Abstract
Axon growth after spinal injury is thought to be limited in part by myelin-derived proteins that act via the Nogo-66 Receptor (NgR). To test this hypothesis, we sought to study recovery from spinal cord injury (SCI) after inhibiting NgR transgenically with a soluble function-blocking NgR fragment. Glial fibrillary acidic protein (gfap) gene regulatory elements were used to generate mice that secrete NgR(310)ecto from astrocytes. After mid-thoracic dorsal over-hemisection injury, gfap∷ngr(310)ecto mice exhibit enhanced raphespinal and corticospinal axonal sprouting into the lumbar spinal cord. Recovery of locomotion is improved in the gfap∷ngr(310)ecto mice. These data indicate that the NgR ligands, Nogo-66, MAG, and OMgp, play a role in limiting axonal growth in the injured adult CNS and that NgR(310)ecto might provide a therapeutic means to promote recovery from SCI.
Introduction
Axonal growth is rapid and extensive during brain and spinal cord development, and proper guidance in many instances derives from selective expression of repellant cues. In the adult mammalian brain and spinal cord, axon extension is very limited even after axotomy. Limited axon regeneration after injury is in part due to a limited expression of those proteins that promote rapid growth during development and during adult peripheral nerve regeneration (Bonilla et al., 2002; Skene, 1989). However, it is also well recognized that the adult CNS affords an inhospitable terrain for axonal extension. Transplantation of a peripheral nerve environment into the brain is documented to provide significant long distance growth of CNS axons into the graft (Benfey and Aguayo, 1982; David and Aguayo, 1981; Richardson et al., 1980). Both glial scar (Bradbury et al., 2002; Davies et al., 1997; Dou and Levine, 1994) and myelin components (Savio and Schwab, 1989; Schwab and Caroni, 1988) of the injured adult CNS have been shown to slow axonal growth in vitro.
Several proteins of CNS myelin possess axon growth-inhibiting properties, notably Nogo-A (GrandPre et al., 2000; Huber and Schwab, 2000; Prinjha et al., 2000), MAG (Fournier et al., 2000; McKerracher et al., 1994; Mukhopadhyay et al., 1994), and OMgp (Wang et al., 2002b). Each recombinant protein can inhibit the growth of postnatal axons in tissue culture systems. We identified a high affinity binding site for an axon-inhibitory Nogo-66 domain of Nogo-A by expression cloning (Fournier et al., 2001; McGee and Strittmatter, 2003). In vitro, the Nogo-66 Receptor (NgR) mediates Nogo-66 inhibition of axon outgrowth (Fournier et al., 2001). This inhibition is transduced by Rho activation (Fournier et al., 2003; Jin and Strittmatter, 1997) and may be mediated via interaction of NgR directly with the p75 low affinity neurotrophin receptor and LINGO-1 (McGee and Strittmatter, 2003; Mi et al., 2004; Wang et al., 2002a; Wong et al., 2002). Remarkably, the NgR protein mediates the inhibitory action of MAG (Domeniconi et al., 2002; Liu et al., 2002) and OMgp (Wang et al., 2002b), as well as Nogo-66.
Since the NgR mediates the action of three independent axon inhibitory myelin-derived ligands, the mechanism of their binding to one protein is of significant interest (Barton et al., 2003; He et al., 2003; McGee and Strittmatter, 2003). NgR protein is composed of eight leucine-rich repeats (LRR) protein flanked by cysteine-rich LRR amino terminal (LRRNT) and LRR carboxyl terminal (LRRCT) domains (Fig. 1A) (Fournier et al., 2001). The LRR cassette is connected at its carboxyl end via a 100 aa stalk to a GPI anchorage site on the exterior of the plasma membrane. Nogo-66, MAG, and OMgp bind exclusively to the LRRNT/LRR/LRRCT region (Barton et al., 2003; Fournier et al., 2002; He et al., 2003; Liu et al., 2002). The 100 aa carboxyl linker region is required for signaling but not for ligand binding (Fournier et al., 2002). The GPI anchorage localizes NgR to lipid rafts, but raft localization is not essential for receptor function (Fournier et al., 2002). Previously, we demonstrated that the soluble LRRNT/LRR/LRRCT ectodomain of NgR can block the action of either Nogo-66 or CNS myelin on axon outgrowth (Barton et al., 2003; Fournier et al., 2002; Liu et al., 2002).
Fig. 1.
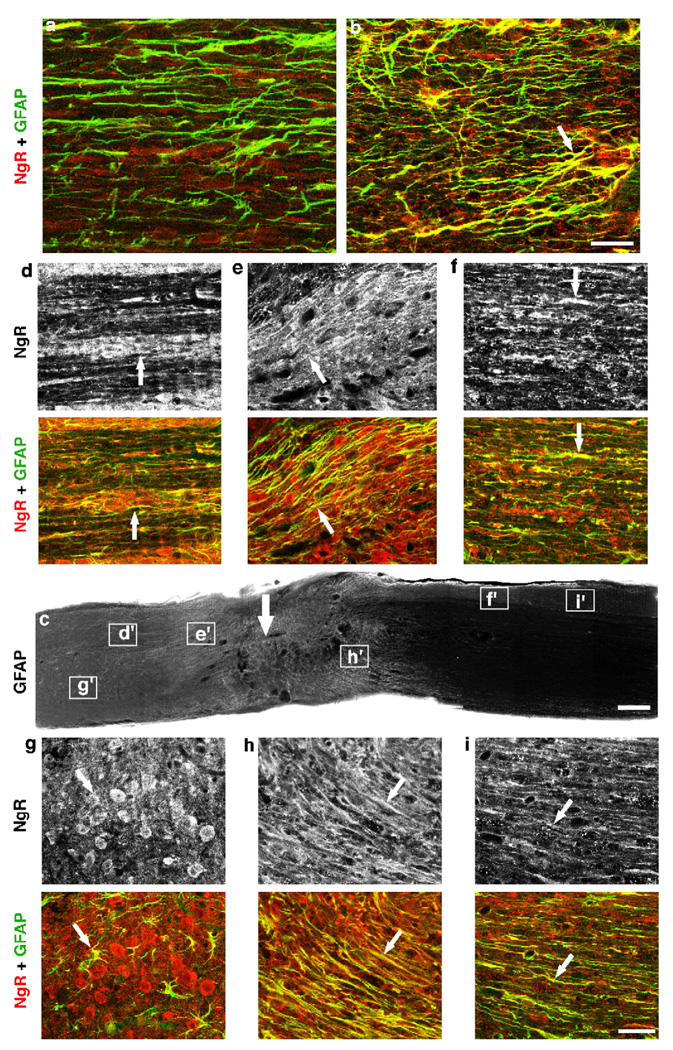
Expression of a general NgR antagonist after CNS injury in transgenic mice. (A) Schematic of WT NgR and the NgR cassette for transgenic mouse production. SS, signal sequence; LRR, leucine-rich Repeat; LRRNT, LRR amino terminal cysteine-rich region; LRRCT, LRR carboxyl terminal cysteine-rich region; TM/GPI, transmembrane/glycophosphatidylinositol anchorage site; gfap, glial fibrillary acidic protein. (B) Immunoblot analysis demonstrates the expression of NgR(310)ecto protein in cortex, spinal cord, and injured spinal cord. The secreted NgR(310)-ecto protein of 37 kDa in cortex (CTX) and spinal cord (SC) is seen in transgenic mice from both Line 08 and Line 01, but not in wild-type (WT) mice. There are no differences in the expression of 81 kDa membrane-associated endogenous NgR between WT and transgenic mice. After SCI, the soluble NgR(310)ecto level is increased in injured spinal cord compared with uninjured SC in transgenic mice. The densitometric measurements of the immunoblot optical density (arbitrary units) are displayed under each band. (C–H) Cortical sections double stained for NgR and GFAP near an injury area display a higher diffuse signal for NgR in transgenic mice (F) than in WT mice (C) consistent with secreted NgR. NgR staining is also co-localized with astrocytic marker GFAP in transgenic mice (arrows). Neuronal cell body NgR is detected in both WT and transgenic samples (*). (I and J) Sagittal spinal cord sections double staining for NgR and GFAP around over-hemisection display the stronger staining for NgR ecto in transgenic mice (J) than in WT mice (I) and the NgR staining is partly co-localized with astrocytes (J). Scale bar: 50 µm (C–J).
While in vitro experiments have clearly documented the existence of a myelin/NgR inhibitory cascade, the in vivo significance of an NgR inhibitory system and of the relative importance of Nogo, MAG, and OMgp as ligands have remained less than clear. Studies supporting Nogo as an important inhibitor of axon regeneration began with studies of IN-1 antibody administration to spinal-injured rats (Schnell and Schwab, 1990; Thallmair et al., 1998) and have included overexpression of Nogo in peripheral Schwann cells (Kim et al., 2003a; Pot et al., 2002). Different targeted mutations of various splice forms of the nogo gene have yielded conflicting results, with significant corticospinal (CST) axon growth after spinal cord injury (SCI) in some experiments and no CST growth in others (Kim et al., 2003b; Simonen et al., 2003; Zheng et al., 2003). The basis of these discrepancies is not resolved. A small fragment of Nogo-A potently blocks Nogo-66, but not MAG, action at the NgR, and this peptide yields CST sprouting and functional recovery after SCI (GrandPre et al., 2002). However, this NgR antagonist peptide does not block the inhibitory action of a second Nogo domain located in the amino terminal region of the Nogo-A isoform (Fournier et al., 2001). Viral expression of a dominant-negative version of the NgR enhances optic nerve regeneration, especially when an enhanced growth state is induced by lens injury (Fischer et al., 2004). For MAG, null mutations do not permit CST regeneration after SCI but do enhance peripheral nerve regeneration on certain genetic backgrounds (Bartsch et al., 1995; Schafer et al., 1996). In vivo studies of OMgp function have not been reported.
With this complicated background, we sought to assess the effect of blocking the action of all ligands at the NgR on the ability of CNS axons to grow after SCI. Because the extracellular LRRNT/LRR/LRRCT domain of the NgR actively binds Nogo-66, MAG, and OMgp it blocks CNS myelin action in vitro (Barton et al., 2003; Fournier et al., 2002; Liu et al., 2002). We delivered this protein to mice via a transgenic promotor and studied SCI outcomes. The soluble NgR(310)ecto protein induced evidence of CST and raphespinal axon growth. The correlation of this growth with functional recovery demonstrates that the NgR plays a substantial role in regulating axonal growth in the injured adult CNS.
Results
Transgenic expression of NgR(310)ecto
To express soluble NgR(310)ecto protein in vivo, we chose the gfap gene regulatory elements (Fig. 1A) to allow diffuse CNS secretion from quiescent astrocytes as well as enhanced secretion from reactive astrocytes at site of injury (Johnson et al., 1995). Two NgR(310)ecto-expressing lines were established from five founder mice. To test whether the GFAP-positive cells express and secrete NgR(310)ecto protein in heterozygous-transgenic mice, homogenates of cerebral cortex and spinal cord were examined by Western blot analysis using antibody raised against NgR. This antibody detects an ~80-kDa protein species in homogenates of cortex and spinal cord as in the NgR-transfected HEK293T cells (Domeniconi et al., 2002; Fournier et al., 2002). As expected, the secreted 37-kDa NgR(310)ecto protein is detected in detergent-free soluble extracts of cortex and spinal cord from the two transgenic lines Tg08 and Tg01 without injury (Fig. 1B), but little if any soluble NgR protein at 37 or 81 kDa is present in littermate wild-type (WT) mice. Expression in the two transgenic lines is similar. Examination of the particulate fractions demonstrates comparable levels of endogenous GPI-anchored NgR in both WT and transgenic mice. To test the effect of CNS injury on NgR(310)ecto expression in transgenic sample, a dorsal over-hemisection injury was performed. Ten days after lesion, the level of secreted NgR(310)ecto protein is increased approximately twofold in soluble extracts of injured spinal cord of transgenic mice (Fig. 1B).
To examine the cellular expression of NgR(310)ecto protein in injured CNS, we immunostained the injured brain (Figs. 1C–H) and spinal cord (Figs. 1I–J) containing the lesion area with antibodies against NgR and GFAP. The general morphology of reactive astrocytic glia does not differ between WT and transgenic mice (Figs. 1D, G, I, and J), but the density of NgR immunoreactivity in the astrocytes is high in the NgR(310)ecto-transgenic mice (Fig. 1F) and absent in WT mice (Fig. 1C). As shown in merged images (Figs. 1E–J), NgR protein is co-localized with the astrocytic marker GFAP only in the transgenic mice (Figs. 1H–J), demonstrating the selectivity of GFAP-driven expression of NgR(310)ecto in reactive astrocytes around the lesion. There is also a greatly enhanced diffuse extracellular staining in the transgenic samples, consistent with NgR(310)ecto in the extracellular space (Figs. 1F and J). Neuronal cell body NgR staining is similar in WT and transgenic mice. The region of NgR up-regulation near an injury site corresponds to the region of GFAP upregulation (Fig. 2), as is expected for secreted NgR ecto protein expression by the gfap promoter. A potentially confounding factor in the analysis of these mice would be compensatory upregulation of Nogo-A. However, in either intact or injured cortex and spinal cord, the expression of Nogo-A is similar in WT and transgenic mice (Fig. 3).
Fig. 2.
Distribution of NgR(310)ecto in the injured spinal cord of transgenic mice. (a and b) Parasagittal sections double stained for NgR and GFAP from uninjured mice display a higher signal for NgR in transgenic mice (b) than in WT mice (a). NgR staining is co-localized with astrocytic marker GFAP in transgenic mice (arrow), but not in wild-type mice. (c) Sagittal section from a spinal cord injured transgenic animal displays the increased GFAP signal at lesion areas, consistent with the upregulation of this protein in reactive astrocytes. (d–i) Higher magnification of several areas in c double stained with GFAP and NgR illustrates the high NgR signals (upper panels) at transected site, with maximal level at the center of lesion. NgR staining is co-localized with GFAP (arrows, lower panels) and is diffusely distributed in the extracellular space consistent with secreted protein. Scale bar: 50 µm (a and b), 250 µm (c); 50 µm (d–i).
Fig. 3.
Expression of Nogo A after spinal cord injury in NgR(310)ecto-transgenic mice. (A) Immunoblot analysis demonstrates the expression of Nogo A protein of 210 kDa in spinal cord and injured spinal cord. There are no differences in the expression of Nogo A protein after SCI. (B–E) Sagittal spinal cord sections stained for Nogo A (red) and GFAP (green) near a dorsal over-hemisection site illustrate the similar staining for Nogo A in white matter (arrow in B and D) and gray matter (arrow in C and E) for WT (B and C) and transgenic (D and E) mice. Nogo A staining of oligodendrocytic (B and D) and neuronal (C and E) cell bodies is seen in both WT and transgenic samples at a similar level. Scale bar: 25 µm (B and D); 50 µm (C and E).
Transgenic secreted NgR(310)ecto induces CST growth in the lesioned spinal cord
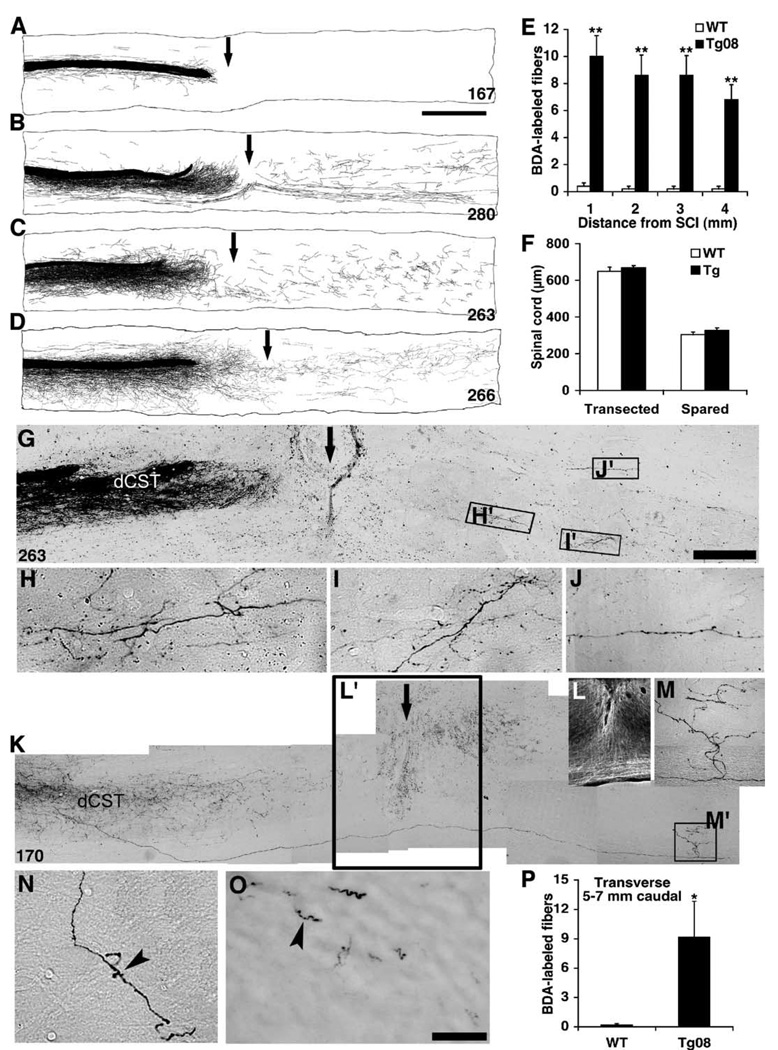
Transgenic secretion of the soluble NgR(310)ecto protein, which blocks the binding of myelin-derived inhibitors to neuronal surface NgR (Fournier et al., 2002; Liu et al., 2002), may stimulate the growth of injured axons after SCI. To test this, the integrity of descending corticospinal tracts (CST) was investigated by injecting the anterograde tracer biotin dextran amine (BDA) into the right motor cortex after mid-thoracic dorsal over-hemisection SCI (Li and Strittmatter, 2003). The BDA-labeled prominent dorsal CST (dCST) is tightly bundled rostral to the lesion in littermate WT mice. In contrast, the sections rostral to the dorsal over-hemisection from injured NgR(310)ecto-transgenic mice exhibit a high density of BDA-labeled sprouting outside of prominent dCST (Fig. 4). This pattern is not present in uninjured transgenic mice (Fig. 4A). The density of CST fibers outside of the dCST and dorsolateral CST (dlCST) is increased more than 10 fold in the transgenic animals (Figs. 4C–E).
Fig. 4.
CST sprouting rostral to SCI in NgR(310)ecto-transgenic mice. (A) Transverse sections rostral to lesion display a similar degree of dorsal CST labeling in both wild-type and NgR(310)ecto-transgenic mice. These transverse sections were obtained 5 mm rostral to a dorsal over-hemisection site from eight wild-type (WT) mice and 16 transgenic gfap∷ngr-ecto mice as indicated. The midline is to the left and dorsal is up in all sections. Also note the increased density of ectopic sprouts lateral to the dCST in the transgenic animals. The bottom row illustrates the dCST pattern at the same level of the spinal cord in transgenic mice without spinal injury. Note that there is little dCST sprouting, similar to the injured control and distinct from the injured transgenic mice. Mouse identifying number is at the bottom right of each panel. (B) Schematic of transverse spinal cord section illustrating the dCST and the location of the high magnification images in C and D. (C and D) BDA-labeled CST fiber in gray matter adjacent to the dCST from a wild-type mouse (C) and gfap∷NgR(310)ecto mouse (D). (E) Ectopic CST fibers outside of the dCST and dlCST and ≥100 µm in length are counted from transverse sections 5–7 mm rostral to over-hemisection. Means ± SEM are reported from 7 to 9 determinations. The indicated values in the presence of the inhibitor were statistically different from control binding without inhibitor (**P ≤ 0.01; Student’s t test).
Are sprouting fibers observed in the caudal spinal cord where they might contribute to functional recovery? Camera lucida drawings of all consecutive parasagittal sections from each animal across a T6 dorsal over-hemisection injury site display the overall distribution pattern of CST fibers (Figs. 5A–D). Sections from WT mice show very few or no CST fibers extending caudal to the injury site (Fig. 5A). Similar sections from NgR(310)ecto-transgenic mice display numerous CST fibers in the distal gray and white matter areas with a highly branched pattern (Figs. 5B–D). Immediately rostral to over-hemisection, a high density of BDA-labeled CST sprouts originating from the dCST project near the lesion area (Figs. 5B–D), but most CST sprouts fail to pass the transection area, where scar formation is obvious. A small fraction of the branched axons is visible at the level of the injury in ventral and ventrolateral gray and white matter. In the region immediately caudal to the lesion, the course of CST fibers in NgR(310)ecto mice is typically tortuous with longitudinal branching in gray matter (Figs. 5B–D and G–J) and quite distinct from the normally straight fibers in the uninjured CST. Collaterals and arborized fibers are most frequently seen in gray matter area of caudal spinal cord. In one case, the origin of a caudal arborization in the transgenic mice could be traced back to the rostral dCST, verifying that axonal regeneration around the lesion had occurred in this particular case (Figs. 5K–M). In other cases, sprouting from uninjured fibers may account for the increase in caudal CST fibers.
Fig. 5.
Transgenic NgR(310)ecto induces CST fiber growth in the caudal spinal cord. (A–D) Camera lucida reconstructions of all consecutive parasagittal sections around the lesion site. The injury site is indicated with an arrow. WT animals show no axons in the caudal cord (A). In contrast, soluble NgR(310)ecto induces a high density of sprouting from lesioned dorsal CST fibers (B–D). A subset of CST fibers project into caudal spinal cord, particularly into gray matter areas. Mouse identifying number is at the bottom right of each panel. (E) Quantification of CST fibers (total number of fibers per animal) is illustrated at various distances caudal to the injury site from transgenic (n = 6 mice) and WT (n = 6 mice) groups. (F) Dorsal–ventral linear measurements of lesioned spinal cord from parasagittal sections demonstrate a similar degree of transected and spared tissue at the injury site from wild-type and transgenic mice (n = 6 in each group). (G–J) Parasagittal section containing the transection site (arrow) from an NgR(310) ecto-transgenic animal illustrates the transection of BDA-labeled dCST fibers and some branched, sprouting fibers around transection site. Higher magnification of these areas in H′, I′, and J′ displays the meandering course of the sprouting CST fibers in both the gray matter (H and I) and white matter area (J). (K–M) Composite parasagittal sections around the lesion site (arrow) from an NgR(310)ecto-transgenic animal demonstrate a BDA-labeled regenerating axon from the transected rostral dCST bypassing the transection area and projecting into the caudal spinal cord gray matter (M). Immunostaining with GFAP displays the transection injury in this mouse (L). (N and O) Transverse sections at a level 5–7 mm caudal to the lesion illustrate some CST fibers with branching patterns in the gray matter (N) and white matter (O) of spinal cord. (P) CST fiber counts (number of fibers/section) at a level of 5–7 mm caudal to the lesion from transverse sections indicate a greater number in transgenic group (n = 7 mice) than in control group (n = 7 mice). Means ± SEM are reported. The values from transgenic mice are statistically different from the WT mice (*P < 0.05; **P < 0.01; Student’s t test). Scale bar, 1 mm (A–D, L); 250 µm (G and K); 25 µm (H–J, M–O).
The reconstructions demonstrate 5–15 BDA-labeled fibers coursing in the rostral–caudal axis at any level 1–4 mm caudal to the lesion in each transgenic mouse (Fig. 5E). For transverse sections 5–7 mm caudal to dorsal over-hemisection, BDA-labeled CST axons are seen in both the gray matter (Fig. 5N) and white matter areas (Fig. 5O) in each transgenic mouse. The fiber counts from different caudal levels from the transgenic mice reveal extension of most BDA-labeled CST fibers for more than 7 mm caudal to the lesion (Figs. 5E and P). To ensure that the observation of caudal CST fibers in the transgenic mice could not be attributed to a lesser lesion in this group of animals, the depth of the injury was measured. In both wild-type and transgenic animals, the over-hemisection injury involved the dorsal 70% of the spinal cord (Fig. 5F). It is also clear that the differences between groups cannot be explained by differences in BDA labeling intensity since the rostral CST is labeled to a similar extent in all animals (Fig. 4A).
In addition to CST fibers, other descending tracts, such as raphespinal fibers, contribute to locomotor function in mice. Serotonin immunohistology of parasagittal sections reveals the high density of serotonergic fibers in the rostral spinal cord of both WT (Figs. 6A–C) and transgenic mice (Figs. 6F–H). In the spinal cord distal to transection site, the density of 5-HT fibers is higher in transgenic (Figs. 6F, I, and J) than in the WT mice (Figs. 6A, D, and E). Examination of transverse sections from 5 to 7 mm caudal to SCI indicates that a majority of the serotonergic fibers are severed, decreasing the density of these fibers by approximately 80% in the lumbar ventral horn (Fig. 6M). Analysis of total length of serotonin fibers in the ventral horn of caudal spinal cord demonstrates a much greater value in transgenic mice than in the WT group (Figs. 6K–M). This increase may be due either to local sprouting from uninjured fibers or to long distance growth of axotomized 5-HT fibers. Thus, the growth-promoting effects of NgR(310)ecto protein in transgenic mice are not limited to one descending pathway.
Fig. 6.
Serotoninergic fiber sprouting in NgR(310)ecto-transgenic mice. (A–E) Parasagittal spinal cord sections from WT group double immunostained with anti-5-HT (red) and anti-GFAP (green) antibodies illustrate a large number of serotonin fibers (A–C, white arrow) rostral to the lesion (black arrow), but only few serotonin fibers are seen at the cord caudal to SCI (A, D, E). (F–J) Longitudinal sections from transgenic mice display a high density of serotonin fibers (white arrows) in both the rostral (F–H) and caudal spinal cord (F, I, J) to SCI. (K–L) Transverse spinal cord sections 5–7 mm distal to transection immunostained with anti-5-HT antibodies exhibit only a few serotonin fibers in ventral horn from WT mice (K), but a large number of 5-HT fibers are observed in the ventral horn from transgenic mice (L). (M) Immunoreactive serotonin fiber length in the ventral horn per transverse section at a level 5–7 mm rostral or 5–7 mm caudal to injury site was measured (n = 7 mice in each group). Means ± SEM are reported. The values from transgenic group are statistically different from the WT mice (**P < 0.01; Student’s t test). Scale bar, 1 mm (A, F); 25 µm (B–E, G–L).
Transgenic NgR(310)ecto protein improves locomotor recovery
The CST axon tracing and serotonergic fiber analysis indicate that the NgR(310)ecto protein released from astrocytes in transgenic mice stimulates significant anatomical growth of descending axons in the spinal cord. To determine whether this fiber growth benefits functional recovery, we examined several behavioral tests. An open field locomotor scoring system was employed to assess outcome (Basso et al., 1996; Joshi and Fehlings, 2002). The WT mice partially recover locomotor function during a 4-week period of survival. At 4 weeks post-injury, most WT mice recover to a level characterized by consistent plantar stepping with consistent weight support, but they exhibit only occasional to frequent forelimb–hindlimb coordination, with a rotation of predominant paw position when making initial contact with surface. In contrast, the BBB scores of NgR(310)ecto-transgenic mice from both lines Tg08 and Tg 01 are significantly higher than control group throughout the 7- to 28-day observation period (Fig. 7A). At 28 days after SCI, most transgenic mice show consistent forelimb–hindlimb coordination, and the predominant paw position is parallel to the body. Tg 08 mice express slightly more NgR(310)ecto than do Tg01 mice (Fig. 1B), but the behavioral results are similar in the two lines. The lack of behavioral differences in recovery could reflect either a “ceiling effect” for the benefit of NgR(310)ecto or to the fact that expression differs by only a minor degree.
Fig. 7.
Behavioral recovery from SCI in gfap∷NgR(310)ecto-transgenic mice. (A and B) Locomotor BBB score is reported as a function of time after dorsal over-hemisection in mice injured at 2 months of age (A) from WT (n = 9 mice), Line 08 (n = 9) or Line 01 (n = 7) or mice injured at 4–6 months of age (B; WT, n = 8; Tg, n = 11). For the specific mice in which tissue sparing was measured in Fig. 5F, the results were indistinguishable. For example, the 21-day BBB wild-type score was 12.2 ± 0.9 and the Tg08 score was 16.2 ± 0.2 (P < 0.01). (C) The maximal tolerated inclined plane angle is plotted as a function of time after SCI for WT (n = 9 mice) and transgenic (n = 9 mice) animals injured at 2 months of age. (D) Hindlimb errors during inclined grid climbing are reported as a function of post-SCI time from both groups (n = 9 mice in each group, age 2 months). In all the graphs, means ± SEM are reported. The values from transgenic group are statistically different from the WT mice (**P < 0.01; Student’s t test).
Two other behavioral tests were employed to further characterize the performance of NgR(310)ecto-transgenic mice. First, we measured the maximal angle to which a board could be tilted without the mouse losing its grip within 5 s. Before dorsal over-hemisection injury, both transgenic and WT mice can sustain their posture on a board angled at 558. On days 7–28 after SCI, the sustainable angle is reduced in all mice, but the angles sustainable by the transgenic mice are significantly greater than those for the control group (Fig. 7C). In another behavioral test, mice climbed a grid placed at a 45° angle to vertical and excursions of the hindlimbs below the plane of the grid were counted (Metz et al., 2000). No mice made errors on this test during the pre-injury training. There are numerous foot fault errors with only minimal improvement in WT mice during the period 2–4 weeks post-injury. In contrast, the NgR(310)ecto-transgenic mice exhibit a progressive improvement in grid climbing during this period, with the majority of improvement occurring between 1 and 3 weeks post-injury (Fig. 7D). Thus, transgenic mice secreting soluble NgR(310)ecto from astrocytes exhibit CST fiber growth, raphespinal sprouting, and improved motor function after thoracic spinal over-hemisection.
In our studies of mice lacking Nogo-A/B, we noted that caudal CST fiber growth was present in young adult (8 weeks) but not is some older mice (14 weeks) (Kim et al., 2003b). To examine if the effect of soluble NgR(310)ecto was age dependent, we examined mice of 4–6 months age in the same dorsal over-hemisection model. While the difference between transgenic and wild-type mice was of lesser magnitude, the older NgR(310)ecto mice recovered open field locomotion more completely than did wild-type littermates (Fig. 7B).
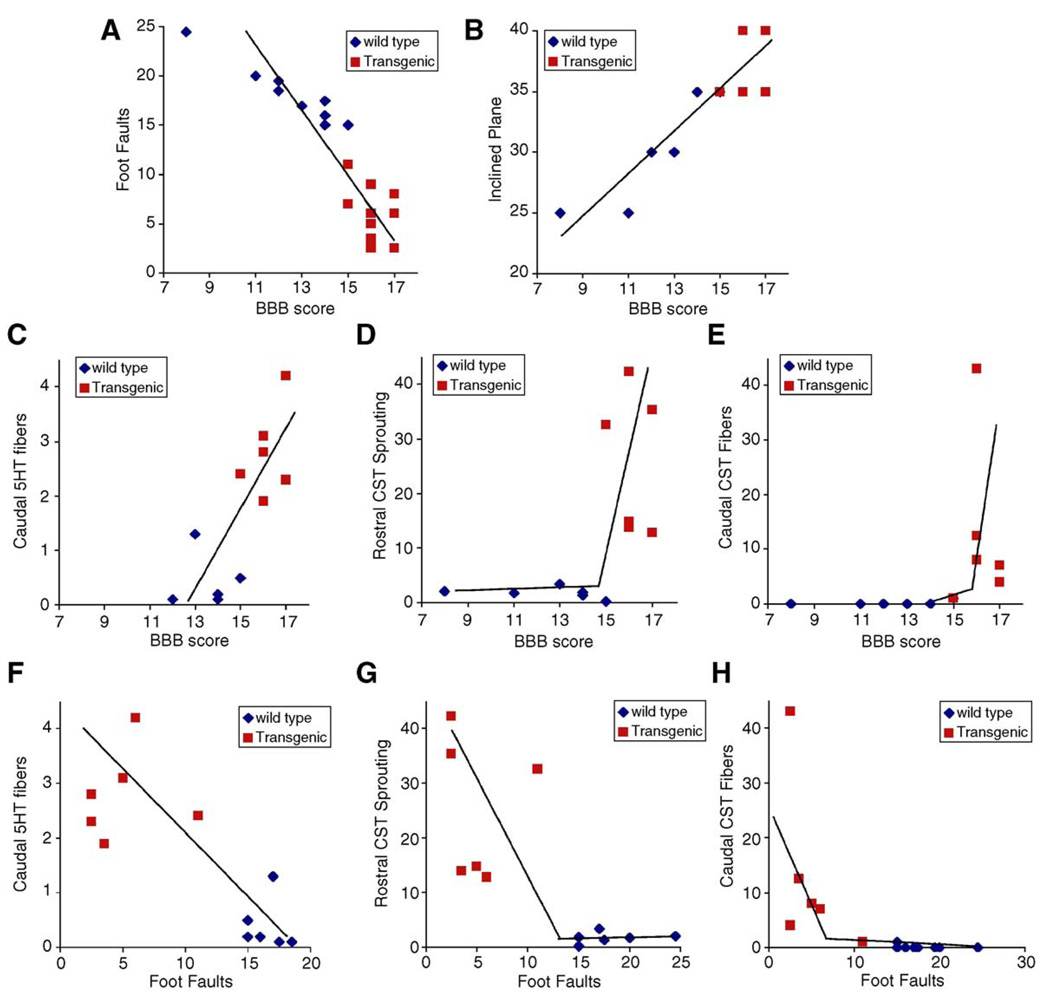
The NgR(310)ecto-transgenic mice exhibit both increased axonal fiber counts and improved behavioral measurements after SCI. We suggest that enhanced growth in one or more axonal pathways accounts for the improved behavior. To the extent that this hypothesis is true, there should be a correlation between anatomical and behavioral outcomes on a mouse-by-mouse basis, as well as a correlation on the basis of genotype. Scatter plots demonstrate that performance values on the various behavioral tests (grid climbing, open field BBB, and inclined plane) are closely correlated with one another (Figs. 8A and B). More interestingly, each of the axonal measurements of caudal 5HT fibers, rostral CST sprouting, and caudal CST fibers is tightly correlated, on a mouse-by-mouse basis, with the BBB scores and the grid climbing performance (Figs. 8C–H). These correlations support the notion that one or more of these of axonal systems is responsible for improved behavior. The data do not provide an indication as to whether rostral CST sprouting versus caudal CST fibers versus caudal 5HT fibers play different roles in different behavioral tests.
Fig. 8.
Correlation between anatomical and behavioral outcomes. (A and B) Behavioral scores from the experiments in Figs. 7A, C, and D are plotted against one another with each point representing the data from one mouse of the indicated genotype at 21 days post-SCI. (C–H) Axonal measurements of caudal 5HT fiber length from Fig. 6M or rostral CST sprouting density from Fig. 4E or caudal CST fiber counts from Fig. 5P are plotted against BBB scores from Fig. 7A or grid climbing errors from Fig. 7D on a mouse-by-mouse basis. Behavioral scores are from 21 days post-SCI.
While the NgR(310)ecto-transgenic mice score better on several locomotor tasks after SCI, it is possible that axonal sprouting would result in abnormal but adaptive gait strategies. To more fully assess gait in the SCI mice, an automated treadmill system with video-assisted analysis of stride parameters was utilized. At a speed of 25 cm/s, uninjured mice display a regular right fore/left hind alternating with left fore/right hind gait at a stride length of 6.5 cm (Figs. 9A and F). Four weeks after injury, all of the NgR(310)ecto-transgenic mice tested were able to walk at this speed and the gait pattern was regular with a stride length indistinguishable from uninjured animals (Figs. 9C–F). In marked contrast, the majority of wild-type littermate SCI mice could not attain this normal walking speed (Fig. 9E). In the subset of control SCI mice that did manage to produce regular 25 cm/s strides, there was a breakdown of forelimb/hindlimb coordination (Fig. 9B) and a reduced stride length (Fig. 9F). The measure of forelimb stride variation between steps was significantly increased in the wild-type SCI mice as compared to the NgR(310)ecto SCI mice (Fig. 9G). This analysis confirms that the gait of transgenic mice after SCI is significantly more effective than that of wild-type injured mice and more closely resembles that of uninjured mice.
Fig. 9.
Gait analysis after SCI in gfap∷NgR(310)ecto-transgenic mice. (A–D) Gait was imaged from below animals walking on a transparent treadmill belt moving at a speed of 25 cm/s. Uninjured mice (A), wild-type injured mice (B), or NgR(310)ecto-transgenic mice (C and D). Paw area in contact with the treadmill surface is plotted as a function of time for each paw. Records were obtained 4 weeks after SCI from 4- to 6-month-old female mice. (E) The fraction of injured mice of the indicated genotypes able to walk at 25 cm/s is plotted. Wild-type, n = 8; NgR(310)ecto-transgenic (TG), n = 11. (F) The average stride length calculated from all four paws for the genotypes and injury status is plotted. Data are collected from records as in A–D. The wild-type injury data are from the 3 of 8 mice able to walk at 25 cm/s and the transgenic data are from 11 mice. (G) The average variability in forelimb stride length is plotted for the indicated groups of mice. Means ± SEM are reported. *P < 0.05; Student’s t test.
Discussion
Transgenic expression of a secreted function-blocking NgR protein has profound effects after SCI. In the dorsal over-hemisection model tested here, both CST and raphespinal fiber growth is stimulated in the injured spinal cord. For CST fibers, wild-type animals exhibit little or no growth. NgR inhibition leads to a highly significant increase of CST fiber growth into the caudal spinal cord. However, the density of CST fibers in the lumbar cord is a small fraction of the pre-injury density. Despite this low density of CST fibers, gait is dramatically improved with cadence and stride lengths nearly resembling uninjured mice. One explanation for the greater functional than CST recovery in response to NgR(310)ecto is the possibility that only a few CST fibers are required functionally. An alternative and more likely explanation is that other fiber systems grow more robustly in this injury model in response to NgR(310)ecto secretion from astrocytes. In particular, the serotonergic innervation of the caudal spinal cord returns to near normal levels in the injured transgenic mice and may contribute substantially to motor recovery. NgR(310)ecto expression may also enhance the natural tendency to form new intraspinal circuits after SCI (Bareyre et al., 2004).
We find many more CST fibers in the caudal spinal of NgR(310)ecto mice than wild-type mice after dorsal over-hemisection. This is not due to any difference in lesion severity and must be attributed to axonal growth stimulated by the presence of transgenic soluble NgR(310)ecto-Fc. CST fiber growth appears to occur in numerous locations. There is local gray matter sprouting from the injured dCST rostral to the lesion site. It is likely that there is enhanced sprouting from the rare ventral CST fibers which are spared by dorsal over-hemisection. At least occasionally, cut dCST fibers grow around the lesion site to reach the caudal spinal cord. There is not extensive growth in the glial scar at the injury site itself. This is consistent with specificity of NgR(310)ecto for blockade of myelin but not glial scar inhibition. It suggests that a combination of NgR blockade with perturbation of CSPG-mediated axon inhibition may be most effective for therapeutic intervention after SCI.
Nogo-A in myelin contains both the Nogo-66 inhibitory domain that binds to NgR and a second Amino-Nogo inhibitory domain that does not act via NgR (Fournier et al., 2001, 2003). Null mutations in the nogo-A gene should remove both of these inhibitory domains from oligodendrocytes. The regeneration phenotype of nogo-A−/− mice has been at odds in different studies, so a clear comparison with the present study is difficult (Kim et al., 2003b; Simonen et al., 2003; Zheng et al., 2003). Those mice with the most robust regeneration (Kim et al., 2003b) show CST fiber growth after SCI that exceeds that observed with selective NgR blockade. Mice with loss of the Amino-Nogo but not the Nogo-66 domain show a minor degree of CST sprouting (Simonen et al., 2003). However, some Nogo-A/B-deficient mice exhibit no CST fiber growth (Zheng et al., 2003). While a firm conclusion is uncertain from the nogo gene knockout studies, the current data tend to suggest some modest contribution of the Amino-Nogo domain to the limitation of axon sprouting in the adult CNS after SCI and a more significant contribution of Nogo-66.
Previous studies in mice with the systemic Nogo-66-selective antagonist peptide, NEP1-40, demonstrated CST axon growth, 5HT axon growth and improved walking after dorsal over-hemisection (Li and Strittmatter, 2003). In the current study, we utilized a transgenic delivery method for an NgR blocker that should reduce MAG and OMgp function as well as Nogo-66. The number of CST fibers extending into the caudal spinal cord and the density of serotonergic fibers in the lumbar cord are enhanced to a very similar degree in the two experiments. Functional improvements in the BBB score and the inclined grid climb are slightly greater in the NgR(310)ecto-transgenic mice than in NEP1-40-treated mice. This suggests that there is a moderate contribution of MAG and OMgp to limiting axonal growth after SCI. The extent of this contribution can be addressed most directly in animals in which NgR(310)ecto protein and NEP1-40 peptide are delivered by identical methodology. This can accomplished pharmacologically in rats via intrathecal cannula placement (GrandPre et al., 2002). Recent descriptions of intrathecal NgR(310)ecto-Fc treatment of rats and of mice lacking NgR protein support the conclusions of the current study (Kim et al., 2004; Lee et al., 2004; Li et al., 2004).
In summary, the current study reveals significant increases in CST and raphespinal growth after injury in a strain of mice expressing a secreted function-blocking form of the NgR. This is correlated with improved locomotion and gait. The level of improvement after SCI with this genetic perturbation of NgR slightly exceeds that induced by Nogo-66-selective antagonist pharmacological treatment. Soluble NgR protein may provide a therapeutic means to promote recovery after SCI.
Experimental methods
Generation of gfap∷ngr(310)ecto-transgenic mice
The mouse NgR(310)ecto cDNA (corresponding to aa 1–310) was subcloned into the NotI site of the C-3123 vector containing the 2.0-kb GFAP promoter, a gift from Dr. Lennart Mucke (Gladstone Institute of Neurological Disease) (Johnson et al., 1995). This construct was released from the pGEM-11Z backbone by sequential digestion with AatII and SfiI, and the resulting 3.4 kb fragment was microinjected into embryos to generate transgenic mice. Transgene integration was verified by PCR and five founders were identified. Two founder lines with the highest expression levels were crossed to C57BL/6J mice. Wild-type littermates were used as controls in all experiments.
Surgical procedures and corticospinal fiber tracing in transgenic mice
All surgical procedures and postoperative care were performed in accordance with guidelines of the Yale Animal Care and Use Committee. Adult female heterozygous-transgenic or littermate wild-type mice (2–6 months of age) were deeply anesthetized with intramuscular ketamine (100 mg/kg) and intraperitoneal xylazine (15 mg/kg). A complete laminectomy was performed and dorsal part of spinal cord was fully exposed at T6 and T7 levels. A dorsal over-hemisection was performed at T6 with a 30-gauge needle and a pair of microscissors to completely sever the dorsal and dorsolateral CSTs. The depth of lesion (1.0 mm) was assured by passing the marked needle across dorsal part of the spinal cord several times. The lesion was bilaterally symmetric and extended to a depth about 2/3 of the dorsal/ventral axis of the spinal cord, as measured in Fig. 5F. Both the right and left dorsal CSTs and both dorsolateral CSTs were completely severed but the ventral CST was spared. For serotonin fibers, fibers on both sides were severed and the intermediolateral area was included in the lesion. Only serotonin fibers in the ventral 1/3 of the cord were spared. The muscle layers over the laminectomies were sutured, and the skin on the back was closed with surgical staples. To trace the corticospinal tracts, a burr hole overlying cerebral cortex on the right side was made into the skull 14 days after spinal cord injury. Tracer BDA (MW 10,000, 10% in PBS) (Molecular Probes, Eugene, OR) was applied into four injection sites (0.3 µl/site) at a depth of 0.7 mm from the cortical surface (coordinates: 0.5–1.5 mm posterior to bregma, 0.5–1.5 mm lateral to bregma). Four weeks after SCI, the mice were perfused transcardially with PBS, followed by 4% paraformaldehyde. To examine the developmental distribution of CST axons in the transgenic mice, BDA injections were conducted in four adult mice without receiving dorsal over-hemisection injury.
Some SCI mice were used for NgR(310)ecto and Nogo A protein expression experiments. These mice did not receive tracer injection 2 weeks after SCI. For immunoblot analysis, the spinal cord at a level between T3 and L3 was collected without perfusion 10 days after SCI. Mice used for NgR and Nogo A immunohistochemical staining were perfused with 4% paraformaldehyde 14 days after over-hemisection, and the injured spinal cord was removed for sectioning. To examine NgR ecto protein expression in the injured brain of transgenic and WT mice, a stab injury of the cerebral cortex was performed with a number 11 scalpel blade held in a stereotaxic apparatus (David Kopf, Tujunga, CA). A 4-mm parasagittal cut was made, 0.5 mm posterior to bregma, 1.5 mm laterally from the midline, and 3.5 mm deep.
Detection of NgR in transgenic mice
The cortex, spinal cord, and injured spinal cord were homogenized in Tris-buffered saline supplemented with protease inhibitors (Roche) and centrifuged at 100,000 rpm for 20 min at 4°. The particulate fraction from the initial spin was homogenized in RIPA buffer (1% Triton X-100 + 0.5% sodium deoxycholate + 0.1% SDS in PBS) by sonication. After a second centrifugation, the second supernatant was collected as the detergent-soluble particulate fraction. To enhance antibody specificity, the samples (20 µg protein) were treated with 4% paraformaldehyde for 20 min and then dialyzed prior to immunoblot with 1:2000 anti-NgR rabbit antiserum (Domeniconi et al., 2002; Fournier et al., 2001). Immunoreactivity was visualized by incubation with alkaline phosphatase-conjugated anti-rabbit IgG and NBT/BCIP AP substrates.
Histology for BDA tracing, lesion depth, and serotonin fiber staining
For transgenic mice, the spinal cord 4 mm rostral to and 4 mm caudal to the lesion (an 8-mm segment) was embedded in a glutaraldehyde–polymerized albumin matrix, and cut parasagittally on a vibratome (30 µm thick). Transverse sections (50 µm) were collected from the spinal cord 5–7 mm rostral to and 5–7 mm caudal to the injury site. The sections were incubated with avidin–biotin–peroxidase complex and the BDA tracer was visualized by nickel-enhanced diaminobenzidine HRP reaction or with Avidin-Alexa594 (GrandPre et al., 2002). To verify the origin of caudal CST fibers in a subset of animals, the path of individual axons near the transection area was traced from a complete set of consecutive parasagittal sections. Some sections from other animals were processed for serotonin immunohistochemistry (anti-5-HT anti-body, Immunostar) by indirect immunofluorescence. To visualize the lesion area, some sections were double stained with antibody directed against GFAP (Sigma, St. Louis, MO). The sections were mounted, dehydrated, and covered with mounting medium. The transection depth was measured from GFAP staining and differential interference contrast photographs from all consecutive parasagittal sections for each animal. The dorsal–ventral linear depth of spared and transected tissue were measured. Serotonin fiber length in the ventral horn of transverse mouse sections rostral or caudal to lesion site was measured with NIH image software (Li and Strittmatter, 2003). Three sections were analyzed for each animal, and the values from these sections are averaged. For anti-NgR/anti-GFAP immunohistology all images were collected as matched sets of transgenic and non-transgenic samples with identical exposures and processing during image collection.
Behavioral analysis
For mice, a modified Basso–Beattie–Bresnahan (BBB) locomotor rating scale (Joshi and Fehlings, 2002) with 21 as normal and 0 as complete hindlimb paralysis was used to score locomotion in the open field after SCI (Basso et al., 1996; Joshi and Fehlings, 2002; Kim et al., 2003b; Ma et al., 2001; Noble et al., 2002; Wells et al., 2003; Zheng et al., 2003). Another BBB modification developed to score mice as opposed to rats collapses values from 16 to 21 of the original rat scoring system to scores of 16–17 due to the difficulties in making assessments at the upper end of the scoring range in mice (Dergham et al., 2002). Since all injured mice had BBB scores of 16 or less in the current study, this second mouse BBB modification is irrelevant to measurements of the injured mice in this study. Inclined plane test and the inclined grid walking tests were also used to monitor mice after SCI (Li and Strittmatter, 2003). For the inclined plane test, we measured the maximal angle to which a 50 × 60 cm board could be tilted for 5 s without the mouse sliding down. For inclined grid walking, the mice were trained to climb a wire grid (35 cm long with 2.54 cm squares) at a slope of 458. The number of instances in which the hindpaw dropped below the grid plane was scored for each excursion from bottom to top.
Treadmill gait analysis
The DigiGait system was utilized for gait analyses (Hampton et al., 2004). Briefly, digital images of paw placement were recorded at 80 Hz through a clear treadmill from below the animal. Mice were tested without pre-training in one session at a treadmill speed of 25 cm/s. Plotting the area of each digital paw print imaged sequentially in time provides a dynamic gait signal, representing the temporal record of paw placement relative to the treadmill belt. Stride length was calculated from the fixed walking speed divided by the measured stride frequency (strides per second). Additional gait indices were determined as described (Hampton et al., 2004).
Acknowledgments
We thank Yiguang Fu and Ji Liao for expert technical assistance with in vivo studies. This work was supported by grants to S.L. from the Christopher Reeve Paralysis Foundation and the Institut International de Recherche en Paraplegie, Geneva, and to S.M.S. from the NIH.
References
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Barton WA, Liu BP, Tzvetkova D, Jeffrey PD, Fournier AE, Sah D, Cate R, Strittmatter SM, Nikolov DB. Structure and Axon Outgrowth Inhibitor Binding of the Nogo-66 Receptor and Related Proteins. EMBO J. 2003;22:3291–3302. doi: 10.1093/emboj/cdg325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch U, Bandtlow CE, Schnell L, Bartsch S, Spillmann AA, Rubin BP, Hillenbrand R, Montag D, Schwab ME, Schachner M. Lack of evidence that myelin-associated glycoprotein is a major inhibitor of axonal regeneration in the CNS. Neuron. 1995;15:1375–1381. doi: 10.1016/0896-6273(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC, Anderson DK, Faden AI, Gruner JA, Holford TR, Hsu CY, Noble LJ, Nockels R, Perot PL, Salzman SK, Young W. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter animal spinal cord injury study. J. Neurotrauma. 1996;13:343–359. doi: 10.1089/neu.1996.13.343. [DOI] [PubMed] [Google Scholar]
- Benfey M, Aguayo AJ. Extensive elongation of axons from rat brain into peripheral nerve grafts. Nature. 1982;296:150–152. doi: 10.1038/296150a0. [DOI] [PubMed] [Google Scholar]
- Bonilla IE, Tanabe K, Strittmatter SM. Small proline-rich repeat protein 1A is expressed by axotomized neurons and promotes axonal outgrowth. J. Neurosci. 2002;22:1303–1315. doi: 10.1523/JNEUROSCI.22-04-01303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390:680–683. doi: 10.1038/37776. [DOI] [PubMed] [Google Scholar]
- Dergham P, Ellezam B, Essagian C, Avedissian H, Lubell WD, McKerracher L. Rho signaling pathway targeted to promote spinal cord repair. J. Neurosci. 2002;22:6570–6577. doi: 10.1523/JNEUROSCI.22-15-06570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domeniconi M, Cao Z, Spencer T, Sivasankaran R, Wang K, Nikulina E, Kimura N, Cai H, Deng K, Gao Y, He Z, Filbin M. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35:283–290. doi: 10.1016/s0896-6273(02)00770-5. [DOI] [PubMed] [Google Scholar]
- Dou CL, Levine JM. Inhibition of neurite growth by the NG2 chondroitin sulfate proteoglycan. J. Neurosci. 1994;14:7616–7628. doi: 10.1523/JNEUROSCI.14-12-07616.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D, He Z, Benowitz LI. Counteracting the Nogo receptor enhances optic nerve regeneration if retinal ganglion cells are in an active growth state. J. Neurosci. 2004;24:1646–1651. doi: 10.1523/JNEUROSCI.5119-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier AE, Nakamura F, Kawamoto S, Goshima Y, Kalb RG, Strittmatter SM. Semaphorin3A enhances endocytosis at sites of receptor-F-actin colocalization during growth cone collapse. J. Cell Biol. 2000;149:411–422. doi: 10.1083/jcb.149.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- Fournier AE, Gould GC, Liu BP, Strittmatter SM. Truncated soluble Nogo receptor binds Nogo-66 and blocks inhibition of axon growth by myelin. J. Neurosci. 2002;22:8876–8883. doi: 10.1523/JNEUROSCI.22-20-08876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier AE, Takizawa BT, Strittmatter SM. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J. Neurosci. 2003;23:1416–1423. doi: 10.1523/JNEUROSCI.23-04-01416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GrandPre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- GrandPre T, Li S, Strittmatter SM. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417:547–551. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- Hampton TG, Stasko MR, Kale A, Amende I, Costa AC. Gait dynamics in trisomic mice: quantitative neurological traits of down syndrome. Physiol. Behav. 2004;82:381–389. doi: 10.1016/j.physbeh.2004.04.006. [DOI] [PubMed] [Google Scholar]
- He XL, Bazan JF, M G, Park JB, Wang K, Tessier-Lavigne M, He Z, Garcia KC. Structure of the Nogo receptor ectodomain: a recognition module implicated in myelin inhibition. Neuron. 2003;38:177–185. doi: 10.1016/s0896-6273(03)00232-0. [DOI] [PubMed] [Google Scholar]
- Huber AB, Schwab ME. Nogo-A, a potent inhibitor of neurite outgrowth and regeneration. Biol. Chem. 2000;381:407–419. doi: 10.1515/BC.2000.053. [DOI] [PubMed] [Google Scholar]
- Jin Z, Strittmatter SM. Rac1 mediates collapsin-1-induced growth cone collapse. J. Neurosci. 1997;17:6256–6263. doi: 10.1523/JNEUROSCI.17-16-06256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WB, Ruppe MD, Rockenstein EM, Price J, Sarthy VP, Verderber LC, Mucke L. Indicator expression directed by regulatory sequences of the glial fibrillary acidic protein (GFAP) gene: in vivo comparison of distinct GFAP-lacZ transgenes. Glia. 1995;13:174–184. doi: 10.1002/glia.440130304. [DOI] [PubMed] [Google Scholar]
- Joshi M, Fehlings MG. Development and characterization of a novel, graded model of clip compressive spinal cord injury in the mouse: Part 1. Clip design, behavioral outcomes, and histopathology. J. Neurotrauma. 2002;19:175–190. doi: 10.1089/08977150252806947. [DOI] [PubMed] [Google Scholar]
- Kim JE, Bonilla IE, Qiu D, Strittmatter SM. Nogo-C is sufficient to delay nerve regeneration. Mol. Cell. Neurosci. 2003a;23:451–459. doi: 10.1016/s1044-7431(03)00076-9. [DOI] [PubMed] [Google Scholar]
- Kim JE, Li S, GrandPre T, Qiu D, Strittmatter SM. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron. 2003b;38:187–199. doi: 10.1016/s0896-6273(03)00147-8. [DOI] [PubMed] [Google Scholar]
- Kim JE, Liu BP, Park JH, Strittmatter SM. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron. 2004;44:439–451. doi: 10.1016/j.neuron.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Lee JK, Kim JE, Sivula M, Strittmatter SM. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J. Neurosci. 2004;24:6209–6217. doi: 10.1523/JNEUROSCI.1643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Strittmatter SM. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J. Neurosci. 2003;23:4219–4227. doi: 10.1523/JNEUROSCI.23-10-04219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Liu BP, Budel S, Li M, Ji B, Walus L, Li W, Jirik A, Rabacchi S, Choi E, Worley D, Sah DW, Pepinsky B, Lee D, Relton J, Strittmatter SM. Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J. Neurosci. 2004;24:10511–10520. doi: 10.1523/JNEUROSCI.2828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BP, Fournier A, GrandPre T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- Ma M, Basso DM, Walters P, Stokes BT, Jakeman LB. Behavioral and histological outcomes following graded spinal cord contusion injury in the C57Bl/6 mouse. Exp. Neurol. 2001;169:239–254. doi: 10.1006/exnr.2001.7679. [DOI] [PubMed] [Google Scholar]
- McGee AW, Strittmatter SM. The Nogo-66 receptor: focusing myelin inhibition of axon regeneration. Trends Neurosci. 2003;26:193–198. doi: 10.1016/S0166-2236(03)00062-6. [DOI] [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- Metz GA, Merkler D, Dietz V, Schwab ME, Fouad K. Efficient testing of motor function in spinal cord injured rats. Brain Res. 2000;883:165–177. doi: 10.1016/s0006-8993(00)02778-5. [DOI] [PubMed] [Google Scholar]
- Mi S, Lee X, Shao Z, Thill G, Ji B, Relton J, Levesque M, Allaire N, Perrin S, Sands B, Crowell T, Cate RL, McCoy JM, Pepinsky RB. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat. Neurosci. 2004;7:221–228. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J. Neurosci. 2002;22:7526–7535. doi: 10.1523/JNEUROSCI.22-17-07526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pot C, Simonen M, Weinmann O, Schnell L, Christ F, Stoeckle S, Berger P, Rulicke T, Suter U, Schwab ME. Nogo-A expressed in Schwann cells impairs axonal regeneration after peripheral nerve injury. J. Cell Biol. 2002;159:29–35. doi: 10.1083/jcb.200206068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinjha R, Moore SE, Vinson M, Blake S, Morrow R, Christie G, Michalovich D, Simmons DL, Walsh FS. Inhibitor of neurite outgrowth in humans. Nature. 2000;403:383–384. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- Richardson PM, McGuinness UM, Aguayo AJ. Axons from CNS neurons regenerate into PNS grafts. Nature. 1980;284:264–265. doi: 10.1038/284264a0. [DOI] [PubMed] [Google Scholar]
- Savio T, Schwab ME. Rat CNS white matter, but not gray matter, is nonpermissive for neuronal cell adhesion and fiber outgrowth. J. Neurosci. 1989;9:1126–1133. doi: 10.1523/JNEUROSCI.09-04-01126.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer M, Fruttiger M, Montag D, Schachner M, Martini R. Disruption of the gene for the myelin-associated glycoprotein improves axonal regrowth along myelin in C57BL/Wlds mice. Neuron. 1996;16:1107–1113. doi: 10.1016/s0896-6273(00)80137-3. [DOI] [PubMed] [Google Scholar]
- Schnell L, Schwab ME. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990;343:269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Caroni P. Oligodendrocytes and CNS myelin are nonpermissive substrates for neurite growth and fibroblast spreading in vitro. J. Neurosci. 1988;8:2381–2393. doi: 10.1523/JNEUROSCI.08-07-02381.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonen M, Pedersen V, Weinmann O, Schnell L, Buss A, Ledermann B, Christ F, Sansig G, van der Putten H, Schwab ME. Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-a improves regenerative and plastic responses after spinal cord injury. Neuron. 2003;38:201–211. doi: 10.1016/s0896-6273(03)00226-5. [DOI] [PubMed] [Google Scholar]
- Skene JH. Axonal growth-associated proteins. Annu. Rev. Neurosci. 1989;12:127–156. doi: 10.1146/annurev.ne.12.030189.001015. [DOI] [PubMed] [Google Scholar]
- Thallmair M, Metz GA, Z’Graggen WJ, Raineteau O, Kartje GL, Schwab ME. Neurite growth inhibitors restrict plasticity and functional recovery following corticospinal tract lesions. Nat. Neurosci. 1998;1:124–131. doi: 10.1038/373. [DOI] [PubMed] [Google Scholar]
- Wang KC, Kim JA, Sivasankaran R, Segal R, He Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002a;420:74–78. doi: 10.1038/nature01176. [DOI] [PubMed] [Google Scholar]
- Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002b;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- Wells JE, Hurlbert RJ, Fehlings MG, Yong VW. Neuro-protection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain. 2003;126:1628–1637. doi: 10.1093/brain/awg178. [DOI] [PubMed] [Google Scholar]
- Wong ST, Henley JR, Kanning KC, Huang KH, Bothwell M, Poo MM. A p75(NTR) and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nat. Neurosci. 2002;5:1302–1308. doi: 10.1038/nn975. [DOI] [PubMed] [Google Scholar]
- Zheng B, Ho C, Li S, Keirstead H, Steward O, Tessier-Lavigne M. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron. 2003;38:213–224. doi: 10.1016/s0896-6273(03)00225-3. [DOI] [PubMed] [Google Scholar]