Abstract
BACKGROUND
Associations of pathophysiologic calf muscle characteristics with functional decline in people with lower extremity peripheral arterial disease (PAD) are unknown.
METHODS AND RESULTS
Three hundred seventy participants with PAD underwent baseline measurement of calf muscle area, density, and percent fat using computed tomography. Participants were followed annually for two years. The outcome of mobility loss was defined as becoming unable to walk ¼ mile or walk up and down one flight of stairs without assistance, among those without baseline mobility limitations. Additional outcomes were ≥ 20% decline in six-minute walk distance and becoming unable to walk for six minutes continuously among participants who walked continuously for six minutes at baseline. Adjusting for age, sex, race, body mass index, the ankle brachial index, smoking, physical activity, relevant medications, and comorbidities, lower calf muscle density (p trend < 0.001) and lower calf muscle area (p trend =0.039) were each associated with increased mobility loss rates. Compared to participants in the highest baseline tertiles, participants in the lowest tertile of calf muscle percent fat had a hazard ratio of 0.18 for incident mobility loss (95% CI = 0.06–0.55, p=0.003), and participants in the lowest tertile of muscle density had a 3.50 hazard ratio for incident mobility loss (95% CI= 1.28–9.57, p=0.015). No significant associations of calf muscle characteristics with six-minute walk outcomes were observed.
CONCLUSION
Our findings suggest that interventions to prevent mobility loss in PAD should focus on reversing pathophysiologic findings in calf muscle.
Keywords: Intermittent claudication, mobility, peripheral arterial disease, physical functioning
Chronic lower extremity arterial ischemia is associated with adverse calf muscle characteristics. Histopathologic data demonstrate that lower extremity ischemia is associated with apoptosis, Type II muscle fiber atrophy and proliferation of connective tissue in the gastrocnemius muscle (1–3). Computed tomography (CT) imaging demonstrates that lower extremity ischemia is associated with smaller calf muscle area and increased calf muscle percent fat, compared to absence of ischemia (4).
Men and women with PAD have greater functional impairment and faster rates of functional decline compared to persons without PAD (4,5). However, associations of ischemia-related pathophysiologic calf muscle characteristics and decline in lower extremity functional performance are unknown. If pathophysiologic calf muscle characteristics are associated with more rapid functional decline in PAD, then reversal of these adverse calf muscle characteristics may be important for preserving functional performance in PAD.
This study identified associations of computed tomography-measured calf muscle characteristics with mobility loss and decline in six-minute walk performance among men and women with PAD participating in the Walking and Leg Circulation Study (WALCS) II (6). We hypothesized that lower calf muscle density, greater calf muscle percent fat, and smaller calf muscle area would be associated with a higher incidence of mobility loss and faster functional decline compared to more favorable calf muscle characteristics in PAD. To determine whether these associations were specific to PAD, we also studied associations of these calf muscle characteristics with functional decline in persons without PAD.
METHODS
Study Overview
The institutional review boards of Northwestern University and Catholic Health Partners Hospital approved the protocol. Participants gave written informed consent.
Participants were part of the WALCS II cohort, a prospective, observational study designed to identify mechanisms of functional decline in PAD (6,7). Participants underwent baseline measures and returned annually for follow-up. Participants unable to return for follow-up were interviewed by telephone for the mobility outcome measure.
Participant Identification
PAD participants were age 59 and older and were identified from among consecutive patients diagnosed with PAD in Chicago-area non-invasive vascular laboratories (6,7). Approximately half of non-PAD participants were identified consecutively from patients with normal lower extremity arterial studies in the same vascular laboratories. The remainder was identified from consecutive patients in a general medicine practice at Northwestern. A small number of PAD participants were identified from among consecutive patients in the general internal medicine with a low ABI at their study visit. PAD was defined as ABI < 0.90 (1–4). Absence of PAD was defined as ABI ≥ 0.90 and ≤ 1.30 (6,7).
Participation rates and exclusion criteria for the WALCS II cohort have been described (6,7). Patients with dementia were excluded because of their inability to answer questions accurately. Nursing home residents, wheelchair-bound patients, and patients with foot or leg amputations were excluded because they have severely impaired functioning. Non-English-speaking patients were excluded because investigators were not fluent in non-English languages. Patients with recent major surgery were excluded.
Ankle Brachial Index Measurement
After participants rested for five minutes supine, a hand-held Doppler probe (Nicolet Vascular Pocket Dop II; Nicolet Biomedical Inc, Golden, Colo) was used to obtain systolic pressures in the right and left brachial, dorsalis pedis, and posterior tibial arteries (4–7). Each pressure was measured twice. The ABI was calculated by dividing the mean of the dorsalis pedis and posterior tibial pressures in each leg by the mean of the 4 brachial pressures (4–7). Zero values for the dorsalis pedis and posterior tibial pulses were excluded. Average brachial pressures in the arm with highest pressure were used when 1 brachial pressure was higher than the opposite brachial pressure in both measurement sets and the 2 brachial pressures differed by 10 mm Hg or more in at least one measurement set, since in these participants subclavian stenosis was possible (8). The lowest leg ABI was used in analyses.
Measuring Calf Skeletal Muscle Characteristics
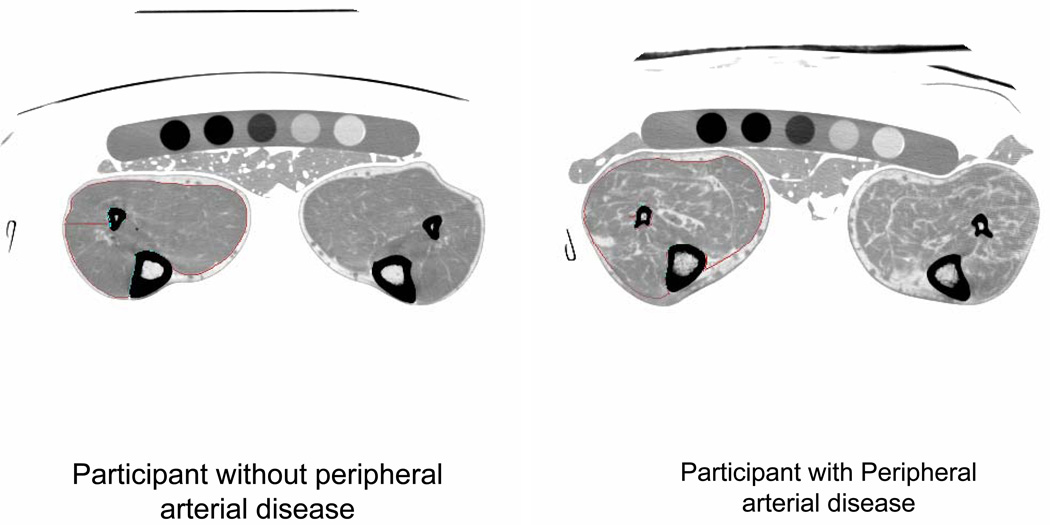
Using a CT scanner (LightSpeed, General Electric Medical Systems, Waukesha, WI, USA), 2.5 mm cross-sectional images of the calves were obtained at 66.7% of the distance from the distal to the proximal tibia (6) (See Figure). Images were analyzed using BonAlyse (BonAlyse Oy, Jyvaskyla- Finland), a software for processing CT images that identifies muscle tissue, fat, and bone (6). The muscle outline was traced manually, excluding subcutaneous fat, and bone. When quantifying muscle area, the Bon Alyse software quantifies voxels within a range corresponding to muscle density (9 to 271 mg/cm3) and excludes voxels corresponding to fat density (−270 to 8 mg/cm3). Intra-muscular fat is quantified by summing voxels corresponding to fat within muscle tissue. Cadaver studies demonstrate that these methods provide an estimate of muscle area that is highly correlated with direct anatomic measures (9). Because larger individuals require greater muscle mass to support their frame, muscle area was adjusted for the square of individual tibia length. Muscle density measures the quantity of muscle per volume, within the voxel range corresponding to muscle (9 to 271 mg/cm3) and is a measure of muscle quality.
Figure. Calf images from a participant with and without peripheral arterial disease.*.
* A phantom and gel packs are shown below the legs. A marker indicates the right lower extremity. In this example, the right calf muscle in each participant is outlined with specially designed software, allowing quantification of calf muscle characteristics.
Functional Outcomes
Outcome measures were mobility loss, becoming unable to walk for six-minutes continuously without stopping, and a decline in six-minute walk performance of ≥ 20% (10). These outcomes were assessed annually and were selected because they represent discrete endpoints and avoid a “floor” effect that may occur, for example, when a participant becomes unable to walk and cannot further deteriorate in functional performance.
Six-minute walk
The six-minute walk was administered at baseline and at each annual follow-up visit. Following a standardized protocol (11), participants walk up and down a 100-foot hallway for six minutes after instructions to cover as much distance as possible. The test administrator records whether the participant stopped during the six-minute walk. Participants who stopped at baseline were excluded from analyses for the outcome of becoming unable to walk for six minutes continuously.
Mobility Measures
A critical factor in an older person’s ability to function independently in the community is mobility, defined as the ability to walk or climb stairs without assistance (12). Older people who lose mobility have higher morbidity and mortality and experience a poorer quality of life (12,13). At baseline and at each follow-up visit, participants were asked to indicate whether they could walk ¼ mile and whether they could climb up and down one flight of stairs a) on their own; b) with assistance; or c) not at all (10,12,13). Mobility loss was defined as becoming unable to walk up and down one flight of stairs or walk ¼ mile without assistance among those without mobility limitations at baseline (10,12,13).
Comorbidities
Comorbidities assessed were diabetes, angina, myocardial infarction, heart failure, cancer, chronic lung disease, lower extremity arthritis, spinal stenosis, spinal disk disease, and stroke. Disease-specific algorithms that combine data from patient report, medical record review, medications, laboratory values, and a questionnaire completed by the participant’s primary care physician were used to verify and document baseline comorbidities, based on criteria previously developed (14). American College of Rheumatology criteria were used to document presence of knee and hip osteoarthritis (15,16).
Other Measures
Height and weight were measured at baseline. Body mass index (BMI) was calculated as weight (kilograms)/(height (meters))2. Cigarette smoking history was determined with patient report. At baseline, participants were asked to report the number of blocks they walked during the previous week using a questionnaire validated previously (17). Participants were asked to bring their medications to each study visit and each medication was recorded. The principal investigator (MMM) identified statin, pentoxifylline, and cilostazol use, blinded to other participant characteristics. At each follow-up visit, we used patient report, a primary care physician questionnaire, and medical record review to identify lower extremity revascularizations.
Statistical Analyses
Participants with and without PAD, respectively, were categorized according to baseline tertiles of calf muscle area, calf muscle percent fat, and calf muscle density. Baseline characteristics of PAD participants across tertiles of each muscle characteristic were compared using general linear models for continuous variables and chi-square tests for categorical variables.
Cox regression analyses were used to compare rates of each functional outcome across tertiles of baseline calf muscle characteristic, separately for participants with and without PAD, adjusting for confounders. A priori, we categorized participants into tertiles to ensure a reasonable sample size per category. For the outcome of mobility loss, participants with mobility limitations at baseline were excluded. For the outcome of becoming unable to walk for six minutes continuously, participants unable to walk for six-minutes without stopping at baseline were excluded. Calf muscle characteristics were entered as continuous variables into the Cox regression analyses. Time to events was entered as “1” or “2” corresponding to the first or second annual follow-up visit. Participants who died or who underwent lower extremity revascularization during follow-up were censored at their last visit prior to these events. For PAD participants, four models were performed for each outcome. Model I adjusted for age, sex, and race. Model II adjusted for covariates in Model I and ABI, BMI, smoking, and comorbidities (diabetes, angina, myocardial infarction, heart failure, cancer, lung disease, knee arthritis, hip arthritis, spinal stenosis, disk disease, and stroke). Model III adjusted for physical activity in addition to covariates in Model II. Model IV adjusted for statin, pentoxifylline and cilostazol use in addition to covariates in Model III. For non-PAD participants, only the first three models were performed. There were no significant deviations from the proportional hazards assumption for each outcome, using martingale residuals based methods (18). Statistical significance was defined as p<0.05. The study had 80% power to detect a hazard ratio of 0.45 (or 2.22) for mobility loss between the highest and lowest calf muscle tertiles. Analyses were performed using SAS statistical software (version 9.2, SAS Institute Inc, Cary, NC).
RESULTS
Four-hundred thirty-nine participants in WALCS II had a baseline ABI < 0.90 and underwent a CT scan. Of these, 370 (84%) met inclusion criteria for at least one outcome. Two-hundred ninety-two participants in WALCS II had a baseline ABI of 0.90 to 1.30 and no history of lower extremity revascularization, consistent with PAD. Of these, 238 (90%) met inclusion criteria for at least one outcome.
Compared to those without PAD, PAD participants were older, had a lower BMI, and included higher proportions of men, current and former smokers, and individuals with diabetes and angina. Compared to those without PAD, PAD participants had lower calf muscle area, lower calf muscle density, and poorer functional performance (Table 1).
Table 1.
Baseline Characteristics of Participants with and without Peripheral Arterial Disease
| PAD (n=370) |
No PAD (n=238) |
P Value | |
|---|---|---|---|
| Age (years) | 74.5±7.86 | 71.6±7.57 | <0.001 |
| Male sex (%) | 55.41 | 43.28 | 0.004 |
| Black race (%) | 15.41 | 20.17 | 0.13 |
| Current or former smoker (%) | 78.1% | 57.6% | <.001 |
| ABI | 0.63±0.16 | 1.09±0.09 | <0.001 |
| Body mass index (kg/M2) | 28.0±4.97 | 29.1±5.65 | 0.013 |
| Diabetes Mellitus (%) | 32.97 | 23.53 | 0.013 |
| Angina (%) | 33.24 | 20.17 | <0.001 |
| Calf muscle area (mm2) | 5,542±1,149 | 6,031±1,535 | <0.001 |
| Calf muscle percent fat (%) | 11.1±12.3 | 9.6±11.8 | 0.15 |
| Calf muscle density gm/cm3 | 32.7±4.1 | 33.9±3.9 | <0.001 |
| Baseline six-minute walk performance (feet) | 1137±384 | 1437±415 | <0.001 |
| Pentoxifylline or cilostazol use (%) | 5.14 | 0.42* | 0.013 |
| Percent stopping during the six-minute walk at baseline | 28.22 | 7.56 | <.001 |
One participant without PAD was taking pentoxifylline for Raynaud’s disease.
Table 2 shows characteristics of PAD participants by tertiles of each calf muscle characteristic. Lower calf muscle density was associated with older age, higher BMI, and higher prevalences of diabetes mellitus, pulmonary disease, and hip arthritis. Higher calf muscle percent fat was associated with older age, higher BMI, and higher prevalences of diabetes mellitus, pulmonary disease, and disk disease. Lower calf muscle area was associated with older age, lower ABI, higher BMI, fewer blocks walked during the prior week, and higher prevalences of males and African-Americans.
Table 2.
Baseline Characteristics Associated with Pathophysiologic Calf Skeletal Muscle Measures in Men and Women with Peripheral Arterial Disease.
| 1st Tertile (20.1– 31.3 gm/cm3) |
2nd Tertile (31.3–34.6 gm/cm3) |
3rd Tertile (34.7–41.4 gm/cm3) |
P-trend | |
|---|---|---|---|---|
| CALF MUSCLE DENSITY | ||||
| N | 123 | 124 | 123 | |
| Age (years) | 75.6 (7.8) | 75.2 (8.1) | 72.8 (7.4) | 0.005 |
| ABI | 0.64 (0.16) | 0.63 (0.16) | 0.64 (0.15) | 0.85 |
| BMI (Kg/M2) | 29.6 (5.7) | 27.3 (4.4) | 27.1 (4.4) | <.001 |
| Blocks walked in past 7 days | 25.0 (54.1) | 28.2 (64.6) | 37.4 (51.6) | 0.090 |
| Male (%) | 52.9 | 53.2 | 60.2 | 0.25 |
| African American (%) | 16.3 | 14.5 | 15.5 | 0.86 |
| Current or former smoker (%) | 74.8 | 79.8 | 79.7 | 0.35 |
| Angina (%) | 30.9 | 37.9 | 30.9 | 1.00 |
| Myocardial infarction (%) | 26.8 | 28.2 | 22.8 | 0.47 |
| Diabetes mellitus (%) | 45.5 | 30.7 | 22.8 | <.001 |
| Pulmonary disease (%) | 49.6 | 45.2 | 35.0 | 0.021 |
| Hip arthritis (%) | 7.3 | 3.2 | 1.6 | 0.031 |
|
1st Tertile (0.1– 0.2 mm2) |
2nd Tertile 0.2–0.2 mm2) |
3rd Tertile (0.2– 0.3 mm2) |
P-trend | |
| CALF MUSCLE AREA* | ||||
| N | 123 | 123 | 123 | |
| Age (years) | 76.4 (8.0) | 74.81 (7.6) | 72.54 (7.5) | <.001 |
| ABI | 0.61 (0.17) | 0.63 (0.14) | 0.66 (0.15) | 0.008 |
| BMI (Kg/M2) | 26.32 (4.4) | 27.67 (4.6) | 29.94 (5.3) | <.0001 |
| Blocks walked in past 7 days | 20.93 (48.1) | 27.58 (63.7) | 42.27 (57.2) | 0.003 |
| Male (%) | 43.9 | 52.9 | 69.1 | <.0001 |
| African American (%) | 20.3 | 16.3 | 9.8 | 0.023 |
| Current or former smoker (%) | 67.5 | 82.9 | 83.7 | 0.002 |
| Angina (%) | 32.5 | 28.5 | 39.0 | 0.28 |
| Myocardial infarction (%) | 25.2 | 20.3 | 32.5 | 0.19 |
| Diabetes mellitus (%) | 26.8 | 35.0 | 36.6 | 0.104 |
| Pulmonary disease (%) | 45.5 | 43.9 | 40.7 | 0.44 |
| Knee arthritis (%) | 18.7 | 12.2 | 10.6 | 0.067 |
|
1st Tertile (1.2–5.1%) |
2nd Tertile (5.1–10.1%) |
3rd Tertile (10.2–87.1%) |
P-trend | |
| CALF MUSCLE PERCENT FAT | ||||
| N | 123 | 124 | 123 | |
| Age (years) | 73.6 (7.8) | 74.2 (8.0) | 75.8 (7.6) | 0.030 |
| ABI | 0.63 (0.1) | 0.64 (0.2) | 0.62 (0.2) | 0.61 |
| BMI (Kg/M2) | 25.81 (3.9) | 27.88 (4.7) | 30.25 (5.2) | <.0001 |
| Blocks walked (in past 7 days) | 36.50 (51.1) | 26.05 (38.1) | 28.08 (75.9) | 0.25 |
| Male (%) | 64.2 | 50.0 | 52.0 | 0.055 |
| African American (%) | 11.4 | 16.1 | 18.7 | 0.11 |
| Current or former smoker (%) | 81.3 | 80.7 | 72.4 | 0.09 |
| Angina (%) | 34.2 | 39.5 | 26.0 | 0.18 |
| Myocardial infarction (%) | 26.0 | 25.8 | 26.0 | 1.00 |
| Diabetes mellitus (%) | 22.8 | 32.3 | 43.9 | <.001 |
| Pulmonary disease (%) | 36.6 | 41.9 | 51.2 | 0.021 |
| Knee arthritis (%) | 11.4 | 11.3 | 18.7 | 0.098 |
Data shown are means (standard deviations).
Calf muscle area tertiles are standardized for tibia length.
Table 3 shows correlations between muscle characteristics among men and women with and without PAD, respectively.
Table 3.
Correlation Coefficients between Muscle Characteristics*
| Women with PAD | Men with PAD | Women without PAD | Men without PAD | |
|---|---|---|---|---|
| Calf muscle area and calf muscle density | ||||
| Correlation coefficient | 0.25 | 0.30 | 0.15 | 0.32 |
| P value | 0.001 | <0.001 | 0.092 | 0.001 |
| Calf muscle area and calf muscle percent fat | ||||
| Correlation coefficient | −0.31 | −0.31 | −0.18 | −0.27 |
| P value | <0.001 | <0.01 | 0.037 | 0.006 |
| Calf muscle density and calf muscle percent fat | ||||
| Correlation coefficient | −0.79 | −0.79 | −0.74 | −0.79 |
| P value | 0.001 | <0.001 | <0.001 | <0.001 |
Spearman correlation coefficients. PAD = peripheral arterial disease.
Calf Muscle Density and Functional Decline in PAD Participants
Table 4 shows associations of baseline muscle density with mobility loss at two-year follow-up among 332 PAD participants with intact mobility at baseline. Lower calf muscle density was associated with increased mobility loss, adjusting for age, sex, and race (Model 1, Table 4, p trend < 0.001). Even after additional adjustment for ABI, BMI, comorbidities, and smoking, lower calf muscle density remained associated with an increased rate of mobility loss (Model 2, Table 4, p trend ≤0.001). Associations of lower calf muscle density with increased mobility loss remained statistically significant even after additional adjustment for physical activity (Model 3, Table 4, p trend = 0.0001) and medication use (Model 4, Table 4, p trend <0.0001).
Table 4.
Calf muscle density and functional outcomes among persons with peripheral arterial disease.
| Tertile 1 | Tertile 2 | Tertile 3 | Trend P value | |
|---|---|---|---|---|
| MOBILITY LOSS (n=332) | ||||
| Tertile Definition | 20.10–31.55 gm/cm3 | 31.62–35.11 gm/cm3 | 35.12–41.10 gm/cm3 | |
| Number of participants | N=110 | N=111 | N=111 | |
| Number with mobility loss during follow-up | N=28 | N=15 | N=6 | |
| Model 1 | 5.10 (2.03–12.82)1 | 2.19(0.82–5.85) | 1.0(Reference) | <.001 |
| Model 2 | 3.64(1.36–9.71)2 | 1.62(0.59–4.50) | 1.0(Reference) | <0.001 |
| Model 3 | 3.53(1.28–9.68)3 | 1.49(0.53–4.21) | 1.0(Reference) | <0.001 |
| Model 4 | 3.50(1.28–9.57)3 | 1.47 (0.52–4.19) | 1.0(Reference) | <.001 |
| BECOMING UNABLE TO WALK FOR SIX MINUTES CONTINUOUSLY (n=237) | ||||
| Tertile Definition | 20.10–32.01 gm/cm3 | 32.01–35.49 gm/cm3 | 35.51–41.26 gm/cm3 | |
| Number of participants | N=79 | N=79 | N=79 | |
| Number who stopped during the six-minute walk at follow-up. | N=22 | N=21 | N=11 | |
| Model 1 | 2.01(0.92–4.41) | 1.77(0.80–3.91) | 1.0(Reference) | 0.40 |
| Model 2 | 2.00(0.84–4.77) | 1.65(0.72–3.83) | 1.0(Reference) | 0.62 |
| Model 3 | 1.99(0.83–4.78) | 1.58(0.68–3.66) | 1.0(Reference) | 0.57 |
| Model 4 | 2.06 (0.85–4.99) | 1.56 (0.67–3.63) | 1.0(Reference) | 0.53 |
Analyses for mobility loss exclude participants with mobility limitation at baseline. Analyses for becoming unable to walk for six-minutes continuously exclude participants who stopped during the six-minute walk test at baseline. Because of sample size differences, tertiles are distinct for each outcome.
Data shown are hazard ratios (95% confidence intervals). Model 1 adjusts for age, sex, and race. Model 2 adjusts for variables in Model 1 and the ankle brachial index, body mass index, smoking, and comorbidities. Model 3 adjusts for covariates in Model 2 and physical activity level. Model 4 adjusts for covariates in Model 3 and time-dependent use of statin, cilostazol, and pentoxifylline use. The reference group is participants in the highest calf muscle density tertile at baseline.
p=0.005 relative to reference.
p=0.010 relative to reference.
p=0.015 relative to reference.
Among the 237 PAD participants able to walk for six minutes continuously at baseline, there were no significant associations of higher calf muscle density with becoming unable to walk for six minutes continuously at two-year follow-up, adjusting for confounders (Table 4). Among all PAD participants, there were no significant associations of calf muscle density with ≥ 20% decline in six-minute walk performance (data not shown).
Calf Muscle Area and Functional Decline in PAD Participants
Table 5 shows associations of baseline calf muscle area with mobility loss at two-year follow-up among 331 PAD participants with intact mobility at baseline. Lower calf muscle area was associated with increased mobility loss, adjusting for age, sex, and race (Model 1, Table 5, p trend = 0.005). Even after additional adjustment for ABI, BMI, comorbidities, and smoking, lower calf muscle area remained associated with increased mobility loss (Model 2, Table 5, p trend =0.004). This association remained statistically significant even after additional adjustment for physical activity (Model 3, Table 5, p trend = 0.02) and even after additional adjustment for medication use (Model 4, Table 5, p trend =0.039). Among the 237 PAD participants able to walk for six minutes continuously at baseline, there were no significant associations of higher calf muscle area with becoming unable to walk for six minutes continuously at two-year follow-up, adjusting for confounders (Table 5). Among PAD participants, there were no significant associations of calf muscle area with a ≥ 20% decline in six-minute walk performance (data not shown).
Table 5.
Calf muscle area and functional outcomes among persons with peripheral arterial disease.
| Tertile 1 (lowest calf muscle area) |
Tertile 2 | Tertile 3 (highest calf muscle area) |
Trend P value | |
|---|---|---|---|---|
| MOBILITY LOSS (n=331) | ||||
| Tertile Definition** | 0.073–0.194 | >0.194–0.215 | >0.215–0.293 | |
| Number of participants | N=110 | N=111 | N=110 | |
| Number with mobility loss | N=28 | N=10 | N=11 | |
| Model 1 | 2.53 (1.18 – 5.40)1 | 0.74 (0.30 – 1.80) | 1.0 (Reference) | 0.005 |
| Model 2 | 3.29 (1.40 – 7.73)2 | 0.90 (0.35 – 2.32) | 1.0 (Reference) | 0.004 |
| Model 3 | 2.45 (0.98 – 6.13) | 0.70 (0.26 – 1.86) | 1.0 (Reference) | 0.023 |
| Model 4 | 2.46(0.97–6.24) | 0.74 (0.27–2.00) | 1.0 (Reference) | 0.039 |
| BECOMING UNABLE TO WALK FOR SIX MINUTES CONTINUOUSLY (n=236) | ||||
| Tertile Definition** | 0.072–0.194 | >0.194–0.213 | 0.214–0.275 | |
| Number of participants | N=78 | N=79 | N=79 | |
| Number who stopped during the six-minute walk at follow-up. | N=21 | N=15 | N=18 | |
| Model 1 | 1.04 (0.51 – 2.11) | 0.64 (0.30 – 1.34) | 1.0 (Reference) | 0.66 |
| Model 2 | 1.67 (0.74 – 3.79) | 0.95 (0.43 – 2.11) | 1.0 (Reference) | 0.086 |
| Model 3 | 1.47 (0.63 – 3.43) | 0.84 (0.37 – 1.90) | 1.0 (Reference) | 0.14 |
| Model 4 | 1.46 (0.61–3.47) | 0.83 (0.36–1.89) | 1.0 (Reference) | 0.15 |
Data shown are hazard ratios (95% confidence intervals). Model 1 adjusts for age, sex, and race. Model 2 adjusts for variables in Model 1 and the ankle brachial index, body mass index, smoking, and comorbidities. Model 3 adjusts for covariates in Model 2 and physical activity level. Model 4 adjusts for covariates in Model 3 and time-dependent use of statin, cilostazol, and pentoxifylline use.
The reference group is the tertile with lowest baseline calf muscle area.
Analyses for mobility loss exclude participants with mobility limitations at baseline. Analyses for becoming unable to walk for six-minutes continuously exclude participants who stopped during the six-minute walk test at baseline. Because of sample size differences for each outcome, tertile definitions are distinct for each outcome.
P=0.017 relative to reference;
P=0.006 relative to reference
Tertiles represent standardized tertiles adjusted for tibia length. Muscle area values corresponding to the standardized tertiles are as follows: For mobility loss- 1st standardized tertile- 648–7331 mm2; 2nd standardized tertile- 3,816–7,894 mm2; 3rd standardized tertile- 4,528–9,820 mm2. For becoming unable to walk for six-minutes continuously: 1st standardized tertile- 601.2–6,795 mm2; 2nd standardized tertile- 3,645–7,488 mm2; 3rd standardized tertile- 4,528–9,819 mm2.
Calf Muscle Percent Fat and Functional Decline in PAD Participants
Adjusting for age, sex, and race, lower calf muscle percent fat was associated with lower rates of mobility loss at follow-up (p trend = 0.015) (Table 6, Model 1). This association was no longer statistically significant after additional adjustment for ABI, BMI, comorbidities, and smoking (p trend = 0.074, Table 6, Model 2). However, in a fully adjusted model, participants in the lowest baseline tertile of calf muscle percent fat had lower mobility loss compared to those in the highest baseline tertile of calf muscle fat (p=0.003, Table 6, Model 4). There were no associations of calf muscle percent fat with becoming unable to walk for six-minutes continuously without stopping (Table 6) or with ≥ 20% decline in six-minute walk performance (data not shown).
Table 6.
Calf muscle percent fat and functional outcomes among persons with peripheral arterial disease.
| Tertile 1 | Tertile 2 | Tertile 3 | Trend P value | |
|---|---|---|---|---|
| MOBILITY LOSS (n=332) | ||||
| Tertile Definition | 1.21%–4.94% | 4.97%–9.41% | 9.43%–84.4% | |
| Number of participants | N=110 | N=111 | N=111 | |
| Number with mobility loss during follow-up | N=5 | N=19 | N=25 | |
| Model 1 | 0.18 (0.06 – 0.48)1 | 0.71 (0.37 – 1.37) | 1.0 (Reference) | 0.015 |
| Model 2 | 0.19 (0.06 – 0.59)2 | 0.79 (0.37 – 1.67) | 1.0 (Reference) | 0.074 |
| Model 3 | 0.19 (0.06 – 0.60)2 | 0.79 (0.37 – 1.69) | 1.0 (Reference) | 0.068 |
| Model 4 | 0.18 (0.06–0.55)3 | 0.74 (0.34–1.60) | 1.0 (Reference) | 0.089 |
| BECOMING UNABLE TO WALK FOR SIX MINUTES CONTINUOUSLY (n=237) | ||||
| Tertile Definition | 1.21%–4.80% | 4.87%–9.01% | 9.05%–87.11% | |
| Number of participants | N=79 | N=79 | N=79 | |
| Number who stop during the six-minute walk at follow-up | N=11 | N=22 | N=21 | |
| Model 1 | 0.52 (0.24 – 1.14) | 1.09 (0.57 – 2.11) | 1.0 (Reference) | 0.41 |
| Model 2 | 0.58 (0.23 – 1.47) | 1.12 (0.53 – 2.38) | 1.0 (Reference) | 0.54 |
| Model 3 | 0.55 (0.22 – 1.40) | 1.09 (0.51 – 2.31) | 1.0 (Reference) | 0.45 |
| Model 4 | 0.52 (0.20–1.35) | 1.09 (0.51–2.34) | 1.0 (Reference) | 0.49 |
Data shown are hazard ratios (95% confidence intervals). Model 1 adjusts for age, sex, and race. Model 2 adjusts for variables in Model 1 and the ankle brachial index, body mass index, smoking, and comorbidities. Model 3 adjusts for covariates in Model 2 and physical activity level. Model 4 adjusts for covariates in Model 3 and time dependent use of statin, cilostazol, and pentoxifylline use.
For all analyses, the reference group is the tertile of PAD participants with highest calf percent fat.
p<0.001 relative to the reference value.
p=0.004 relative to the reference value.
p=0.003 relative to the reference value.
Analyses for mobility loss exclude participants with mobility limitation at baseline. Analyses for becoming unable to walk for six-minutes continuously exclude participants who stopped during the six-minute walk test at baseline. Because of sample size differences for each outcome, tertile definitions are distinct for each outcome.
Participants without PAD
Among participants without PAD, lower calf muscle density was associated significantly with increased mobility loss (p trend = 0.0003) and higher calf muscle percent fat was associated with increased mobility loss (p trend = 0.036), adjusting for age, sex, race, BMI, comorbidities, and smoking. Only the association of lower calf muscle density with increased mobility loss remained statistically significant after additional adjustment for physical activity levels (p trend = 0.0005). Lower calf muscle area (p trend = 0.009) and higher calf muscle percent fat (p trend = 0.04) were each associated significantly with a ≥ 20% decline in six-minute walk distance, adjusting for age, sex, race, BMI, and comorbidities. After additional adjustment for physical activity, only calf muscle area (p trend = 0.012) remained associated significantly with a ≥ 20% decline in six-minute walk performance. There were no significant associations of calf muscle density with functional decline in participants without PAD.
DISCUSSION
Histopathologic studies demonstrate that lower extremity ischemia is associated with calf muscle apoptosis, atrophy and loss of Type II muscle fibers, and increased connective tissue (1–3). Among participants included in the longitudinal analyses reported here, PAD was associated with significantly lower calf muscle area and lower calf muscle density, but not with significantly higher calf muscle percent fat compared to absence of PAD. However, prior study shows that PAD patients with significant ABI discrepancies between their right and left legs have lower calf muscle area and higher calf muscle percent fat in the leg with lowest ABI (6). Together, these data indicate that lower extremity ischemia is associated with pathophysiologic changes in calf muscle (1–3,6). The etiology of pathophysiologic calf muscle findings in PAD is likely multi-factorial. Reduced physical activity, neuropathy, genetic factors, exercise activity, and comorbid diseases may all contribute to calf muscle pathophysiologic findings in persons with PAD. To our knowledge, associations of adverse calf muscle characteristics with prospectively measured decline in functional performance have not been reported previously in men and women with PAD.
Among PAD participants, we found that lower calf muscle density, lower calf muscle area, and higher calf percent fat were associated with increased mobility loss at two year follow-up. Associations of calf muscle density and calf muscle area with mobility loss remained statistically significant even after adjustment for multiple potential confounders, including physical activity and PAD-related medications. PAD participants in the most adverse baseline tertiles of calf muscle density and calf muscle percent fat had higher rates of mobility loss compared to those in the most favorable calf muscle tertiles at baseline. These findings demonstrate that calf muscle pathophysiologic findings are associated independently with mobility loss in persons with PAD.
Among participants without PAD, lower calf muscle density was associated with increased mobility loss and lower calf muscle area was associated with a ≥ 20% decline in six-minute walk performance, even after adjusting for confounders including physical activity. Higher calf muscle percent fat was associated with increased mobility loss and with an increased risk of declining ≥ 20% in six-minute walk distance, but these associations were no longer statistically significant after additional adjustment for physical activity. Our findings indicate that adverse calf muscle characteristics are associated with functional decline in persons both with and without PAD. However, since chronic lower extremity ischemia contributes to the muscle pathophysiologic findings, the calf muscle pathology is potentially more clinically significant in PAD.
This study has limitations. First, the study is observational. Associations reported here cannot be construed as causal. Second, analyses for mobility loss were limited to participants without baseline mobility disability and analyses for becoming unable to walk for six-minutes continuously at follow-up were limited to those who walked for six minutes continuously at baseline. These characteristics and our exclusion criteria limit the generalizability of our findings. Third, muscle biopsy data were not available in our study cohort. Fourth, our study had limited statistical power to detect differences less than hazard ratios of 0.45 or 2.22 between highest vs. lowest tertiles of calf muscle characteristics.
Mechanisms of functional impairment and decline in PAD are not well understood. For example, changes in the ABI over time are minimal even while patients with PAD experience significant functional decline (5,19). Our findings suggest that interventions to reverse mobility loss in PAD should focus on reversing adverse calf muscle characteristics. While one small prior study demonstrated that leg strengthening increased muscle fiber density in PAD with intermittent claudication (20), to our knowledge no other studies have identified interventions that reverse adverse calf muscle characteristics in PAD.
SYNOPSIS.
Men and women with lower extremity peripheral arterial disease (PAD) have smaller calf muscle area, lower calf muscle density, and increased calf muscle percent fat as compared to people without PAD. However, the prognostic significance of these calf muscle changes in PAD is unclear. This observational, prospective study measured calf muscle area, calf muscle density, and calf muscle percent fat using computed tomography in 370 participants with PAD. At two-year follow-up, participants with lower baseline calf muscle area, lower baseline calf muscle density, and higher baseline calf muscle percent fat had higher rates of mobility loss. These findings also suggest that interventions to prevent mobility loss in PAD should focus on reversing pathophysiologic findings in calf muscle.
Acknowledgments
Funding Sources: Supported by grants #R01-HL58099, R01-HL64739, R01-HL071223, and R01-HL076298 from the National Heart Lung and Blood Institute and by grant #RR-00048 from the National Center for Research Resources, NIH. Supported in part by the Intramural Research Program, National Institute on Aging, NIH.
Footnotes
Disclosures
None of the authors have any conflicts of interest with the manuscript topic.
REFERENCES
- 1.Regensteiner JG, Wolfel EE, Brass EP, Carry MR, Ringel SP, Hargarten ME, Stamm ER, Hiatt WR. Chronic changes in skeletal muscle histology and function in peripheral arterial disease. Circulation. 1993;87:413–421. doi: 10.1161/01.cir.87.2.413. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell RB, Duscha BD, Robbins JL, Redfern SI, Chung J, Bensimhon DR, Kraus WE, Hiatt WR, Regensteiner JG, Annex BH. Increased levels of apoptosis in gastrocnemius muscle in patients with peripheral arterial disease. Vascular Medicine. 2007;12:285–290. doi: 10.1177/1358863X07084858. [DOI] [PubMed] [Google Scholar]
- 3.Hedberg B, Angquist KA, Henriksson-Larsen K, Sjostrom M. Fibre loss and distribution in skeletal muscle from patients with severe peripheral arterial insufficiency. Eur J Vasc Surg. 1989;3:315–322. doi: 10.1016/s0950-821x(89)80067-2. [DOI] [PubMed] [Google Scholar]
- 4.McDermott MM, Greenland P, Liu K, Guralnik JM, Celic L, Criqui MH, Chan C, Martin GJ, Schneider J, Pearce WH, Taylor LM, Clark E. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study. Ann Intern Med. 2002;136:873–883. doi: 10.7326/0003-4819-136-12-200206180-00008. [DOI] [PubMed] [Google Scholar]
- 5.McDermott MM, Liu K, Greenland P, Guralnik JM, Criqui MH, Chan C, Pearce WH, Schneider JR, Ferrucci L, Celic L, Taylor LM, Vonesh E, Martin GJ, Clark E. Functional decline in peripheral arterial disease: Associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–461. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 6.McDermott MM, Hoff F, Ferrucci L, Pearce WH, Guralnik JM, Tian L, Liu K, Schneider JR, Sharma L, Tan J, Criqui MH. Lower extremity ischemia, calf skeletal muscle characteristics, and functional impairment in peripheral arterial disease. J Am Geriatr Soc. 2007;55:400–406. doi: 10.1111/j.1532-5415.2007.01092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDermott MM, Sufit R, Nishida T, Guralnik JM, Ferrucci L, Tian L, Liu K, Tan J, Pearce WH, Schneider JR, Sharma L, Criqui MH. Lower extremity nerve function in patients with lower extremity ischemia. Arch Intern Med. 2006;166:1986–1992. doi: 10.1001/archinte.166.18.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shadman R, Criqui MH, Bundens WP, Fronek A, Denenberg JO, Gamst AC, McDermott MM. Subclavian artery stenosis: Prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004;44:618–623. doi: 10.1016/j.jacc.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 9.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and Computerized Tomography. J Appl Physiol. 1999;86(3):1097–1098. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 10.McDermott MM, Guralnik JM, Tian L, Liu K, Ferrucci L, Liao Y, Sharma L, Criqui MH. Associations of borderline and low normal ankle brachial index values with functional decline at five-year follow-up: The Walking and Leg Circulation Study. J Am Coll Cardiol. 2009;53:1056–1062. doi: 10.1016/j.jacc.2008.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDermott MM, Ades PA, Dyer A, Guralnik JM, Kibbe M, Criqui MH. Corridor-based functional performance measures correlate better with physical activity during daily life than treadmill measures in persons with peripheral arterial disease. J Vasc Surg. 2008;48:1231–1237. doi: 10.1016/j.jvs.2008.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel KV, Coppin AK, Manini TM, Lauretani F, Bandinelli S, Ferrucci L, Guralnik JM. Midlife physical activity and mobility in older age. The InCHIANTI study. Am J Prev Med. 2006;31:217–224. doi: 10.1016/j.amepre.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fried LP, Guralnik JM. Disability in older adults: evidence regarding significance, etiology, and risk. J Am Geriatr Soc. 1997;45:92–100. doi: 10.1111/j.1532-5415.1997.tb00986.x. [DOI] [PubMed] [Google Scholar]
- 14.Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME. Bethesda, MD: National Institute on Aging; 1995. The Women’s Health and Aging Study: Health and social characteristics of older women with disability. NIH publication No. 95-4009, Appendix E. [Google Scholar]
- 15.Altman R, Alarcon G, Appelrouth D. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991;34:505–514. doi: 10.1002/art.1780340502. [DOI] [PubMed] [Google Scholar]
- 16.Altman R, Alarcon G, Appelrouth D, Bloch D, Borenstein D, Brandt K for the American College of Rheumatology. Development of criteria for the classification and reporting of osteoarthritis. Arthritis Rheum. 1986;29:1039–1049. [Google Scholar]
- 17.Garg PK, Tian L, Criqui MH, Liu K, Ferrucci L, Guralnik JM, Tan J, McDermott MM. Physical activity during daily life and mortality in patients with peripheral arterial disease. Circulation. 2006;114:242–248. doi: 10.1161/CIRCULATIONAHA.105.605246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale residuals. Biometrika. 1993;80:557–572. [Google Scholar]
- 19.Smith FB, Lee AJ, Price JF, van Wijk MCW, Fowkes FGR. Changes in ankle brachial index in symptomatic and asymptomatic subjects in the general population. J Vasc Surg. 2003;38:1323–1330. doi: 10.1016/s0741-5214(03)01021-8. [DOI] [PubMed] [Google Scholar]
- 20.McGuigan MRM, Bronks R, Newton RU, Sharman MJ, Graham JC, Cody DV, Kraemer WJ. Resistance training in patients with peripheral arterial disease: Effects on myosin isoforms, fiber type distribution, and capillary supply to skeletal muscle. Journal of Gerontology Biological Sciences. 2001;56A:B302–B310. doi: 10.1093/gerona/56.7.b302. [DOI] [PubMed] [Google Scholar]