Abstract
Extracellular matrix (ECM) secreted from the resident cell of tissue is an ideal biomaterial evolved by nature. Cartilage is also built from well-organized ECM components in a gel-like structure with a high collagen and proteoglycan content. Here, we explored cartilage tissue engineering using ECM scaffolds seeded with stem cells. Both scaffolds and stem cells were isolated from human adipose tissue, which is abundant and easily harvested in the human body. The human ECM scaffolds contained various endogenous bioactive factors, including transforming growth factor-beta1 (TGF-β1, 8782±4989 pg/g, dry ECM), insulin growth factor-1 (13319±1388 pg/g, dry ECM), basic fibroblast growth factor (82373±9572 pg/g, dry ECM), and vascular endothelial growth factor (25647±2749 pg/g, dry ECM). A composite of ECM and stem cells was prepared and cultured in chondrogenic medium (with 10 ng/mL TGF-β1 or not) for 45 days. The volumes and weights of the composites increased during culture and the surface gradually became smooth. Cell viability remained high throughout the 45 days of in vitro culture. Composites showed the formation of cartilage-like tissue with the synthesis of cartilage-specific proteins such as collagen and glycosaminoglycan. Important chondrogenic markers were expressed including Sox-9, aggrecan, and collagen type II and XI. These results demonstrate that a cell/ECM composite containing endogenous bioactive factors could provide biochemical cues for the promotion of cartilage formation.
Introduction
Cartilage is a highly specialized tissue comprising a network of extracellular matrix (ECM) components, which is organized in a gel-like structure with a high water content.1,2 Cartilage is avascular and displays a very poor capacity for self-repair; hence, even small defects may require surgery.3 Tissue engineering approaches using autologous cells and various scaffolds are becoming a promising alternative to conventional surgery. Many scaffolds fabricated from natural or synthetic biomaterials have been proposed for cartilage tissue engineering. Choosing a proper biomaterial is important because the behaviors and fates of cells are strongly influenced by the structure and component of the biomaterial.4
Tissue-derived biomaterials are functionally superior to synthetic polymers. They provide ECM and endogenous factors that promote adhesion, growth, and differentiation of various cell types.5 Many tissue-derived biomaterials have been successfully used in tissue engineering,6–8 and several decellularized tissues, including human dermis, porcine small intestinal submucosa, and porcine urinary bladder, have received approval for use in humans.9 Decellularized calf and human cadaver cartilage-derived matrices have been studied as scaffolds for cartilage regeneration.10,11
Human adipose-derived ECM is a highly attractive tissue-derived biomaterial. Adipose tissue is abundant, easily harvested, and manipulated, and contains numerous ECM components and endogenous factors named “adipokines.” In addition, adipose tissue contains stem cells that have the ability to differentiate into multiple lineages.12–14 Adipose-derived stem cells (ASCs) have significant potential for chondrogenic differentiation when cultured in medium containing growth factors such as transforming growth factor-beta1 (TGF-β1), insulin growth factor-1 (IGF-1), bone morphogenic growth factor-6 (BMP-6), and basic fibroblast growth factor (bFGF) via pellet culture or three-dimensional culture using scaffolds.15–17
Previously, we developed human adipose-derived ECM scaffolds fabricated into different shapes such as sponges, sheets, cubes, and powders.18–20 Human adipose-derived ECM scaffolds are mainly composed of collagen, elastin, glycosaminoglycan (GAG), and well-preserved adipokines. Human ECM (hECM) scaffolds seeded with human ASCs (hASCs) were applied to adipose tissue engineering. hECM scaffolds showed good biocompatibility for cell adhesion and proliferation in vitro, and supported new adipose tissue formation in vivo. In this study, we hypothesized that even though hECM scaffolds are derived from adipose tissue, they might support formations of tissue other than adipose, such as cartilage. Bioactive factors in the hECM scaffolds such as TGF-β1, IGF-1, bFGF, and vascular endothelial growth factor (VEGF) were quantified to assess the potential for inducing the chondrogenesis of hASCs, and then hECM/hASC composites of high-cell density were prepared and cultured in vitro for 45 days. We expected that hECM gel-like scaffolds would provide not only structural guidance for cell adhesion and growth, but that they would also function in cartilage tissue engineering.
Materials and Methods
Preparation of ECM gel-like scaffolds from human adipose tissue
hECM was prepared from adipose tissue as described previously.18–20 Briefly, human adipose tissue was obtained with informed consent from healthy female donors between 20 and 40 years of age who had undergone liposuction using a single combined machine (Lipokit; Medikan Inc., Seoul, Korea) at the Kangnam Plastic Surgery Clinic (Seoul, Korea). Adipose tissue was washed three times with distilled water to remove blood components, and the tissue/water mixture was homogenized at 12,000 rpm for 5 min using a homogenizer (T 18 basic ULTRA-TURAX, IKA®-Werke GmbH & Co. KG, Staufen, Germany). The tissue suspension was centrifuged at 1800 g for 5 min, and the upper layer containing oil components was discarded. This process was repeated several times. The viscous suspension (hECM) was treated with a buffered 1 M sodium chloride (NaCl, diluted 1:1) for 2 h at 37°C and subsequently incubated in buffered 0.5% sodium dodecyl sulfate (Sigma, St. Louis, MO) for 1 h at room temperature in a shaking water bath (Personal-11EX; TAITEC, Tokyo, Japan). hECM was treated with a mixture of 0.2% DNase (2000 U; Sigma) and 200 μg/mL RNase (Sigma) for 1 h at 37°C. Before proceeding to each subsequent step, products were centrifuged and thoroughly washed with distilled water for removal of residual reagents. The final hECM gel-like scaffold was sterilized with ethanol and stored in sterile phosphate-buffered saline (PBS) at 4°C until further use.
Extraction and identification of growth factors
Growth factors were extracted from the decellularized hECM using three different types of extraction reagents (2 M NaCl, 2 M urea, and 0.5 M acetic acid). The 25 mg ECM was suspended in 1 mL of 2.5% (w/v) basal buffer (50 mM Tri-HCl, pH 7.4 and 0.1× protease inhibitor) containing one of three extraction reagents and stirred at 4°C for 3 days. The mixture was centrifuged at 12,000 g for 30 min at 4°C to remove insoluble materials, and the supernatant was dialyzed extensively in dialysis tubing (MWCO12000-14000; Fisher Scientific, Pittsburgh, PA) against 30 volumes of PBS at 4°C for 2 days. The dialysate was centrifuged at 12,000 g for 30 min at 4°C, and the supernatant was used immediately or lyophilized for long-term storage. The protein concentrations extracted in the different buffers were measured using a bicinchoninic acid (BCA) assay (Pierce, Rockford, IL). Protein samples (50 μL) were added to 200 μL BCA reagent in a microplate reader, and bovine serum albumin (BSA) served as a standard. Growth factors in extractions were quantified via enzyme-linked immunosorbent assay (ELISA) for TGF-β1, IGF-1, nerve growth factor (NGF), bFGF, and VEGF according to the manufacturer's protocol (KOMA BIOTECH, Inc., Seoul, Korea). Optical density was measured at 450 nm using a microplate spectrophotometer (PowerWave XS; Bio-Tek Instruments, Winooski, VT).
Isolation, culture, and characterization of hASC
According to a modified procedure,21 adipose tissue was washed with PBS containing 5% penicillin/streptomycin (P/S) (Gibco-BRL, Life Technologies Inc., Carlsbad, CA). After removing red blood cells, tissue was digested in PBS supplemented with 0.01% (w/v) collagenase type II (Gibco-BRL) for 1 h at 37°C. Digested tissue was filtered through a 100-μm mesh to remove aggregated tissue and debris. The filtered suspension was centrifuged at 200 g for 7 min and the resulting stromal vascular fraction (SVF) pellet was washed several times in PBS. SVF cells were incubated in Dulbecco's modified Eagle's medium (DMEM; Gibco-BRL) supplemented with 10% fetal bovine serum (FBS; Gibco-BRL) and 1% P/S at 37°C under 5% CO2. Cells were maintained until passage 6. For flow cytometry analysis, cell samples were washed in cold PBS, re-suspended at 1×105 cells/mL, and incubated at 4°C for 1 h with fluorochrome-conjugated antibodies against surface markers or an isotype control. Antibodies were purchased from BioLegend (San Diego, CA): APC anti-human CD29 (Catalog No. 303007), fluorescein isothiocyanate (FITC) anti-human CD34 (Catalog No. 316405), and FITC anti-mouse/human CD44 (Catalog No. 103021). Cells were washed, re-suspended in PBS, and analyzed with a FACSCalibur™ flow cytometer (BD Biosciences, San Jose, CA).
Chondrogenic differentiation of hASCs in high-density pellet cultures
For chondrogenic differentiation, a pellet culture system was used.22 A total of 2×105 cells/0.5 mL hASCs (passage 6) were centrifuged at 500 g for 10 min in a 15-mL polypropylene conical tube, and cultured in chondrogenic medium: DMEM-high glucose (Gibco-BRL), 5% FBS (Gibco-BRL), 1% P/S (Gibco-BRL), 50 μg/mL ascorbate-2-phosphate (Sigma), 0.1 μM dexamethasone (Sigma), and 1% ITS+1 Premix (Sigma; 6.25 μg/mL insulin, 6.25 μg/mL transferrin, 6.25 ng/mL selenious acid, 1.25 μg/mL BSA, and 5.35 mg/mL linoleic acid). Pellets were cultured in two experimental groups: with or without 10 ng/mL recombinant human TGF-β1 (Peprotech, Inc., Recky Hill, NJ). The cell pellet was incubated at 37°C under 5% CO2 for 45 days. The media was changed every 3 days.
Cell seeding and culture with hECM scaffolds
Decellularized hECM (40 mg/ECM, wet weight) was washed several times in PBS and soaked in chondrogenic medium. The hECM was transferred to a 15-mL polypropylene conical tube, mixed with 500 μL cell suspension containing 2×105 hASCs (passage 6) in chondrogenic medium, and incubated for 30 min. The hASC/hECM mixture was centrifuged at 500 g for 10 min to prepare a high cell-density hASC/hECM composite. After centrifugation, the medium was removed, and 1 mL chondrogenic medium was added to each tube. The hASC/hECM composites were cultured in two experimental groups: with or without 10 ng/mL recombinant human TGF-β1. The hASC/hECM composites were incubated at 37°C under 5% CO2 for 45 days. The media were changed every 3 days.
Scanning electron microscopy
The inner structure of the hASC/hECM composite was observed using a scanning electron microscope (SEM; Hitachi S-4800 FE-SEM, Tokyo, Japan). All samples were washed with PBS, fixed in 2.5% glutaraldehyde, dehydrated through a graded ethanol series, frozen at −70°C, and freeze-dried. Samples were fixed to metal stubs and coated with platinum by a sputter at an accelerating voltage of 15 kV.
Cell viability assay
Samples were stained using a commercial Live/Dead® Viability/Cytotoxicity kit (Molecular Probes, Eugene, OR). Samples were cross-sectioned, washed in PBS, and incubated in PBS containing calcein AM and ethidium homodimer for 30 min at 37°C. After staining, samples were observed using a fluorescence microscope (IX81; Olympus Corporation, Tokyo, Japan) equipped with a digital camera.
DNA and protein quantification
The hASC/hECM composites were washed with PBS and digested with 0.1 M phosphate buffer (pH 6.8) containing 125 μg/mL papain (Sigma), 10 mM cystein hydrochloride (Sigma), and 2 mM EDTA (Sigma) at 60°C for 24 h. DNA in hASC/hECM composites was quantified fluorometrically using Hoechst Dye 33258 solutions (Bio-Rad Laboratories, Inc., Hercules, CA) with purified calf thymus DNA as a standard (Bio-Rad Laboratories, Inc.). Briefly, the digested solutions (10 μL) were incubated with 0.1 μg/mL Hoechst 33258 solutions for 10 min at room temperature in a 96-well black plate. Fluorescence was determined using a Synergy™ HT Multi-Mode Microplate Reader (BioTek instruments, Winooski, VT) at 360 nm excitation and 460 nm emission.
Total protein was determined using a BCA protein assay as above. Digested solutions were incubated with BCA reagents for 30 min at 60°C. Absorbance was measured at 562 nm in a microplate reader (PowerWave XS). Protein concentration in each sample was normalized to the DNA content in μg/μg.
Biochemical analyses
Biochemical assays were performed for the quantification of acid/pepsin-soluble collagen and sulfated glycosaminoglycan (sGAG) in hASC/hECM composites. All contents were normalized to the DNA content in μg. Collagen type I (rat tail) and chondroitin 4-sulfate (bovine trachea) were used as standards.
Acid/pepsin-soluble collagen content was measured using a Sircol soluble collagen assay kit (Biocolor Ltd., Carrickfergus, Northern Ireland). For extraction of acid/pepsin-soluble collagen, samples were digested with 0.5 M acetic acid containing 1% (w/v) pepsin (P7012; Sigma) at room temperature for 24 h. The digested suspension was centrifuged at 10,000 g for 10 min. The supernatant was collected and incubated with 1 mL Sircol dye reagent for 30 min at room temperature. The collagen-dye complex was precipitated via centrifugation at 10,000 g for 10 min and the supernatant was removed. Pellets were dissolved in 1 mL alkali reagent, and relative absorbance was measured in a 96-well plate at 540 nm using a microplate reader.
Sulfated GAG content was measured using a Blyscan sulfated GAG assay kit (Biocolor Ltd., Carrickfergus, Northern Ireland). Samples were digested with 0.1 M phosphate buffer (pH 6.8) containing 125 μg/mL papain (Sigma), 10 mM cystein hydrochloride (Sigma), and 2 mM EDTA (Sigma) at 60°C for 48 h. The suspension was centrifuged at 15,000 g for 30 min. The supernatant was mixed with 1 mL Blyscan dye and shaken for 30 min. The precipitate was collected via centrifugation for 10 min and then dissolved in 1 mL dissociation reagent. Absorbance was measured in a 96-well plate at 656 nm using a microplate reader.
Histological and immunofluorescence staining
Samples were fixed in 4% paraformaldehyde, embedded in paraffin, and sliced at 6 to 10 μm using a microtome. Sections were deparaffinized, dehydrated, and stained with hematoxylin and eosin (H&E) (Sigma) for normal histological evaluation. For detection of cartilage formation, Alcian blue and Safranin-O stainings were performed according to standardized protocols. Sections were stained with 1% Alcian blue 8GX (Sigma), pH 1.0 for 15 min and counterstained with neutral red (Sigma) to detect proteoglycan. For detection of cartilage matrix, sections were stained with Weigert's iron hematoxylin for 10 min and washed in tap water for 5 min. Subsequently, sections were stained with 0.001% fast green (Sigma) for 5 min and 0.1% Safranin-O (Sigma) for 5 min. Stained sections were rinsed in absolute alcohol, cleared in xylene, and mounted in Permount® (Fisher Scientific, Fair Lawn, NJ).
For detection of type II collagen, sections were incubated with a 10% (w/v) BSA blocking agent for 30 min to inhibit nonspecific binding of IgG, followed by incubation in collagen type II (Santa Cruz Biotechnology, INC., Santa Cruz, CA) primary monoclonal antibody for 2 h at room temperature, and washed three times in PBS. Sections were incubated with FITC-conjugated secondary antibody (Santa Cruz Biotechnology) for 1 h at room temperature in the dark and were washed three times in PBS. Sections were counterstained with 1 μg/mL DAPI for 1 min and then observed with a fluorescence microscope (IX81; Olympus Corporation).
Reverse-transcription polymerase chain reaction analysis
Total RNA was isolated from cells using an easy-BLUE™ total RNA extraction kit (iNtRON Biotechnology, Seongnam, Kyunggi, Korea). RNA concentration was quantified using a Nanodrop® ND-1000 spectrophotometer (Nanodrop Technologies, Inc., Wilmington, DE). Extracted RNA was reverse-transcribed using a first-strand cDNA synthesis system with avian myeloblastosis virus reverse transcriptase for polymerase chain reaction (PCR) (iNtRON Biotechnology) and the cDNA was used as a template for PCR analysis using primers described in Table 1. Conventional PCR was performed using a TaKaRa PCR Thermal Cycler Dice™ (TaKaRa Bio, Inc., Shiga, Japan) instrument using the reaction profile: initial denaturation at 95°C for 5 min and 30 to 35 cycles of denaturation at 95°C for 1 min, annealing at 55 to 65°C for 1 min, polymerization at 72°C for 1 min, and extension at 72°C for 7 min. As an internal control, expression of the housekeeping gene GAPDH was assessed. PCR products were electrophoresed in 1.5% agarose gels and analyzed using a gel imaging system (Gel Doc XR; Bio-Rad Laboratories, Inc.).
Table 1.
Polymerase Chain Reaction Primer Sequences for Analyzing Chondrogenic Genes
| Gene | Forward and reverse primer sequences | Annealing temperature | Product size (base pair) | Gene bank accession no. |
|---|---|---|---|---|
| Housekeeping gene | ||||
| GAPDH | 5′-GGG CTG CTT TTA ACT CTG GT-3′ | 56°C | 702 bp | NM002046 |
| 5′-GCA GGT TTT TCT AGA CGG-3′ | ||||
| Chondrogenic gene | ||||
| Sox9 | 5′-ATC TGA AGA AGG AGA GCG A-3′ | 58°C | 264 bp | NM000346 |
| 5′-TCA GAA GTC TCC AGA GCT TG-3′ | ||||
| AGN | 5′-TGA GGA GGG CTG GAA CAA GTA CC-3′ | 64°C | 350 bp | NM013227 |
| 5′-GGA GGT GGT AAT TGC AGG GAA CA-3′ | ||||
| Col II α1 | 5′-CAG GTC AAG ATG GTC-3′ | 52°C | 370 bp | NM001844 |
| 5′-TGC AGC ACC TGT CTC ACC A-3′ | ||||
| Col X α1 | 5′-CAC CAG GCA TTC CAG GAT TCC-3′ | 60°C | 825 bp | NM 000493 |
| 5′-AGG TTT GTT GGT CTG ATA GCT C-3′ | ||||
| Col XI α1 | 5′-GGA AAG GAC GAA GTT GGT CTG C-3′ | 62°C | 584 bp | NM001168249 |
| 5′-TTC TTC TCC ACG CTG ATT GCT ACC C-3′ | ||||
GAPDH, glyceraldehyde 3-phosphate dehydrogenase; Sox9, transcription factor; AGN, aggrecan; Col II α1, collagen type II alpha 1; Col X α1, collagen type X alpha 1; Col XI α1, collagen type XI alpha 1.
Statistical analysis
Experimental data were expressed as means±standard deviation (SD). Student's two-tailed t-test with SPSS 17.0 statistical software (SPSS, Chicago, IL) was used for comparison, and statistical significance was accepted at p<0.05 or p<0.01.
Results
Preparation and characterization of hECM gel-like scaffolds
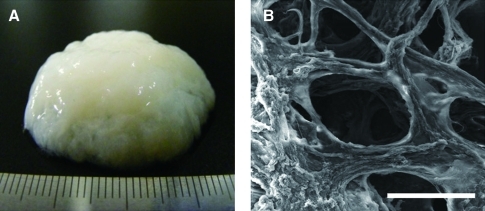
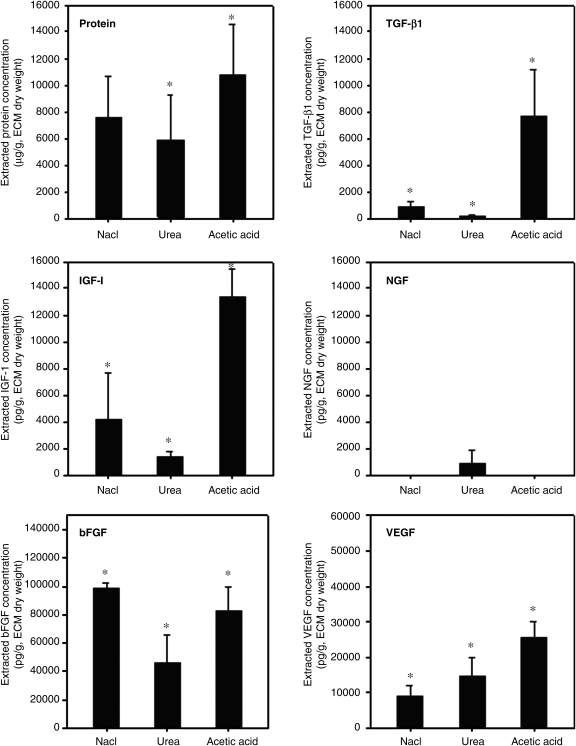
hECM was extracted from adipose tissue as described previously.18,19 Cellular components were removed through successive physical, chemical, and enzymatic treatments. The extracted hECM had a whitish gel-like appearance with bundles of ECM fibers (Fig. 1A). An SEM image shows an inner microstructure with a network of ECM fibers in a hECM scaffold (Fig. 1B). In our previous studies, we demonstrated that the hECM scaffold consisted mostly of collagen and elastin, and contained various adipokines. In this study, to assess the bioactive properties, several bioactive factors were extracted and identified. Depending on the extraction buffer, the quantity of the bioactive factors varied, as shown in Figure 2. The results of ELISA demonstrated that the mixture extracted with the acetic acid extraction buffer system had high concentrations of total protein and bioactive factors including TGF-β1 (8782±4989 pg/g), IGF-1 (13319±1388 pg/g), bFGF (82373±9572 pg/g), and VEGF (25647±2749 pg/g) (p<0.01). The small amount of NGF was detected in the urea-based extraction system but not in the NaCl- or acetic acid-based extraction system. The results imply that the hECM gel-like scaffolds containing ECM components and endogenous bioactive factors could provide biochemical signals for chondrogenesis of hASCs and, thus might be favorable for cartilage regeneration.
FIG. 1.
Macroscopic (A) and scanning electron microscopy (B) images of an hECM gel-like scaffold. Scale bars represent 100 μm. hECM, human extracellular matrix. Color images available online at www.liebertonline.com/tea
FIG. 2.
Quantification of growth factors extracted from hECM scaffolds. Three different types of extraction buffers (containing either 2 M sodium chloride, 2 M urea, or 0.5 M acetic acid) were used. Data are shown as means±standard deviations with significance at *p<0.01.
Characterization of hASCs
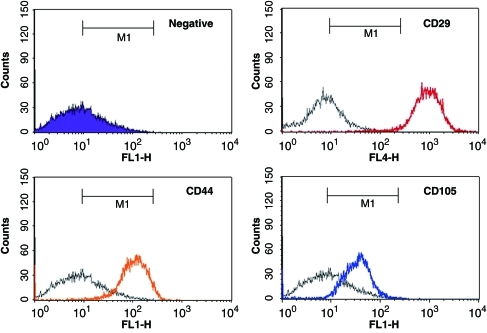
hASCs were isolated from a portion of adipose tissue via an enzymatic digestion method using collagenase, and were expanded until passage 6. Representative flow histograms for the hASC are shown in Figure 3. The hASCs expressed high levels of mesenchymal stem cell-related surface markers such as CD29, CD44, and CD105, suggesting that the isolated hASCs had the capacity of stem cells under controlled conditions in vitro.
FIG. 3.
Flow cytometry histograms for mesenchymal stem cells markers (CD29, CD44, and CD105) were displayed on hASCs at passage 6. Each color line (red, orange, blue) indicates positive staining cells, whereas the purple area and black line indicate the isotype-matched monoclonal antibody control. hASC, human adipose-derived stem cell. Color images available online at www.liebertonline.com/tea
Cell culture with hECM scaffolds
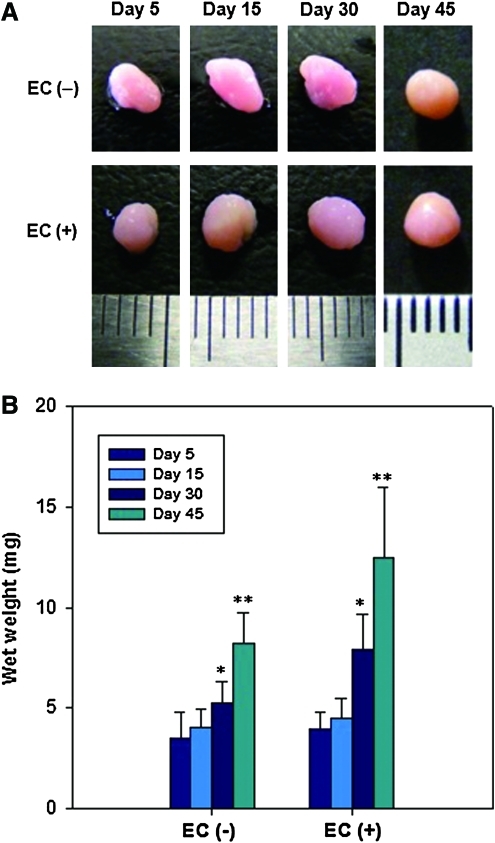
The hASC/hECM composites were cultured in chondrogenic medium for 45 days with addition of TGF-β1 (EC [+]) or without TGF-β1 (EC [−]). The composites matured over time and formed a spherical cartilage-like tissue, appearing the pinkish-orange color (Fig. 4A). The volumes of the composites increased with culture time and the surfaces smoothed gradually. Figure 4B shows that the wet weights of the composites continuously increased during culture in both EC (−) and EC (+). The composite wet weights of the two groups were not much different for the first 15 days, but were significantly different at 30 and 45 days (p<0.05).
FIG. 4.
(A) Gross morphologies and (B) wet weights of hASC/hECM composites on day 5, 15, 30, and 45. Scales of a ruler represent 1 mm. EC (+), with addition of 10 ng/mL TGF-β1; EC (−), without TGF-β1. Data are shown as means±standard deviations (n=5) with significance at *,**p<0.05. TGF-β1, transforming growth factor beta1. Color images available online at www.liebertonline.com/tea
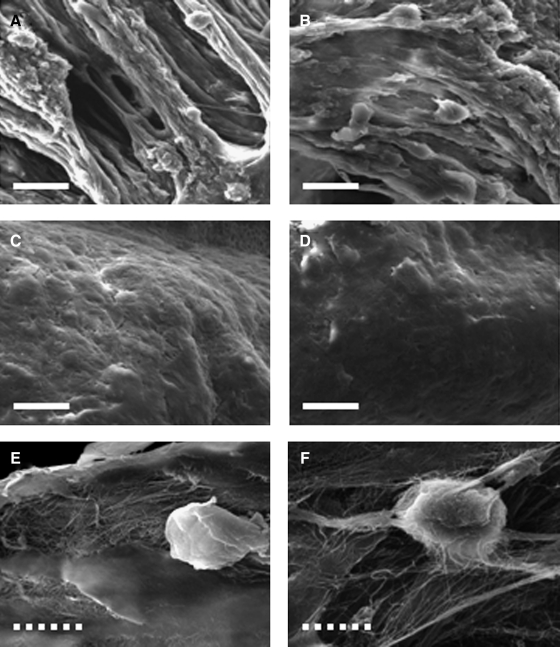
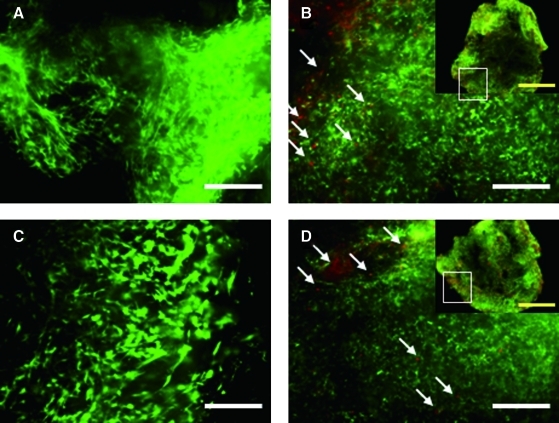
On day 1, SEM images of both composites show that many hASCs were attached and well-spread on the fibrous hECM scaffold (Fig. 5A, B). On day 45, cells and hECM scaffolds seemed to be well integrated (Fig. 5C, D) on the surface of the composite and ECM secreted by hASC was observed (Fig. 5E, F). The hASCs cultured with hECM scaffolds showed good viability and distribution, as confirmed through a live/dead assay. On day 1, most hASCs in both composites were alive (green) and spindle-shaped cells were homogeneously distributed (Fig. 6A, C). On day 45, most cells had a round morphology and showed good viability, although some dead cells (red color) were observed in both composites (Fig. 6B, D).
FIG. 5.
Scanning electron microscopy micrographs of hASC/hECM composites on day 1 (A, B) and day 45 (C–F). Composites were maintained in chondrogenic medium without (A, C, E) or with TGF-β1 (B, D, F). Scale bars represent 20 μm (white bold line) and 10 μm (white dotted line).
FIG. 6.
Fluorescence micrographs of hASC/hECM composites on day 1 (A, C) and day 45 (B, D). Composites were maintained in chondrogenic medium without (A, B) or with TGF-β1 (C, D). Cells were stained by calcein-AM (green=live) and ethidium homodimer (red=dead). The white arrows indicate dead cells. Scale bars represent 200 μm (white bold line) and 1 mm (yellow). Color images available online at www.liebertonline.com/tea
Biochemical analyses
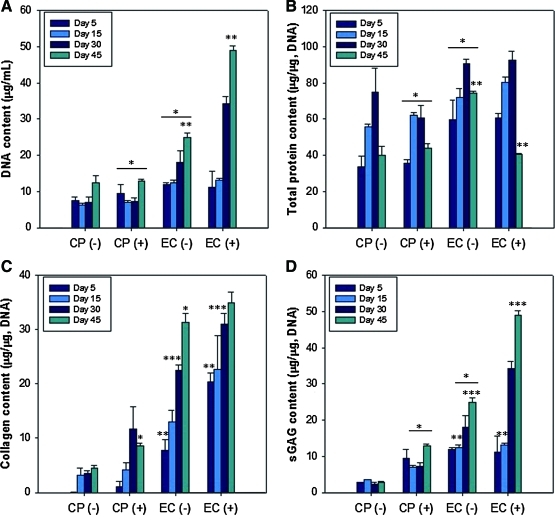
The DNA contents of hASC/hECM composites (EC [+] and EC [−]) were compared to those of conventional cell pellet culture with addition of TGF-β1 (CP [+]) or without (CP [−]) for 45 days (Fig. 7A). No significant changes with culture time were found in the two cell pellet culture systems (CP [+] and CP [−]), while the DNA contents of hASC/hECM composites in both EC (+) and EC (−) increased significantly (p<0.01). As indications of cartilage-specific matrix synthesis, the protein, collagen, and sGAG contents were quantified (Fig. 7B–D). All biochemical analyses were normalized to DNA content, and hECM scaffolds without cells were used as a negative control. In all groups, total protein content normalized to DNA content increased up to 30 days, and decreased at 45 days (Fig. 7B). The collagen content gradually increased with time in all groups (Fig. 7C). On day 45, the collagen content was 8.61±0.54 μg/μg in CP (+), 31.33±1.62 μg/μg in EC (−), and 34.97±1.89 μg/μg in EC (+). It is notable that there is a significant difference between CP (+) and EC (−) (p<0.01). The sGAG content normalized to DNA content significantly increased with time in EC (−) and EC (+) (Fig. 7D). The sGAG content was 7.37±0.41 μg/μg in CP (+), 10.45±0.27 μg/μg in EC (−), and 13.14±0.22 μg/μg in EC (+) on day 45, respectively. Even without additional TGF-β1, the hASC/hECM composites showed much higher sGAG contents at all time points than did the conventional cell pellet culture with additional TGF-β1.
FIG. 7.
DNA (A), protein (B), total collagen (C), and sGAG (D) contents in cell pellets (CP) and hASC/hECM composites (EC). CP (+) and EC (+), with addition of 10 ng/mL TGF-β1. CP (−) and EC (−), without TGF-β1. All contents were normalized to DNA content in each condition. Data are shown as means±standard deviations with significance at *,**,***p<0.01 between the groups. sGAG, sulfated glycosaminoglycan. Color images available online at www.liebertonline.com/tea
Histological and immunofluorescence staining
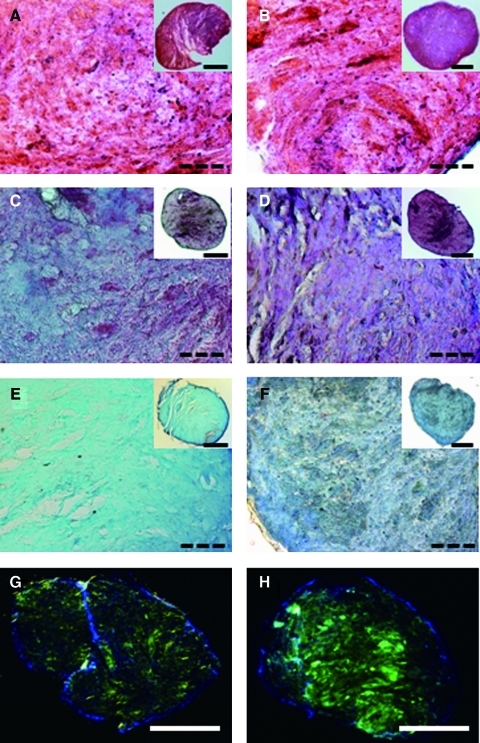
After 45 days of culture, H&E, Safranin-O, and Alcian blue staining were used to assess histological appearance and proteoglycan accumulation (Fig. 8). H&E staining showed that the hASCs were uniformly distributed in EC (−) and EC (+), where the black spots were the cell nuclei (Fig. 8A, B). The cartilage-specific sGAG stained positive in both groups by Safranin-O (Fig. 8C, D) and Alcian blue (Fig. 8E, F). Positive staining for Safranin-O (dark purple) was partially observed in EC (−), whereas strong positive staining was observed throughout EC (+). The sGAG staining due to Alcian blue was distributed homogeneously in both composites, but the intensity was higher in EC (+) than it was in EC (−). Collagen type II is the main ECM protein in cartilage composition. On day 45, strong positive collagen type II staining was observed in both composites. Notably, although the intensity in EC (−) was slightly weaker than that in EC (+), a homogeneous distribution of type II collagen was found in EC (−). In addition, a few sporadic lacuna-like structures were observed in both composites after 45 days of in vitro culture.
FIG. 8.
Histological and immunofluorescence characterization of hASC/hECM composites (n=5) after 45 days of in vitro culture. (A, C, E, G) EC (−) and (B, D, F, H) EC (+). Cell nuclei (black spot) and cytoplasm (pink) were stained by hematoxylin and eosin (A, B). Sulfated proteoglycans were identified via Safranin-O (dark purple; C, D), and Alcian blue (blue; E, F) staining. Collagen type II was stained by a monoclonal collagen type II-conjugated fluorescein isothiocyanate (green). Cell nuclei in the composites were counterstained using DAPI (blue) (G, H). Scale bars represent 1 mm (bold line) and 400 μm (dotted line). Color images available online at www.liebertonline.com/tea
Chondrogenic gene expression
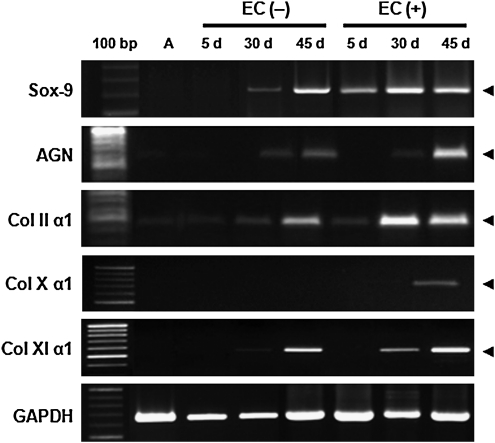
The expressions of the chondrogenic genes including Sox-9, aggrecan (AGN), and collagen type II and XI were evaluated using reverse-transcription-PCR over 45 days. Sox-9 is one of earliest markers expressed in cells undergoing precartilagous condensation. Sox-9 is also a transcriptional factor required for the expression of collagen type II and AGN, which are expressed during cell condensation until differentiation into chondrocytes.22 Sox-9 was clearly detected in most conditions. On day 45, collagen type XI was strongly expressed in both composites, collagen type II and AGN were weakly expressed in EC (−), but strongly expressed in EC (+). Collagen X, a marker of hypertrophic chondrocytes, was slightly expressed only in EC (+) on day 45.
Discussion
Biomaterials for cartilage tissue engineering should provide biochemical stimuli and a structural environment, which are important for inducing chondrogenesis and retaining the phenotypes of differentiated stem cells.23 Many synthetic or naturally derived polymers have been extensively studied in cartilage tissue engineering, such as biodegradable polyesters (e.g., poly(lactic-co-glycolic acid)), polyurethane, poly(3-hydroxybutyrate), hyaluronic acid, collagen, fibrin and gelatin.24–28 Recently, decellularized tissues have gained increasing attention for their excellent biocompatibility and bio-inductive properties.29–37 ECM containing endogenous bioactive factors, derived from a variety of intact tissues, has been applied as an allograft or xenograft material for tissue engineering, including for adipose,19 liver,38 urethral,39 heart,7 skin,40 and cartilage,8 Indeed, ECM secreted from the resident cells of tissue is an ideal biomaterial evolved by nature. ECM plays numerous critical roles in the lives of cells. ECM provides mechanical support for cells and tissues, integrate cells into tissues, influences cell shape and movement, influences mechanical and chemical signaling pathways, and coordinates the behavior of different cells in tissues.9
We successfully fabricated a composite of hECM and stem cells containing various bioactive factors. An important consideration in tissue engineering scaffold design is the provision of a framework for cellular events. The hECM scaffold exhibited viscous gel-like appearances and a fibrous microstructure (Fig. 1). From a structural viewpoint for scaffolds, a fibrous and gel-like structure is highly desirable. A porous and fibrous structure provides surfaces for cell attachment and initial biomechanical stability.41,42 In particular, hydrogels mimic the microenvironment found in cartilage.43,44 More importantly, the hECM scaffold contained bioactive factors such as TGF-β1, IGF-1, bFGF, and VEGF, which could act as major biochemical cues for chondrogenesis (Fig. 2). These growth factors are known to induce chondrogenic differentiation of stem cells and to aid in the maintenance of phenotype.45,46 In particular, major cartilage modulating growth factors are TGF-β1 and IGF-1. TGF-β1 is a potent factor in proliferation and differentiation of stem cells into chondrocytes. IGF-1 is synthesized by chondrocytes and prevents chondrocyte apoptosis. In addition, both TGF-β1 and IGF-1 is well known for the ability to stimulate production of cartilage matrix, including collagen, proteoglycan, and hyaluronan (HA).46,47 Therefore, the structural and biochemical properties of the hECM scaffolds could help enhance the adhesion, proliferation, and chondrogenic differentiation of stem cells. High-cell density hASC/hECM composites may be favorable for promoting the cell-matrix and cell-cell contacts.
Biomaterials in combination with autologous adult stem cells have been studied to regenerate defected cartilage.1 Multipotent mesenchymal stem cells are attractive because of their self-renewing potential and multipotentiality. In particular, hASCs obtained from autologous adipose tissue are useful because adipose tissue is abundant, readily accessible, and easily expandable.48 hASCs can be induced toward a chondrogenic phenotype by exogenous growth factors, including TGF-β1, IGF-1, and BMP-4.15,16 When hASCs were cultured in combination with hECM scaffolds for 45 days, the composites formed spherical cartilage-like tissue. The composite size and weight gradually increased with culture time (Fig. 4). SEM showed that the hASCs were well-attached to the fibrous ECM and formed dense aggregation after 45 days, and a live/dead assay also indicates that most cells in the composite remained viable (Figs. 5 and 6). These results suggest that the hECM scaffold provides a good microenvironment favorable to the adhesion of cells and the maintenance of viability. Moreover, the rapid increase in DNA content also indicated that the scaffold well supported the growth of hASCs (Fig. 7A). The synthesis of cartilage-specific proteins, including collagen and sGAG, as a key marker of chondrogenesis, substantially increased with time when the hASCs were cultured with the hECM scaffold in chondrogenic medium (Fig. 7C, D). Although all of the cartilage-related proteins were expressed in higher levels when TGF-β1 was added, the chondrogenesis of hASCs was successfully induced without additional TGF-β1. Therefore, our results imply that the hECM gel-like scaffold could promote the synthesis and deposition of cartilage-specific proteins in vitro, highlighting the importance of hECM scaffolds in the differentiation of hASCs toward chondrogenesis.
Chondrogenesis involves the formation of cartilage tissue through stem cell differentiation and is tightly controlled by regulation of gene expression and cell-cell or cell-ECM interactions. Condensation and proliferation of stem cells is one of the earliest events in chondrogenesis, and condensed stem cells begin up-regulating the transcription factor Sox-9 and producing cartilage-specific ECM proteins including AGN, proteoglycans, HA, and collagen type II, VI, IX, XI.49,50 Collagen type X is expressed later, when chondrocytes acquire the hypertrophic phenotype, form calcified cartilage, or enter endochondral ossification. There is a concern on terminal differentiation into hypertrophic chondrocytes, which may lead to inter-regional osteophytes during in vivo cartilage transplantation.3 In this study, the expressions of chondrogenic markers including Sox-9, AGN, and collagen type II, XI were promoted in hASC/hECM composites, as were increase in sGAG and collagen contents. In contrast, collagen X mRNA was slightly expressed only in hASC/hECM composites with TGF-β1 on day 45 (Fig. 9). These results confirm that the hECM scaffold derived from human adipose tissue was able to trigger and support chondrogenic differentiation of hASCs.
FIG. 9.
Reverse-transcription-polymerase chain reaction analysis of gene expression for chondrogenic differentiation in hASC/hECM composites. hASC undifferentiated cells (A) were used as a negative control (lane 2). Each expressed gene was analyzed using human-origin primers designed for Sox-9, AGN, collagen type II α1 (Col IIα1), collagen type X (Col X α1), and collagen type XI α1 (Col XIα1). GAPDH was used for normalization.
Conclusions
A hECM gel-like scaffold containing endogenous bioactive factors, derived from adipose tissue, provided a highly favorable microenvironment for in vitro chondrogenic differentiation of hASCs. Throughout 45 days of in vitro culture in chondrogenic medium, the cell viability remained high. The volumes and weights of hASC/hECM composites continuously increased with culture time. The composites formed a spherical cartilage-like tissue with the synthesis of cartilage-specific proteins such as collagen and sGAG. Our findings demonstrate that even though the hECM scaffolds were derived from adipose tissue, they could serve as an efficient biomaterial for tissue engineering in addition to adipose tissue.
Acknowledgments
This work was supported by the Basic Science Research Program (Grant No. 2009-0075546) and the Engineering Research Center Program (Grant No. R11-2008-044-02001-0) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology. This work was also supported by the Human Resources Development of the Korea Institute of Energy Technology Evaluation and Planning (Grant No. 20104010100620) grant funded by the Ministry of Knowledge Economy, Republic of Korea.
Disclosure Statement
No competing financial interests exist.
References
- 1.Chen F.H. Rousche K.T. Tuan R.S. Technology insight: adult stem cells in cartilage regeneration and tissue engineering. Nat Clin Pract Rheumatol. 2006;2:373. doi: 10.1038/ncprheum0216. [DOI] [PubMed] [Google Scholar]
- 2.Ateshian G.A. Artificial cartilage: weaving in three dimensions. Nat Mater. 2007;6:89. doi: 10.1038/nmat1830. [DOI] [PubMed] [Google Scholar]
- 3.Hunziker E.B. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 4.Adams J.C. Watt F.M. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117:1183. doi: 10.1242/dev.117.4.1183. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert T.W. Sellaro T.L. Badylak S.F. Decellularization of tissues and organs. Biomaterials. 2006;27:3675. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Flynn L. Prestwich G.D. Semple J.L. Woodhouse K.A. Adipose tissue engineering with naturally derived scaffolds and adipose-derived stem cells. Biomaterials. 2007;28:3834. doi: 10.1016/j.biomaterials.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Ott H.C. Matthiesen T.S. Goh S.K. Black L.D. Kren S.M. Netoff T.I. Taylor D.A. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 8.Cheng N.C. Estes B.T. Awad H.A. Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells by a porous scaffold derived from native articular cartilage extracellular matrix. Tissue Eng Part A. 2009;15:231. doi: 10.1089/ten.tea.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badylak S.F. Freytes D.O. Gilbert T.W. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 2009;5:1. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Yang Q. Peng J. Guo Q. Huang J. Zhang L. Yao J. Yang F. Wang S. Xu W. Wang A. Lu S. A cartilage ECM-derived 3-D porous acellular matrix scaffold for in vivo cartilage tissue engineering with PKH26-labeled chondrogenic bone marrow-derived mesenchymal stem cells. Biomaterials. 2008;29:2378. doi: 10.1016/j.biomaterials.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 11.Elder B.D. Eleswarapu S.V. Athanasiou K.A. Extraction techniques for the decellularization of tissue engineered articular cartilage constructs. Biomaterials. 2009;30:3749. doi: 10.1016/j.biomaterials.2009.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakajima I. Yamaguchi T. Ozutsumi K. Aso H. Adipose tissue extracellular matrix: newly organized by adipocytes during differentiation. Differentiation. 1998;63:193. doi: 10.1111/j.1432-0436.1998.00193.x. [DOI] [PubMed] [Google Scholar]
- 13.Fraser J.K. Wulur I. Alfonso Z. Hedrick M.H. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Lago F. Dieguez C. Gómez-Reino J. Gualillo O. Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol. 2007;3:716. doi: 10.1038/ncprheum0674. [DOI] [PubMed] [Google Scholar]
- 15.Kim B.S. Kang K.S. Kang S.K. Soluble factors from ASCs effectively direct control of chondrogenic fate. Cell Prolif. 2010;43:249. doi: 10.1111/j.1365-2184.2010.00680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim H.J. Im G.I. Chondrogenic differentiation of adipose tissue-derived mesenchymal stem cells: greater doses of growth factors are necessary. J Orthop Res. 2009;27:612. doi: 10.1002/jor.20766. [DOI] [PubMed] [Google Scholar]
- 17.Merceron C. Vinatier C. Clouet J. Colliec-Jouault S. Weiss P. Guicheux J. Adipose-derived mesenchymal stem cells and biomaterials for cartilage tissue engineering. Joint Bone Spine. 2008;75:672. doi: 10.1016/j.jbspin.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Choi J.S. Yang H.J. Kim B.S. Kim J.D. Kim J.Y. Yoo B. Park K. Lee H.Y. Cho Y.W. Human extracellular matrix (ECM) powders for injectable cell delivery and adipose tissue engineering. J Control Release. 2009;139:2. doi: 10.1016/j.jconrel.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 19.Choi J.S. Yang H.J. Kim B.S. Kim J.D. Lee S.H. Lee E.K. Park K. Cho Y.W. Lee H.Y. Fabrication of porous extracellular matrix scaffolds from human adipose tissue. Tissue Eng Part C Methods. 2010;16:387. doi: 10.1089/ten.TEC.2009.0276. [DOI] [PubMed] [Google Scholar]
- 20.Choi J.S. Kim B.S. Kim J.Y. Kim J.D. Choi Y.C. Yang H.J. Park K. Lee H.Y. Cho Y.W. Decellularized extracellular matrix derived from human adipose tissue as a potential scaffold for allograft tissue engineering. J Biomed Mater Res A. 2011;97:292. doi: 10.1002/jbm.a.33056. [DOI] [PubMed] [Google Scholar]
- 21.Bunnell B.A. Flaat M. Gagliardi C. Patel B. Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45:115. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Estes B.T. Diekman B.O. Gimble J.M. Guilak F. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc. 2010;5:1294. doi: 10.1038/nprot.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonzani I.C. George J.H. Stevens M.M. Novel materials for bone and cartilage regeneration. Curr Opin Chem Biol. 2006;10:568. doi: 10.1016/j.cbpa.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed T.A. Giulivi A. Griffith M. Hincke M. Fibrin glues in combination with mesenchymal stem cells to develop a tissue-engineered cartilage. Tissue Eng Part A. 2011;17:323. doi: 10.1089/ten.TEA.2009.0773. [DOI] [PubMed] [Google Scholar]
- 25.Lee C.T. Huang C.P. Lee Y.D. Biomimetic porous scaffolds made from poly(L-lactide)-g-chondroitin sulfate blend with poly(L-lactide) for cartilage tissue engineering. Biomacromolecules. 2006;7:2200. doi: 10.1021/bm060451x. [DOI] [PubMed] [Google Scholar]
- 26.Kim H.J. Lee J.H. Im G.I. Chondrogenesis using mesenchymal stem cells and PCL scaffolds. J Biomed Mater Res A. 2010;92:659. doi: 10.1002/jbm.a.32414. [DOI] [PubMed] [Google Scholar]
- 27.Li Z. Kupcsik L. Yao S.J. Alini M. Stoddart M.J. Chondrogenesis of human bone marrow mesenchymal stem cells in fibrin-polyurethane composites. Tissue Eng Part A. 2009;15:1729. doi: 10.1089/ten.tea.2008.0247. [DOI] [PubMed] [Google Scholar]
- 28.Ye C. Hu P. Ma M.X. Xiang Y. Liu R.G. Shang X.W. PHB/PHBHHx scaffolds and human adipose-derived stem cells for cartialge tissue engineering. Biomaterials. 2009;30:4401. doi: 10.1016/j.biomaterials.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Dawson J.I. Wahl D.A. Lanham S.A. Kanczler J.M. Czernuszka J.T. Oreffo R.O.C. Development of specific collagen scaffolds to support the osteogenic and chondrogenic differentiation of human bone marrow stromal cells. Biomaterials. 2008;29:3105. doi: 10.1016/j.biomaterials.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 30.Haberhauer M. Zernia G. Deiwick A. Pösel C. Bader A. Huster D. Schulz R.M. Cartilage tissue engineering in plasma and whole blood scaffolds. Adv Mater. 2008;20:2061. [Google Scholar]
- 31.Wu S.C. Chang J.K. Wang C.K. Wang G.J. Ho M.L. Enhancement of chondrogenesis of human adipose derived stem cells in a hyaluronan-enriched microenvironment. Biomaterials. 2010;31:631. doi: 10.1016/j.biomaterials.2009.09.089. [DOI] [PubMed] [Google Scholar]
- 32.Fan H. Zhang C. Li J. Bi L. Qin L. Wu H. Hu Y. Gelatin microspheres containing TGF-beta3 enhance the chondrogenesis of mesenchymal stem cells in modified pellet culture. Biomacromolecules. 2008;9:927. doi: 10.1021/bm7013203. [DOI] [PubMed] [Google Scholar]
- 33.Ito H. Steplewski A. Alabyeva T. Fertala A. Testing the utility of rationally engineered recombinant collagen-like proteins for applications in tissue engineering. J Biomed Mater Res A. 2006;76:551. doi: 10.1002/jbm.a.30551. [DOI] [PubMed] [Google Scholar]
- 34.Betre H. Setton L.A. Meyer D.E. Chilkoti A. Characterization of a genetically engineered elastin-like polypeptide for cartilaginous tissue repair. Biomacromolecules. 2002;3:910. doi: 10.1021/bm0255037. [DOI] [PubMed] [Google Scholar]
- 35.Peel S.A.F. Chen H. Renlund R. Badylak S.F. Kandel R.A. Formation of a SIS-cartilage composite graft in vitro adn its use in the repair of articular cartilage defects. Tissue Eng. 1998;4:143. [Google Scholar]
- 36.Diekman B.O. Rowland C.R. Lennon D.P. Caplan A.I. Guilak F. Chondrogenesis of adult stem cells from adipose tissue and bone marrow: induction by growth factors and cartilage-derived matrix. Tissue Eng Part A. 2009;16:523. doi: 10.1089/ten.tea.2009.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin C.Z. Choi B.H. Park S.R. Min B.H. Cartilage engineering using cell-derived extracellular matrix scaffold in vitro. J Biomed Mater Res A. 2010;92:1567. doi: 10.1002/jbm.a.32419. [DOI] [PubMed] [Google Scholar]
- 38.Lin P. Chan W.C.W. Badylak S.F. Bhatia S.N. Assessing porcine liver-derived biomatrix for hepatic tissue engineering. Tissue Eng. 2004;10:1046. doi: 10.1089/ten.2004.10.1046. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y. Bharadwaj S. Lee S.J. Atala A. Zhang Y. Optimization of a natural collagen scaffold to aid cell-matrix penetration for urologic tissue engineering. Biomaterials. 2009;30:3865. doi: 10.1016/j.biomaterials.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Brown-Etris M. Cutshall W.D. Hiles M.C. A new biomaterial derived from small intestine submucosa, developed into a wound matrix device. Wounds. 2002;14:150. [Google Scholar]
- 41.Jeong C.G. Hollister S.J. A comparison of the influence of material on in vitro cartilage tissue engineering with PCL, PGS, and POC 3D scaffold architecture seeded with chondrocytes. Biomaterials. 2010;31:4304. doi: 10.1016/j.biomaterials.2010.01.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moutos F.T. Guilak F. Composite scaffold for cartilage tissue engineering. Biorheology. 2008;45:501. [PMC free article] [PubMed] [Google Scholar]
- 43.Fan J. Gong Y. Ren L. Varshney R R. Cai D. Wang D.A. In vitro engineered cartilage using synovium-derived mesenchymal stem cells with injectable gellan hydrogels. Acta Biomater. 2010;6:1178. doi: 10.1016/j.actbio.2009.08.042. [DOI] [PubMed] [Google Scholar]
- 44.Emans P.J. van Rhijn L.W. Welting T.J. Cremers A. Wijnands N. Spaapen F. Voncken J.W. Shastri V.P. Autologous engineering of cartilage. Proc Natl Acad Sci USA. 2010;107:3418. doi: 10.1073/pnas.0907774107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vinatier C. Mrugala D. Jorgensen C. Guicheux J. Noël D. Cartilage engineering: a crucial combination of cells, biomaterials and biofactors. Trends Biotechnol. 2009;27:307. doi: 10.1016/j.tibtech.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Gaissmaier C. Koh J.L. Weise K. Growth and differentiation factors for cartilage healing and repair. Injury. 2008;39:88. doi: 10.1016/j.injury.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 47.Davies L.C. Blain E.J. Gilbert S.J. Caterson B. Duance V.C. The potential of IGF-1 and TGF beta1 for promoting “adult” articular cartilage repair: an in vitro study. Tissue Eng Part A. 2008;14:1251. doi: 10.1089/ten.tea.2007.0211. [DOI] [PubMed] [Google Scholar]
- 48.Gimble J.M. Katz A.J. Bunnell B.A. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quintana L. zur Nieden N.I. Semino C.E. Morphogenetic and regulatory mechanisms during developmental chondrogenesis: new paradigms for cartilage tissue engineering. Tissue Eng Part B. 2009;15:29. doi: 10.1089/ten.teb.2008.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hardingham T. Tew S. Murdoch A. Tissue engineering: chondrocytes and cartilage. Arthritis Res. 2002;4:S63. doi: 10.1186/ar561. [DOI] [PMC free article] [PubMed] [Google Scholar]