Abstract
Aims: Vitamin K epoxide reductase complex, subunit 1 (VKORC1) is a critical participant in the production of active forms of reduced vitamin K and is required for modification of vitamin K–dependent proteins. Homologues of VKORC1 (VKORH) exist throughout evolution, but in bacteria they appear to function in oxidative protein folding as well as quinone reduction. In the current study we explore two questions: Do VKORHs function in the mammalian vitamin K cycle? Is the pair of loop cysteines—C43 and C51 in human VKORC1—conserved in all VKORC1s, essential for the activity of vitamin K epoxide reduction? Results: We used our recently developed cell-based assay to compare the function of VKORHs to that of human VKORC1 in mammalian cells. We identified for the first time a VKORH (from Mycobacterium tuberculosis [Mt-VKORH]) that can function in the mammalian vitamin K cycle with vitamin K epoxide or vitamin K as substrate. Consistent with our previous in vitro results, the loop cysteines of human VKORC1 are not essential for its activity in vivo. Moreover, the corresponding loop cysteines of Mt-VKORH (C57 and C65), which are essential for its activity in disulfide bond formation during protein folding in Escherichia coli, are not required in the mammalian vitamin K cycle. Innovation and Conclusions: Our results indicate that VKORC1 in eukaryotes and Mt-VKORH in bacteria, that is, in their respective native environments, employ apparently different mechanisms for electron transfer. However, when Mt-VKORH is in the mammalian cell system, it employs a mechanism similar to that of VKORC1. Antioxid. Redox Signal. 16, 329–338.
Introduction
Vitamin K epoxide reductase complex, subunit 1 (VKORC1) is an endoplasmic reticulum (ER) integral membrane protein (18, 23). It is responsible for the conversion of vitamin K epoxide (KO, a quinone epoxide) to vitamin K (a quinone), and can also convert vitamin K to vitamin K hydroquinone (KH2). This series of quinone reductase reactions is an essential part of a redox cycle known as the vitamin K cycle (3). The active form of the vitamin, KH2, is a cofactor for posttranslational modification of proteins essential for blood coagulation, bone homeostasis, signal transduction, and cell proliferation (6, 29).
VKORC1 is a member of a large family of homologues (VKORH) widely distributed among vertebrates, invertebrates, plants, bacteria, and archaea (12). All of these enzymes are quinone reductases, but in bacteria they also participate in disulfide bond formation during protein folding. The significance of VKORC1's role in the latter process in eukaryotes remains unclear (33). In bacteria, VKORHs are present especially in strains lacking the quinone reductase, DsbB (7). At least some of the bacterial VKORHs can complement DsbB in Escherichia coli DsbB deletion mutant strains (7, 27, 35).
DsbB and VKORHs have two pairs of conserved cysteines that are necessary for catalysis (30, 36): the active site, CXXC, which reduces ubiquinone and a second pair, the loop cysteines, which oxidizes DsbA to its active form (16). In DsbB the loop cysteines shuttle electrons from DsbA to reduce the disulfide-bonded CXXC to free cysteines. In vitro enzymatic activity assays and cell-based E. coli complementation studies show that all four conserved cysteine residues in bacterial VKORH are required for activity (19, 35). Based on these results, an intramolecular electron transfer pathway between the two pairs of conserved cysteines, similar to that of DsbB, has been proposed for the bacterial VKORHs as well as for mammalian VKORC1 (19, 25).
While the electron flow in DsbB is well established, the mechanism for active site regeneration in VKORC1 is less clear. Experimental data from site-directed mutagenesis confirm that two conserved cysteine residues, C132 and C135, comprise the CXXC redox center in human VKORC1 (15, 22, 34). But reports of the function of the other pair of conserved loop cysteine residues, C43 and C51, do not allow a consistent interpretation for their role (15, 21, 22). It appears that VKORC1's active site can be reduced in at least two ways. Results with thioredoxin (Trx)/Trx reductase as reductant suggest that the loop cysteines are important for activity (21). On the other hand, with dithiothreitol (DTT) as reductant the loop cysteines do not appear to be essential (15, 22, 34). This latter observation is also true with the Synechococcus VKORH (19). This would indicate that the active site can be reduced directly by DTT or by the loop cysteines that accept electrons from a physiologic reductant.
Innovation.
Vitamin K epoxide reductase complex, subunit 1 (VKORC1) is an enzyme of the endoplasmic reticulum that supports posttranslational modification of glutamate to 4-carboxy glutamate of numerous proteins whose importance spans several physiologic areas including blood coagulation, bone metabolism, and signal transduction. VKORC1 is a member of a widely distributed family of enzymes found throughout evolution. Characterizations of the human enzyme and homologues from bacteria have yielded a large amount of structure–function information, but some are contradictory. This is particularly true of results about the role of the conserved loop cysteines as well as the membrane structure. In the current study, we used our recently developed cell-based assay to compare the function of homologues of VKORC1 (VKORHs) to that of human VKORC1 in mammalian cells. We identified for the first time a VKORH (from Mycobacterium tuberculosis [Mt-VKORH]) that can function in the mammalian vitamin K cycle. Moreover, the conserved loop cysteines of Mt-VKORH, which are essential for electron transfer in disulfide bond formation during oxidative protein folding in Escherichia coli, are not required in the mammalian vitamin K cycle. Our results indicate that VKORC1 and VKORH in their native milieu employ fundamentally different mechanisms in quinone reduction. However, in mammalian cells they employ a similar mechanism.
Although DsbB, VKORH, and VKORC1 are functionally similar enzymes, there is limited sequence identity among them. The DsbB has almost no identity with the others. VKORC1 and VKORHs have amino acid identity between 20% and 25%. Interestingly, the sequence identities among the VKORHs are as divergent as those among VKORHs and VKORC1; for example, Synechococcus VKORH and Mycobacterium VKORH are 20% identical and both are ∼20% identical to human VKORC1. This is important for at least two reasons. First, residues conserved among VKORC1s and VKORHs are candidates for functional importance. On the other hand, the low similarity would suggest that the tertiary structure could be significantly different, and therefore results with either group should be interpreted carefully when applied to the other.
In addition, there appear to be significant differences in the reactions the VKORH versus VKORC1 catalyze. While the VKORH oxidation of DsbA, as well as quinone reduction, is essential for protein folding and electron transport, it is not obvious that VKORC1's participation in any function other than the vitamin K cycle, and quinone reduction, is essential in the eukaryotic system. If this were not the case, then it seems that drugs such as warfarin, a VKORC1 inhibitor, would cause widespread physiologic changes unrelated to the known functions of vitamin K.
Recently we described a cell-based assay that allows us to study the mammalian vitamin K cycle in the cellular milieu (31). This approach has the advantage of allowing us to assess functionality of VKORC1 or a homologue in an environment that requires the enzyme to interact with its physiologic reduction partner and other in vivo components of the vitamin K cycle. We use two cell lines for the experiments depending on the question we are asking. The HEK293 cells have endogenous KO to vitamin K (VKOR) activity that is warfarin sensitive and vitamin K to KH2 (VKR) activity that is warfarin resistant. On the other hand, AV12 cells do not have warfarin-resistant enzyme(s) for VKOR or VKR activity. These cell lines are, therefore, a useful tool for expressing warfarin-resistant VKORC1 or its homologues for studying either VKOR or VKR activity depending on whether we supply KO or vitamin K as substrate.
Taking advantage of this assay system, in our current study, we expressed five VKORHs in HEK293 and AV12 cell lines. We then determined whether any of these enzymes could function in the mammalian vitamin K cycle leading to carboxylation of a chimeric reporter protein, protein C, with its gla domain exchanged with factor IX (FIXgla-PC) (31). We hoped that if a given homologue is active then we will be able to find clues as to the features in VKORC1 important for its function. In addition, we extended our earlier in vitro studies concerning the roles of the loop cysteines in VKORC1 to this in vivo assay. The results indicate that at least three bacterial homologues of VKORC1 can support carboxylation. Interestingly these enzymes are warfarin resistant and have both VKOR and VKR activity. Finally in our cell-based assay system, the loop cysteines are not essential for activity of VKORC1 or the Mt-VKORH.
Results
Investigation of VKORHs' ability to support in vivo carboxylation with KO as substrate
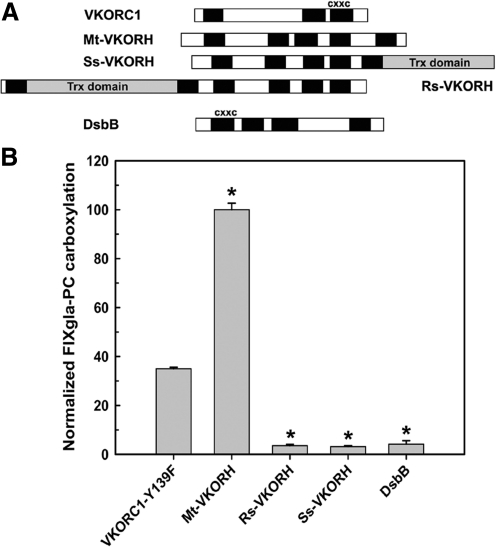
Some VKORH have certain structural and functional characteristics similar to those of human VKORC1 (19, 25). We used our cell-based assay to investigate whether these similarities allow some VKORHs to participate in the mammalian vitamin K cycle. We initially tested VKORHs from three organisms (Fig. 1A): Synechococcus sp. (Ss-VKORH), which has VKR activity and a Trx-like domain at its C-terminus and whose crystal structure has been reported (19); Roseiflexus sp. RS-1 (Rs-VKORH), which has an N-terminal Trx-like domain; and Mycobacterium tuberculosis (Mt-VKORH), which has no Trx-like domain but is active in E. coli protein folding and is reported to be warfarin sensitive (8). We transiently expressed these enzymes in HEK293 cells that stably express the reporter protein and cultured the cells in the presence of 5 μM KO and 4 μM warfarin. The HEK293 cells have warfarin-resistant VKR activity, but warfarin-sensitive VKOR activity. Therefore, the carboxylated reporter protein can result only if the exogenously expressed VKORH has warfarin-resistant VKOR activity. Of the VKORH tested, only Mt-VKORH supported carboxylation of the reporter protein (Fig. 1B). Mt-VKORH has 2.5-fold higher activity than human VKORC1-Y139F. Since VKORC1-Y139F has ∼40% of wild-type activity (17, 24), this result suggests that Mt-VKORH has activity similar to that of wild-type human VKORC1.
FIG. 1.
Reduction of KO to vitamin K by VKORHs to support carboxylation in HEK293 cells. (A) Schematic representation of the VKORHs and controls used in this study. The solid bars indicate the TMD predicted by TOPCONS (4) or experimentally determined TMD for human VKORC1 (32) and DsbB (14). The gray bars indicate the Trx-like domain. The alignment of VKORHs is based on the TMD-containing CXXC redox center. (B) Cell-based activity assay of VKORHs to reduce KO to vitamin K. VKORHs and controls (warfarin-resistant human VKORC1-Y139F and DsbB) were transiently expressed in FIXgla-PC/HEK293 cells. Cells were cultured in complete medium containing 5 μM KO and 4 μM warfarin for 48 h. The concentration of carboxylated FIXgla-PC in the cell culture medium was measured by ELISA and normalized by luciferase activity as described in the Materials and Methods section. Data are presented as mean±SD (n=3). *p<0.001, compared with human VKORC1-Y139F. FIXgla-PC, protein C with its gla domain exchanged with factor IX; VKORC1, vitamin K epoxide reductase complex, subunit 1; VKORHs, homologues of VKORC1; Trx, thioredoxin; TMD, transmembrane domain.
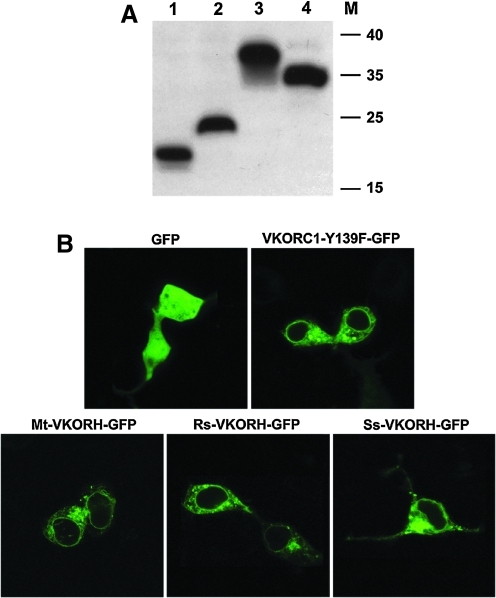
To confirm that VKORHs that lack activity are actually expressed and targeted to the correct cellular location, we fused a HPC4 epitope (peptide epitope from human protein C comprising residues EDQVDPRLIDGK) tag or a green fluorescence protein (GFP) tag at the C-terminus of these proteins. Western blot results show that Rs-VKORH and Ss-VKORH, which are inactive in the cell-based assay, are expressed at a level similar to those of VKORC1-Y139F and Mt-VKORH (Fig. 2A). Confocal fluorescence microscopy results show that when GFP alone is expressed, it is present throughout the cell (Fig. 2B). However, like the VKORC1-Y139F-GFP fusion, all the VKORH-GFP fusions appear to reside in the ER membrane; thus, the subcellular location of the three VKORHs tested is similar to that of VKORC1.
FIG. 2.
Expression and subcellular location of VKORC1 and its homologues in HEK293 cells. (A) Western blot detection of the expression of VKORC1 and its bacterial homologues in HEK293 cells. HPC4-tagged proteins were transiently expressed in HEK293 cells for 48 h. Cell lysates were directly used for SDS-NuPAGE and protein bands were transferred to PVDF membrane. HPC4-tagged protein bands were probed with anti-HPC4 monoclonal antibody and visualized by ECL Western blot reagents. Lane 1, VKORC1-Y139F; lane 2, Mt-VKORH; lane 3, Rs-VKORH; lane 4, Ss-VKORH; M, marker, kDa. (B) Subcellular localization of VKORC1 and its bacterial homologues in HEK293 cells. GFP-tagged proteins were transiently expressed in HEK293 cells for 48 h. Cell images were collected from emission at 493–530 nm after excitation at 488 nm. GFP, green fluorescence protein; HPC4, peptide epitope from human protein C comprising residues EDQVDPRLIDGK; Rs-VKORH, Roseiflexus sp. RS-1 vitamin K epoxide reductase homologue; SDS-NuPAGE, Sodium dodecyl sulfate neutral polyacrylamide gel electrophoresis; Ss-VKORH, Synechococcus sp. vitamin K epoxide reductase homologue.
Reduction of vitamin K to KH2 by VKORHs in AV12 cells
Since HEK293 cells have an unidentified warfarin-resistant vitamin K reductase (26, 31), the results presented in the previous section with HEK293 cells indicate only that Mt-VKORH can reduce KO to vitamin K. In contrast to HEK293 cells, AV12 cells have almost no warfarin-resistant vitamin K reductase (31), and in the presence of warfarin, they produce no carboxylated reporter protein. Previously we showed that VKORC1-Y139F can efficiently catalyze the VKOR but not the VKR reaction to an extent necessary for efficient carboxylation. Thus, AV12 cells stably expressing VKORC1-Y139F are a good model for investigating the ability of an exogenous enzyme's ability to reduce vitamin K to KH2 (31).
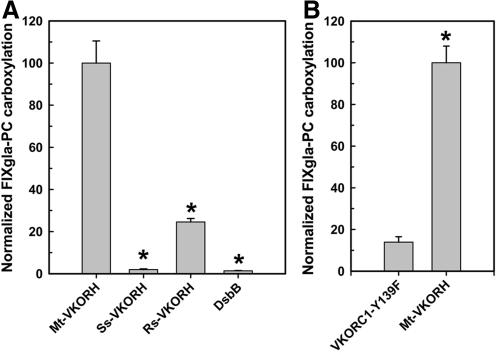
To determine whether VKORHs can efficiently reduce vitamin K to KH2, and thus supports carboxylation, we transiently expressed the VKORHs in FIXgla-PC/AV12 cells stably expressing VKORC1-Y139F. As described in the previous paragraph, if we introduce an enzyme that can efficiently reduce vitamin K to KH2, the resulting cell line should produce carboxylated reporter protein when the cells are cultured with KO and warfarin. Of the cell lines transiently expressing VKORHs, only those expressing Mt-VKORH and Rs-VKORH have significant activity (Fig. 3A). VKORH from Synechococcus can reduce vitamin K in vitro (10, 19), but it has no significant activity in our in vivo system.
FIG. 3.
Reduction of vitamin K to KH2 by VKORHs to support carboxylation in AV12 cells. (A) VKORH from different species was transiently expressed in FIXgla-PC/VKORC1-Y139F/AV12 cell line and the enzymatic activity was determined as described in the legend of Figure 1. Data are presented as mean±SD (n=3). *p<0.001, compared with Mt-VKORH. (B) Warfarin-resistant human VKORC1-Y139F and Mt-VKORH were transiently expressed in the FIXgla-PC/AV12 cell line and the enzymatic activity was determined as described in the legend of Figure 1. Data are presented as mean±SD (n=3). *p<0.01, compared with human VKORC1-Y139F. KH2, vitamin K hydroquinone.
To further confirm that Mt-VKORH can reduce both KO to vitamin K and vitamin K to KH2, we transiently expressed either Mt-VKORH or VKORC1-Y139F in FIXgla-PC/AV12 cells that were not expressing VKORC1-Y139F. We cultured the cells in 5 μM KO and 4 μM warfarin. Since in AV12 cells warfarin inhibits both the VKOR and VKR endogenous activities, we reasoned that only cells with an exogenously expressed enzyme that can reduce both KO and vitamin K will produce carboxylated reporter protein. As we showed in our previous publication, expression of VKORC1-Y139F did not support carboxylation of the reporter protein (31). In contrast, expression of Mt-VKORH produced significant carboxylated reporter protein (Fig. 3B). This result further supports the conclusion that Mt-VKOKH can reduce both KO and vitamin K. It also supports our earlier conclusions that the main function of human VKORC1 in the vitamin K cycle is to reduce KO to vitamin K (31).
The effect of cysteine mutations on Mt-VKORH ability to support in vivo carboxylation
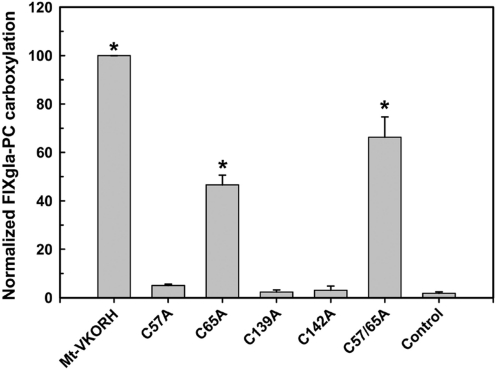
In order to examine the role of cysteine residues in Mt-VKORH's function in the vitamin K cycle, we used HEK293 cells stably expressing the reporter protein. In that cell line, we transiently expressed mutant Mt-VKORH with conserved cysteines changed to alanine (C57A, C65A, C139A, C142A, and C57A/C65A). The cells were then cultured in the presence of 5 μM KO and 4 μM warfarin. Mutation of either of the cysteines (C139 or C142) in the CXXC redox center abolishes its ability to support carboxylation (Fig. 4). These mutations also abolish the ability of Mt-VKORH to complement DsbB deletion in E. coli (35). Mt-VKORH C57A mutant has <10% carboxylation activity (Fig. 4). On the other hand, C65A and C57A/C65A mutants retain 50%–70% activity of the wild-type Mt-VKORH. Since mutation of either C57 or C65 abolishes Mt-VKORH activity for complementing DsbB in E. coli and is absolutely required for its activity (35), this result suggests that loop cysteines C57/C65 are not essential for the reduction of KO in mammalian cells.
FIG. 4.
Reduction of KO to vitamin K by Mt-VKORH cysteine mutants to support carboxylation in HEK293 cells. Mt-VKORH and its cysteine mutants were transiently expressed in FIXgla-PC/HEK293 cells and the enzymatic activity was determined as described in the legend of Figure 1. The control is transfection of the expression vector into the above cell line. Data are presented as mean±SD (n=3). *p<0.001, compared with control.
The effect of cysteine mutations on human VKORC1 activity in vivo
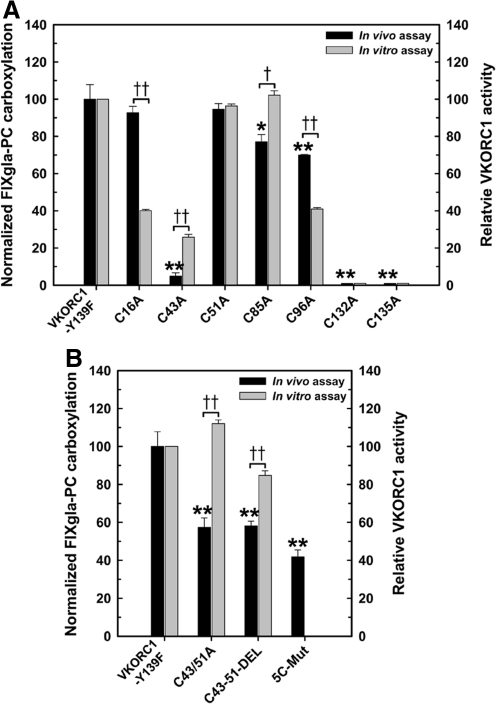
We previously reported that only the CXXC active site cysteines are essential for human VKORC1 activity by the in vitro assay (15). In that study we used purified VKORC1 and its cysteine mutants. In the current study, to test these same VKORC1 mutants in vivo, we employed the cell-based assay using the same cell system described in the previous paragraph. To accomplish this, we changed the cysteines to alanines in the warfarin-resistant VKORC1-Y139F molecule and transiently expressed these enzymes in FIXgla-PC/HEK293. The in vivo results (Fig. 5A) are similar to our previous in vitro results except that the C16A and C96A mutations have 75%–100% activity in the in vivo assay but have only ∼40% activity in the in vitro assay. One possible reason for the activity difference could be the lipid composition differences between the in vitro and in vivo assays since both C16 and C96 are located in a hydrophobic region. Another possibility for the lower activity in the in vitro assay could be part of the purified enzyme is inactive because the mutation of cysteine renders the molecule less stable in vitro.
FIG. 5.
Reduction of KO to vitamin K by VKORC1 cysteine mutants to support carboxylation in HEK293 cells. (A) Individual cysteines in warfarin-resistant VKORC1-Y139F were mutated to alanine. These mutant proteins were transiently expressed in FIXgla-PC/HEK293 cells and the enzymatic activity was determined as described in the legend of Figure 1. The results are shown as black bars with the Y-axis on the left. The VKORC1-Y139F activity was normalized to 100%. The gray bars represent our previous in vitro results (15). Wild-type VKORC1 activity was normalized to 100%. (B) Activity assays of the double mutation C43A and C51A (C43/51A), deletion of these two cysteines and the residues between them (C43-51-DEL), and mutation of five cysteines (C16A, C43A, C51A, C85A, and C96A) simultaneously (5C-Mut). Data are presented as mean±SD (n=3). *p<0.05, **p<0.001, compared with VKORC1-Y139F. †p<0.05, ††p<0.001, compared with the in vitro results.
Only mutations of the active site residues, C132 and C135, reduce activity to background levels. The C43A has ∼30% activity in the in vitro assay but ∼5% activity in the in vivo assay. The C51A has activity similar to that of wild-type VKORC1 in both assays. To confirm the role of C43 and C51, we mutated both the cysteines to alanine, or deleted both the cysteines and the sequences between them. As shown (Fig. 5B), in either case, the molecule still retains ∼60% activity. In addition, mutating all five nonactive site cysteines simultaneously to alanine results in a molecule with ∼40% activity. These results together suggest that the conserved loop cysteines, C43 and C51, are not required for VKOR activity, which agrees with our previous in vitro results (15).
Cysteine residues 57 and 65 of Mt-VKORH are located in the ER lumen
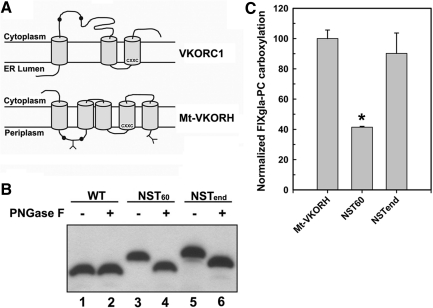
Membrane topology studies of Mt-VKORH in E. coli indicate that C57 and C65 are located in the periplasm, the same side of the bacterial inner membrane as the CXXC redox center (35). A similar membrane topology has been proposed for human VKORC1 (19, 25). However, our previously published in vitro results and our recent in vivo studies (unpublished results) place the corresponding conserved pair of cysteines 43 and 51 of human VKORC1 in the cytoplasm. If true this means that these cysteines are on the opposite side of the ER membrane from the CXXC redox center (32) (Fig. 6A). The function of C57 and C65 of Mt-VKORH in reducing KO in mammalian cells appears to be similar to that of C43/C51 of human VKORC1, but different than its function for DsbB complementation in E. coli. One reason for this might be that Mt-VKORH has a different membrane topology in E. coli than in mammalian cells.
FIG. 6.
Localization with respect to the ER membrane of the conserved loop cysteines 57 and 65 and the C-terminus of Mt-VKORH by N-linked glycosylation mapping. (A) Schematic representation of the proposed membrane topology of human VKORC1 (32) and Mt-VKORH (35). Conserved loop cysteines are indicated by black dots and Y indicates the introduced N-linked glycosylation site. The CXXC redox center is located near the N-terminus of the third TMD in human VKORC1 and the fourth TMD in Mt-VKORH; both face the ER lumen. (B) Localization of the introduced glycosylation site in Mt-VKORH by Western blot assay. HPC4-tagged Mt-VKORH (WT) and its glycosylation mutants (NST60: glycosylation site was introduced between cysteine 57 and 65; NSTend: glycosylation site was introduced at the C-terminus) were transiently expressed in HEK293 cells. Cell lysate was treated with or without PNGase F before being subjected to SDS-NuPAGE. HPC4-tagged protein bands were probed with anti-HPC4 monoclonal antibody and visualized by ECL Western blot reagents. (C) Cell-based activity assay of Mt-VKORH glycosylation mutants. Mt-VKORH and its glycosylation mutants were transiently expressed in FIXgla-PC/HEK293 cells and the enzymatic activity was determined as described in the legend of Figure 1. Data are presented as mean±SD (n=3). *p<0.001, compared with wild-type Mt-VKORH. ER, endoplasmic reticulum.
To test this possibility, we used the N-linked glycosylation mapping technique (5) to determine the Mt-VKORH membrane topology in HEK293 cells. Our results show that introducing glycosylation sites between the conserved loop cysteines or at the C-terminus of the molecule causes Mt-VKORH to migrate slower than wild-type enzyme in sodium dodecyl sulfate neutral polyacrylamide gel electrophoresis (SDS-NuPAGE) (Fig. 6B, lanes 3 and 5). These higher-molecular-weight molecules are sensitive to endoglycosidase digestion (Fig. 6B, lanes 4 and 6), indicating that the introduced sites are glycosylated. Therefore, the cysteine pair C57/C65 and the C-terminus of Mt-VKORH are located in the ER lumen, which is consistent with the periplasmic location in bacteria (35). Glycosylation at the C-terminus does not affect Mt-VKORH activity, while glycosylation in the loop between C57 and C65 decreases activity 50% compared with that of the wild-type enzyme (Fig. 6C). This result, together with the cysteine mutation results of human VKORC1 (Fig. 5), further suggests that whether the conserved loop cysteine pair is located in the cytoplasm or ER lumen, they are not required for the reduction of the CXXC redox center.
Reduction of KO and vitamin K by other bacterial VKORHs with sequences similar to that of Mt-VKORH
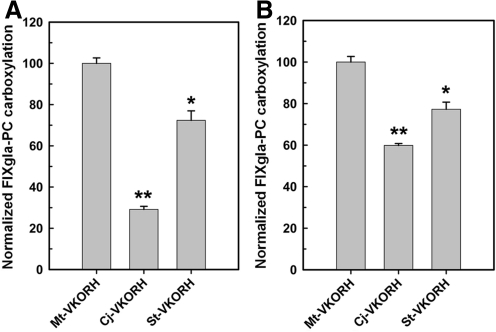
Our results suggest that VKORHs without the Trx domain may be more likely to catalyze both the reduction of KO and vitamin K. In contrast to VKORC1, the dipeptide sequence between the active site cysteines in Mt-VKORH is PY, which is a common sequence in the glutaredoxin family's CXXC redox center. The PY sequence causes a pKa <4 for the N-terminal nucleophilic cysteine and a high reducing potential (11). Therefore, the dipeptide PY may also contribute to VKOR and VKR activity. To test this hypothesis, we selected two bacterial VKORHs with higher sequence identity (35%–50%) to Mt-VKORH and PY as the dipeptide: Cj-VKORH (from Corynebacterium jeikeium K411) and St-VKORH (from Salinispora tropica CNB-440). With KO as substrate, St-VKORH and Cj-VKORH have 30% and 70% activity relative to Mt-VKORH, respectively (Fig. 7A). To determine whether these enzymes can reduce vitamin K to KH2 to support carboxylation, we expressed them in FIXgla-PC/VKORC1-Y139F/AV12 cell lines. As is the case with Mt-VKORH, these two VKORHs also have significant VKR activity (Fig. 7B).
FIG. 7.
VKOR and VKR activity assay of bacterial VKORHs with sequences similar to Mt-VKORH. (A) Cell-based VKOR activity assay of Cj-VKORH and St-VKORH in FIXgla-PC/HEK293 cells. VKORHs were transiently expressed in FIXgla-PC/HEK293 cells and the enzymatic activity was determined as described in the legend of Figure 1. (B) Cell-based VKR activity assay of the same bacterial VKORHs as in (A) in FIXgla-PC/VKORC1-Y139F/AV12 cells. VKORHs were transiently expressed and the enzymatic activity was determined as described in the legend of Figure 1. Data are presented as mean±SD (n=3). *p<0.05, **p<0.001, compared with Mt-VKORH. Cj-VKORH, Corynebacterium jeikeium vitamin K epoxide reductase homologue; St-VKORH, Salinispora tropica vitamin K epoxide reductase homologue; VKOR, the reduction of vitamin K epoxide to vitamin K; VKR, the reduction of vitamin K to KH2.
Discussion
Characterizations of the human VKORC1 and its homologues from bacteria and plants have yielded important structure–function information about this family of enzymes (7, 8, 19, 27, 35). We are primarily interested in how the human VKORC1 functions in the mammalian vitamin K cycle, and how it interacts with the other components of that cycle. Identifying apparent functional differences and similarities among the various homologues should shed light on the significance of structural differences. The problem is that comparing the results until now has been difficult because the studies were done using a variety of experimental models. We believe that studying VKORC1 and its homologues in the same in vivo system provides valuable insights toward our goal of understanding structure–function relationships in VKORC1. Our objective in the current study was to use our previously described cell-based assay (31) to identify similarities and differences of function between VKOR homologues and VKORC1.
Initially we investigated three bacterial VKORHs (Mt-VKORH, Ss-VKORH, and Rs-VKORH), one of which, Ss-VKORH, has been shown to reduce vitamin K in vitro (2, 13). We set out to investigate whether these enzymes could participate in the mammalian vitamin K cycle and support carboxylation in vivo. We had previously shown that, while VKORC1 can support VKR reaction in vitro, in our cell-based system, human VKORC1-Y139F does not support the reduction of vitamin K efficiently enough for reporter protein carboxylation (31). According to our results, of the three VKORHs tested, only Mt-VKORH can employ KO in mammalian cells to support carboxylation of our reporter protein (Fig. 1). But with vitamin K as substrate, both Mt-VKORH and Rs-VKORH (although to a lesser extent) can support carboxylation (Fig. 3). This result is particularly interesting. First, Mt-VKORH is the only noneukaryotic enzyme shown to reduce KO to vitamin K; second, as opposed to VKORC1, it efficiently uses either KO or vitamin K as substrate; third, the Mt-VKORH is resistant to warfarin at concentrations that completely inhibit VKORC1 in our system; and finally, the active sites of both Mt-VKORH can be reduced in vivo by the physiologic reductant for VKORC1.
Mt-VKORH's activity in the DsbB complementation assay in E. coli requires both the active site (CPYC) cysteines and the loop cysteines (C57 and C65). Mutations of any of these four cysteines abolish Mt-VKORH activity (35). In contrast, our results indicate that, like VKORC1 (Fig. 5), only the active site cysteines are required for Mt-VKORH function in mammalian cell carboxylation (Fig. 4). Although in both enzymes mutation of the first loop cysteine (C57 in Mt-VKORH or C43 in VKORC1) significantly decreases VKOR activity, mutation of the second loop cysteine (C65 in Mt-VKORH or C51 in VKORC1) or of both loop cysteines has a minor effect on VKOR activity (Fig. 5). These results are consistent with those presented in our earlier publication with purified VKORC1 (15).
One possible explanation for these results is that the first loop cysteine (C57 and C43) in both enzymes is absolutely required for electron transfer. It could form a mixed disulfide with one of the active site (CXXC) cysteines. In the wild-type enzyme, the second loop cysteine (C65 and C51) would then reduce this mixed disulfide to activate the enzyme. If this hypothesis was correct, then mutating C57 or C43 would prevent the formation of the mixed disulfide and inactivate the enzyme. On the other hand, with the C65 or C51 mutant, perhaps the mixed disulfide is reduced by an unidentified intracellular component to reactivate the enzyme's CXXC. If this is the case, one would expect that when the active site is directly reduced by a reductant, such as DTT, mutating the loop cysteines should not affect the activity. Our results show that with either DTT (in vitro results) or the physiological reductant (in vivo results) the C43A VKORC1 mutant has significantly decreased activity (Fig. 5). This suggests that the mixed disulfide pathway does not apply to the VKORC1 reduction of KO. Since we do not have in vitro results for Mt-VKORH, we cannot rule this mechanism out for Mt-VKORH. However, the loop cysteine mutants of Ss-VKORH do have full activity with DTT as reductant in the in vitro assay where the mixed disulfide mechanism apply (19). As proposed previously (22), an alternative explanation for the significant activity decrease caused by the C43A mutant is that it is important for maintaining the active enzyme structure.
While the membrane topology of human VKORC1 is controversial (19, 32), according to our previous results, the human VKORC1 has three transmembrane domains (TMDs) (32) and the loop cysteines are in the cytoplasm. On the other hand, according to Wang et al. the VKORC1 domain of Mt-VKORH has four TMDs (35) with the loop cysteines located in the periplasm of E. coli - which is analogous to the ER lumen in eukaryotic cells. Since the loop cysteines of Mt-VKORH are essential for activity in E. coli, but not in our mammalian system, it is conceivable that a different location of the loop cysteine in E. coli and mammalian cell might contribute to this difference. Our results show this is not the case (Fig. 6); consistent with the E. coli results the loop cysteines are in the ER lumen. This result indicates that there are at least two mechanisms by which the active site of the reductases can be reduced.
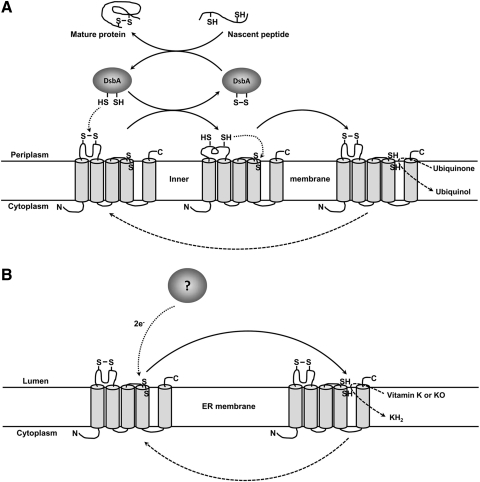
In the bacteria periplasm, the loop of the VKORH interacts with DsbA to support disulfide bond formation during folding of proteins necessary for cell motility. This leaves the loop cysteines in the sulfhydryl form so they can in turn reduce the active site CXXC to activate VKORH for quinone reduction. On the other hand, in the mammalian system, the loop of VKORC1 or VKORH apparently plays only a secondary role in any reducing cycle other than that of vitamin K. If this was not the case, it seems likely that warfarin inhibition of the enzyme might affect important cellular functions other than vitamin K production and cause negative physiological effects. In addition, if this was not the case, the murine knockout of VKORC1 would not be viable when fed with vitamin K (28). This suggests that in the mammalian system reduction of the active site CXXC must be catalyzed directly and intermolecularly by an as yet unidentified reducing protein/molecule (Fig. 8).
FIG. 8.
Proposed reaction mechanism of Mt-VKORH in the reduction of ubiquinone and vitamin K. (A) Proposed reaction mechanism of Mt-VKORH in Escherichia coli protein disulfide bond formation. When a nascent peptide folds to a disulfide-linked mature protein, a disulfide bond in the active site of DsbA is reduced to two free cysteines. These cysteines reduce the disulfide bond of the conserved loop cysteines (C57 and C65) of Mt-VKORH and, in turn those sulfhydryls participate in the intramolecular reduction of the CXXC redox center. When ubiquinone is reduced to ubiquinol by Mt-VKORH, the CXXC active site cysteines are oxidized back to a disulfide bond. (B) Proposed reaction mechanism of Mt-VKORH for reducing vitamin K in mammalian cells. The Mt-VKORH disulfide bond of the CXXC active site is directly reduced by an unknown reductant. The reduced form of the enzyme can apparently reduce both KO and vitamin K for carboxylation in mammalian cells.
In addition to shedding light on the role of cysteines in the mammalian vitamin K cycle, our results show that VKORHs lacking Trx-like domains (Mt-VKORH, St-VKORH, and Cj-VKORH) can support carboxylation utilizing both KO and vitamin K. This indicates that in our system the Trx-like domain is not necessary for either the VKOR or VKR reaction. We believe that sequence differences and similarities among these enzymes will lead to identification of structural features that contribute to the functional differences and likely lead to a better understanding of structure–function in VKORC1.
In summary, we have shown that VKORH from M. tuberculosis, C. jeikeium, and S. tropica can reduce both KO and vitamin K to support carboxylation in mammalian cells. In contrast to Mt-VKORH's function in oxidative protein folding in E. coli, the conserved loop cysteines are apparently dispensable for supporting carboxylation in mammalian cells. If the three TMD structures of human VKORC1 are correct, then our results with Mt-VKORH suggest that as long as the active site of the enzyme is facing the lumen of the ER, the location of the loop cysteines does not affect activity in the vitamin K cycle. These results indicate that Mt-VKORH can function by two physiologically relevant mechanisms depending on its cellular environment. Based on comparison of VKORHs' sequences to that of the VKORC1, our results should also provide a platform for identifying residues in VKORC1 that are likely important for its function.
Materials and Methods
Materials
Vitamin K1 and warfarin were obtained from Sigma-Aldrich (St. Louis, MO). Luciferase substrate coelenterazine was from NanoLight Technology (Pinetop, AZ). Mammalian expression vector pIRES2 DsRed-Express2, secreted Metridia luciferase containing vector pBI-CMV5, and Xfect transfection reagent were from Clontech Laboratories, Inc. (Mountain View, CA). HEK293 and AV12 cell lines were from ATCC (Manassas, VA). All cell culture medium were from Invitrogen Corp. (San Diego, CA). The cDNA sequences coding the VKORHs and DsbB used in the study were optimized for mammalian cell expression and chemically synthesized by Blue Heron Biotechnology (Bothell, WA). Mouse anticarboxylated factor IX gla domain monoclonal antibody (BC2) was a gift from GlaxoSmithKline (Philadelphia, PA) and Green Mountain Antibodies (Burlington, VT) (1, 9). Horseradish peroxidase–conjugated affinity purified sheep antihuman Protein C IgG was from Affinity Biologicals, Inc. (Ancaster, ON Canada). Anti-HPC4 monoclonal antibody was from Dr. Charles T. Esmon (Oklahoma Medical Research Foundation, Oklahoma City, OK).
DNA manipulations and plasmid constructions
Mammalian expression vector pIRES2 DsRed-Express2 was used as the basic cloning vector. The red fluorescent protein DsRed-Express2 was replaced by secreted Metridia luciferase that was used as the internal control for normalizing the transient transfection efficiency. The resulting vector pIRES2-Met.Luc was used for expressing all the molecules used in this report. This vector permits both the target protein and the secreted Metridia luciferase to be translated from a single mRNA transcript with a ribosome re-entry site.
All VKORHs and warfarin-resistant human VKORC1 (Y139F) and their mutants were subcloned into the EcoRI site of the pIRES2-Met.Luc vector under control of the cytomegalovirus promoter. For Western blot detection, a HPC4 tag was fused to the C-terminus of all the VKORHs and VKORC1. To localize the expression of VKORC1 and its homologues, a GFP tag was introduced to the C-terminus of the protein with a (GGSGG)6 flexible linker. To create the N-linked glycosylation site in the first periplasmic loop of Mt-VKORH, residues P61 and I62 were mutated to S61 and T62, respectively. Together with residue N60, this introduces an Tripeptide Asn-Ser-Thr of N-Linked glycosylation consensus site (NST) sequence between the pair cysteines C57 and C65. Due to the short carboxyl terminus of Mt-VKORH and the requirement of at least 12 amino acid residues between the glycosylation site and the membrane interface (20), an N-linked glycosylation consensus sequence (NST) with a flexible extension linker (GGSGGSGGS) was introduced at the carboxyl terminus of Mt-VKORH. All the constructs for glycosylation studies have a HPC4 tag at their carboxyl terminus for Western blot detection.
In vivo VKOR and VKR activity assay
VKOR and VKR in vivo activity was determined with our cell-based assay as described previously (31). It is based on the ability of VKORC1 or its homologue to convert sufficient KO to vitamin K or vitamin K to KH2 in mammalian cells to support the carboxylation of a reporter protein, FIXgla-PC. VKORHs or warfarin-resistant VKORC1-Y139F was transiently expressed into HEK293 or AV12 cell lines that stably express the reporter protein in a 24-well plate. Metridia luciferase was coexpressed as the internal control from the same mRNA transcript as VKORC1 or VKORHs. Forty-eight hours posttransfection, carboxylated FIXgla-PC in the cell culture medium was measured by ELISA (31). Luciferase activity was determined by injecting 50 μl of coelenterazine solution (2 μM in PBS with 300 mM NaCl) to 50 μl of cell culture medium directly. Luminescence emission (relative light units [RLU]) from the mixture was recorded at 480 nm with a delay of 6 s and integration time of 1 s. VKOR or VKR activity was expressed as normalized carboxylated FIXgla-PC (ng/ml/RLU). Mt-VKORH activity was normalized to 100% unless otherwise stated. Before normalization, carboxylated FIXgla-PC in the cell culture medium varies from ∼80 to ∼200 ng/ml by transient expression of wild-type Mt-VKORH. One-way analysis of variance was used to assess significant differences as indicated. The criterion for statistical significance was set as p<0.05 or 0.001.
Western blot and confocal fluorescence microscope
To analyze the protein expression level and glycosylation modification of Mt-VKORH, HPC4-tagged proteins were transiently expressed in HEK293 cells. Forty-eight hours posttransfection, cells were harvested and lysed in 300 μl PBS with 1% Triton X-100 in the presence of protease inhibitor cocktail. For protein expression detection, the amount of cell lysate loaded to SDS-NuPAGE was normalized by luciferase activity. For deglycosylation, 1 μl PNGase F (500 U/μl) was added to a 50 μl aliquot of the cell lysate and incubated for 30 min at 37°C. Deglycosylated or nontreated samples were directly subjected to SDS-NuPAGE under reducing condition. Western blot analysis was performed as described previously (32).
For subcelluar localization of VKORC1 and its homologues, GFP-tagged proteins were transiently expressed in HEK293 cells in a chambered cover glass. Forty-eight hours posttransfection, the chambered cover glass was placed directly on the microscope stage for image collection. Confocal microscopy was performed on a Zeiss LSM710 confocal laser scanning microscope (Carl Zeiss Microimaging, Thornwood, NY). Images were collected using a 40X/1.2NA C-Apochromat objective lens. Visualization of the GFP was achieved by use of a 488 nm Argon laser line for excitation and the detector was set to collect emission at 493–530 nm.
Abbreviations Used
- Cj-VKORH
Corynebacterium jeikeium vitamin K epoxide reductase homologue
- DTT
dithiothreitol
- ER
endoplasmic reticulum
- FIXgla-PC
protein C with its gla domain exchanged with factor IX
- GFP
green fluorescence protein
- HPC4
peptide epitope from human protein C comprising residues EDQVDPRLIDGK
- KH2
vitamin K hydroquinone
- KO
vitamin K epoxide
- Mt-VKORH
Mycobacterium tuberculosis vitamin K epoxide reductase homologue
- NST
Tripeptide Asn-Ser-Thr of N-Linked glycosylation consensus site
- RLU
relative light units
- Rs-VKORH
Roseiflexus sp. RS-1 vitamin K epoxide reductase homologue
- SDS-NuPAGE
sodium dodecyl sulfate neutral polyacrylamide gel electrophoresis
- Ss-VKORH
Synechococcus sp. vitamin K epoxide reductase homologue
- St-VKORH
Salinispora tropica vitamin K epoxide reductase homologue
- TMD
transmembrane domain
- Trx
thioredoxin
- VKOR
the reduction of vitamin K epoxide to vitamin K
- VKORC1
vitamin K epoxide reductase complex, subunit 1
- VKORH
vitamin K epoxide reductase homologue
- VKR
the reduction of vitamin K to KH2
Acknowledgments
This work was supported by National Institutes of Health Grants HL077740, HL048318, and HL06350 (to D.W.S.). The authors thank Dr. David Straight for critical discussions and for helping to write and organize the manuscript. The authors thank Dr. Gilles Basset from University of Nebraska-Lincoln for useful discussions and providing the cDNA of VKORH from Arabidopsis thalania. The authors also thank Dr. Christian Klatt from Montana State University and Dr. Abhay Singh from Washington University at St. Louis who provided the genomic DNA from Roseiflexus and Synechocystis and for helpful discussions. The authors thank Dr. Tony Perdue for the help on confocal fluorescence microscopy.
Author Disclosure Statement
The authors declare no competing financial interests exist.
References
- 1.Aktimur A. Gabriel MA. Gailani D. Toomey JR. The factor IX gamma-carboxyglutamic acid (Gla) domain is involved in interactions between factor IX and factor XIa. J Biol Chem. 2003;278:7981–7987. doi: 10.1074/jbc.M212748200. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett GJ. Porter CT. Borkakoti N. Thornton JM. Analysis of catalytic residues in enzyme active sites. J Mol Biol. 2002;324:105–121. doi: 10.1016/s0022-2836(02)01036-7. [DOI] [PubMed] [Google Scholar]
- 3.Bell RG. Matschiner JT. Warfarin and the inhibition of vitamin K activity by an oxide metabolite. Nature. 1972;237:32–33. doi: 10.1038/237032a0. [DOI] [PubMed] [Google Scholar]
- 4.Bernsel A. Viklund H. Hennerdal A. Elofsson A. TOPCONS: consensus prediction of membrane protein topology. Nucleic Acids Res. 2009;37:W465–W468. doi: 10.1093/nar/gkp363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chavez RA. Hall ZW. The transmembrane topology of the amino terminus of the alpha subunit of the nicotinic acetylcholine receptor. J Biol Chem. 1991;266:15532–15538. [PubMed] [Google Scholar]
- 6.Danziger J. Vitamin K-dependent proteins, warfarin, and vascular calcification. Clin J Am Soc Nephrol. 2008;3:1504–1510. doi: 10.2215/CJN.00770208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dutton RJ. Boyd D. Berkmen M. Beckwith J. Bacterial species exhibit diversity in their mechanisms and capacity for protein disulfide bond formation. Proc Natl Acad Sci U S A. 2008;105:11933–11938. doi: 10.1073/pnas.0804621105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dutton RJ. Wayman A. Wei JR. Rubin EJ. Beckwith J. Boyd D. Inhibition of bacterial disulfide bond formation by the anticoagulant warfarin. Proc Natl Acad Sci U S A. 2010;107:297–301. doi: 10.1073/pnas.0912952107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feuerstein GZ. Patel A. Toomey JR. Bugelski P. Nichols AJ. Church WR. Valocik R. Koster P. Baker A. Blackburn MN. Antithrombotic efficacy of a novel murine antihuman factor IX antibody in rats. Arterioscler Thromb Vasc Biol. 1999;19:2554–2562. doi: 10.1161/01.atv.19.10.2554. [DOI] [PubMed] [Google Scholar]
- 10.Furt F. Oostende C. Widhalm JR. Dale MA. Wertz J. Basset GJ. A bimodular oxidoreductase mediates the specific reduction of phylloquinone (vitamin K(1)) in chloroplasts. Plant J. 2010;64:38–46. doi: 10.1111/j.1365-313X.2010.04305.x. [DOI] [PubMed] [Google Scholar]
- 11.Gan ZR. Sardana MK. Jacobs JW. Polokoff MA. Yeast thioltransferase—the active site cysteines display differential reactivity. Arch Biochem Biophys. 1990;282:110–115. doi: 10.1016/0003-9861(90)90093-e. [DOI] [PubMed] [Google Scholar]
- 12.Goodstadt L. Ponting CP. Vitamin K epoxide reductase: homology, active site and catalytic mechanism. Trends Biochem Sci. 2004;29:289–292. doi: 10.1016/j.tibs.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Holliday GL. Mitchell JB. Thornton JM. Understanding the functional roles of amino acid residues in enzyme catalysis. J Mol Biol. 2009;390:560–577. doi: 10.1016/j.jmb.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Jander G. Martin NL. Beckwith J. Two cysteines in each periplasmic domain of the membrane protein DsbB are required for its function in protein disulfide bond formation. EMBO J. 1994;13:5121–5127. doi: 10.1002/j.1460-2075.1994.tb06841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin DY. Tie JK. Stafford DW. The conversion of vitamin K epoxide to vitamin K quinone and vitamin K quinone to vitamin K hydroquinone uses the same active site cysteines. Biochemistry. 2007;46:7279–7283. doi: 10.1021/bi700527j. [DOI] [PubMed] [Google Scholar]
- 16.Kadokura H. Beckwith J. Mechanisms of oxidative protein folding in the bacterial cell envelope. Antioxid Redox Signal. 2010;13:1231–1246. doi: 10.1089/ars.2010.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lasseur R. Longin-Sauvageon C. Videmann B. Billeret M. Berny P. Benoit E. Warfarin resistance in a French strain of rats. J Biochem Mol Toxicol. 2005;19:379–385. doi: 10.1002/jbt.20104. [DOI] [PubMed] [Google Scholar]
- 18.Li T. Chang CY. Jin DY. Lin PJ. Khvorova A. Stafford DW. Identification of the gene for vitamin K epoxide reductase. Nature. 2004;427:541–544. doi: 10.1038/nature02254. [DOI] [PubMed] [Google Scholar]
- 19.Li W. Schulman S. Dutton RJ. Boyd D. Beckwith J. Rapoport TA. Structure of a bacterial homologue of vitamin K epoxide reductase. Nature. 2010;463:507–512. doi: 10.1038/nature08720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nilsson IM. von Heijne G. Determination of the distance between the oligosaccharyltransferase active site and the endoplasmic reticulum membrane. J Biol Chem. 1993;268:5798–5801. [PubMed] [Google Scholar]
- 21.Rishavy MA. Usubalieva A. Hallgren KW. Berkner KL. Novel insight into the mechanism of the vitamin K oxidoreductase (VKOR): electron relay through Cys43 and Cys51 reduces VKOR to allow vitamin K reduction and facilitation of vitamin K-dependent protein carboxylation. J Biol Chem. 2011;286:7267–7278. doi: 10.1074/jbc.M110.172213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rost S. Fregin A. Hunerberg M. Bevans CG. Muller CR. Oldenburg J. Site-directed mutagenesis of coumarin-type anticoagulant-sensitive VKORC1: evidence that highly conserved amino acids define structural requirements for enzymatic activity and inhibition by warfarin. Thromb Haemost. 2005;94:780–786. doi: 10.1160/TH05-02-0082. [DOI] [PubMed] [Google Scholar]
- 23.Rost S. Fregin A. Ivaskevicius V. Conzelmann E. Hortnagel K. Pelz HJ. Lappegard K. Seifried E. Scharrer I. Tuddenham EG. Muller CR. Strom TM. Oldenburg J. Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature. 2004;427:537–541. doi: 10.1038/nature02214. [DOI] [PubMed] [Google Scholar]
- 24.Rost S. Pelz HJ. Menzel S. MacNicoll AD. Leon V. Song KJ. Jakel T. Oldenburg J. Muller CR. Novel mutations in the VKORC1 gene of wild rats and mice—a response to 50 years of selection pressure by warfarin? BMC Genet. 2009;10:4. doi: 10.1186/1471-2156-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schulman S. Wang B. Li W. Rapoport TA. Vitamin K epoxide reductase prefers ER membrane-anchored thioredoxin-like redox partners. Proc Natl Acad Sci U S A. 2010;107:15027–15032. doi: 10.1073/pnas.1009972107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shearer MJ. Barkhan P. Vitamin K1 and therapy of massive warfarin overdose. Lancet. 1979;1:266–267. doi: 10.1016/s0140-6736(79)90786-4. [DOI] [PubMed] [Google Scholar]
- 27.Singh AK. Bhattacharyya-Pakrasi M. Pakrasi HB. Identification of an atypical membrane protein involved in the formation of protein disulfide bonds in oxygenic photosynthetic organisms. J Biol Chem. 2008;283:15762–15770. doi: 10.1074/jbc.M800982200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spohn G. Kleinridders A. Wunderlich FT. Watzka M. Zaucke F. Blumbach K. Geisen C. Seifried E. Muller C. Paulsson M. Bruning JC. Oldenburg J. VKORC1 deficiency in mice causes early postnatal lethality due to severe bleeding. Thromb Haemost. 2009;101:1044–1050. [PubMed] [Google Scholar]
- 29.Stafford DW. The vitamin K cycle. J Thromb Haemost. 2005;3:1873–1878. doi: 10.1111/j.1538-7836.2005.01419.x. [DOI] [PubMed] [Google Scholar]
- 30.Tapley TL. Eichner T. Gleiter S. Ballou DP. Bardwell JC. Kinetic characterization of the disulfide bond-forming enzyme DsbB. J Biol Chem. 2007;282:10263–10271. doi: 10.1074/jbc.M611541200. [DOI] [PubMed] [Google Scholar]
- 31.Tie JK. Jin DY. Straight DL. Stafford DW. Functional study of the vitamin K cycle in mammalian cells. Blood. 2011;117:2967–2974. doi: 10.1182/blood-2010-08-304303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tie JK. Nicchitta C. von Heijne G. Stafford DW. Membrane topology mapping of vitamin K epoxide reductase by in vitro translation/cotranslocation. J Biol Chem. 2005;280:16410–16416. doi: 10.1074/jbc.M500765200. [DOI] [PubMed] [Google Scholar]
- 33.Wajih N. Hutson SM. Wallin R. Disulfide-dependent protein folding is linked to operation of the vitamin K cycle in the endoplasmic reticulum. A protein disulfide isomerase-VKORC1 redox enzyme complex appears to be responsible for vitamin K1 2,3-epoxide reduction. J Biol Chem. 2007;282:2626–2635. doi: 10.1074/jbc.M608954200. [DOI] [PubMed] [Google Scholar]
- 34.Wajih N. Sane DC. Hutson SM. Wallin R. Engineering of a recombinant vitamin K-dependent gamma-carboxylation system with enhanced gamma-carboxyglutamic acid forming capacity: evidence for a functional CXXC redox center in the system. J Biol Chem. 2005;280:10540–10547. doi: 10.1074/jbc.M413982200. [DOI] [PubMed] [Google Scholar]
- 35.Wang X. Dutton RJ. Beckwith J. Boyd D. Membrane topology and Mutational analysis of Mycobacterium tuberculosis VKOR, a protein involved in disulfide bond formation and a homologue of human vitamin K epoxide reductase. Antioxid Redox Signal. 2011;14:1413–1420. doi: 10.1089/ars.2010.3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Y. Cierpicki T. Jimenez RH. Lukasik SM. Ellena JF. Cafiso DS. Kadokura H. Beckwith J. Bushweller JH. NMR solution structure of the integral membrane enzyme DsbB: functional insights into DsbB-catalyzed disulfide bond formation. Mol Cell. 2008;31:896–908. doi: 10.1016/j.molcel.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]