues is compounded by the anomeric glycosylation selectivity (see below).
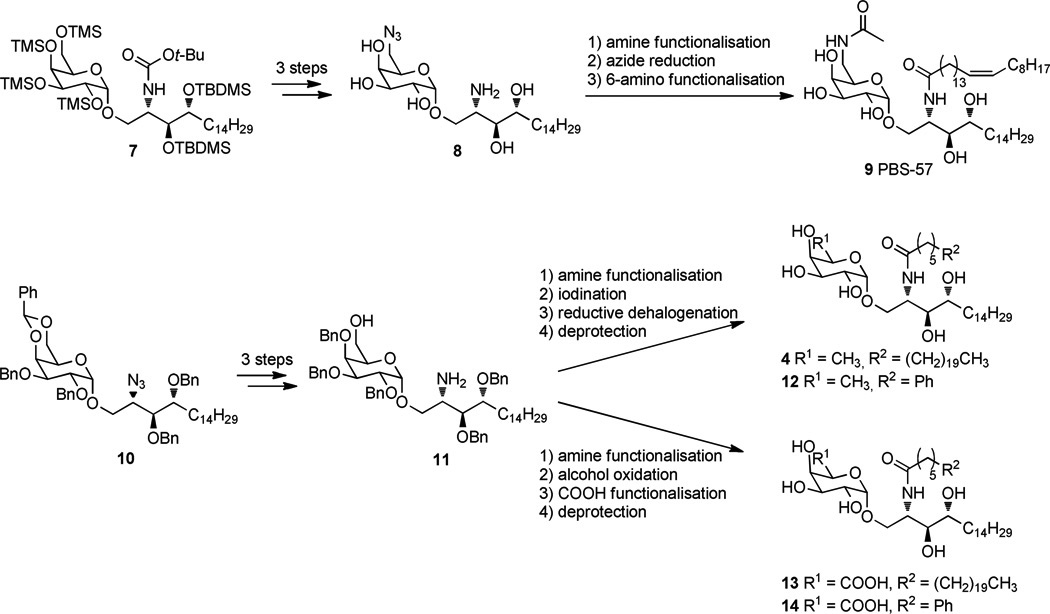
Recently, Cox/Besra and co-worker reported a practical procedure to synthesize 6”-N-derivatized α-GalCer analogues, relying on the orthogonal protection of the 2- and 6”-amino groups (Scheme 1, top).25 Selective 6”-TMS-ether cleavage nicely allowed introduction of the 6”-azide group. Prompted by this report, we want to disclose an alternative divergent approach towards 6”-modified analogues (Scheme 1, bottom), in which the difficult galacturonic acid glycosylation was circumvented by the use of a protected galactose donor which 4”,6”-benzylidene protecting group not only contributed to selective glycosylation,26,27,28 but also provided a handle for selective 6”-OH deprotection. In contrast to the Cox/Besra method, the nature of the chemistry involved did not allow global deprotection before functionalisation. To demonstrate the versatility of our method, it was first used to gain access to the known α-d-fucopyranosyl analogue 4, the galacturonic analogue 13,29 and two derivatives in which the conventional acyl moiety has been replaced by a 6-phenylhexanoyl (C6Ph) moiety known to considerably enhance the overall production of both Th1 and Th2 type cytokines and to skew the balance toward a Th1 type response.30 Compound 13 (Scheme 1) represents an analogue of GSL-1’ (6) in which the ceramide part was replaced by that found in α-GalCer.29 GSL-1’ was shown to induce a more Th1-based immunity and to suppress tumor growth and prolong survival of mice bearing lung cancer more effectively than α-GalCer at equal doses. In contrast to GSL-1’, i.v. administration of compound 13 results in a drastically lower secretion of INF-γ than that caused by α-GalCer administration. Despite this fact, it is more effective towards lung and breast cancer in mice compared to α-GalCer.
Scheme 1.
The synthesis of KRN7000 analogues with late-stage functionalisation of the sphingosine amino group and sugar 6”-position. Top: the Cox/Besra method illustrated for a 6”-amino analogue PBS-57; and bottom: our proposed method, illustrated for fucosyl and galacturonic acid-modified analogues.
Results and Discussion
Chemistry
The selective ring opening of 4,6-O-benzylidene protected carbohydrates is a well-described method for obtaining protected saccharides having a free 6-OH group.31 In addition, glycosylation of 4,6-O-benzylidene protected galactosyl donors is known to be highly α-stereoselective due to fact that the cis-decalin ring system with the equatorial phenyl group prevents attack from the β-face.27, 32 We decided to exploit the selectivity of both reactions for the synthesis of KRN7000 derivatives functionalised at the galactose 6”-position, in particular galacturonic acid, ester, amide and amino derivatives, as well as 6”-deoxy (fucosyl) KRN7000.
An added beneficial aspect of this strategy concerns the glycosylation of galacturonic acid donors, which surprisingly is not well-precedented.12,33,34 This may be due to the deactivating effect of the electron-withdrawing C-5 carboxylic group, making the galacturonidation particularly challenging.35 In addition, in their efforts to assess the influence of a pyranosyl C-5 carboxylate ester on the stereochemical outcome of glycosylation reactions, van der Marel and coworkers demonstrated that a C-5 ester is 1,5-cis or β-directing as opposed to C-5 methylene oxybenzyl, which induces little selectivity.36 This is reflected in the synthesis of the Sphingomonas glycolipid 6 by Seeberger et al., who found that even after extensive optimization (including galactose protecting groups), glycosylation was achieved with a maximum 4.2:1 ratio of the α- and β-anomers.34
The synthesis of the α-d-fucosyl analogue RCAI-58 by Mori and coworkers involved glycosylation under Mukaiyama conditions.20 Unfortunately, neither yield nor anomeric ratio were mentioned. In contrast to the scarce reports about d-fucosylation, l-fucosylation is more prevalent, with reasonable α-selectivities.37,38,39 Neglecting the potential influence on the α/β ratio due to the asymmetry of the sphingosine, glycosylation of this acceptor with d- and l-fucose is expected to proceed with the same stereoselectivity.
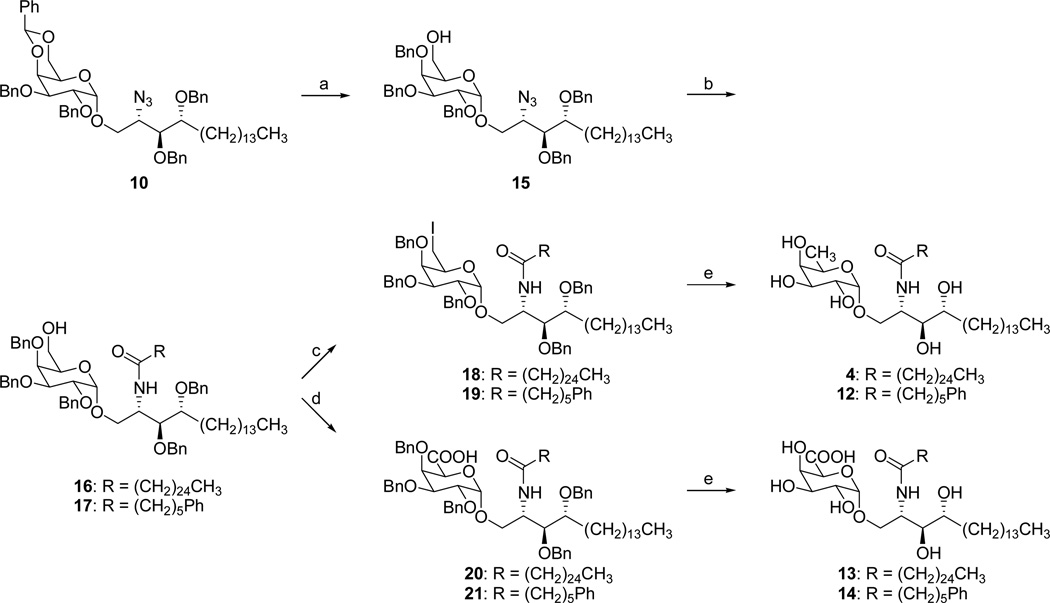
So, 10 was synthesized by Schmidt glycosidation,27 forming only a negligible amount of the β-anomer which could be easily removed by flash chromatography. Starting from the glycosidation product 10, Scheme 2 summarizes the divergent route followed for the synthesis of both types of target analogues. In order to selectively address the C-6” position for further modifications, a regioselective opening of the benzylidene ring was required. This key step could be realized by treating 10 with BH3.THF and Cu(OTf)2.31 The regioselective opening was confirmed by an HSQC experiment, which unambiguously proved that the carbon attached to the hydroxyl group possesses two protons, indicating that the free hydroxy-group was connected to C-6”. Staudinger reduction of the azide followed by acylation of the resulting amino group with the appropriate acids gave the versatile intermediates 16 and 17.
Scheme 2.
Reagents and conditions: (a) BH3.THF, Cu(OTf)2, CH2Cl2, 78%; (b) PMe3, THF, NaOH, EDC, RCOOH, CH2Cl2, 71–81%; (c) PPh3, I2, imidazole, toluene, 85%; (d) TEMPO, BAIB, CH2Cl2, H2O, 81–97%; (e) Pd black, H2, 49–86%.
Towards the deoxygenation of the 6”-OH, 16 and 17 were first converted to the corresponding iodo analogues 18 and 19 upon treatment with triphenylphosphine, imidazole and iodine. Our plan was to carry out the reductive dehalogenation and debenzylation in a single step. However, upon treatment with palladium black under hydrogen atmosphere, the reaction stopped at the stage of the deiodinated products still containing all benzyl groups, probably due to poisoning of the catalyst. After flash chromatography of the reaction mixture, the deiodinated intermediates were again subjected to catalytic hydrogenation (same conditions) to afford the target compounds 4 and 12.
The galacturonic acid derivatives were prepared upon oxidation of the 6”-OH groups of 16 and 17 into the corresponding carboxylic acids 20 and 21 via a TEMPO/BAIB oxidation.40 Final deprotection was accomplished by Pd-catalyzed hydrogenation to afford the desired compounds 13 and 14.
Biological Evaluation
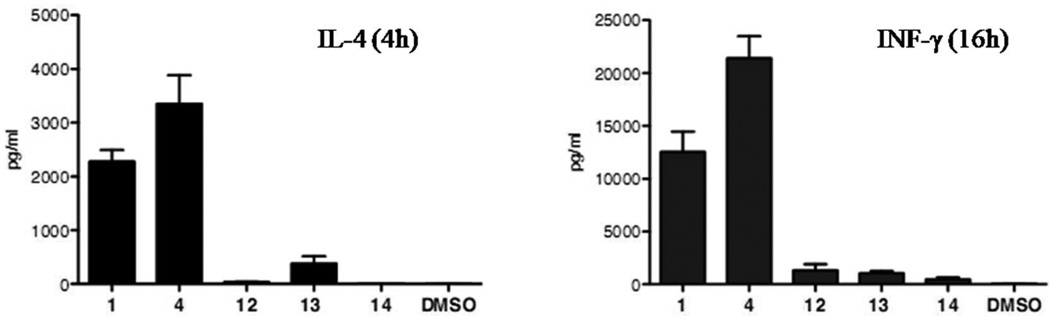
To assess the biological activity of the galacturonic acid and α-d-fucopyranosyl analogues, serum levels of INF-γ and IL-4 were measured after intraperitoneal injection of 5 µg of the corresponding glycolipids in mice. The cytokine secretion induced by these compounds is presented in Figure 4. Consistent with the results of Mori,20 6”-deoxy-analogue 3 is a superior INF-γ inducer. However, where Mori and coworkers only reported the INF-γ secretion, we measured the IL-4 levels as well, revealing a strong Th2 response, hence resulting in no polarization. In combination with a C6Ph modified acyl chain, fucosyl analogue 12 induces only a weak cytokine secretion. So, combination of the two modifications known to enhance antigenic activity results in a remarkably decreased activity. In contrast to the in vitro response in human NKT cells,29 galacturonic acid 13 shows a minor cytokine secretion in vivo in mice. Combination with the C6Ph modified acyl chain in compound 14 abolishes all activity.
The low cytokine release of the C6Ph analogues 12 and 14 is surprising. Wong et al. demonstrated that this and related acyl moieties enhanced the stability for mCD1d, a finding that was further substantiated by cocrystal structures with that protein.41 Furthermore, introduction of this acyl group in α-GalCer afforded a compound that produced more INF-γ and less IL-4 from human NKT cells compared to α-GalCer. It remains to be investigated if the very low antigenicity found for 12 and 14 is due to the fact that this acyl moiety negatively influences cytokine secretion in the mouse system (despite good affinity for mCD1d). Alternatively, our results may also indicate that modification of the carbohydrate moiety can significantly alter the influence of the lipid moiety of the ligand on CD1d presentation and iNKT cell responses. This is in accordance with observations made by Besra and coworkers.42
We also determined the equilibrium binding affinities of the mouse Vα14Vβ8.2 NKT TCR toward the four CD1d-glycolipid complexes using surface plasmon resonance. Purified and biotinylated mouse CD1d was loaded with the four individual glycolipid ligands and coated on a Biacore sensor chip. Increasing concentrations of TCR were simultaneously passed over each flow channel of the chip to measure real time binding kinetics. Interestingly, the binding phase (kass) of the TCR to each of the CD1d-presented glycolipids is similar, ranging from 0.8×105–1.3×105 (M−1s−1) while the dissociation of the TCR (kdiss) can differ up to 10-fold between KRN7000 1 and 13 (compare 1.45×10−3 s−1 with 16.6×10−3 s−1), resulting in over 10-fold different binding affinities (KD=11.2nM–165nM).
These data indicate that modifications of the galactose moiety and the acyl chain do affect NKT TCR affinity but not to a degree that has been seen for weak microbial antigens, such as borrelial α-galactosyl diacylglycerolipids (KD=6.2µM). As a result, the apparent disconnect between relatively high affinity TCR interaction in vitro and weak NKT cell activation in vivo, likely is a result of different pharmacokinetics of the lipids inside cells, rather than attributed by altered TCR binding kinetics.
Conclusions
Modification of the 6”-position of the prototypical NKT-cell agonist KRN7000, has resulted in a number of analogues with interesting biological properties. In this report we describe a practical synthetic route to modify the 6”-position after the glycosidation, featuring the regioselective opening of a 4”,6”-O-benzylidene ring as the key step. As a proof-of-concept this method was employed to prepare the 6”-deoxy-analogue of KRN7000, as well as the otherwise not so accessible galacturonic acid analogue. The carboxylate group of the latter may be suitable for flexible substitution through an amide linkage. An additional advantage of the reported procedure is that it allows to introduce alternative acyl moieties in the phytosphingosine moiety in the final stages of the synthesis.
Experimental Section
Synthesis
General
Precoated Macherey-Nagel SIL G/UV254 plates were used for TLC, and spots were examined under UV light at 254 nm and further visualized by sulfuric acid-anisaldehyde spray. Column chromatography was performed on Biosolve silica gel (63–200 µm, 60 Å). NMR spectra were obtained with a Varian Mercury 300 Spectrometer or a Bruker Avance II 500 spectrometer. Chemical shifts are given in ppm (δ) relative to the residual solvent signals, in the case of CDCl3: δ = 7.26 ppm for 1H and δ = 77.4 ppm for 13C, in the case of DMSO-d6: δ = 2.54 ppm for 1H and δ = 40.5 ppm for 13C, and in the case of pyridine-d5: δ = 8.71, 7.56 and 7.18 ppm for 1H and δ = 149.9, 135.5 and 123.5 ppm for 13C. Exact mass measurements were performed on a Waters LCT Premier XE TOF equipped with an electrospray ionization interface and coupled to a Waters Alliance HPLC system. Samples were infused in a in a CH3CN/HCOOH (1000:1) mixture at 10 mL/min. The purity of the target compounds was assessed by HPLC. An Agilent Technologies 1100 HPLC system (Agilent Technolgies, Waldbronn, Germany) was coupled to a Varian 385-LC Evaporative Light Scattering Detector (ELSD)(Agilent Technologies). The compounds were subjected to an Xbridge Shield C18 column (Waters, Millford, MA, USA) operated at 65°C. Mobile phases, delivered at a flow rate of 2 mL/min, consisted of 25 mM ammonium formate pH 5 (solvent A) and methanol (solvent B). A linear gradient from 70% B to 100% B in 10 min. was applied, followed by elution with 100% B during 3 min. The target compounds were dissolved in pyridine and further diluted a hundred fold in acetonitrile prior to injecting 2 µl. Final concentrations were 92 ng/µl, 97 ng/µl, 75 ng/µl and 20 ng/µl for 4, 12, 13 and 14, respectively.
2-azido-3,4-di-O-benzyl-1-O-(2,3-di-O-benzyl-4,6-O-benzylidene-α-d-galactopyranosyl) octadecane-1,3,4-triol (10)
A solution of 2-azido-3,4-di-O-benzyl-1-hydroxy-octadecane-1,2,4-triol (400 mg, 0.76 mmol) in THF (10 mL) was added to a solution of 2,3-di-O-benzyl-4,6-O-benzylidene-α-d-galactopyranosyl trichloroacetimidate (680 mg, 1.15 mmol) in THF (5 mL) followed by dropwise addition of TMSOTf (0.02 mL, 0.11 mmol) at −20 °C. After stirring for 1h at −20°C, the reaction mixture was neutralized with Et3N and evaporated to dryness. The residue was purified by column chromatography (hexanes/EtOAc: 9/1 + 1 V% Et3N) to afford compound 10 (486 mg, 67%) as a white solid.
1H NMR (300 MHz, CDCl3): δ 7.53-7.51 (m, 2H, arom. H), 7.41-7.21 (m, 23H, arom. H), 5.45 (s, 1H, O-CHPh-O), 4.97 (d, J = 3.2 Hz, 1H, H-1’), 4.86 (d, J = 12.0 Hz, 1H, CH2-Ph), 4.81 (d, J = 13.2 Hz, CH2-Ph), 4.77 (d, J = 10.4 Hz, 1H, CH2-Ph), 4.74 (d, J = 12.3 Hz, 1H, CH2-Ph), 4.67 (d, J = 11.7 Hz, 1H, CH2-Ph), 4.60 (d, J = 11.4 Hz, 1H, CH2-Ph), 4.58 (d, J = 11.7 Hz, 1H, CH2-Ph), 4.50 (d, J = 11.7 Hz, 1H, CH2-Ph), 4.16 (app. d, J = 3.5 Hz, 1H, H-4’), 4.14-4.07 (m, 2H, H-2’, H-6’), 4.04-3.99 (m, 2H, H-3’, H-1), 3.88 (dd, J = 12.6 Hz and 1.3 Hz, 1H, H-6’), 3.73-3.69 (m, 3H, H-1, H-2, H-3), 3.65-3.60 (m, 1H, H-4), 3.57 (app. s, 1H, H-5’), 1.65-1.26 (m, 26H, CH2), 0.88 (t, J = 6.6 Hz, 3H, CH3).
13C NMR (75 MHz, CDCl3): δ 139.00, 138.60, 138.26, 138.07, 129.90, 129.10, 128.62, 128.59, 128.51, 128.47, 128.34, 128.15, 128.04, 128.00, 127.94, 127.91, 127.85, 127.75, 127.70, 126.58, 121.12, 101.30, 99.38, 79.65, 79.20, 76.04, 75.69, 74.91, 74.02, 73.74, 72.44, 72.31, 72.28, 69.56, 68.69, 63.21, 62.04, 54.71, 53.64, 32.16, 30.26, 30.01, 29.94, 29.92, 29.90, 29.88, 29.85, 29.72, 29.60, 29.47, 25.70, 22.92, 21.58, 14.35.
Exact mass (ESI-MS) for C59H75N3O8 [M+Na]+ found, 976.5492; calcd, 976.5507.
2-azido-3,4-di-O-benzyl-1-O-(2,3,4-tri-O-benzyl-6-hydroxy-α-d-galactopyranosyl) octadecane-1,3,4-triol (15)
To a solution of 10 (50 mg, 0.05 mmol) in anhydrous CH2Cl2 (1.6 mL) under argon atmosphere was added cupper(II)triflate (3 mg, 0.008 mmol) and BH3.THF (0.26 mL, 0.26 mmol). After stirring for 1.5h at room temperature, the brown reaction mixture was quenched with methanol. Subsequently the mixture was diluted with EtOAc and washed with sat. NaHCO3, water and brine. The organic layer was dried over Na2SO4 and concentrated under reduced pressure. Purification by column chromatography (hexanes/EtOAc: 8.5/1.5) yielded 15 (37 mg, 73%) as a colorless oil.
1H NMR (300 MHz, DMSO-d6): δ 7.38-7.7.22 (m, 25H, arom. H), 4.97 (d, J = 2.25 Hz, 1H, H-1’), 4.82 (d, J = 11.4 Hz, 1H, CH2-Ph), 4.79-4.69 (m, 5H, CH2-Ph, OH), 4.60-5.54 (m, 4H, CH2-Ph), 4.44 (d, J = 11.6 Hz, 1H, CH2-Ph), 4.08 (br.s, 1H, H-3’), 3.97-3.88 (m, 3H, H-1, H-3, H-2’), 3.79-3.73 (m, 3H, H-2, H-4’, H-5’), 3.67-3.62 (m, 2H, H-1, H-4), 3.52-3.46 (m, 2H, H-6’), 2.49-1.20 (m, 26H, CH2) 0.85 (t, J = 6.6 Hz, 3H, CH3).
13C NMR (75 MHz, CDCl3): δ 138.96, 138.88, 138.63, 138.43, 138.30, 128.82, 128.72, 128.66, 128.63, 128.61, 128.54, 128.22, 128.13, 128.09, 128.00, 127.98, 127.86, 127.84, 127.78, 98.84, 79.56, 79.46, 79.06, 77.72, 77.30, 76.87, 76.69, 75.43, 74.73, 73.96, 73.69, 73.48, 72.30, 71.12, 68.68, 62.70, 62.25, 32.19, 30.27, 30.02, 29.97, 29.93, 29.89, 29.87, 29.63, 25.61, 22.96, 14.39.
Exact mass (ESI-MS) for C59H77N3O8 [M+Na]+ found, 978.5695; calcd, 978.5603
3,4-di-O-benzyl-1-O-(2,3,4-tri-O-benzyl-6-hydroxy-α-d-galactopyranosyl)-2-hexacosylamino-octadecane-1,3,4-triol (16)
To a solution of 15 (157 mg, 0.16 mmol) in THF (1.6 mL) was added dropwise trimethylphosphine (0.8 mL, 0.82 mmol). After stirring for 2.5h, a NaOH solution (3 mL, 1 M) was added and the mixture was allowed to stir for an additional 2.5h. The reaction mixture was extracted with EtOAc and the organic layer was washed with brine, dried over Na2SO4 and concentrated under reduced pressure. The crude amine was dissolved in CH2Cl2 (2 mL) and added to a solution of EDC (63 mg, 0.33 mmol) and hexacosanoic acid (97 mg, 0.25 mmol) in CH2Cl2 (0.5 mL). This reaction mixture was stirred overnight at room temperature after which it was extracted with CH2Cl2. The organic layer was washed with brine and dried over Na2SO4. After evaporation of the organic solvent, the residue was purified by column chromatography (hexanes/EtOAc: 6/4) affording compound 16 (167 mg, 78%) as a yellow oil.
1H NMR (300 MHz, CDCl3): δ 7.39-7.25 (m, 25H, arom. H), 5.83 (d, J = 9.0 Hz, 1H, NH), 4.94 (d, J = 11.5 Hz, 1H, CH2-Ph), 4.88 (d, J = 3.8 Hz, 1H, H-1’), 4.82 (d, J = 12.7 Hz, 1H, CH2-Ph), 4.79 (d, J = 11.8 Hz, 1H, CH2-Ph), 4.73 (d, J = 11.8 Hz, 1H, CH2-Ph), 4.71 (d, J = 11.8 Hz, 1H, CH2-Ph), 4.64 (d, J = 12.1 Hz, 1H, CH2-Ph), 4.62 (d, J = 11.8 Hz, 1H, CH2-Ph), 4.58 (d, J = 11.8 Hz, 1H, CH2-Ph), 4.50 (d, J = 11.5 Hz, 1H, CH2-Ph), 4.47 (d, J = 11.8 Hz, 1H, CH2-Ph), 4.41-4.33 (m, 1H, H-2), 4.03 (dd, J = 3.7 Hz and 9.6 Hz, 1H, H-2’), 3.95 (dd, J = 7.6 Hz and 11.6 Hz, 1H, H-1), 3.85-3.82 (m, 3H, H-1, H-3’, H-4’), 3.70-3.62 (m, 3H, H-3, H-5’, H-6’), 3.55-3.50 (m, 1H, H-4), 3.48-3.42 (m, 1H, H-6’), 2.39 (t, 1H, OH), 1.92-1.87 (m, 2H, COCH2), 1.69-1.14 (m, 72H, CH2), 0.88 (t, J = 6.8 Hz, 6H, CH3)
13C NMR (75 MHz, CDCl3): δ 173.37, 138.86, 138.79, 138.67, 138.65, 138.48, 128.66, 128.64, 128.60, 128.17, 128.13, 128.06, 127.96, 127.94, 127.83, 127.67, 100.31, 80.68, 79.46, 77.66, 77.44, 77.24, 76.91, 76.81, 75.09, 74.77, 73.77, 73.43, 73.32, 72.08, 71.50, 70.04, 62.70, 50.90, 37.05, 32.16, 30.43, 29.95, 29.93, 29.89, 29.83, 29.66, 29.59, 26.01, 26.93, 22.92, 14.43, 14.35.
Exact mass (ESI-MS) for C85H129NO9 [M+H]+ found, 1308.9780; calcd, 1308.9746
3,4-di-O-benzyl-1-O-(2,3,4-tri-O-benzyl-6-hydroxy-α-d-galactopyranosyl)-2-(6-phenylhexanoyl)amino-octadecane-1,3,4-triol (17)
To a solution of 15 (120 mg, 0.13 mmol) in THF (1.3 mL) was added dropwise trimethylphosphine (0.6 mL, 0.63 mmol). After stirring for 2.5h, a NaOH solution (2.3 mL, 1 M) was added and the mixture was allowed to stir for an additional 2.5h. The reaction mixture was extracted with EtOAc and the organic layer was washed with brine, dried over Na2SO4 and concentrated under reduced pressure. The crude amine was dissolved in CH2Cl2 (1.4 mL) and added to a solution of EDC (48 mg, 0.25 mmol) and 6-phenylhexanoic acid (36 mg, 0.19 mmol) in CH2Cl2(0.5 mL). This reaction mixture was stirred overnight at room temperature after which it was extracted with CH2Cl2. The organic layer was washed with brine and dried over Na2SO4. After evaporation of the organic solvent, the residue was purified by column chromatography (hexanes/EtOAc: 7/3) affording compound 17 (107 mg, 77%) as a colorless oil.
1H NMR (300 MHz, CDCl3): δ 7.38-7.15 (m, 30H, arom. H), 5.84 (d, J = 8.6 Hz, 1H, NH), 4.94 (d, J = 11.4 Hz, 1H, CH2-Ph), 4.86 (d, J = 3.7 Hz, 1H, H-1’), 4.83 (d, J = 11.7 Hz, 1H, CH2-Ph), 4.80 (d, J = 11.7 Hz, 1H, CH2-Ph), 4.74 (d, J = 11.7 Hz, 1H, CH2-Ph), 4.69 (d, J = 11.7 Hz, 1H, CH2-Ph), 4.66 (d, J = 11.7 Hz, 1H, CH2-Ph), 4.60 (d, J = 11.7 Hz, 1H, CH2-Ph), 4.55 (d, J = 11.4 Hz, 1H, CH2-Ph), 4.52 (d, J = 11.7 Hz, 1H, CH2-Ph), 4.48 (d, J = 11.4 Hz, 1H, CH2-Ph), 4.44 (d, J = 11.4 Hz, 1H, CH2-Ph), 4.37 (m, 1H, H-2), 4.02 (dd, J = 9.9 and 3.7 Hz, 1H, H-2’), 3.94 (dd, J = 11.4 and 7.7 Hz, 1H, H-1), 3.85-3.82 (m, 3H, H-1, H-3’and H-4’), 3.70-3.62 (3H, H-3, H-5’ and H-6’), 3.55-3.41 (m, 2H, H-4, H-6’), 2.57 (t, J = 7.1 Hz, 2H, CH2-Ph), 1.97-1.80 (m, 2H, COCH2), 1.69-1.26 (m, 34H, CH2), 0.88 (t, J = 6.6 Hz, 3H, CH3).
13C NMR (75 MHz, CDCl3): δ 173.26, 142.68, 138.84, 138, 76, 138.66, 138.47, 128.68, 128.65, 128.62, 128.60, 128.51, 128.17, 128.14, 128.09, 127.98, 127, 95, 127.86, 127.84, 127.66, 125.93, 100.23, 80.57, 79.50, 79.43, 77.67, 77.45, 77.25, 76.82, 75.04, 74.76, 73.79, 73.40, 73.33, 72.06, 71.51, 69.98, 62.69, 51.08, 50.89, 36.87, 35.95, 32.16, 31.36, 31.16, 30.44, 30.02, 29.96, 29.92, 29.60, 29.12, 26.01, 25.73, 22.93, 14.36
Exact mass (ESI-MS) for C71H93NO9 [M+H]+ found, 1104.7063; calcd, 1104.6923
3,4-di-O-benzyl-1-O-(2,3,4-tri-O-benzyl-6-iodo-α-d-galactopyranosyl)-2-hexacosylamino-octadecane-1,3,4-triol (18)
PPh3 (18 mg, 0.07 mmol) was added to a solution of 16 (74 mg, 0.06 mmol) in toluene (0.4 mL) under argon followed by refluxing during 10 minutes. The mixture was cooled down to 80 °and imidazole (11 mg, 0.17 mmol) and I2 (19 mg, 0.07 mmol) were added. After refluxing for 20 minutes, the solution was concentrated under reduced pressure. The resulting residue was diluted with EtOAc and washed with a saturated Na2S2O3 solution and water. The organic layer was dried on Na2SO4 and evaporated to dryness. Purification by column chromatography (hexanes/EtOAc: 9/1) yielded 18 (68 mg, 85%) as a white solid.
1H NMR (300 MHz, CDCl3): δ 7.40-7.21 (m, 25H, arom. H), 5.84 (d, J = 8.6 Hz, 1H, NH), 5.03 (d, J = 11.2 Hz, 1H, CH2-Ph), 4.84 (d, J = 11.7 Hz, 1H, CH2-Ph), 4.83 (d, J = 3.4 Hz, 1H, H-1’), 4.79-4.72 (m, 3H, CH2-Ph), 4.65-4.58 (m, 3H, CH2-Ph), 4.52 (d, J = 11.7 Hz, 1H, CH2-Ph), 4.49 (d, J = 11.7 Hz, 1H, CH2-Ph), 4.30 (m, 1H, H-2), 4.08 (m, 1H, H-4’), 4.02 (dd, J = 10.0 Hz and 3.5 Hz, 1H, H-2’), 3.91-3.87 (m, 2H, H-1, H-3’), 3.84-3.77 (m, 3H, H-1, H-3, H-5’), 3.53 (ddd, J = 7.2 Hz, and 3.6 Hz, 1H, H-4), 3.18 (dd, J = 9.9 Hz and 7.1 Hz, 1H, H-6’), 3.09 (dd, J = 9.9 Hz and 7.0 Hz, 1H, H-6’), 2.02-1.86 (m, 2H, COCH2), 1.72-1.06 (m, 72H, CH2), 0.88 (t, J = 6.7 Hz, 6H, CH3).
13C NMR (75 MHz, CDCl3): δ 169.41, 135.12, 135.05, 135.00, 134.87, 134.74, 124.95, 124.90, 124.87, 124.39, 124.37, 124.30, 124.23, 124.16, 124.12, 124.08, 123.93, 101.28, 95.36, 76.43, 75.71, 75.61, 73.94, 73.94, 73.72, 73.52, 73.10, 72.70, 71.88, 71.63, 69.97, 69.80, 69.70, 68.44, 64.81, 46.66, 33.29, 28.44, 26.63, 26.37, 26.24, 26.21, 26.17, 26.12, 25.97, 25.91, 25.89, 25.87, 22.46, 22.26, 19.21, 10.63.
Exact mass (ESI-MS) for C85H128INO8 [M+H]+ found, 1418.8851; calcd, 1418.8757
3,4-di-O-benzyl-1-O-(2,3,4-tri-O-benzyl-6-iodo-α-d-galactopyranosyl)-2-(6-phenylhexanoyl)amino-octadecane-1,3,4-triol (19)
PPh3 (20 mg, 0.08 mmol) was added to a solution of 17 (70 mg, 0.06 mmol) in toluene (0.4 mL) under argon followed by refluxing during 10 minutes. The mixture was cooled down to 80 °C and imidazole (13 mg, 0.19 mmol) and I2 (21 mg, 0.08 mmol) were added. After refluxing for 20 minutes, the solution was concentrated under reduced pressure. The resulting residue was diluted with EtOAc and washed with a saturated Na2S2O3 solution and water. The organic layer was dried on Na2SO4 and evaporated to dryness. Purification by column chromatography (hexanes/EtOAc: 8/2) yielded 19 (67 mg, 85%) as a yellow oil.
1H NMR (300 MHz, CDCl3): δ 7.42-7.16 (m, 30H, arom. H), 5.86 (d, J = 8.6 Hz, 1H, NH), 5.05 (d, J = 11.2 Hz, 1H, CH2-Ph), 4.86 (d, J = 3.6 Hz, 1H, H-1’), 4.85 (d, J = 11.7 Hz, 1H, CH2-Ph), 4.79 (d, J = 11.7 Hz, 1H, CH2-Ph), 4.78 (d, J = 11.8 Hz, 1H, CH2-Ph), 4.76 (d, J = 11.5 Hz, 1H, CH2-Ph), 4.66 (d, J = 11.1 Hz, 1H, CH2-Ph), 4.65 (d, J = 10.0 Hz, 1H, CH2-Ph), 4.62 (d, J = 12.0 Hz, 1H, CH2-Ph), 4.54 (d, J = 11.5 Hz, 1H, CH2-Ph), 4.52 (d, J = 11.7 Hz, 1H, CH2-Ph), 4.34 (m, 1H, H-2), 4.11-4.10 (m, 1H, H-4’), 4.05 (dd, J = 10.0 Hz and 3.5 Hz, 1H, H-2’), 3.95-3.89 (m, 2H, H-1, H-3’), 3.87-3.85 (m, 1H, H-5’), 3.83-3.79 (m, 2H, H-1, H-3), 3.59-3.54 (m, 1H, H-4), 3.21 (dd, J = 10.0 Hz and 7.2 Hz, 1H, H-6’), 3.12 (dd, J = 10.0 Hz and 6.9 Hz, 1H, H-6’), 2.60 (t, J = 7.6 Hz, 2H, CH2-Ph), 2.03-1.86 (m, 2H, COCH2), 1.73-1.26 (m, 32H, CH2), 0.91 (t, J = 6.7 Hz, 3H, CH3).
13C NMR (75 MHz, CDCl3): δ 169.14, 138,98, 135.09, 135.02, 134.97, 134.84, 134.72, 124.93, 124.88, 124.85, 124.76, 124.38, 124.36, 124.33, 124.29, 124.21, 124.14, 124.11, 124.07, 123.90, 122.17, 95.31, 76.35, 75.71, 75.57, 73.96, 73.54, 73.12, 72.69, 71.83, 71.60, 69.95, 69.75, 69.63, 68.39, 64.75, 56.87, 45.58, 33.11, 32.24, 28.42, 27.67, 26.62, 26.35, 26.21, 26.16, 25.86, 25.42, 22.42, 22.00, 19.19, 17.53, 10.69, 10.63.
Exact mass (ESI-MS) for C71H92INO8 [M+K]+ found, 1252.5511; calcd, 1252,5499
3,4-di-O-benzyl-1-O-(2,3,4-tri-O-benzyl-α-d-galactopyranosyluronate)-2-hexacosylamino-octadecane-1,3,4-triol (20)
TEMPO (22 mg, 0.14 mmol) and BAIB (578 mg, 1.80 mmol) were added to a mixture of 16 (940 mg, 0.72 mmol) in CH2Cl2 (4.7 mL) and H2O (2.4 mL). The emulsion was vigorously stirred overnight at room temperature and the reaction was quenched with Na2S2O3. After extraction of the aqueous layer with EtOAc, the organic layer was washed with sat. NaHCO3 and brine, dried over Na2SO4 and evaporated. The residue was submitted to column chromatography (CH2Cl2/MeOH: 24/1 with 1% formic acid), affording compound 20 (823 mg, 87%) as a yellow oil.
1H NMR (300 MHz, CDCl3): δ 7.32-7.19 (m, 25H, arom. H), 4.97 (app. s, 1H, H-1’), 4.86-4.28 (m, 13H, CH2-Ph, H-2, H-4’, H-5’), 4.02 (dd, J = 3.4 Hz and 9.9 Hz, 1H, H-2’), 3.90 (dd, J = 2.6 Hz and 10.0 Hz, 1H, H-3’), 3.88-3.83 (m, 1H, H-1), 3.76-3.69 (m, 2H, H-1 and H-3), 3.53-3.50 (m, 1H, H-4), 1.93-1.74 (m, 2H, COCH2), 1.59-1.08 (m, 72H, CH2), 0.86 (t, J = 6.8 Hz, 6H, CH3).
13C NMR (75 MHz, CDCl3): δ 173.30, 169.69, 138.59, 138.55, 138.38, 138.16, 128.70, 128.66, 128.47, 128.38, 128.21, 128.14, 128.13, 128.09, 128.01, 127.98, 127.66, 99.32, 79.65, 79.57, 78.14, 77.67, 77.45, 77.25, 76.82, 76.47, 75.84, 75.55, 74.07, 73.23, 73.10, 72.16, 71.21, 68.97, 50.15, 36.90, 32.17, 30.47, 30.04, 29.97, 29.94, 29.91, 29.89, 29.87, 29.84, 29.81, 29.67, 29.61, 29.60, 29.54, 25.99, 25.90, 22.93, 14.36.
Exact mass (ESI-MS) for C85H127NO10 [M+Na]+ found, 1344.9396; calcd, 1344.9352
3,4-di-O-benzyl-1-O-(2,3,4-tri-O-benzyl-α-d-galactopyranosyluronate)-2-(6-phenylhexanoyl)amino-octadecane-1,3,4-triol (21)
TEMPO (3 mg, 0.02 mmol) and BAIB (77 mg, 0.2 mmol) were added to a mixture of 17 (106 mg, 0.10 mmol) in CH2Cl2 (0.6 mL) and H2O (0.3 mL). The emulsion was vigorously stirred overnight at room temperature and the reaction was quenched with Na2S2O3. After extraction of the aqueous layer with EtOAc, the organic layer was washed with sat. NaHCO3 and brine, dried over Na2SO4 and evaporated. The residue was submitted to column chromatography (CH2Cl2/MeOH: 29/1 with 1% formic acid), affording compound 21 (104 mg, 97%) as a yellow oil.
1H NMR (300 MHz, CDCl3): δ 7.34-7.14 (m, 30H, arom. H), 5.80 (d, J = 8.2 Hz, 1H, NH), 4.95 (d, J = 3.4 Hz, 1H, H-1’), 4.91-4.42 (m, 12H, CH2-Ph, H-4’, H-5’), 4.42-4.31 (m, 1H, H-2), 4.06 (dd, J = 10.0 and 3.5 Hz, 1H, H-2’), 3.93 (dd, J = 10.1 and 2.6 Hz, 1H, H-3’), 3.89 (dd, J = 10.5 and 4.9 Hz, 1H, H-1), 3.80 (m, 2H, H-1, H-3), 3.54 (m, 1H, H-4), 2.67 (t, J = 7.7 Hz, 2H, CH2-Ph), 1.92-1.77 (m, 2H, COCH2), 1.67-1.19 (m, 34H, CH2), 0.90 (t, J = 6.7 Hz, CH3).
13C NMR (75 MHz, CDCl3): δ 173.33, 170.50, 142.73, 138.57, 138.55, 138.44, 138.42, 138.25, 128.71, 128.70, 128.68, 128.66, 128.62, 128.52, 128.46, 128.32, 128.25, 128.18, 128.16, 128.10, 128.02, 128.00, 127.96, 127.92, 127.64, 125.93, 99.93, 79.64, 79.45, 78.23, 77.72, 77.29, 76.87, 76.18, 75.95, 75.43, 74.07, 73.22, 73.07, 72.12, 71.17, 69.03, 50.23, 36.74, 35.98, 32.18, 31.38, 30.47, 30.06, 29.98, 29.93, 29.63, 29.12, 25.98, 25.72, 22.95, 14.39.
Exact mass (ESI-MS) for C71H97NO10 [M+H]+ found, 1118.6841; calcd, 1118.6716
1-O-(6-deoxy-α-d-galactopyranosyl)-2-hexacosylamino-octadecane-1,3,4-triol (4)
A solution of compound 18 (65 mg, 0.05 mmol) in MeOH (2.5 mL) was hydrogenated under atmospheric pressure in the presence of palladium black (20 mg). After consumption of the starting material, 1 spot was visible on TLC, corresponding with the deiodinated product. After a quick purification by column chromatography (Hexanes/EtOAc: 7/3), the product was dissolved in MeOH (3 mL) and hydrogenated under atmospheric pressure in the presence of palladium black (15 mg). Upon completion of the reaction, the mixture was diluted with MeOH and filtered through celite. The filter cake was rinsed with MeOH and the filtrate was evaporated to dryness. After purification by column chromatography (CH2Cl2/MeOH:8/2), compound 4 (18 mg, 47%) was afforded as a white powder.
1H NMR (300 MHz, pyridine-d5): δ 8.43 (d, J = 8.7 Hz, 1H, NH), 6.94 (d, J = 4.8 Hz, 1H, OH), 6.53 (d, J = 5.8 Hz, 1H, OH), 6.42 (d, J = 6.5 Hz, 1H, OH), 6.16 (d, J = 4.5 Hz, 1H, OH), 6.11 (d, J = 5.8 Hz, 1H, OH), 5.48 (d, J = 3.7 Hz, 1H, H-1’), 5.31 (m, 1H, H-2), 4.67 (dd, J = 10.5 Hz and 5.3 Hz, 1H, H-1), 4.59-4.56 (m, 1H, H-2’), 4.42-4.29 (m, 5H, H-1, H-3, H-4, H-3’, H-5’), 4.09-4.04 (m, 1H, H-4’), 2.47 (t, J = 7.43, COCH2), 2.31-1.27 (m, 72H, CH2), 1.11 (t, J = 7.1 Hz, 3H, CH3), 0.89 (t, J = 6.7 Hz, CH3).
13C NMR (75 MHz, pyridine-d5): δ 171.88, 100.25, 75.62, 72.08, 71.39, 70.44, 68.72, 67.34, 66.32, 59.09, 50.02, 35.63, 33.30, 30.94, 29.20, 28.96, 28.85, 28.83, 28.75, 28.74, 28.69, 28.64, 28.57, 28.44, 28.43, 25.31, 25.23, 21.76, 19.63, 16.03, 13.10.
Exact mass (ESI-MS) for C50H99NO8 [M+H]+ found, 842.7474; calcd, 842,7444
1-O-(6-deoxy-α-d-galactopyranosyl)-2-hexacosylamino-octadecane-1,3,4-triol (12)
A solution of compound 19 (66 mg, 0.05 mmol) in MeOH (2.5 mL) was hydrogenated under atmospheric pressure in the presence of palladium black (20 mg). After consumption of the starting material, 1 spot was visible on TLC, corresponding with the deiodinated product. After a quick purification by column chromatography (Hexanes/EtOAc: 7/3), the product was dissolved in MeOH (2.5 mL) and hydrogenated under atmospheric pressure in the presence of palladium black (15 mg). Upon completion of the reaction, the mixture was diluted with MeOH and filtered through celite. The filter cake was rinsed with MeOH and the filtrate was evaporated to dryness. After purification by column chromatography (CH2Cl2/MeOH: 9.2/0.8), compound12 (22 mg, 70%) was afforded as a white powder.
1H NMR (300 MHz, CD3OD): δ 7.88 (d, J = 8.9 Hz, 1H, NH), 7.26-7.10 (m, 5H, arom. H), 4.79 (app. s, 1H, H-1’), 4.22-4.17 (m, 1H, H-2), 3.95 (dd, J = 13.3 Hz and 6.7 Hz, 1H, H-5’), 3.84 (dd, J = 10.5 Hz and 4.4 Hz, 1H, H-1), 3.77-3.70 (m, 2H, H-2’, H-3’), 3.65-3.52 (m, 4H, H-1, H-3, H-4, H-4’), 2.61 (t, J = 7.7 Hz, 2H, CH2-Ph), 2.21 (t, J = 7.5 Hz, 2H, COCH2), 1.69-1.18 (m, 32H, CH2), 0.89 (t, J = 6.7 Hz, 3H, CH3).
13C NMR (75 MHz, CD3OD): δ 174.47, 142.55, 128.21, 128.11, 125.52, 99.86, 74.20, 72.42, 71.77, 70.50, 68.78, 66.81, 66.60, 50.59, 35.98, 35.57, 31.90, 31.85, 31.31, 29.64, 29.59, 29.31, 28.77, 25.82, 25.77, 22.56, 15.52, 13.28.
Exact mass (ESI-MS) for C36H63NO8 [M+H]+ found, 638.4659; calcd, 638.4626
1-O-(α-d-galactopyranosyluronate)-2-hexacosylamino-octadecane-1,3,4-triol (13)
A solution of compound 20 (105 mg, 0.08 mmol) in CHCl3 (1.3 mL) and EtOH (3.8 mL) was hydrogenated under atmospheric pressure in the presence of palladium black (15 mg). Upon reaction completion, the mixture was diluted with pyridine and filtered through celite. The filter cake was rinsed with CHCl3 and EtOH and the filtrate was evaporated to dryness. After purification by column chromatography (CH2Cl2/MeOH:8/2), compound 13 (54 mg, 78%) was afforded as a white powder.
1H NMR (500 MHz, pyridine-d5): δ 8.46 (d, J = 8.7 Hz, 1H, NH), 5.64 (d, J = 3.4 Hz, 1H, H-1’), 5.27-5.26 (m, 1H, H-2), 5.15 (app. s, 1H, H-5’), 5.02 (app.s, 1H, H-4’), 4.69-4.66 (m, 2H, H-2’, H-1), 4.50 (dd, J = 9.9 Hz and 3.1 Hz, 1H, H-3’), 4.37 (dd, J = 10.6 Hz and 4.5 Hz, 1H, H-3), 4.30 (app. s, 2H, H-1, H-4), 2.46-2.41 (m, 2H, COCH2), 2.26-1.23 (m, 72H, CH2), 0.85 (t, J = 6.7 Hz, CH3).
13C NMR (75 MHz, pyridine-d5): δ 171.93, 171.07, 152.04, 149.40, 105.00, 100.53, 75.48, 71.51, 71.34, 70.99, 70.05, 68.64, 68.08, 49.98, 35.61, 33.18, 30.94, 29.48, 29.20, 28.97, 28.86, 28.83, 28.76, 28.74, 28.69, 28.64, 28.60, 28.45, 28.43, 25.31, 25.22, 21.76, 13.10.
Exact mass (ESI-MS) for C50H97NO10 [M−H]− found, 870.7089; calcd, 870.7039
1-O-(α-d-galactopyranosyluronate)-2-(6-phenylhexanoyl)amino-octadecane-1,3,4-triol (14)
A solution of compound 21 (35 mg, 0.03 mmol) in MeOH (2 mL) was hydrogenated under atmospheric pressure in the presence of palladium black (5 mg). Upon reaction completion, the mixture was diluted with MeOH and filtered through celite. The filter cake was rinsed with MeOH and the filtrate was evaporated to dryness. After purification by column chromatography (CH2Cl2/MeOH:8/2), compound 14 (14 mg, 68%) was afforded as a white powder.
1H NMR (500 MHz, DMSO-d6): δ 7.78 (d, J = 6.25 Hz, 1H, NH), 7.28-7.14 (m, 5H, arom. H), 4.78 (d, J = 3.4 Hz, 1H, H-1’), 4.07-3.93 (m, 3H, H-2, H-4’, H-5’), 3.74 (app. s, 1H, H-1), 3.62-3.38 (m, 5H, H-2’, H-3’, H-1, H-3, H-4), 2.57-2.52 (m, 4H, COCH2, CH2-Ph), 1.59-1.23 (m, 32H, CH2), 0.86 (t, J = 6.9 Hz, CH3).
13C NMR (75 MHz, DMSO-d6): δ 172.65, 142.97, 128.90, 128.86, 126.22, 100.10, 75.03, 73.65, 71.45, 71.31, 70.51, 68.55, 67.90, 50.44, 36.09, 35.78, 32.86, 31.98, 31.59, 30.05, 29.95, 29.86, 29.82, 29.79, 29.77, 29.71, 29.40, 29.12, 28.91, 26.09, 25.90, 22.78, 14.64.
Exact mass (ESI-MS) for C36H61NO10 [M−H]− found, 666.4213; calcd, 666.4223
Surface Plasmon Resonance studies
Recombinant mouse CD1d and TCR preparations, glycolipid loading and SPR studies were performed as reported previously43. Briefly, glycolipids were dissolved in DMSO at 1mg/ml and loaded to biotinylated CD1d by incubating o/n at RT in 100mM Tris/HCl, pH 7.0, 0.08% Tween 20 buffer. Approximately 300 response units (RU) of 3 different CD1d-glycolipid complexes were immobilized onto flow channels 2–4 of a SA capture chip (GE), while biotinylated CD1d incubated in buffer without lipid was bound to flow channel 1 for background substraction. After increasing concentrations of TCR were passed over the individual flow channels simultaneously, kinetic parameters were calculated using a simple Langmuir 1:1model in the BIA evaluation software version 4.1. Experiments were performed 2–3 times for each glycolipid, using 2 different TCR preparations.
Supplementary Material
Figure 3.
IL-4 and INF-γ secretion, measured at respective 4h and 16h, after intraperitoneal injection of 5 µg of the glycolipids in mice.
Table 1.
Equilibrium binding affinity measurements by surface plasmon resonance.
| Glycolipid | Kass(M−1s−1) | Kdiss(s−1) | KD(Kdiss/Kass) |
|---|---|---|---|
| KRN7000 (1) | 1.3E+05 ± 1E+03 | 1.45E−03 ± 4.5E−05 | 11.2 ± 0.2 nM |
| 4 | 0.8E+05 ± 3E+03 | 5.26E−03 ± 2.9E−04 | 64.8 ± 6.0 nM |
| 12 | 1.0E+05 ± 2E+04 | 6.48E−03 ± 1.8E−03 | 74.4 ± 25.0 nM |
| 13 | 1.0E+05 ± 9E+03 | 16.6E−03 ± 5.5E−03 | 165 ± 38.3 nM |
| 14 | 1.1E+05 ± 6E+03 | 8.11E−03 ± 1.2E−03 | 75.3 ± 15.2 nM |
Acknowledgment
S.V.C. and D.E. received support of the Belgian Stichting tegen Kanker and the FWO Flanders. D.M.Z is recipient of an Investigator award from the Cancer Research Institute and supported by NIH grant AI074962.
Abbreviations
- α-GalCer
α-galactosylceramide
- i.v.
intravenous
- MHC
Major Histocompatibility Complex
- NK
Natural Killer
- NKT
Natural Killer T
- S.A.R.
Structure-Activity Relationship
- TCR
T Cell Receptor
- Th
T helper
Footnotes
Electronic supplementary information (ESI) available: 1H-spectra and 13C-spectra and chromatograms of final compounds 4, 12, 13 and 14.
References
- 1.Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. Curr. Opin. Immunol. 2008;20:358–368. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeng Z-H, Castano AR, Segelke BW, Stura EA, Peterson PA, Wilson IA. Science. 1997;277:339–345. doi: 10.1126/science.277.5324.339. [DOI] [PubMed] [Google Scholar]
- 3.Kronenberg M. Annu. Rev. Immunol. 2005;26:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 4.(a) Natori T, Morita M, Akimoto K, Koezuka Y. Tetrahedron. 1994;50:2771–2784. [Google Scholar]; (b) Morita M, Motoki K, Akimoto K, Natori T, Sakai T, Sawa E, Yamaji K, Koezuka Y, Kobayashi E, Fukushima H. J. Med. Chem. 1995;38:2176–2187. doi: 10.1021/jm00012a018. [DOI] [PubMed] [Google Scholar]
- 5.(a) Ishikawa A, Motohashi S, Ishikawa E, Fuchida H, Higashino K, Otsuji M, Iizasa T, Nakayama T, Tanigushi M, Fujisawa T. Clin. Cancer Res. 2005;11:1910–1917. doi: 10.1158/1078-0432.CCR-04-1453. [DOI] [PubMed] [Google Scholar]; (b) Giaccone G, Punt CJ, Ando Y, Ruijter R, Nishi N, Peters M, von Blomberg BM, Scheper RJ, van der Vliet HJ, van den Eertwegh AJ, Roelvink M, Beijnen J, Zwierzina H, Pinedo HM. Clin. Cancer Res. 2002;8:3702–3709. [PubMed] [Google Scholar]
- 6.Berkers CR, Ovaa H. Trends Pharmacol. Sci. 2005;26:252–257. doi: 10.1016/j.tips.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Banchet-Caddedu A, Hénon E, Dauchez M, Renault J-H, Monneaux F, Haudrechy A. Org. Biomol. Chem. 2011;9:3080–3104. doi: 10.1039/c0ob00975j. [DOI] [PubMed] [Google Scholar]
- 8.Goff RD, Gao Y, Mattner J, Zhou D, Yin N, Cantu C, III, Teyton L, Bendelac A, Savage PB. J. Am. Chem. Soc. 2004;126:13602–13603. doi: 10.1021/ja045385q. [DOI] [PubMed] [Google Scholar]
- 9.Chen G, Chien M, Tsuji M, Franck RW. ChemBioChem. 2006;7:1017–1022. doi: 10.1002/cbic.200500386. [DOI] [PubMed] [Google Scholar]
- 10.Costantino V, Fattorusso E, Imperatore C, Mangoni A. Tetrahedron. 2002;58:369–375. [Google Scholar]
- 11.Barbieri L, Costantino V, Fattorusso E, Mangoni A, Aru E, Parapini S, Taramelli D. Eur. J. Org. Chem. 2004:468–473. [Google Scholar]
- 12.Wu D, Xing G-W, Poles MA, Horowitz A, Kinjo Y, Sullivan B, Bodmer-Narkevitch V, Plettenburg O, Kronenberg M, Tsuji M, Ho DD, Wong C-H. Proc. Natl. Acad. Sci. U.S.A. 2005;102:1351–1356. doi: 10.1073/pnas.0408696102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raju R, Castillo B, Richardson SK, Thakur M, Severins R, Kronenberg M, Howell AR. Bioorg. Med. Chem. Lett. 2009;19:4122–4125. doi: 10.1016/j.bmcl.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbieri L, Costantino V, Fattorusso E, Mangoni A, Basilico N, Mondani M, Taramelli D. Eur. J. Org. Chem. 2005:3279–3285. [Google Scholar]
- 15.(a) Motoki K, Morita M, Kobayashi E, Uchida T, Akimoto K, Fukushima H, Koezuka Y. Biol. Pharm. Bull. 1995;18:1487. doi: 10.1248/bpb.18.1487. [DOI] [PubMed] [Google Scholar]; (b) Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 16.Xing G-W, Wu D, Poles MA, Horowitz A, Tsuji M, Ho DD, Wong C-H. Bioorg. Med. Chem. 2005;13:2907–2916. doi: 10.1016/j.bmc.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Zhou XT, Forestier C, Goff RD, Li C, Teyton L, Bendelac A, Savage PB. Org. Lett. 2002;4:1267–1270. doi: 10.1021/ol025565+. [DOI] [PubMed] [Google Scholar]
- 18.a) Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]; b) Zhou D, Mattner J, Cantu C, III, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu Y-P, Yamashita T, Teneberg S, Wang D, Proia Richard L, Levery Steven B, Savage Paul B, Teyton L, Bendelac A. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]; c) Prigozy TI, Naidenko O, Qasba P, Elewaut D, Brossay L, Khurana A, Natori T, Koezuka Y, Kulkarni A, Kronenberg M. Science. 2001;291:664–667. doi: 10.1126/science.291.5504.664. [DOI] [PubMed] [Google Scholar]
- 19.Ebensen T, Link C, Riese P, Schulze K, Morr M, Guzmán CA. J. Immunol. 2007;179:2065–2073. doi: 10.4049/jimmunol.179.4.2065. [DOI] [PubMed] [Google Scholar]
- 20.Tashiro T, Nakagawa R, Inoue S, Shiozaki M, Watarai H, Taniguchi M, Mori K. Tetrahedron Lett. 2008;49:6827–6830. [Google Scholar]
- 21.Trappeniers M, Van Beneden M, Decruy T, Linclau B, Elewaut D, Van Calenbergh S. J. Am. Chem. Soc. 2008;130:16468–16469. doi: 10.1021/ja8064182. [DOI] [PubMed] [Google Scholar]
- 22.Aspeslagh S, Li YL, Yu ED, Pauwels N, Trappeniers M, Girardi E, Decruy T, Van Beneden K, Venken K, Drennan M, Leybaert L, Wang J, Franck RW, Van Calenbergh S, Zajonc DM, Elewaut D. EMBO J. 2011;30:2294–2305. doi: 10.1038/emboj.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borg NA, Wun KS, Kjer-Nielsen L, Wilce MCJ, Pellicci DG, Koh R, Besra GS, Bharadwaj M, Godfrey DI, McCluskey J, Rossjohn J. Nature. 2007;448:44–49. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 24.a) Siram V, Du W, Gervay-Hague J, Brutkiewicz R. Eur. J. Immunol. 2005;35:1692–1701. doi: 10.1002/eji.200526157. [DOI] [PubMed] [Google Scholar]; b) Kinjo Y, Wu D, Kim G, Xing G-W, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong C-H, Kronenberg M. Nature. 2005;434:520. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 25.Jervis PJ, Cox LR, Besra GS. J. Org. Chem. 2011;76:320–323. doi: 10.1021/jo102064p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plettenburg O, Bodmer-Narkevitch V, Wong C-H. J. Org. Chem. 2002;67:4559–4564. doi: 10.1021/jo0201530. [DOI] [PubMed] [Google Scholar]
- 27.Figueroa-Pérez S, Schmidt RR. Carbohydr. Res. 2000;328:95–102. doi: 10.1016/s0008-6215(00)00092-6. [DOI] [PubMed] [Google Scholar]
- 28.Risseeuw MDP, Berkers CR, Ploegh HL, Ovaa H. Tetrahedron Lett. 2006;47:3677–3679. [Google Scholar]
- 29.Chang Y-J, Huang J-R, Tsai Y-C, Hung J-T, Wu D, Fujio M, Wong C-H, Yu A-L. Proc. Natl. Acad. Sci U.S.A. 2007;104:10299–10304. doi: 10.1073/pnas.0703824104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujio M, Wu D, Garcia-Navarro R, Ho DD, Tsuji M, Wong CH. J. Am. Chem. Soc. 2006;128:9022–9023. doi: 10.1021/ja062740z. [DOI] [PubMed] [Google Scholar]
- 31.Shie C-R, Tzeng Z-H, Kulkarni SS, Uang B-J, Hsu C-Y, Hung S-C. Angew. Chem. Int. Ed. 2005;44:1665–1668. doi: 10.1002/anie.200462172. [DOI] [PubMed] [Google Scholar]
- 32.Yule JE, Wong TC, Gandhi SS, Qiu D, Riopel MA, Koganty RR. Tetrahedron Lett. 1995;36:6839–6842. [Google Scholar]
- 33.(a) Nolting B, Boye H, Vogel C. J. Carbohydrate Chem. 2001;20:585–610. [Google Scholar]; (b) Reiffarth D, Reimer KB. Carbohydrate Res. 2008;343:179–188. doi: 10.1016/j.carres.2007.10.030. [DOI] [PubMed] [Google Scholar]; (c) Nemati N, Karapetyan G, Nolting B, Endress H-U, Vogel C. Carbohydrate Res. 2008;343:1730–1742. doi: 10.1016/j.carres.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 34.Oberli MA, Bindschädler P, Werz DB, Seeberger PH. Org. Lett. 2008;10:905–908. doi: 10.1021/ol7030262. [DOI] [PubMed] [Google Scholar]
- 35.Wadouachi A, Kovensky J. Molecules. 2011;16:3933–3968. [Google Scholar]
- 36.Codée JDC, van der Bos JJ, de Jong A-R, Dinkelaar J, Ladder G, Overkleeft H-S, van der Marel GA. J. Org. Chem. 2009;74:38–42. doi: 10.1021/jo8020192. [DOI] [PubMed] [Google Scholar]
- 37.Okamoto N, Kanie O, Huang Y-Y, Fujii R, Watanabe H, Shimamura M. Chemistry and Biology. 2005;12:677–683. doi: 10.1016/j.chembiol.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 38.Fan GT, Pan Y-S, Lu K-C, Cheng Y-P, Lin W-C, Lin S, Lin C-H, Wong C-H, Fang J-M, Lin C-C. Tetrahedron. 2005;61:1855–1862. [Google Scholar]
- 39.Veerapen N, Reddington F, Bricard G, Porcelli SA, Besra GS. Bioorg. Med. Chem. Lett. 2010;20:3223–3226. doi: 10.1016/j.bmcl.2010.04.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Mico A, Margarita R, Parlanti L, Vescovi A, Piancatelli G. J. Org. Chem. 1997;62:6974–6977. [Google Scholar]
- 41.a) Schiefner A, Fujio M, Wu D, Wong C-H, Wilson IA. J. Mol. Biol. 2009;394:71–82. doi: 10.1016/j.jmb.2009.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liang P-H, Imamura M, Li X, Wu D, Fujio M, Guy RT, Wu B-C, Tsuji M, Wong C-H. J. Am. Chem. Soc. 2008;130:12348–12354. doi: 10.1021/ja8012787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veerapen N, Reddington F, Bricard G, Porcelli SA, Besra GS. Bioorg. Med. Chem. Lett. 2010;20:3223–3226. doi: 10.1016/j.bmcl.2010.04.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, Li Y, Kinjo Y, Mac T-T, Gibson D, Painter GF, Kronenberg M, Zajonc DM. Proc. Natl. Acad. Sci U.S.A. 2010;107:1535–1540. doi: 10.1073/pnas.0909479107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.