Abstract
Purpose
To determine the impact of brachytherapy implant quality on outcome among cervical cancer patients treated on RTOG prospective trials 0116 and 0128.
Materials and Methods
All enrolled patients received concurrent chemo-radiation followed by brachytherapy. Individual brachytherapy parameters, including the symmetry of ovoids in relation to the tandem, displacement of ovoids in relation to the cervical os, tandem bisecting the ovoids, tandem in the mid-pelvis, and appropriateness of packing, were scored for each implant. Multivariate (MV) Cox proportional hazard models were constructed for each parameter for local recurrence (LR), regional recurrence (RR), distant recurrence (DR), disease-free survival (DFS) and overall survival (OS).
Results
Records for 103 patients were analyzed. The median follow-up time was 24.5 months. Patients with unacceptable symmetry of ovoids to the tandem had a significantly higher risk of LR than patients in the acceptable group (HR=2.67, 95% C.I. = [1.11, 6.45], p=0.03). Patients with displacement of ovoids in relation to the cervical os had a significantly increased risk of LR (HR=2.50, 95% C.I. = [1.05, 5.93], p=0.04) and a lower DFS rate (HR=2.28, 95% C.I. = [1.18, 4.41], p=0.01). Inappropriate placement of packing resulted in a lower DFS rate (HR=2.06, 95% C.I. = [1.08, 3.92], p=0.03).
Conclusion
Assessment of the quality of a brachytherapy implant is imperative, as proper placement impacts patient disease-free survival. If feasible, inappropriate placements should be corrected prior to treatment initiation. Brachytherapy applicators for cervical cancer should preferably be placed and assessed by experienced practitioners.
Keywords: brachytherapy quality, cervical cancer
Introduction
Brachytherapy, the placement of radioactive sources directly in or in close proximity to a malignant tumor, may successfully eradicate localized disease in many sites. Brachytherapy results in central regions of high dose directly in the tumor adjacent to the radioactive sources and is thus a form of very localized dose escalation. In contrast, other forms of dose escalation with external-beam radiation may give significantly higher doses to the normal tissues, such as the rectum, sigmoid, bladder, and small bowel, that surround the cervix. Women with locally advanced FIGO Stages IB–IVA cervical cancer treated with tandem-based brachytherapy after external-beam radiation have a significantly increased survival rate compared to those treated with external-beam radiation alone.1, 2 The recognition that the high dose from the tandem-based applicator results in better tumor control3, 4 and that local control in cervical cancer is critical for survival1, 2 underscore the critical importance of brachytherapy. In order to treat the appropriate region, the applicator must be properly placed in order to focus the dose around the areas harboring disease.3, 5, 6 One may also conjecture that experienced practitioners are more likely to properly place the applicators, and that their experience would thus have direct impact on the survival of their patients. Indeed, the Patterns of Care study concluded that centers treating fewer than 500 patients per year are significantly more likely to underdose cervical cancer patients.7–9
The dose distribution around a tandem-based brachytherapy implant is determined by three factors: applicator position, source location and source strength. Furthermore, attenuation factors, such as shields, may intentionally alter the distribution. In one analysis, significant factors related to dose points (A, bladder and rectum) included mg of radium in the ovoids, mg of radium in the tandem, lateral displacement of the ovoids in the frontal plane, vertical separation between the ovoid and tandem sources, and anterior-posterior displacement of the ovoids relative to the tandem.6 Standard practice dictates that sources should be loaded in each ovoid and to the tip of the tandem unless a spacer is required to protect bowel situated close to the uterine fundus.10 For applicator alignment, general principles include that the tandem should be positioned midline in the pelvis, the ovoids should be symmetric, and the packing should not cross over the top of the ovoids, pushing the cervix away from dose.11 Proper placement of the applicator homogeneously distributes dose throughout the pelvis, ensuring adequate tumor and nodal coverage.
Several randomized trials demonstrate that chemotherapy administered concurrently with radiation for locally advanced cervical cancer significantly increases survival.12–15 To date, no trial has quantitatively assessed brachytherapy parameters in a prospective fashion in order to assess the impact of placement on survival outcomes, particularly in the era of chemo-radiation. The purpose of this study was to determine the impact of proper applicator placement on overall survival; disease-free survival; local, regional, para-aortic, and distant failures; and acute and late toxicities among patients with cervical cancer treated with brachytherapy and concurrent chemotherapy in the Phase II RTOG clinical trials 0116 and 0128.
Materials and Methods
The Phase II RTOG trials 0116 and 0128 accrued women with Stages IB to IVA cervical squamous carcinoma or adenocarcinoma. Trial 0116 only enrolled patients with positive common iliac or positive para-aortic lymph nodes and therefore treated patients with radiation to an extended field. External-beam borders and dose and the chemotherapy regimens have been previously described.16–18
All patients received brachytherapy. Either high-dose-rate (HDR) or low-dose-rate (LDR) options could be selected based on availability and physician preference. For patients treated with HDR, tandem and ovoid, tandem and ring or tandem and cylinder (for lower vaginal extension) were acceptable. For LDR patients, tandem and ovoid was preferable, although tandem and cylinder or interstitial methods could be used for patients with initial vaginal extension at diagnosis, with a prescription goal of 85 Gy to point A.
The first LDR intracavitary application was given within two weeks of the completion of external irradiation. If tumor and normal-tissue anatomy permitted acceptable intracavitary geometry, brachytherapy could be initiated during external-beam therapy. The interval allowed between the two LDR applications was one to three weeks, with the total course of treatment to be completed in less than eight weeks. Plain x-ray films were required immediately after insertion for all LDR or HDR insertions.
HDR brachytherapy could start as early as week two (0116) or week three (0128), with 5 fractions of 6.0 Gy specified at Point A. When HDR brachytherapy was started before the end of external beam, at least one insertion was performed per week with no external-beam therapy or chemotherapy given on the day of the insertion. If most of the external-beam radiation was finished prior to initiating brachytherapy, then two insertions per week could be done, separated by at least 48 hours, in order to complete all treatment within eight weeks. Chemotherapy could be given with one HDR fraction delivered after the completion of all the external-beam irradiation.
For LDR, the dose tolerances were: bladder ICRU point, 80 Gy; rectum ICRU point, 75 Gy; vaginal surface lateral surface of ovoid or cylinder, 135 Gy. For HDR, in order to stay below an LDR equivalent of 75 Gy to the rectum for six HDR insertions, including the 45-Gy contribution from the external-beam radiation, the rectum was to receive less than 70% of the prescribed dose (4.1 Gy per fraction) to Point A. The dose to the bladder was limited to less than 80% of the prescribed dose (4.6 Gy per fraction) to Point A. As in LDR brachytherapy, every attempt should have been made to deliver tumoricidal doses, even if the late-responding tissues received a slightly higher dose.
Brachytherapy quality was scored after patients finished treatment based on films obtained by the study principal investigators who evaluated the overall brachytherapy quality and scored as per protocol, variation acceptable, or deviation unacceptable. In this study, the scores were grouped as acceptable (A: per protocol) or unacceptable (U: variation acceptable/deviation unacceptable). The individual parameters evaluated were: symmetry of ovoids to tandem, displacement of ovoids in relation to the cervical os, position of tandem in mid pelvis on lateral film, tandem bisecting ovoids on lateral film, and appropriateness of packing. Each parameter was scored as: 0, not applicable; 1, acceptable; 2, unacceptable; or 9, not evaluated. In this study, if a patient had a score of acceptable across all their implants for each individual parameter then they were grouped as “all implants acceptable” (A) for that particular parameter. If a patient had any other score besides acceptable for any of their implants then they were grouped as “unacceptable/not evaluated” (U). The data were collected prospectively, as patients were enrolled on prospective studies. However, the scoring was not performed in real time and hence no modifications were able to be made to the treatment course. The investigators performed the human investigations after approval by a local Human Investigations Committee and in accord with an assurance filed with and approved by the Department of Health and Human Services, where appropriate.
Statistical Analysis
All analyses were performed using SAS/STATR® Version 9.2. Chi-squared tests were used to compare pretreatment characteristics between brachytherapy quality groups. Actuarial estimates for overall survival (OS) and disease-free survival (DFS) were calculated using Kaplan-Meier methods19 and the cumulative incidence method20 was used to estimate the local, regional, para-aortic, and distant failure rates. All time events were measured from the date of study registration to the date of their occurrence or last follow-up. All pre-treatment characteristics were dichotomized except for age. Cox21 proportional hazards models were used to determine if there was any correlation between overall brachytherapy quality or an individual brachytherapy parameter and OS; DFS; or local (LR), regional (RR), and/or distant (DR. Logistic regression models were used to determine if there was any correlation between overall brachytherapy quality or an individual brachytherapy parameter and grade ≥3 acute non-hematologic toxicities, grade ≥3 acute gastrointestinal (GI)/genitourinary (GU) toxicities, any grade ≥3 acute toxicities, grade ≥3 late GI/GU toxicities, or any late grade ≥3 toxicities. Acute toxicities, within 90 days of treatment start, were scored with the National Cancer Institute Common Toxicity Criteria (CTC) version 2.0 and late RT toxicities, > 90 days from treatment start, with the Radiation Therapy Oncology Group (RTOG)/European Organization for Research and Treatment of Cancer (EORTC) Late RT Morbidity Scoring Scheme.22
The following variables were assessed in the multivariate (MV) Cox and logistic models: overall brachytherapy quality (A vs. U) or individual brachytherapy parameter (A vs. U), age (continuous), FIGO stage (IA–IIB vs. IIIA–IVB) or T stage (T3/T4 vs. other), pelvic/iliac nodes positive (yes vs. no), para-aortic nodes positive (yes vs. no), Zubrod performance status (1 or 2 vs. 0), histology (squamous vs. other), smoking currently or in the past (yes vs. other), insertion type (LDR vs. HDR), RT total dose (continuous), number of chemotherapy cycles (continuous), number of brachytherapy insertions (continuous), and brachytherapy duration in days (continuous). All variables were coded such that a hazard ratio (HR) or odds ratio (OR) >1 indicated an increased risk of failure or odds of toxicity for the worse prognostic group compared to the better prognostic group (i.e., an increased risk of failure for IIIA/IIIB/IVA/IVB compared to IA/IB/IIA/IIB). If an individual brachytherapy parameter was associated with an outcome, then one additional variable from the list above was added to the model along with the brachytherapy parameter in order to construct a two-variable model. Those variables that showed an association with the outcomes in the two-variable models were then included in a multivariable model. At most, 5 variables (including the brachytherapy parameter) were investigated in the multivariable models. These models were built with stepwise and backwards selection procedures using α = 0.05 as inclusion and/or exclusion criteria. These approaches were both used to confirm the validity of the models built.
Results
Patient characteristics and treatment
A total of 119 analyzable patients were enrolled on RTOG 0116 (n=41) and 0128 (n=78). Patients were excluded from this analysis if they did not have an implant (n=9), were not evaluable (n=1), or had a tandem/cylinder or interstitial (n=6). Therefore, a total of 103 patients were included in this analysis (36 patients from 0116 and 67 patients from 0128). Patient characteristics are shown in Table 1. The majority of patients had Zubrod performance status 0 (71%), early stage disease (78%), and squamous cell histology (89%). The median overall follow-up time for all patients was 24.5 months (range [rg:min-max], 6.1–81.7) and among surviving patients was 26.8 months (rg, 10.4–81.7). Of the 36 patients from RTOG 0116 in this analysis, 3 (8%) did not complete chemotherapy. Of the 67 patients on protocol 0128 in this analysis, 2 (3%) had an unacceptable deviation, and 6 (9%) did not complete chemotherapy.
Table 1.
Characteristics of Patients Entered on RTOG 0116/0128 with an Implant
| (n=103) | ||
|---|---|---|
| Age (years) | ||
| Mean | 47 | |
| Std. Dev. | 11.3 | |
| Median | 46 | |
| Min - Max | 24 – 76 | |
| Q1 – Q3 | 38 – 56 | |
| n | % | |
|
|
||
| Zubrod Performance Status | ||
| 0 | 73 | 71 |
| 1 or 2 | 30 | 29 |
| T stage | ||
| T1 | 26 | 25 |
| T2 | 54 | 52 |
| T3/T4 | 22 | 21 |
| Unknown | 1 | 1 |
| FIGO Stage | ||
| IA–IIB | 80 | 78 |
| IIIA–IVB | 23 | 22 |
| Histology | ||
| Squamous | 92 | 89 |
| Adenocarcinoma | 5 | 5 |
| Adenosquamous | 6 | 6 |
| Pelvic/iliac positive | ||
| Yes | 38 | 37 |
| No | 65 | 63 |
| Para-aortic positive | ||
| Yes | 24 | 23 |
| No | 79 | 77 |
| Smoking | ||
| No | 41 | 40 |
| Former smoker | 31 | 30 |
| Current smoker | 25 | 24 |
| Unknown | 6 | 6 |
All patients received external-beam radiation with a median dose of 50.4 Gy (rg, 25–62.4). The median number of external-beam fractions was 28 (rg, 25–33). A total of 64 patients (62%) received LDR whereas 39 (38%) received HDR. The median total brachytherapy dose to Point A with LDR was 40.1 Gy (rg, 24.5–60.8) and with HDR was 30 Gy (rg, 18–32), and to Point B with LDR was 11.73 Gy (rg, 7–16.5) and with HDR was 7.8 Gy (rg, 4.4–9.4). The median per fraction brachytherapy dose to Point A with LDR was 20.6 Gy (rg, 12.2–40) and with HDR was 6 Gy (rg, 3.8–8), and to Point B with LDR was 6.2 Gy (rg, 3.5–12.1) and with HDR was 1.6 Gy (rg, 0.95–2.2).
Median normal-tissue total brachytherapy doses included: bladder, with LDR 23.5 Gy (rg, 7.1–37.8) and with HDR 17.1 Gy (rg, 9.2–33); rectum, with LDR 23.5 Gy (rg, 10.3–44.8) and with HDR 17.0 Gy (rg, 10.6–25.4); and vaginal surface, with LDR 78 Gy (rg, 4.3–140.4) and with HDR 41.7 Gy (rg, 15.6–64.8). Median normal tissue per fraction doses included: bladder, with LDR 12.5 Gy (rg, 3.6–35.7) and with HDR 3.6 Gy (rg, 2.3–6.6); rectum, with LDR 12.6 Gy (rg, 5.1–26.2) and with HDR 3.6 Gy (rg, 2.5–5.1); and vaginal surface, with LDR 41.3 Gy (rg, 2.2–120.0) and with HDR 8.9 Gy (rg, 3.1–19.4). Fifty-four LDR patients (84%) had 2 insertions and 10 (16%) had only one insertion. For HDR, 33 (85%) had 5 fractions, 5 (13%) had 3 fractions, and 1 (3%) had 6 fractions. The median total number of days from the start of any brachytherapy to the end of any brachytherapy was 15 for LDR (rg, 2–58) and 28 for HDR (rg, 6–49) (median two-sample test: p<0.0001). Treatment time was not related to DFS (HR=0.992, 95% C.I.=[0.97–1.02], p=0.55). The median total number of days from the start of any external-beam radiation/brachytherapy to the end of any external-beam radiation/brachytherapy was 58 for LDR (rg, 44–118) and 51 for HDR (rg, 42–90) (p=0.0005). Though the duration of brachytherapy was not required to fit within a certain time frame based on the protocols, the standard recommendation was for all treatment to be administered within 56 days.
Brachytherapy quality review: overall
Brachytherapy was judged to be “per protocol” in 79 (77%), “variation acceptable” in 14 (14%), and “deviation unacceptable” in 10 (10%) patients. Thus 79 patients were in the A group and 24 in the U group. The distribution of individual quality parameters was similar across A and U implants. Only 12% were U on 3 out of the 5 individual parameters; 21% (22/103) were U on > 2 parameters. For those patients that had more than one insertion, having one implant with U parameters predicted subsequent implant parameters being scored as U. There were no statistically significant differences in age, FIGO stage, pelvic or para-aortic node involvement, histology, smoking status, or median follow-up time between the A and the U groups. On univariate Cox analysis, there were no differences in OS, DFS, LR, RR, or DR, between the overall A and the overall U groups.
Brachytherapy quality review: individual parameters
The individual brachytherapy parameter with the highest frequency of being deemed A was symmetry of ovoids (n=83, 81%). In contrast, the position of the tandem in mid-pelvis on a lateral film was the parameter most frequently found U (n=39, 38%). There were no significant differences in each individual parameter with regard to stage distribution.
Figure 1 demonstrates a case deemed unacceptable due to asymmetrical ovoids and displacement of the ovoids in relation to the tandem.
Figure 1.
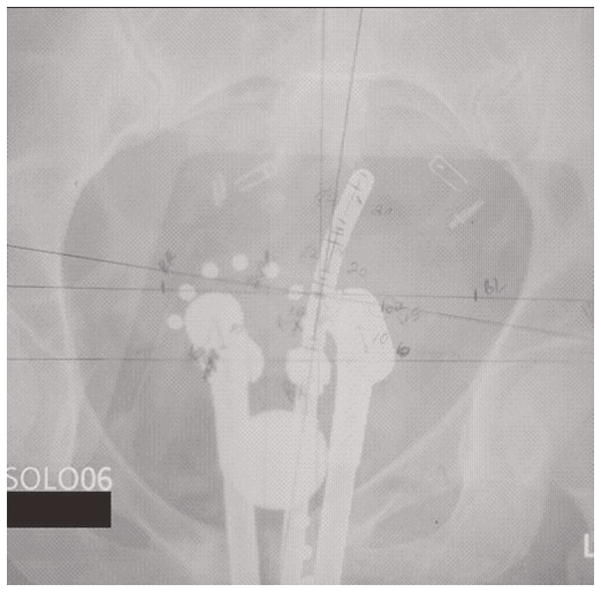
A low-dose-rate tandem and ovoid brachytherapy implant demonstrating unacceptable characteristics including: displacement of the ovoids, asymmetry of the ovoids, and inappropriate packing.
For displacement of ovoids in relation to the cervical os, on univariate analysis, both DFS and LR rates varied significantly between the A and U groups. In terms of DFS, patients in the U group had a 1.9 times greater risk of failure than patients in the A group (HR=1.88, 95% C.I. = [0.99, 3.57], p=0.055). Patients in the U group had a 2.5 times greater risk of failing locally than patients in the A group (HR=2.5, 95% C.I. = [1.05, 5.93], p=0.04). The association with DFS was also seen on MV analysis. Patients in the U group had a 2.3 times greater risk of failure than patients in the A group after adjusting for pelvic/iliac and para-aortic nodal status (HR=2.28, 95% C.I. = [1.18, 4.41], p=0.01).
With regard to the symmetry of ovoids to tandem, the U group contained significantly more patients with positive nodes. There was no significant difference for symmetry of ovoids with regards to unilateral or bilateral parametrial extension. On univariate analysis for DFS, patients in the U group had a 2.5 times greater risk of failure than patients in the A group (HR=2.49, 95% C.I. = [1.29, 4.80], p=0.006). Patients in the U group had a 2.7 times greater risk of failing locally than patients in the A group (HR=2.67, 95% C.I. = [1.11, 6.45], p=0.03). On MV analysis for DFS, this association was not seen after adjusting for pelvic/iliac and para-aortic nodal status in the model. However, on MV analysis, quality review was the only variable to show an association with LR.
Figure 2 depicts a case deemed unacceptable with regard to the position of the tandem in mid pelvis on the lateral film. For this parameter, there were no differences seen in the pretreatment characteristics or in the outcome endpoints between the A and U groups.
Figure 2.
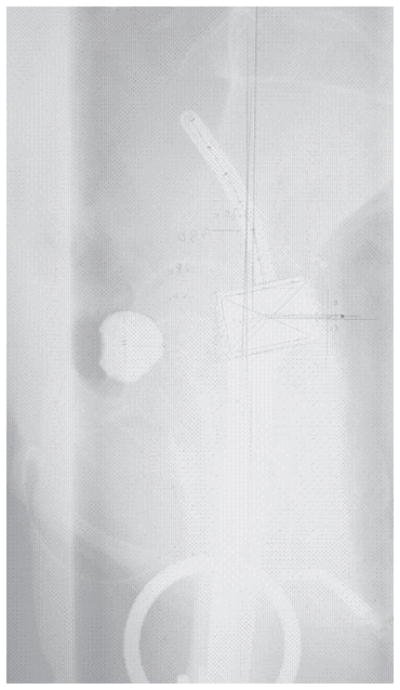
A low-dose-rate tandem and ovoid brachytherapy implant demonstrating the unacceptable characteristic of the tandem being placed too close to the sacrum and not sitting midline on a lateral x-ray film.
For those with the tandem not bisecting ovoids on a lateral film, the U group contained more patients with a Zubrod performance status score of 1 or 2 compared to the A group (42% vs. 22%, p=0.04), but there were no differences seen in the outcome endpoints between the A and U groups.
With regard to the appropriateness of packing, patients in the U group were more likely to have had LDR brachytherapy compared to the A group (81% vs. 52%, p=0.005). On univariate analysis, there were trends toward associations between this parameter and DFS (HR=1.57, 95% C.I. = [0.85, 2.91], p=0.15). The association with appropriateness of packing was strengthened in the MV analysis. In terms of DFS, patients in the U group had a 2 times greater risk of failure than patients in the A group after adjusting for pelvic/iliac and para-aortic nodal status (HR=2.06, 95% C.I. = [1.08, 3.92], p=0.03). To determine if an individual brachytherapy parameter had a stronger association with DFS or LR than any of the others, a model was built using all 5 individual brachytherapy parameters. Symmetry of the ovoids was the only parameter to be statistically significantly associated with DFS (HR=2.33, 95% C.I. = [1.14, 4.75], p=0.02) after adjusting for the other 4 parameters (Table 3).
Table 3.
Multivariate Cox Proportional Hazards Model of Disease-Free Survival (DFS) and all 5 Brachytherapy Parameters (n=103)
| Variable | Comparison | DFS HR (95% C.I.) | DFS p-value† |
|---|---|---|---|
| Symmetry of Ovoids to Tandem | A | 1.00 | |
| U | 2.33 (1.14, 4.75) | 0.02 | |
|
| |||
| Displacement of Ovoids in relation to cervical OS | A | 1.00 | |
| U | 1.81 (0.89, 3.69) | 0.10 | |
|
| |||
| Position of Tandem in Mid Pelvis on Lateral Film | A | 1.00 | |
| U | 0.77 (0.39, 1.50) | 0.44 | |
|
| |||
| Tandem Bisecting Ovoids on Lateral Film | A | 1.00 | |
| U | 0.71 (0.36, 1.38) | 0.31 | |
|
| |||
| Appropriateness of Packing | A | 1.00 | |
| U | 1.13 (0.56, 2.29) | 0.73 | |
Abbreviations: HR, hazard ratio; C.I., confidence interval; A, all implants acceptable (reference group); U, unacceptable/not evaluated
P-value from Chi-square test using the Cox proportional hazards model.
Toxicity
Overall toxicities for RTOG 0128 and 0116 have been previously reported.17, 18, 23 Table 4 shows toxicity endpoints analyzed by brachytherapy quality. Of the individual brachytherapy parameters, only the appropriateness of packing had any association with toxicity. For those with U packing, there was no statistical difference in the median ICRU point bladder dose, but there was a significant different in median total dose to the rectum (A, 18.3Gy vs. U, 24.8 Gy). On univariate analysis, there was a trend toward an association between acute grade ≥3 non-hematologic toxicity and appropriateness of packing. Patients in the U group had a 60% less chance of reporting an acute grade ≥3 non-hematologic toxicity than patients in the A group (OR=0.42, 95% C.I. = [0.17, 1.01], p=0.053).
Table 4.
Toxicity Endpoints by Overall Brachytherapy Quality (n=103)
| Toxicity | Comparison | n | Yes - Grade ≥ 3 Toxicity | OR (95% C.I.) | p- value |
|---|---|---|---|---|---|
| Acute Grade ≥ 3 Non-hematologic Toxicity | A | 79 | 56 | 1.00 | |
| U | 24 | 19 | 1.56 (0.52, 4.68) | 0.43 | |
|
| |||||
| Acute Grade ≥ 3 GI/GU Toxicity | A | 79 | 40 | 1.00 | |
| U | 24 | 10 | 0.70 (0.28, 1.75) | 0.44 | |
|
| |||||
| Any Acute Grade ≥ 3 Toxicity | A | 79 | 69 | 1.00 | |
| U | 24 | 21 | 1.01 (0.26, 4.03) | 0.98 | |
|
| |||||
| Late Grade ≥ 3 GI/GU Toxicity | A | 79 | 10 | 1.00 | |
| U | 24 | 1 | 0.30 (0.04, 2.47) | 0.26 | |
|
| |||||
| Any Late Grade ≥ 3 Toxicity | A | 79 | 20 | 1.00 | |
| U | 24 | 3 | 0.42 (0.11, 1.57) | 0.20 | |
Abbreviations: OR, odds ratio; C.I., confidence interval; A, per protocol (reference group);; U, variation acceptable/deviation unacceptable
Discussion
In this era of increasing complexity, assuring precise and reproducible treatments is critical to achieve the desired outcome. Over the past century, brachytherapy has been tested and confirmed to impart many of the desirable traits sought after with current external-beam systems, namely, the applicator moves with the tumor (natural gating) and it provides central regions of high dose (dose escalation) with lower doses to the surrounding normal tissues (organ sparing). The stability and precision of applicator placement ensure adequate dosing of the tumor and organ sparing. Many errors in brachytherapy occur due to inappropriate insertion or inadequate imaging to identify the misplacement, resulting in inappropriate administration of radiation. This study is the first prospective assessment of the quality of the brachytherapy implant; it identifies proper applicator insertion as an important factor associated with local recurrence and disease-free survival in the modern era of chemoradiation for locally advanced cervical cancer.
Historically, a study by Jampolis et al. published in 1975 demonstrated that 8 of 11 central failures were associated with improper placements of ovoids in a skewed radium system.24 Similarly, Perez et al. observed that inadequate insertions were associated with pelvic failure except in patients with Stage IIB cervical cancer.3 Neither of those reports utilized prospective accrual or multivariable analysis. In 1978 and 1983, prior to the implementation of chemotherapy as standard management for cervical cancer, the Patterns of Care review assessed the technical adequacy of brachytherapy implantation. Corn et al.5 scored the following brachytherapy parameters: the distance between the right ovoid and the distal tandem source, the distance between the left ovoid and the distal tandem source, and the symmetry of the ovoids. Ideal implants (n=8) had satisfactory scores for all three parameters scored, whereas unacceptable implants (n=17) had no satisfactory parameters; all others were labeled adequate (n=41). Due to the small numbers of patients and local failures, separate models were generated for each technical placement factor with regard to treatment time, age, performance status, stage, and total dose. Five-year actuarial local control calculated via Kaplan Meier analysis was significantly better for the ideal and adequate placements compared to the unacceptable (68% vs. 35%, p=0.02). Differences between the 5-year survival rates did not reach statistical significance (61% vs. 42%, p=0.13). Cox regression analysis for local control with technical placement and one additional prognostic variable (i.e., age, Karnofsky performance status, FIGO stage, overall treatment time, and total dose) in the model showed that technical placement was statistically significant in all these models after controlling for the prognostic variable.
In general, physicians who treat large numbers of patients with a highly specialized treatment such as brachytherapy have better patient-related outcomes than those performing fewer procedures. The Patterns of Care studies confirmed the advantage of experience. Physicians at centers that treat approximately 500 patients per year or perform at least 10 brachytherapy implants per year were less likely to underdose patients with <80 Gy to Point A or to have prolonged treatment periods.8 Less experienced physicians are less likely to perform brachytherapy at all, significantly increasing recurrence rates.1, 2, 25 Importantly, physicians who treat only a few cases may be less familiar with detecting nuances in applicator deviations and therefore would be less likely to detect an improperly placed implant.
Over the past 15 years since the publication of the Corn study, advances in the use of imaging, including computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET), have changed the diagnosis and management of patients. The use of CT simulation for visualizing the brachytherapy apparatus has dramatically increased; approximately 70% of practitioners routinely obtain a CT after insertion of the applicator.26 Visualization of the applicator on CT or MRI allows proper assessment of whether the tandem lies within the uterine canal or whether a perforation into the posterior myometrium and through the uterine wall has occurred. In addition, CT or MRI permit assessment of doses to the bladder and rectum using dose-volume histogram analysis.27 With careful analysis on CT or MRI, one may conform dose to the tumor in a much more effective manner than was previously feasible, ideally improving outcomes. Nevertheless, an improperly placed applicator seen on CT or MRI must still be repositioned; optimization of an improperly placed applicator based on the CT or MR images may potentially result in a higher rate of complications.
Of the various quality factors analyzed, the symmetry with relation to the tandem and the displacement of the ovoids appeared to have the strongest association with disease-free survival and local recurrence. Two factors may account for this: the ovoids provide the highest dose to the cervix and the ovoids provide the highest dose to the parametria. If the ovoids are not symmetrically placed, an involved portion of the cervix and/or parametria may not receive an adequate radiation dose and will therefore be at higher risk of recurrence. Tandem placement in this analysis was less critical, likely due to the tandem covering central disease in the cervix adequately, regardless of whether the tandem sat midline between the ovoids or midline in the pelvis as seen on a lateral x-ray. The most important point regarding the tandem is that it should be placed in all patients with locally advanced cervical cancer.
The appropriateness of packing has not been assessed in other studies. This analysis showed a lower rate of disease-free survival for patients who had inappropriate packing. Toxicity was unexpectedly worse in those treated with an acceptable implant, possibly indicating that the packing was allowed to sit in an unacceptable location because it pushed away normal tissue structures. Packing should be used in all cases and assessed for optimal placement, pushing away the bladder and rectum, but not the cervix.
Limitations of this study include the fact that it was not powered to detect differences in toxicity and outcome endpoints for quality of brachytherapy. Though tumor size or response was not collected in the protocols, these factors may impact outcome. We therefore corrected for stage in analyses, but could not correct for all factors and maintain statistical accuracy given the number of recurrences.
The data were collected prospectively, as patients were enrolled on prospective studies, however, the scoring was not performed in real time and hence no modifications were able to be made to the treatment course. In future studies, a checklist may be generated with these parameters, and a patient would receive brachytherapy if the investigator verifies acceptable parameters. The data remain hypothesis-generating; given the ethical implications of intentionally misplacing a brachytherapy applicator, a randomized trial will never be feasible in this area. Nevertheless, data from future studies will provide worthwhile information about the validity of the parameters analyzed.
In conclusion, this analysis of prospective multi-institutional trials RTOG 0116 and 0128 demonstrates that the technical accuracy of brachytherapy implant placement, specifically, the symmetry of the ovoids in relation to the tandem, the displacement of the ovoids in relation to the cervical os, and the appropriateness of packing, affects relapse and disease-free survival rates. These parameters should be assessed for each brachytherapy implant and repacking is recommended for implants that do not meet standards in these areas.
Table 2.
Univariate Cox Proportional Hazards Model of Brachytherapy Quality (n=103)
| Endpoint | Brachytherapy Quality | n | Events | 3-Yr. Survival or Failure Rate (95% C.I.) | HR (95% C.I.) | p- value† |
|---|---|---|---|---|---|---|
| OS | A | 79 | 22 | 67% (52%, 78%) | 1.00 | |
| U | 24 | 7 | 62% (31%, 82%) | 1.10 (0.47, 2.58) | 0.83 | |
|
| ||||||
| DFS | A | 79 | 32 | 57% (45%, 68%) | 1.00 | |
| U | 24 | 10 | 52% (28%, 72%) | 0.95 (0.46, 1.92) | 0.88 | |
|
| ||||||
| LR | A | 79 | 17 | 23% (13%, 33%) | 1.00 | |
| U | 24 | 4 | 19% (1%, 37%) | 0.74 (0.25, 2.21) | 0.59 | |
|
| ||||||
| RR | A | 79 | 11 | 15% (7%, 24%) | 1.00 | |
| U | 24 | 3 | 13% (0%, 27%) | 0.86 (0.24, 3.08) | 0.81 | |
|
| ||||||
| DR | A | 79 | 20 | 27% (17%, 38%) | 1.00 | |
| U | 24 | 5 | 21% (4%, 37%) | 0.82 (0.31, 2.19) | 0.69 | |
Abbreviations: HR, hazard ratio; C.I., confidence interval; A, per protocol; U, variation acceptable/deviation unacceptable; OS, overall survival; DFS, disease-free survival; LR, local; RR, regional; PNR, para-aortic; DR, distant (excluding para-aortic); and DR with PNR, distant (including para-aortic)
P-value from Chi-square test using the Cox proportional hazards model.
Acknowledgments
Supported by RTOG U10 CA21661 and CCOP U10 CA37422 grants from the NCI. This manuscript’s contents are the sole responsibility of the authors and do not necessarily represent the official views of the NCI. Additional support was provided by MedImmune, Inc. and Pfizer, Inc.
Footnotes
Presented as an oral presentation at the American Society of Radiation Oncology, November 4, 2010.
Conflict of interest statement: The authors declare that there are no conflicts of interest.
References
- 1.Lanciano RM, Won M, Coia L, Hanks GE. Pretreatment and treatment factors associated with improved outcome in squamous cell carcinoma of the uterine cervix: A final report of the 1973 and 1978 Patterns of Care Studies. International Journal of Radiation Oncology Biology and Physics. 1991;20:667–76. doi: 10.1016/0360-3016(91)90007-q. [DOI] [PubMed] [Google Scholar]
- 2.Montana GS, Fowler WC, Varia MA, Walton LA, Mack Y, Shemanski L. Carcinoma of the cervix, stage III. Results of radiation therapy Cancer. 1986;57(1):148–54. doi: 10.1002/1097-0142(19860101)57:1<148::aid-cncr2820570130>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 3.Perez CA, Breaux S, Madoc-Jones H, et al. Radiation therapy alone in the treatment of carcinoma of uterine cervix. I. Analysis of tumor recurrence. Cancer. 1983;51(8):1393–402. doi: 10.1002/1097-0142(19830415)51:8<1393::aid-cncr2820510812>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 4.Viswanathan AN, Cormack R, Rawal B, Lee H. Increasing brachytherapy dose predicts survival for interstitial and tandem-based radiation for stage IIIB cervical cancer. Int J Gynecol Cancer. 2009;19(8):1402–6. doi: 10.1111/IGC.0b013e3181b62e73. [DOI] [PubMed] [Google Scholar]
- 5.Corn BW, Hanlon AL, Pajak TF, Owen J, Hanks GE. Technically accurate intracavitary insertions improve pelvic control and survival among patients with locally advanced carcinoma of the uterine cervix. Gynecol Oncol. 1994;53(3):294–300. doi: 10.1006/gyno.1994.1137. [DOI] [PubMed] [Google Scholar]
- 6.Potish RA. The effect of applicator geometry on dose specification in cervical cancer. Int J Radiat Oncol Biol Phys. 1990;18(6):1513–20. doi: 10.1016/0360-3016(90)90329-i. [DOI] [PubMed] [Google Scholar]
- 7.Erickson B, Eifel P, Moughan J, Rownd J, Iarocci T, Owen J. Patterns of brachytherapy practice for patients with carcinoma of the cervix (1996–1999): a patterns of care study. Int J Radiat Oncol Biol Phys. 2005;63(4):1083–92. doi: 10.1016/j.ijrobp.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 8.Eifel PJ, Moughan J, Erickson B, Iarocci T, Grant D, Owen J. Patterns of Radiotherapy Practice for Patients with Carcinoma of the Uterine Cervix: A Patterns of Care Study. Int J Radiation Oncology Biol Phys. 2004;60(4):1144–53. doi: 10.1016/j.ijrobp.2004.04.063. [DOI] [PubMed] [Google Scholar]
- 9.Eifel PJ, Moughan J, Owen J, Katz A, Mahon I, Hanks GE. Patterns of radiotherapy practice for patients with squamous carcinoma of the uterine cervix: patterns of care study. Int J Radiat Oncol Biol Phys. 1999;43(2):351–8. doi: 10.1016/s0360-3016(98)00401-5. [DOI] [PubMed] [Google Scholar]
- 10.Nag S, Chao C, Erickson B, et al. The American Brachytherapy Society recommendations for low-dose-rate brachytherapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2002;52(1):33–48. doi: 10.1016/s0360-3016(01)01755-2. [DOI] [PubMed] [Google Scholar]
- 11.Katz A, Eifel PJ. Quantification of intracavitary brachytherapy parameters and correlation with outcome in patients with carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2000;48(5):1417–25. doi: 10.1016/s0360-3016(00)01364-x. [DOI] [PubMed] [Google Scholar]
- 12.Eifel PJ, Winter K, Morris M, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01. Journal of Clinical Oncology. 2004;22(5):872–80. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- 13.Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. New England Journal of Medicine. 1999;340(15):1144–53. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 14.Whitney CW, Sause W, Bundy BN, et al. A randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stages IIB–IVA carcinoma of the cervix with negative para-aortic lymph nodes. Journal of Clinical Oncology. 1999;17(5):1339–48. doi: 10.1200/JCO.1999.17.5.1339. [DOI] [PubMed] [Google Scholar]
- 15.Keys HM, Bundy BN, Stehman FB, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. New England Journal of Medicine. 1999;340(15):1154–61. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 16.Gaffney DK, Winter K, Dicker AP, et al. A Phase II study of acute toxicity for Celebrex (celecoxib) and chemoradiation in patients with locally advanced cervical cancer: primary endpoint analysis of RTOG 0128. Int J Radiat Oncol Biol Phys. 2007;67(1):104–9. doi: 10.1016/j.ijrobp.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Gaffney DK, Winter K, Dicker AP, et al. Efficacy and patterns of failure for locally advanced cancer of the cervix treated with celebrex (celecoxib) and chemoradiotherapy in RTOG 0128. Int J Radiat Oncol Biol Phys. 2007;69(1):111–7. doi: 10.1016/j.ijrobp.2007.02.050. [DOI] [PubMed] [Google Scholar]
- 18.Small W, Jr, Winter K, Levenback C, et al. Extended-field irradiation and intracavitary brachytherapy combined with cisplatin chemotherapy for cervical cancer with positive para-aortic or high common iliac lymph nodes: results of ARM 1 of RTOG 0116. Int J Radiat Oncol Biol Phys. 2007;68(4):1081–7. doi: 10.1016/j.ijrobp.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan E, Meier P. Nonparameteric estimation from incomplete observations. J Amer Statist Assoc. 1958:457–81. [Google Scholar]
- 20.Kalbfleish J. The statistical analysis of failure time data. New York: John Wiley & Sons; 1980. pp. 167–9. [Google Scholar]
- 21.Cox D. Regression models and life tables. J Royal Stat Soc. 1972;34:187–229. Series B. [Google Scholar]
- 22.Cox J, Stetz J, TP Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European organization for research and treatment of cancer (EORTC) Int Journal of Rad Oncol Biol Phys. 1995;31(5):1341–6. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 23.Small W, Jr, Winter K, Levenback C, et al. Extended Field Irradiation and Intracavitary Brachytherapy Combined with Cisplatin Chemotherapy for Cervical Cancer with Positive Para-aortic or High Common Iliac Lymph Nodes: Results of Arm 2 of RTOG 0116. Int J Radiat Oncol Biol Phys. 2007;69:S5. doi: 10.1016/j.ijrobp.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 24.Jampolis S, Andras EJ, Fletcher GH. Analysis of sites and causes of failures of irradiation in invasive squamous cell carcinoma of the intact uterine cervix. Radiology. 1975;115(3):681–5. doi: 10.1148/15.3.681. [DOI] [PubMed] [Google Scholar]
- 25.Lanciano RM, Pajak TF, Martz K, Hanks G. The influence of treatment time on outcome for squamous cell cancer of the uterine cervix treated with radiation: a Patterns-of-Care Study. International Journal of Radiation Oncology Biology and Physics. 1993;25:391–8. doi: 10.1016/0360-3016(93)90058-4. [DOI] [PubMed] [Google Scholar]
- 26.Viswanathan AN, Erickson BA. Three-Dimensional Imaging in Gynecologic Brachytherapy: A Survey of the American Brachytherapy Society. Int J Radiat Oncol Biol Phys. 2009 doi: 10.1016/j.ijrobp.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 27.Viswanathan AN, Dimopoulos J, Kirisits C, Berger D, Potter R. Computed tomography versus magnetic resonance imaging-based contouring in cervical cancer brachytherapy: results of a prospective trial and preliminary guidelines for standardized contours. Int J Radiat Oncol Biol Phys. 2007;68(2):491–8. doi: 10.1016/j.ijrobp.2006.12.021. [DOI] [PubMed] [Google Scholar]


