Abstract
The natural environment of a neuron is the three-dimensional (3D) tissue. In vivo, embryonic sensory neurons transiently express a bipolar morphology with two opposing neurites before undergoing cytoplasmic and cytoskeletal rearrangement to a more mature pseudo-unipolar axonal arbor before birth. The unipolar morphology is crucial in the adult for correct information transmission from the periphery to the central nervous system. On two-dimensional (2D) substrates this transformation is delayed significantly or absent. We report that a 3D culture platform can invoke the characteristic transformation to the unipolar axonal arbor within a time frame similar to in vivo, overcoming the loss of this essential milestone in 2D substrates. Additionally, 3D substrates alone provided an environment that promoted axonal branching features that reflect morphological patterns observed in vivo. We have also analyzed the involvement of soluble cues in these morphogenic processes by culturing the neurons in the presence and absence of nerve growth factor (NGF), a molecule that plays distinct roles in the development of the peripheral and central nervous systems. Without NGF, both 2D and 3D cultures had significant decreases in the relative population of unipolar neurons as well as shorter neurite lengths and fewer branch points compared to cultures with NGF. Interestingly, branching features of neurons cultured in 3D without NGF resemble those of neurons cultured in 2D with NGF. Therefore, neurons cultured in 3D without NGF lost the ability to differentiate into unipolar neurons, suggesting that this morphological hallmark requires not only presentation of soluble cues like NGF, but also the surrounding 3D presentation of adhesive ligands to allow for realization of the innate morphogenic program. We propose that in a 3D environment, various matrix and soluble cues are presented toward all surfaces of the cell; this optimized milieu allows neurons to elaborate their genuine phenotype and follow programmed instructions that are intrinsic to the neuron, but disrupted when cells were dissected from the embryo. Thus, this study presents quantitative data supporting that 3D substrates are critical for sustaining the in vivo ontogeny of neurons and deciphering signaling mechanisms necessary for designing biomaterial scaffolds for nerve generation and repair.
Introduction
The generation of replacement organs and tissues relies on the design of components that replicate the three-dimensional (3D) structure and physiology. While great strides have been made in the development of new biomaterials and neural stem/progenitor cell biology, tissue engineering efforts focused on nerve repair have been limited by a nascent understanding of how neurons interact with their 3D microenvironment. Neurite process outgrowth, axonal guidance, and synaptogenesis are regulated through interactions between cell-surface receptors and the local extracellular matrix (ECM).1 Differentiation, growth cone motility, and branching events are also influenced by signals from surrounding cells and molecular gradients. In vivo these signals are arranged in a complex extracellular network to create distinct microenvironments.2
Biological studies on two-dimensional (2D) substrates have been invaluable in deciphering the intricacies of mechanisms regulating neuronal behavior; however, a closer approximation to in vivo environments can be attained by growing cells in a 3D configuration. Tissue engineering has provided numerous 3D substrates3–7 that promote neuronal survival and process outgrowth. Yet realized are materials that promote the elaboration of the complex process morphologies observed in vivo. Tailoring cell technologies and nerve repair strategies for specific physiologically relevant morphologies and structures is the next milestone, but to reach this goal, we reason that recapitulation of the physiological morphogenic program is a critical first step to define the molecular mechanisms of biomaterial-cell interactions.
Sensory neurons, with their pseudo-unipolar axons, transmit peripheral information about pain, temperature, and proprioception over long distances to the central nervous system. The majority of DRG neurons possess small diameter soma and are nociceptors and thermoreceptors. During development, these neurons depend on nerve growth factor (NGF) and therefore are sensitive to NGF in culture.8,9 In sensory neurons cultured on 2D surfaces, the NGF/TrkA signaling pathway is extensively characterized and it is established that NGF accelerates survival as well as neurite extension in embryonically derived neurons and branching in adult neurons. In this study we provide insights into NGF-independent regulation of sensory neuron development in 2D and 3D environments, with results supporting the notion that the 3D environment allows elaboration of developmental cellular programs resembling those that occur in vivo.
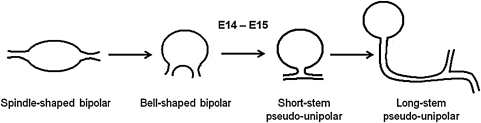
Embryonic DRG neurons undergo a remarkable change in morphology.10 Initially, these neurons display a bipolar morphology, with one neurite extending to the peripheral nervous system and the other toward the spinal cord (Fig. 1). Within 48 h, the cells transform to the canonical pseudo-unipolar morphology that characterizes the perinatal and adult DRG neurons.11–14 In mice, this transition occurs between embryonic (E) day 14 and E15.11 However, in 2D cell culture, the transformation process either does not occur or is delayed at least 1 month.15 Moreover, of the multiple relevant cues tested, only mixed coculture with Schwann cells obtained from postnatal animals for 5 days promotes the conversion of the bipolar neurite processes into the pseudo-unipolar axon.15 These results suggest that in 2D culture, mechanisms regulating this maturation milestone are hindered unless the complex milieu is provided by extrinsic cellular components.
FIG. 1.
Morphological transition of mouse DRG neurons during embryonic development. Before E14 DRG neurons are bipolar, extending one axon each toward the peripheral and central nervous systems. Between E14 and E15, the neurons transform into the canonical pseudo-unipolar morphology found in mature animals. Redrawn by A.R. from Ref.13
A crucial need within the neurobiological and neural tissue engineering fields is culture systems that can invoke the intrinsic morphogenic programs within the in vivo timeframe. We selected a simple common tissue culture substrate, type I collagen, because it is a component of the normal peripheral landscape that the neurons encounter in vivo. The characterization of the physical properties provides a starting point to design the next generation of biomaterial scaffolds. Our experiments demonstrate that DRG neurons sense the dimensionality of their environment and respond to the different arrangement of external signals by modulating neuron morphology, neurite extension, and branching in patterns that closely resemble those that occur during development.
Materials and Methods
Mice
C57Bl/6J mice (Jackson Laboratory) were used. All animal studies were approved by the UMBC and University of Maryland School of Medicine Institutional Animal Care and Use Committees and performed in accordance to NIH guidelines.
Neuronal culture
DRG were dissected from mouse embryos at E13.5–E14.5 following established methods.16 Tissue was dissociated with 0.025% trypsin (Invitrogen) for 20 min, triturated, and filtered through a 25-μm nylon mesh (Sefar) to remove tissue clumps and most non-neuronal cells. Cultures were >95% neurons as assessed by positive TUJ1 and NF160 immunoreactivity.
Neurons were seeded at 1.5×104 cells/cm2 onto 2D collagen-coated coverslips (∼7 μg/cm2, precoated with 0.1 mg/mL with poly-L-Lysine; Sigma) and on top of 2D collagen gels (1 mg/mL, thickness ∼1.5 mm), and at 5×105 cells/mL within 3D collagen gels (1 mg/mL, 20 μL) and cultured for 2 days in 24-well plates. In all cases, rat-tail type I collagen (BD Biosciences) was used. The cell densities have been previously determined to be sufficiently low to minimize cell–cell contact.
Cells were maintained in serum-free medium: Dulbecco's modified Eagle's medium supplemented with 1×N2 supplement, 100 U/mL of penicillin/streptomycin, 20 mM of L-glutamine (Invitrogen), and 50 ng/mL of NGF (Chemicon). For 1 mg/mL collagen gels, 10×Hank's Buffered Salt Solution (Invitrogen), 1 N NaOH, deionized water, collagen, and 50 ng/mL NGF were combined with cells to generate 3D substrates. The collagen/cell solution was allowed to gel in a culture incubator for 30–45 min before addition of culture medium. To study neuronal behavior in the absence of NGF signaling, we withdrew NGF from the medium and collagen/cell solutions.
Immunocytochemistry and image analysis
Samples were fixed in a buffered 4% formaldehyde solution for 20 min and washed three times with phosphate-buffered saline (PBS) and incubated with 10% lamb serum in PBS blocking solution for 30 min (in 2D) and 2 h (in 3D). For 2D culture, the neurons were incubated in primary antibodies to βIII-tubulin (1:500; Sigma) and NF160 (1:200; Sigma) followed by appropriate fluorescently conjugated secondary antibodies (Jackson Immunoresearch) for 30–60 min. 4′,6-Diamidino-2-phenylindole (DAPI; 300 nM; Sigma) was used to observe cell nuclei. Immunocytochemistry procedures for the 3D gels had longer washing steps (>30 min each) and overnight antibody incubations. Gels were processed in the 24-well plates. After staining, coverslips with gels on top were removed from the wells and mounted in a chambered coverglass (diameter vs. thickness=9×1 mm; Sigma). Samples were imaged with confocal microscopy (Leica TCS SP5) and analyzed with LAS AF software (Leica). Growth cone areas were measured using Volocity imaging software (PekinElmer).
Quantitative assessment of neurite outgrowth
Neurolucida software (MFB Bioscience) was used to reconstruct and measure process extension. Sholl analysis was applied to determine branching features.17,18 The quantified outgrowth features consisted of number of primary neurites, total number of branch points, total neurite length, and length of the longest neurite. Primary neurites emerged directly from the soma. Total neurite length was defined as the sum of all neurites plus all the branches deriving from a single neuron. Neurites <15 μm in length or those that contacted other neurites or cell bodies were excluded. Length distributions of ≥15 neurites were analyzed from ≥3 separate experiments (3–4 samples each, n≥50).
Statistical analysis
Statistical analysis was performed using Analyse-it® Statistical software via nonparametric analyses with a minimum significance level set at p<0.05 (>95% confidence level). To determine statistical differences between two non-Gaussian populations the Mann-Whitney test was applied. For comparisons between multiple non-Gaussian populations, the non-parametric Kruskal-Wallis test was used followed by Bonferroni post-hoc analysis.
Results
Effects of culture dimensionality on growth cone morphology
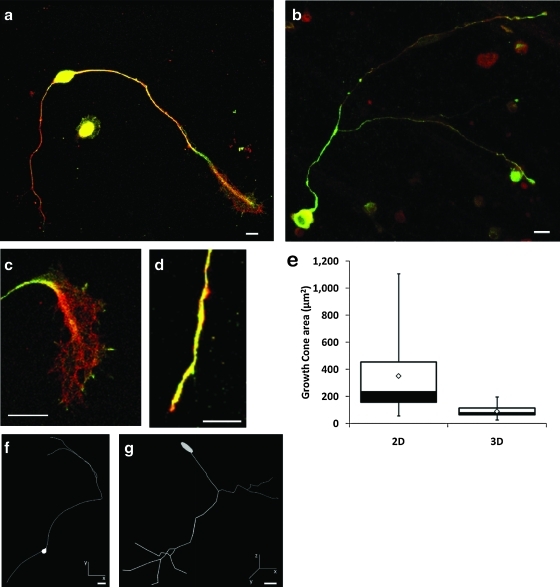
We compared the growth of DRGs in 3D collagen gels and on 2D collagen coated coverslips. After 48 h in culture, the cells were fixed and neurite processes were observed using immunohistochemistry for either neuronal-specific βIII-tubulin (TUJ1) or the 160 kDa neurofilament (NF160). The morphologies of the neurite processes were dramatically different between the substrate dimensionalities (Fig. 2).
FIG. 2.
Sensory neuron morphology is affected by environment dimensionality. Representative confocal images of DRG neurons cultured on two-dimensional (2D) collagen-coated coverslips (a) and within three-dimensional (3D) collagen gels (b). Growth cones in 2D (c) were larger, richer in filopodia, and lamellipodia and more flattened than those in 3D (d). Neurons were co-labeled for βIII-tubulin (red) and NF160 (green). Cell nuclei were labeled with 4′,6-diamidino-2-phenylindole (blue). (e) Quantitative analysis of growth cone projected area (n2D=n3D=40, p<0.01). Boxes enclose 25th (black) and 75th (white) percentiles of each distribution and are bisected by the median; white diamonds represent the mean; whiskers indicate the 5th and 95th percentiles. Neurolucida reconstructions of representative DRG neurons cultured on 2D (f) and within 3D (g) collagen substrates. Scale bars, 10 μm in (a–d), 20 μm in (f, g). Color images available online at www.liebertonline.com/tea
Examination of the leading edges of the neurites revealed growth cone morphologies that were distinct between the neurons cultured on 2D collagen-coated glass substrates and within 3D collagen gels (Fig. 2c, d). Similar to active growth cones in other 2D systems (such as retinal cells on collagen or DRG neurons on laminin19–21) the growth cones on 2D substrates adhered to the substrate, flattened out, and displayed large lamellipodia and numerous filopodia. In the 3D environment, however, the growth cones were much smaller, had fewer filopodia and less lamellipodia, and more closely resembled those found in sensory neurons in vivo.22,23 Quantitative analysis of the projected growth cone areas (Fig. 2e) showed >60% of the neurons grown on 2D substrates exhibited large growth cones that spanned areas >200 μm2 (median=239.4 μm2; maximum=1427 μm2). In 3D cultures, however, 95% of the neurons had growth cones smaller than 200 μm2 and on average were approximately three times smaller than the growth cones of neurons cultured in 2D (median=79.0 μm2, maximum=216.5 μm2) (n2D=n3D=40, p<0.01). In summary, the growth cone morphology varied greatly with the dimension of the substrate.
Quantitative assessment of neurite outgrowth in 2D and 3D environments
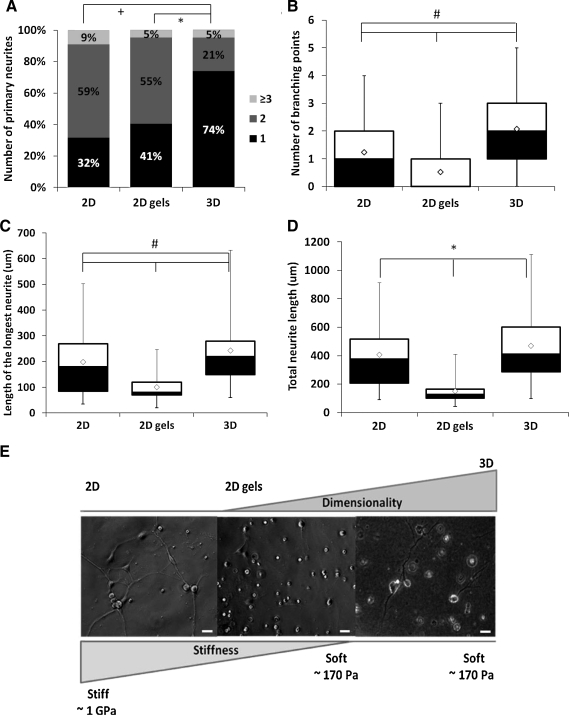
We compared neurite outgrowth on collagen-coated glass coverslips to the response in the 3D collagen gels. We were able to measure process outgrowth in all three dimensions with confocal stacks and the Neurolucida software system. Neurons cultured in 3D were typically unipolar and showed a more branched structure with longer neurites than the bipolar neurons cultured on 2D collagen-coated coverslips (Fig. 2a, b). After 48 h in culture, 74% of the neurons cultured in 3D had a single neurite in comparison to 32% of the neurons cultured on 2D collagen-coated coverslips (Fig. 3A; n2D=87, n3D=90, p<0.01). The bipolar and pseudo-unipolar morphologies are characteristic of embryonic stages of DRG development.11,12 For these experiments, DRGs were obtained between E13.5 and E14.5, during the period of transformation from bipolar to unipolar morphology. After 2 days in culture, the neurons were predicted to adopt morphological features characteristic of E15.5–E16.5 developmental ages. We observed that neurons cultured on 2D substrates resembled bipolar cells found in the E12.5 embryo. In the 3D environment, the majority of neurons adapted the pseudo-unipolar morphology similar to that found in the more mature E15.5–E16.5 sensory ganglion.
FIG. 3.
Effect of environment dimensionality on neurite outgrowth and branching. DRG neurons cultured within 3D substrates (n=90) are predominately unipolar with longer neurites and more branching than neurons cultured on 2D substrates (nglass=87; ngel=65). (A) Number of primary neurites, (B) number of branch points per neurite, (C) length of the longest neurite, and (D) total neurite length. Boxes enclose 25th (black) and 75th (white) percentiles of each distribution and are bisected by the median; white diamonds represent the mean; whiskers indicate the 5th and 95th percentiles. Symbols denote significant differences (p<0.05): *from 2D gels, +from 2D, #between all the culture conditions. (E) A direct comparison was made between the individual effects of substrate stiffness (2D collagen coated glass vs. 2D collagen gel) and environment dimensionality (2D collagen gel vs. 3D collagen gel). Phase contrast images of the DRG neurons cultured for 2 days on 2D collagen-coated glass (left), on top of 2D collagen gel (middle) and within 3D collagen gel (right). Scale bars, 20 μm.
The complexity of the neurite structure elaborated in the 3D substrates reflected the in vivo axonal arbor, which is highly branched to receive information from multiple regions. Greater than 65% of the neurons cultured in 3D had two or more branching points, whereas fewer than 35% of the neurons cultured on collagen-coated coverslips demonstrated this branching pattern (Fig. 3B; n2D=87, n3D=90, p<0.01). Moreover, the length of the longest neurite was observed to be longer for the neurons cultured in 3D (median=220.0 μm) than the neurons cultured in 2D (Fig. 3C; median=180.0 μm, n2D=87, n3D=90, p<0.05). However, no difference in total neurite length per neuron was observed (Fig. 3D; n2D=87, n3D=90, p>0.05). Although the neurons in 3D had an increased number of branches and individually longer neurites, in 2D neurons had more primary neurites contributing to total neurite length. Together, these findings demonstrate that sensory neurons cultured within 3D substrates had a more branched structure and were generally unipolar with significantly longer neurites than those cultured on 2D substrates.
Substrate stiffness and neurite outgrowth
Tissue stiffness influences cell response and morphogenesis24 and substrate mechanical properties actively affect neurite outgrowth in both 2D and 3D culture.25–27 To determine if the stiffness of collagen-coated glass versus collagen gel influenced process morphology, we also cultured the neurons on top of 2D collagen gels (1 mg/mL, same concentration as for the 3D gel substrates). This additional 2D culture condition allowed the decoupling of the effects of substrate stiffness (2D collagen-coated glass, ∼1 GPa28 compared to 2D collagen gel, ∼170 Pa24) and environment dimensionality (2D collagen gel vs. 3D collagen gel) (Fig. 3E).
Sensory neurons cultured on top of 2D collagen gels had a simple structure, typically adapting a bipolar morphology as on 2D collagen-coated glass, and showed significantly fewer branches than both the neurons cultured on 2D collagen-coated glass and those within 3D collagen (p<0.01). The majority of these neurons had two primary neurites (∼55%, Fig. 3A), with ∼90% of these processes having one or no branches (Fig. 3B). The number of cells extending neurites and the length of the neuronal projections were significantly decreased when cultured on top of the collagen gel (p<0.01), with longest neurite and median total lengths reaching only 80.0 and 126.4 μm (Fig. 3C, D) after 2 days in culture. Together, these findings demonstrate that sensory neurons cultured in 3D had a more branched structure and were generally unipolar; moreover, primary neurites grown in 3D were significantly longer than for neurites in 2D culture.
Effects of environment dimensionality on the response of sensory neurons to NGF withdrawal
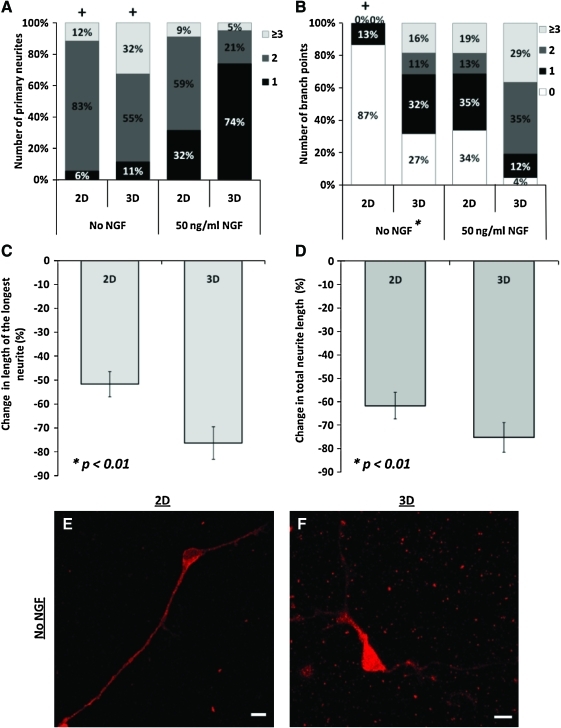
Removal of NGF from culture dramatically impacted DRG neuronal morphology and branching patterns, with effects varying with environment dimensionality (Fig. 4). In the absence of NGF, sensory neurons lost the ability to differentiate into unipolar neurons after 2 days in culture, a morphological transition observed in 3D (Figs. 2 and 3) and that characterizes these neurons in vivo. Only 11% of the neurons cultured in 3D were unipolar in the absence of NGF in comparison to 74% in 3D in the controls (Fig. 4A; 50 ng/mL NGF, n3D=50, p<0.01). In 2D, there was also a significant reduction in the number of unipolar neurons from 32% to 6% (Fig. 4A, n2D=52, p<0.05).
FIG. 4.
Effect of environment dimensionality on DRG neuron response to NGF withdrawal. Without NGF, both 2D and 3D cultures had decreases in the relative population of unipolar neurons (A) and number branch points per neurite (B) compared to cultures with NGF. The neurons exhibited decreased total and individual neurite lengths as a response to NGF withdrawal compared to cultures containing NGF; these data are plotted as % change versus cultures with NGF (C, D). Notably, branching features of neurons cultured in 3D without NGF resemble those of neurons cultured in 2D with NGF [middle two bars in (B)]. Symbols denote significant differences (p<0.05): *between 2D and 3D culture within the same conditions, +from culture with NGF. Representative confocal images of DRG neurons cultured on 2D (E) and within 3D collagen substrates (F) in the absence of NGF. Cultures were stained for βIII-tubulin (red). Scale bars, 10 μm. NGF, nerve growth factor. Color images available online at www.liebertonline.com/tea
The role of NGF in branching enhancement has been previously reported for adult DRG neurons cultured on 2D-coated surfaces.29,30 Thus, not surprisingly, NGF withdrawal influenced arbor formation in the neurons cultured in 2D where the neurons lost the capacity to branch (Fig. 4B; 87% of the neurons have 0 branches, n2D=52, p<0.01). In 3D, there was a slight decrease in branching for the neurons cultured without NGF as 59% of neurons have 0 or 1 branches, when compared to cultures with NGF where 64% of neurons had 2 or more branches (Fig. 4B, n3D=50). However, this decrease was not significant (p>0.05), indicating that NGF does not play a major role in the regulation of branching in embryonic sensory neurons in 3D culture. As a result, in the absence of NGF, neurons branch significantly more in 3D than in 2D (Fig. 4B, n3D=50, p<0.01). Of particular note, branching patterns in 3D in the absence of NGF resembled those observed in 2D cultures in the presence of NGF (Fig. 4B).
Similar to neonatal DRG neurons cultured on 2D poly-l-lysine-coated surfaces,29 withdrawal of NGF signaling resulted in a major decrease in neurite outgrowth (Fig. 4C, D). In 2D, total neurite length and the length of the longest neurites were decreased by 61.7%±2.1% and 51.7%±5.3%, respectively (n2D=52, p<0.01). In 3D, these effects were even more pronounced as total and longest neurite lengths were decreased by 75.2%±6.1% and 76.3%±6.8%, respectively (n3D=50, p<0.01). Altogether, these results suggest that sensory neuron regulation of morphogenic programs through NGF signaling is altered with environment dimensionality.
Discussion
The ECM provides a 3D substrate enriched in ligands that are sensed and remodeled by cells and imposes a spatial restraint to cellular migration and neurite extension.31 During development, DRG neurons innervate sensory targets by extending long axons with multiple branches that are properly distributed within target tissues.23,32 Mechanisms regulating neurite outgrowth and branching in sensory neurons are widely studied, but little is known about the mechanisms that regulate polarization events. Moreover, researchers have successfully induced sensory process outgrowth and indiscriminate branching in a diverse array of conditions, including different substrates in 2D and 3D culture.3,4,25,30,33,34 Remarkably, despite a notable report that bipolar morphology is an artifact of 2D culture,15 the unipolar structure of DRG neurons is usually overlooked.
Our work demonstrates that ontogeny of axonal structure relies on the dimensionality of the biomaterial scaffold. One of the fundamental differences between 2D and 3D culture is the distribution of cell–cell and cell–matrix interactions, which alter signaling mechanisms regulating neuronal biological response and activity.35 Therefore, we hypothesized that neurons sense the dimensionality of their environment and respond by altering their morphology in adaptation to the different arrangement of extracellular signals. Our results demonstrate that environment dimensionality plays a major role in sensory neuron development and regulation of signaling events controlling polarization, branching, growth cone motility, and neurite outgrowth. Neurons sensed the dimensionality of their environment and developed into distinct morphologies in 2D and 3D. The 3D setting also allowed elaboration of the in vivo growth cone morphology, axonal branching pattern, and polarization features within normal ontogeny. These results suggest that a 2D substrate is insufficient to permit completion of the polarization program, whereas, in contrast, the 3D environment supports maturation past the embryonic milestone.
For neurons, as with all cells, morphogenesis occurs in a 3D environment wherein the cell dynamically responds to local environmental cues, such as cell–cell and cell–matrix interactions as well as diffusible molecules.1 These stimuli engage the neuron in a sequence of signaling events that define the characteristic neuronal morphologies and arborization patterns. In a 3D environment, a cell is completely surrounded by stimuli and thus the cell can receive similar external signals from multiple directions. Moreover, as evidenced by the results shown in Figures 2–3 neurons are able to make unrestricted use of use all three dimensions (or degrees of freedom), resulting in longer neurites and more branches than on 2D substrates where integrin receptors must reconfigure to the planar arrangement of ligands.
We have also analyzed the involvement of soluble cues in these morphogenic processes by culturing the neurons in the absence of NGF, a molecule that plays distinct roles in the development of the peripheral and central nervous system. In the developing embryo, NGF is required for the survival of DRG neurons, as the majority of DRG neurons die of apoptosis by E14.5 in mice lacking NGF.8 Moreover, in the absence of NGF/TrkA signaling, embryonic sensory neurons fail to innervate peripheral targets.36 Herein we report that upon withdrawal of NGF, neurite outgrowth was dramatically reduced in 2D culture; this is not surprising as NGF is known to enhance sensory neurite elongation on permissive substrates.29,30,34 We also found that this response to NGF withdrawal was enhanced when the same substrate was presented as an encompassing 3D matrix (Fig. 4C, D).
Many have reported that DRG neurons adapt a bipolar morphology in 2D culture regardless of the addition of growth factors29,30,37–40; we also found this result in 3D culture conditions without NGF (Fig. 4A). Therefore, neurons cultured in 3D without NGF lost the ability to differentiate into unipolar neurons. Since the morphology of DRG neurons in 2D culture is similar with or without NGF, we argue that presentation of soluble cues like NGF but perhaps more importantly the surrounding 3D presentation of adhesive ligands allows for realization of the innate cellular morphogenic program.
The branching patterns of sensory neurons in response to NGF withdrawal were distinct between 2D and 3D culture (Fig. 4B). The effect of removing NGF from 2D culture was dramatic as the majority of neurons had no branches. This result supports the observed link between NGF treatment and the increased complexity of neurite patterns of adult sensory neurons grown in 2D culture.29,30 In 3D, however, DRG neurons cultured without NGF retained the capacity to form complex arbors in the 3D matrix, suggesting that regulation of branching programs in 3D are independent of NGF.
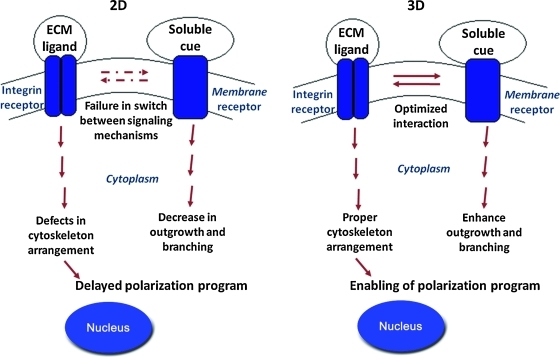
To begin to understand these findings, we propose a hypothetical model (Fig. 5) wherein cells sense and balance cues from their environment. For DRG neurons, the end result of this process is a cell morphology that allows proper relay of sensory information from the periphery to the central nervous system. We propose that in a 3D environment, various matrix and soluble cues are presented toward all surfaces of the cell; this optimized milieu allows neurons to elaborate their genuine phenotype and follow programmed instructions that are intrinsic to the neuron, but disrupted when cells were dissected from the embryo. In other words, the embryonic neurons are competent in a 3D environment to generate mature DRG neurons with complex arbors. On a 2D substrate, matrix and soluble cues are largely segregated by the geometry of a planar system and impair receptor cross-talk and signal integration. As a result, the rearrangement of the cytoskeleton is aberrant and the transformation to a unipolar neuron is delayed for up to a month.15 The suspended transition can be partially overcome in 2D if Schwann cells are included in the culture; however, the time to transformation remains prolonged as compared to in vivo from 1 to 2 days to up to 2 weeks.15 By contrast, the data presented herein support the hypothesis that the axonal growth pattern of the sensory neurons is intrinsic, and in a sufficient environment, the DRG neuron can recapitulate the in vivo maturation program.
FIG. 5.
Model of cell response to culture dimensionality. On a 2D substrate, matrix and soluble cues are segregated and provide an artificial environment that alters the integration of extracellular cues and results in a delayed polarization program. When exposed to a 3D environment, matrix ligands and soluble cues completely surround the cell, and in response, membrane receptors are arranged in an unrestricted configuration. In this setting, signaling events are regulated according to intrinsic programming and the neurons develop into the proper pseudo-unipolar morphology with long and branched axons as found in vivo. Color images available online at www.liebertonline.com/tea
It is also important to recognize that tissue stiffness plays a key role in morphogenesis and that substrate mechanical properties actively affect neurite outgrowth in both 2D and 3D culture. For example, differentiation of mesenchymal stem cells into specific cell lineages is highly regulated by substrate stiffness41 and an increase in matrix stiffness influences cytoskeletal tension in mammary epithelial cells and drives the cells toward a malignant phenotype.24 Matrix stiffness influences neurite outgrowth mechanisms, resulting in different and potentially neuron-type-specific responses in 2D and 3D culture: in 2D culture, neurite outgrowth is greater on stiffer substrates,42 whereas the opposite trend occurs for 3D substrates in which softer gels result in a greater extent of neurite outgrowth.25–27 Our results indicate that DRG neurons cultured on soft 2D collagen gel substrates adapt simplified morphological features similar to those cultured on stiff 2D collagen-coated glass (Fig. 3). However, when the same soft substrate is presented to the cells as a surrounding 3D environment, the cells are able to extend long neurites with significant branching. Thus, after excluding the effects of stiffness in our results, we confirmed that the significant differences in total neurite length, number of branches, and primary neurites found between the 2D and 3D culture conditions are correlated with the changes in dimensionality. Engineered scaffolds (e.g., Refs.43–45) will enable investigation of the effect of scaffold properties in 2D and 3D culture in more detail.
These findings suggest that in a 3D environment, sensory neurons are able to use the limited resources available in culture to realize their innate morphogenic program and thus mimic the phenotypic characteristics observed in vivo. While this study focused on sensory neurons, emerging data hint that the response may be shared by neurons of peripheral and central origin. These findings open new doors for neuronal regeneration and nerve repair strategies that make use of intrinsic cellular mechanisms, in collaboration with engineered environments, to reproduce the natural context and avoid situations that constrain cells into an artificial configuration, leading to temporary or unpredictable biological outcomes. Thus, our ongoing work focuses on defining the signaling mechanisms regulating polarization and axonal arbors in 2D versus 3D culture environments.
Acknowledgments
We thank C. Petty for assistance with confocal microscopy; Dr. A. Puche for assistance with Neurolucida software and analysis; Dr. J. Loehe for advice on statistical analysis; and Dr. D. Bloomberg for generous access to the long working distance objective. This work was supported by NIH-NINDS R01NS065205, the Henry Luce Foundation, a Wyeth Graduate Fellowship (A.R.), and a UMBC Undergraduate Research Award (S.V.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Kollins K.M. Davenport R.W. Branching Morphogenesis. New York: Springer; 2005. [Google Scholar]
- 2.Zhang S. Gelain F. Zhao X. Designer self-assembling peptide nanofiber scaffolds for 3D tissue cell cultures. Semin Cancer Biol. 2005;15:413. doi: 10.1016/j.semcancer.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Bozkurt A. Brook G.A. Moellers S. Lassner F. Sellhaus B. Weis J. Woeltje M. Tank J. Beckmann C. Fuchs P. Damink L.O. Schugner F. Heschel I. Pallua N. In vitro assessment of axonal growth using dorsal root ganglia explants in a novel three-dimensional collagen matrix. Tissue Eng. 2007;13:2971. doi: 10.1089/ten.2007.0116. [DOI] [PubMed] [Google Scholar]
- 4.Deister C. Aljabari S. Schmidt C.E. Effects of collagen 1, fibronectin, laminin and hyaluronic acid concentration in multi-component gels on neurite extension. J Biomater Sci Polym Ed. 2007;18:983. doi: 10.1163/156856207781494377. [DOI] [PubMed] [Google Scholar]
- 5.Luo Y. Shoichet M.S. A photolabile hydrogel for guided three-dimensional cell growth and migration. Nat Mater. 2004;3:249. doi: 10.1038/nmat1092. [DOI] [PubMed] [Google Scholar]
- 6.Gelain F. Bottai D. Vescovi A. Zhang S. Designer self-assembling peptide nanofiber scaffolds for adult mouse neural stem cell 3-dimensional cultures. PLoS ONE. 2006;1:e119. doi: 10.1371/journal.pone.0000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leipzig N.D. Wylie R.G. Kim H. Shoichet M.S. Differentiation of neural stem cells in three-dimensional growth factor-immobilized chitosan hydrogel scaffolds. Biomaterials. 2011;32:57. doi: 10.1016/j.biomaterials.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 8.Wickramasinghe S.R. Alvania R.S. Ramanan N. Wood J.N. Mandai K. Ginty D.D. Serum response factor mediates NGF-dependent target innervation by embryonic DRG sensory neurons. Neuron. 2008;58:532. doi: 10.1016/j.neuron.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levi-Montalcini R. Cohen S. In vitro and in vivo effects of a nerve growth-stimulating agent isolated from snake venom. Proc Natl Acad Sci U S A. 1956;42:695. doi: 10.1073/pnas.42.9.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cajal S. Ramon Association del rnetodo del nitrato de plata con el embyonario. Para el estudio de los focos motores y sensitivos. Trab Lab Invest Biol Univ Madrid. 1904;3:65. [Google Scholar]
- 11.Barber R.P. Vaughn J.E. Differentiation of dorsal root ganglion cells with processes in their synaptic target zone of embryonic mouse spinal cord: a retrograde tracer study. J Neurocytol. 1986;15:207. doi: 10.1007/BF01611657. [DOI] [PubMed] [Google Scholar]
- 12.Matsuda S. Baluk P. Shimizu D. Fujiwara T. Dorsal root ganglion neuron development in chick and rat. Anat Embryol (Berl) 1996;193:475. doi: 10.1007/BF00185878. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi K. Ninomiya T. Morphological changes of dorsal root ganglion cells in the process-forming period. Prog Neurobiol. 1987;29:397. doi: 10.1016/0301-0082(87)90020-7. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez M.A. Avila J. Moya F. Alberto C. Rearrangement of microtubule associated protein parallels the morphological transformation of neurons from dorsal root ganglion. Neuroscience. 1989;29:471. doi: 10.1016/0306-4522(89)90074-2. [DOI] [PubMed] [Google Scholar]
- 15.Mudge A.W. Schwann cells induce morphological transformation of sensory neurones in vitro. Nature. 1984;309:367. doi: 10.1038/309367a0. [DOI] [PubMed] [Google Scholar]
- 16.Banker G. Goslin K. Culturing Nerve Cells. Boston, MA: MIT Press; 1998. [Google Scholar]
- 17.Watson K.K. Jones T.K. Allman J.M. Dendritic architecture of the von economo neurons. Neuroscience. 2006;141:1107. doi: 10.1016/j.neuroscience.2006.04.084. [DOI] [PubMed] [Google Scholar]
- 18.Benson D.L. Cohen P.A. Activity-independent segregation of excitatory and inhibitory synaptic terminals in cultured hippocampal neurons. J Neurosci. 1996;16:6424. doi: 10.1523/JNEUROSCI.16-20-06424.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris W.A. Holt C.E. Smith T.A. Gallenson N. Growth cones of developing retinal cells in vivo, on cultured surfaces, and collagen matrices. J Neurosci Res. 1985;13:101. doi: 10.1002/jnr.490130108. [DOI] [PubMed] [Google Scholar]
- 20.Renaudin A. Lehmann M. Girault J. McKerracher L. Organization of point contacts in neuronal growth cones. J Neurosci Res. 1999;55:458. doi: 10.1002/(SICI)1097-4547(19990215)55:4<458::AID-JNR6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 21.Burden-Gulley S.M. Payne H.R. Lemmon V. Growth cones are actively influenced by substrate-bound adhesion molecules. J Neurosci. 1995;15:4370. doi: 10.1523/JNEUROSCI.15-06-04370.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sann S.B. Xu L. Nishimune H. Sanes J.R. Spitzer N.C. Neurite outgrowth and in vivo sensory innervation mediated by a Ca(V)2.2-laminin beta 2 stop signal. J Neurosci. 2008;28:2366. doi: 10.1523/JNEUROSCI.3828-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozaki S. Snider W.D. Initial trajectories of sensory axons toward laminar targets in the developing mouse spinal cord. J Comp Neurol. 1997;380:215. [PubMed] [Google Scholar]
- 24.Paszek M.J. Zahir N. Johnson K.R. Lakins J.N. Rozenberg G.I. Gefen A. Reinhart-King C.A. Margulies S.S. Dembo M. Boettiger D. Hammer D.A. Weaver V.M. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Balgude A.P. Yu X. Szymanski A. Bellamkonda R.V. Agarose gel stiffness determines rate of DRG neurite extension in 3D cultures. Biomaterials. 2001;22:1077. doi: 10.1016/s0142-9612(00)00350-1. [DOI] [PubMed] [Google Scholar]
- 26.Willits R.K. Skornia S.L. Effect of collagen gel stiffness on neurite extension. J Biomater Sci Polym Ed. 2004;15:1521. doi: 10.1163/1568562042459698. [DOI] [PubMed] [Google Scholar]
- 27.Gunn J.W. Turner S.D. Mann B.K. Adhesive and mechanical properties of hydrogels influence neurite extension. J Biomed Mater Res A. 2005;72:91. doi: 10.1002/jbm.a.30203. [DOI] [PubMed] [Google Scholar]
- 28.Solon J. Levental I. Sengupta K. Georges P.C. Janmey P.A. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys J. 2007;93:4453. doi: 10.1529/biophysj.106.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yasuda T. Sobue G. Ito T. Mitsuma T. Takahashi A. Nerve growth factor enhances neurite arborization of adult sensory neurons; a study in single-cell culture. Brain Res. 1990;524:54. doi: 10.1016/0006-8993(90)90491-s. [DOI] [PubMed] [Google Scholar]
- 30.Tucker B.A. Rahimtula M. Mearow K.M. Integrin activation and neurotrophin signaling cooperate to enhance neurite outgrowth in sensory neurons. J Comp Neurol. 2005;486:267. doi: 10.1002/cne.20518. [DOI] [PubMed] [Google Scholar]
- 31.Friedl P. Zanker K.S. Brocker E.B. Cell migration strategies in 3-D extracellular matrix: differences in morphology, cell matrix interactions, and integrin function. Microsc Res Tech. 1998;43:369. doi: 10.1002/(SICI)1097-0029(19981201)43:5<369::AID-JEMT3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 32.Berg J.S. Farel P.B. Developmental regulation of sensory neuron number and limb innervation in the mouse. Brain Res Dev Brain Res. 2000;125:21. doi: 10.1016/s0165-3806(00)00124-3. [DOI] [PubMed] [Google Scholar]
- 33.Herbert C.B. Nagaswami C. Bittner G.D. Hubbell J.A. Weisel J.W. Effects of fibrin micromorphology on neurite growth from dorsal root ganglia cultured in three-dimensional fibrin gels. J Biomed Mater Res. 1998;40:551. doi: 10.1002/(sici)1097-4636(19980615)40:4<551::aid-jbm6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka M. Kanai H. Hirai S. Effects of nerve growth factor on cultured adult dorsal root ganglion neurons evaluated by enzyme immunoassay for neurofilament protein. Age. 1990;13:57. [Google Scholar]
- 35.Cukierman E. Pankov R. Yamada K.M. Cell interactions with three-dimensional matrices. Curr Opin Cell Biol. 2002;14:633. doi: 10.1016/s0955-0674(02)00364-2. [DOI] [PubMed] [Google Scholar]
- 36.Patel T.D. Jackman A. Rice F.L. Kucera J. Snider W.D. Development of sensory neurons in the absence of NGF/TrkA signaling in vivo. Neuron. 2000;25:345. doi: 10.1016/s0896-6273(00)80899-5. [DOI] [PubMed] [Google Scholar]
- 37.Malgrange B. Delree P. Rigo J.M. Baron H. Moonen G. Image analysis of neuritic regeneration by adult rat dorsal root ganglion neurons in culture: quantification of the neurotoxicity of anticancer agents and of its prevention by nerve growth factor or basic fibroblast growth factor but not brain-derived neurotrophic factor or neurotrophin-3. J Neurosci Methods. 1994;53:111. doi: 10.1016/0165-0270(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 38.Calvet M.C. Calvet J. Teilhac J.R. Drian M.J. Networks formed by dorsal root ganglion neurites within spinal cord explants: a computer-aided analysis of HRP intracellularly labeled neurons. Brain Res. 1992;584:1. doi: 10.1016/0006-8993(92)90871-6. [DOI] [PubMed] [Google Scholar]
- 39.Lerman O. Ben-Zvi A. Yagil Z. Behar O. Semaphorin3A accelerates neuronal polarity in vitro and in its absence the orientation of DRG neuronal polarity in vivo is distorted. Mol Cell Neurosci. 2007;36:222. doi: 10.1016/j.mcn.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Luscher C. Streit J. Quadroni R. Luscher H.R. Action potential propagation through embryonic dorsal root ganglion cells in culture. I. Influence of the cell morphology on propagation properties. J Neurophysiol. 1994;72:622. doi: 10.1152/jn.1994.72.2.622. [DOI] [PubMed] [Google Scholar]
- 41.Engler A.J. Sen S. Sweeney H.L. Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 42.Leach J.B. Brown X.Q. Jacot J.G. Dimilla P.A. Wong J.Y. Neurite outgrowth and branching of PC12 cells on very soft substrates sharply decreases below a threshold of substrate rigidity. J Neural Eng. 2007;4:26. doi: 10.1088/1741-2560/4/2/003. [DOI] [PubMed] [Google Scholar]
- 43.Zustiak S.P. Leach J.B. Hydrolytically degradable poly(ethylene glycol) hydrogel scaffolds with tunable degradation and mechanical properties. Biomacromolecules. 2010;11:1348. doi: 10.1021/bm100137q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong Po Foo C.T. Lee J.S. Mulyasasmita W. Parisi-Amon A. Heilshorn S.C. Two-component protein-engineered physical hydrogels for cell encapsulation. Proc Natl Acad Sci U S A. 2009;106:22067. doi: 10.1073/pnas.0904851106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung J.P. Moyano J.V. Collier J.H. Multifactorial optimization of endothelial cell growth using modular synthetic extracellular matrices. Integr Biol (Camb) 2011;3:185. doi: 10.1039/c0ib00112k. [DOI] [PMC free article] [PubMed] [Google Scholar]