Abstract
Aims: Studies employing transgenic mice indicate that overexpression of superoxide dismutase 1 (SOD1) improves memory during aging. It is unclear whether the improvement is due to a lifetime of overexpression, decreasing the accumulation of oxidized molecules, or if increasing antioxidant enzymes in older animals could reduce oxidative damage and improve cognitive function. We used adeno-associated virus to deliver antioxidant enzymes (SOD1, SOD2, catalase [CAT], and SOD1+CAT) to the hippocampus of young (4 months) and aged (19 months) F344/BN F1 male rats and examined memory-related behavioral performance 1 month and 4 months postinjection. Results: Overexpression of antioxidant enzymes reduced oxidative damage; however, memory function was not related to the level of oxidative damage. Increased expression of SOD1, initiated in advanced age, impaired learning. Increased expression of SOD1+CAT provided protection from impairments associated with overexpression of SOD1 alone and appears to guard against cognitive impairments in advanced age. Innovation: Viral vector gene delivery provides a novel approach to test the hypothesis that increased expression of antioxidant enzymes, specifically in hippocampal neurons, will provide protection from age-related cognitive decline. Further, expression of multiple vectors permits more detailed investigation of mechanistic pathways. Conclusion: Oxidative stress is a likely component of aging; however, it is unclear whether increased production of reactive oxygen species or the accumulation of oxidative damage is the primary cause of functional decline. The results provide support for the idea that altered redox-sensitive signaling rather than the accumulation of damage may be of greater significance in the emergence of age-related learning and memory deficits. Antioxid. Redox Signal. 16, 339–350.
Introduction
The brain is highly sensitive to oxidative stress (22), and the accumulation of damaged molecules may contribute to age-related memory impairments (8, 14, 30, 37, 38). Antioxidant molecules and enzymes balance the biological activity of reactive oxygen species (ROS), superoxide (O2−•), and hydrogen peroxide (H2O2). Superoxide dismutase (SOD) catalyzes O2−• into H2O2 and catalase (CAT) or glutathione peroxidase (GPx) converts H2O2 to water and oxygen. SODs are classified according to their metal cofactors and cellular localization. The Cu/Zn-SOD1 (SOD1) is distributed throughout the cytoplasm, nucleus, and inner membrane space of mitochondria, Cu/Zn-SOD3 (SOD3) is located in the extracellular space, and Mn-SOD (SOD2) is restricted to the mitochondrial matrix (11, 18, 28).
SOD1 overexpression differentially influences brain function over the course of aging (23). Enhanced long-term potentiation (LTP) and spatial memory are observed in aged transgenic SOD1 (tg-SOD1) mice (25, 27). In contrast, tg-SOD2 mice do not exhibit altered synaptic plasticity or memory (23). It is unclear whether increased SOD1 activity is required over the lifespan to prevent the accumulation of oxidative damage; or whether enhanced SOD1 activity, initiated in aged animals, might be therapeutic. To test this hypothesis, we used adeno-associated virus (AAV) to deliver antioxidant enzymes (SOD1, SOD2, CAT, or SOD1+CAT) to the hippocampus of young and aged rats. The results demonstrate a dissociation of learning from measures of oxidative stress and suggest that changes in redox-sensitive signaling may mediate cognitive impairment.
Results
Efficiency-specificity of the viral vectors
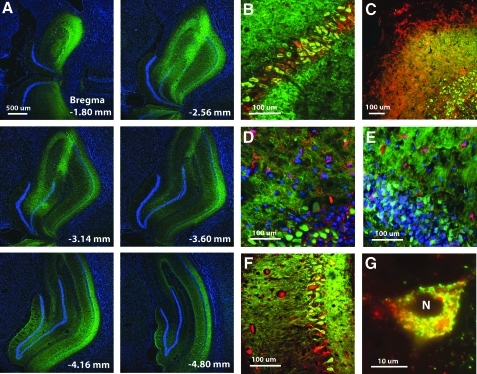
Following behavioral testing, a subset of animals was killed for examination of vector expression. Figure 1 illustrates the pattern of AAV-mediated transfection. Strong expression was observed throughout the dorsal hippocampus, extending >3000 μm along the anterior–posterior axis and included all major cell layers (Fig. 1A). Transduction was limited to the hippocampus in confirmation of our previous work (17) and mainly observed in neurons. Immunostaining for the myc tag of SOD1-myc revealed transduction in neurons identified by neuronal nuclei (NeuN) (Fig. 1B) or MAP2 (Fig. 1C) staining. Myc immunofluorescence was not localized to astrocytes or microglia, assessed by immunostaining for glial fibrillary acidic protein (GFAP) (Fig. 1D) and Iba1 (Fig. 1E). Injections of SOD1+CAT resulted in colocalization within the soma and dendrites of neurons (Fig. 1F). The SOD2 was observed in the soma, but not in the nucleus and it colocalized with OxPhos complex IV subunit I (COX) consistent with mitochondrial localization (Fig. 1G).
FIG. 1.
Neurons are the primary cell type transduced by the adeno-associated virus vectors. (A) Panels show that transduction of myc (green) for superoxide dismutase 1 (SOD1)-myc extended at least 3000 μm through the hippocampus, was observed in all three cell layers, and was limited to the hippocampus. The distance is calculated relative to bregma. (B) Merged images showing colocalization (yellow) of myc (green) for SOD1-myc and neuronal nuclei (red) in neuron cell bodies, and (C) in dendrites determined by MAP2 (red). Myc did not colocalize with (D) glial fibrillary acidic protein (red) or (E) Iba1 (red). (F) Merged figure for the hippocampus of a rat injected with SOD1+CAT and immunostained for myc (green) for SOD1-myc and CAT (red) and colocalization (yellow). (G) Merged figure of a cell in the CA1 pyramidal cell layer of a rat injected with the SOD2 vector shows colocalization of SOD2 (green) with the OxPhos complex IV subunit I (COX) (red). Note the expression is not observed in the nucleus (N) consistent with mitochondrial localization. Cell nuclei in (A), (D), and (E) were stained with 4'-6-diamidino-2-phenylindole (blue). Calibration bars represent 500 μm (A), 100 μm (B–F), and 10 μm (G).
Innovation.
The accumulation of oxidized molecules due to oxidative stress is hypothesized to be a major contributor to age-related cognitive decline, such that antioxidant treatments may be protective against brain aging (36). Viral vector gene delivery provides a novel approach to test the hypothesis that increased expression of antioxidant enzymes, specifically in hippocampal neurons, will provide protection from age-related cognitive decline. Overexpression of antioxidant enzymes in young and aged rats reduced oxidative damage; however, the decrease in oxidative stress was not associated with enhanced cognitive function. Superoxide dismutase 1 (SOD1) overexpression impaired learning in aged rats and increased the expression of glutathione peroxidase. Overexpression of SOD1+catalase (CAT) rescued the learning impairment, indicating that the impairment was likely due to excessive hydrogen peroxide (H2O2). Moreover, SOD1+CAT enhanced spatial learning in aged rats implying the importance of H2O2 and redox state in regulating cognitive function during aging.
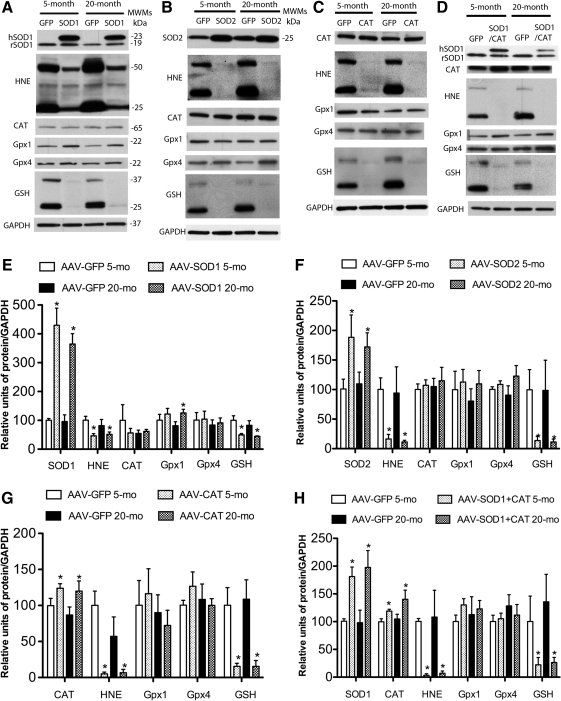
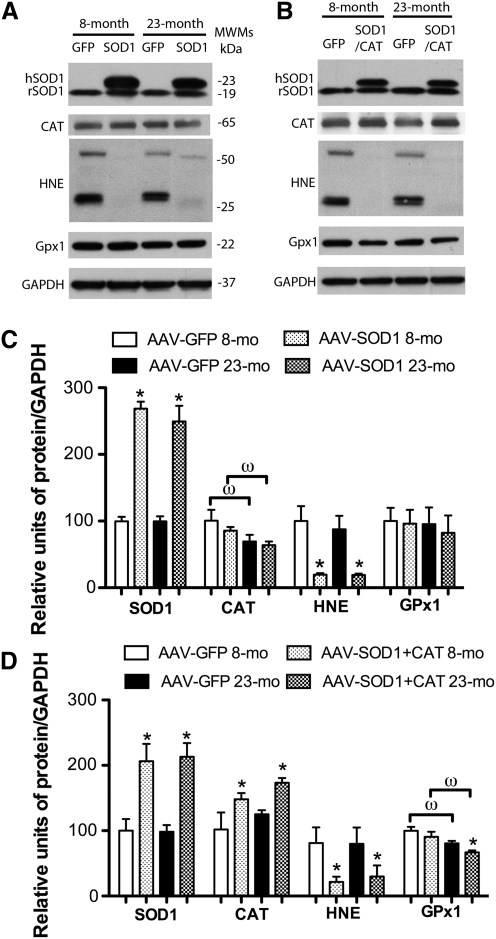
Figure 2 shows the increase in enzyme expression and decrease in oxidative damage associated with viral vector treatment. For quantification, band intensities were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and then normalized to the mean value for young animals. Two SOD1 bands were observed for young and aged SOD1-myc–injected rats (Fig. 2A). The band representing endogenous SOD1 was observed at 19 kDa and a larger band representing the myc-tagged human SOD1 was located at 23 kDa. Densitometry indicated that rats injected with SOD1-myc exhibited a fourfold increase in total SOD1 [F(1,16)=45.59, p<0.0001] (Fig. 2E). Measurement of the 19 kDa band indicated that SOD1-myc expression did not modify the level of endogenous SOD1 (data not shown). An increase in the relevant vector was observed for SOD2 [F(1,11)=7.9, p<0.05] (Fig. 2B, F) and CAT [F(1,8)=7.14, p<0.05] (Fig. 2C, G). Finally, injection of SOD1+CAT increased the expression of SOD1 [F(1,10)=14.54, p<0.01] and CAT [F(1,12)=7.83, p<0.05] (Fig. 2D, H). It should be noted that the level of expression for SOD1 under this condition was reduced relative to injection of SOD1-myc (i.e., two vs. fourfold).
FIG. 2.
Overexpression of antioxidant enzymes in the hippocampus reduces markers of oxidative stress. Western blot analysis of hippocampal lysates from young and aged rats injected with viral vectors to express (A, E) SOD1-myc, (B, F) SOD2, (C, G) CAT, or (D, H) SOD1+CAT. For quantification, band intensities were normalized to the expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and this value was normalized to the mean value of young controls from the same blot. In each case, transduction resulted in an increase in the expression of the antioxidant enzyme. For SOD1-myc (A, D), two bands were observed representing endogenous rat SOD1 (rSOD1) and the human myc–tagged SOD1 (hSOD1). Lipid peroxidation (4-hydroxy-2-nonenal [HNE]) and S-glutathionylated proteins (glutathione [GSH]) were decreased by enzyme overexpression. To determine whether overexpression of antioxidant enzymes would influence expression of downstream antioxidant enzymes, immunostaining was performed for glutathione peroxidase 1 (GPx1), GPx4, and CAT. Overexpression of SOD1-myc in aged rats was associated with an increase in GPx1 (E). Each bar represents the normalized mean+SEM (n=3–6). Asterisks indicate significant (p<0.05) differences determined by Fisher's protected least significant difference (PLSD) post hoc test.
As shown in Figure 2, SOD1, SOD2, CAT, or SOD1+CAT overexpression was associated with reduced 4-hydroxy-2-nonenal (HNE) staining. Quantification confirmed a decrease in HNE-reactive protein for SOD1 [F(1,8)=9.26, p<0.05], SOD2 [F(1,12)=11.45, p<0.01], CAT [F(1,8)=18.53, p>0.01], and SOD1+CAT [F(1,8)=16.53, p<0.01]. A decrease in protein S-glutathionylation was observed for rats overexpressing SOD1 [F(1,12)=15.17, p<0.005], SOD2 [F(1,12)=7.83, p<0.05], CAT [F(1,8)=22.54, p<0.01], and SOD1+CAT [F(1,8)=7.3, p<0.05]. Examination of SOD activity indicated that SOD1-myc rats exhibited a twofold increase in SOD activity compared with green fluorescence protein (GFP)–injected rats (p<0.05; n=4/group, data not shown).
SOD overexpression can increase the level of H2O2 and the expression of downstream antioxidant enzymes (13, 19, 29, 33). Western blots indicate that GPx4 and CAT levels did not differ among groups; however, there was a tendency for a treatment effect for GPx1 [F(1,15)=3.46, p=0.08] and post hoc comparisons indicated GPx1 expression significantly increased in aged SOD1 rats (Fig. 2E).
Age-dependent influence of enzyme overexpression on spatial learning
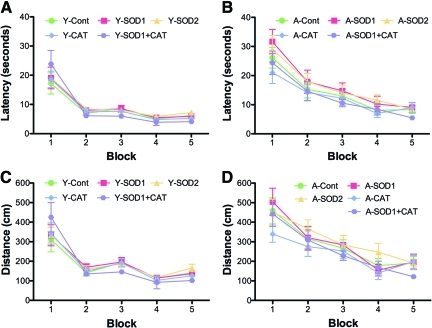
Spatial learning in the water maze was tested 1 month following virus injections. Figure 3 shows the mean escape latency and escape path length across the five training blocks during cue discrimination training. All groups exhibited a decrease in escape latency and path length during training, and young animals exhibited superior latency and path length relative to aged rats. An analysis of variance (ANOVA) on latency indicated an effect of training [F(4,404)=107.21, p<0.0001] and an age effect [F(1,404)=34.71, p<0.0001] in the absence of a treatment effect (Fig. 3A, B). Similarly, an ANOVA on path length indicated an effect of training [F(4,404)=71.23, p<0.0001] and age [F(1,404)=40.084, p<0.0001] in the absence of a treatment effect (Fig. 3C, D).
FIG. 3.
Overexpression of antioxidant enzymes did not affect cue discrimination in water maze. All groups learned to reach the visible platform, as indicated by a significant overall decrease in latency (A, B) and distance (C, D) across blocks. Y=5-month-old and A=20-month-old rats injected with SOD1, SOD2, CAT, or SOD1+CAT. Age-matched controls (Cont) included rats injected with GFP and no-surgery controls. (To see this illustration in color the reader is referred to the Web version of this article at www.liebertonline.com/ars).
For spatial discrimination, an ANOVA on escape latency indicated significant main effects of training [F(5,505)=68.03, p<0.0001] and age [F(1,505)=37.35, p<0.0001], with a tendency for a treatment effect [F(4,505)=2.10, p=0.08] (Fig. 4A, B). Analysis of escape path length indicated effects of training [F(5,505)=81.19, p<0.0001] and age [F(1,505)=28.83, p<0.0001], with a tendency for a treatment effect [F(4,505)=2.27, p=0.06] (Fig. 4C, D). To localize treatment effects, the data for the last two training blocks were averaged and an ANOVA was run within each age group. No treatment effects were found in young rats. A tendency [F(4,50)=1.92, p=0.12] for a treatment effect on latency was observed for aged rats, and post hoc analyses indicated that aged SOD1+CAT rats exhibited significantly less time to reach platform compared with aged rats expressing SOD2 or SOD1 alone (Fig. 4E). The results were confirmed for the distance (Fig. 4F). An ANOVA on the mean for the last two blocks revealed tendency for a treatment effect in aged rats [F(4,50)=2.5, p=0.05] and post hoc analysis indicated that aged SOD1+CAT rats had a shorter path length compared with aged SOD2 or SOD1 rats.
FIG. 4.
Overexpression of SOD1 impaired spatial learning in aged rats. Behavioral measures for young and aged rats during training on the spatial version of the water escape task. Mean latency (A, B) and mean path length (C, D) to escape during spatial discrimination training. No treatment effects were found for young rats. Examination of the mean latency (E) and mean path length (F) averaged across the last two training blocks in aged animals indicated an effect of treatment. The SOD1+CAT group performed better relative to the SOD1 and SOD2 groups. Asterisk indicates a significant (p<0.05) difference. (To see this illustration in color the reader is referred to the Web version of this article at www.liebertonline.com/ars).
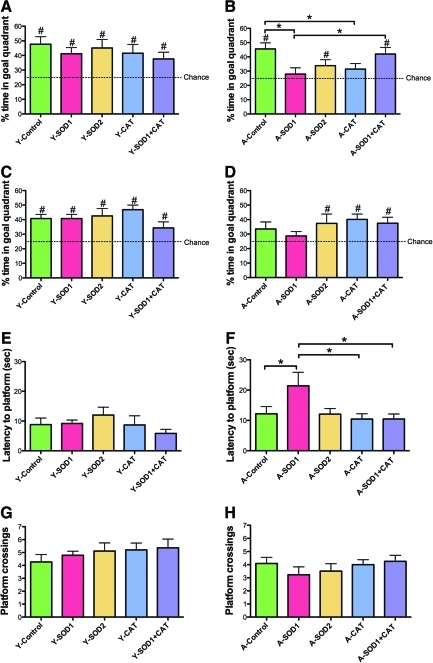
A 60 s probe trial was delivered between training blocks 5 and 6. Analyses were performed on two consecutive 30 s segments. An ANOVA on the percent time in the goal quadrant during the first 30 s indicated that young rats spent more time in the goal quadrant than aged rats [F(1,100)=4.82, p<0.05] and there was a tendency for a treatment effect [F(4,100)=2.07, p=0.08] (Fig. 5A, B). ANOVAs within each age group revealed a treatment effect for aged animals [F(4,50)=3.07, p<0.05] and post hoc comparisons indicated that aged control rats spent more time in the goal quadrant compared with aged rats with SOD1 or CAT overexpression. Aged SOD1+CAT rats spent significantly more time in the goal quadrant relative to aged SOD1 rats. The percent time in the goal quadrant was compared with that expected by chance (i.e., 25%). For the first 30 s of the probe trial, all groups except for aged SOD1 or CAT performed above chance. Examination of time spent searching the goal quadrant for the last 30 s of the probe trial indicated a tendency for an age effect [F(1,100)=5.06, p=0.08] in the absence of a treatment effect (Fig. 5C, D). An examination of the percent time in the goal quadrant indicated that all groups except aged controls and aged SOD1 rats spent significantly greater time in the goal quadrant relative to chance (Fig. 5C, D). The fact that aged SOD1 rats did not spend a significant portion of their search behavior in the goal quadrant for either segment of the probe trial indicates that these animals did not acquire a spatial search strategy.
FIG. 5.
Overexpression of SOD1 impaired acquisition of a spatial search strategy in aged rats. A single probe trial was given between block 5 and block 6 of spatial training. Percent time spent searching the goal quadrant during the first 30 s for (A) young and (B) aged rats. The search behavior of aged rats with overexpression of SOD1 or CAT was not different from chance levels (25% dashed line). Aged SOD1 rats spent significantly less time compared with aged control and aged rats with overexpression of SOD1+CAT. Aged rats with overexpression of CAT spent significantly less time than aged control rats. Examination of the percent of time spent in goal quadrant during the second 30 s of probe trial for (C) young and (D) aged rats indicated that aged rats with overexpression of SOD1 continued to perform at chance levels. Examination of the latency for first platform crossing for (E) young and (F) aged rats indicated a treatment effect for aged animals due to an extended latency for the SOD1 group. Age differences were observed for total number of platform crossings between (G) young and (H) aged rats. Asterisk indicates group difference (p<0.05). Pound sign (#) indicates a significant (p<0.05) difference from chance. (To see this illustration in color the reader is referred to the Web version of this article at www.liebertonline.com/ars).
The aged SOD1 impairment was confirmed by measuring the latency to the first platform crossing (Fig. 5E, F). An ANOVA indicated an age [F(1,100)=10.95, p<0.01] and treatment [F(4,100)=2.52, p<0.05] effect. ANOVAs within each age group indicated a tendency for a treatment effect in aged animals [F(4,49)=2.38, p=0.06] and post hoc comparisons indicated that aged SOD1 rats required a longer time to cross the platform location compared with age-matched controls, CAT, and SOD1+CAT expressing groups. There was a tendency (p=0.08) for a difference between SOD1 and SOD2 groups. An ANOVA on the total number of platform crossing indicated an age difference [F(1,100)=13.15, p<0.001] (Fig. 5G, H), in the absence of a treatment effect. In sum, the results indicate that SOD1 overexpression for 1 month was associated with impaired spatial learning in aged rats. The fact that the SOD1+CAT exhibited superior performance relative to SOD1 group indicates that the impairment maybe rescued to co-overexpressing CAT.
Overexpression of SOD1+CAT for 4 months improves spatial learning
Our results contrast with studies in tg-SOD1 mice, which indicate that young tg-SOD1 mice exhibit impaired spatial learning while aged tg-SOD1 mice show enhanced spatial memory (20, 27). The difference may result from an interaction of age and the duration of overexpression. To examine this possibility, a subset of animals tested at 1 month was retested at 4 months postinjection. The experimental groups included young rats (8 months) injected with SOD1 (n=6), SOD1+CAT (n=10), GFP (n=2), no-surgery controls (n=4), and aged rats (23 months) injected with SOD1 (n=6), SOD1+CAT (n=10), GFP (n=2), or no-surgery controls (n=4).
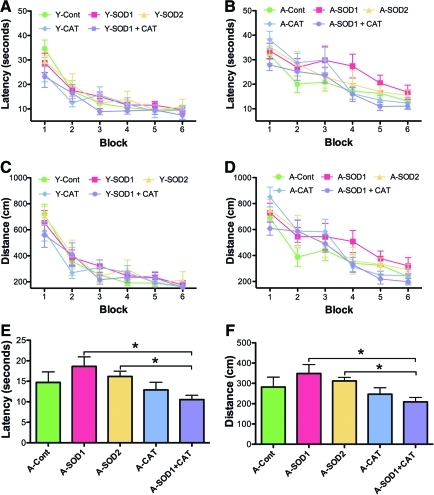
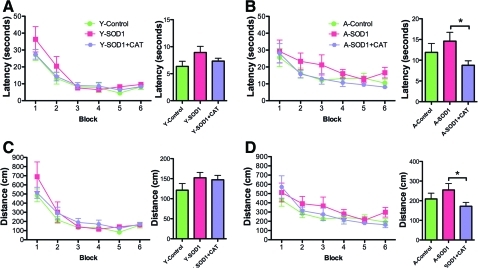
For examination of spatial learning 4 months after viral vector injections, the platform location in the maze was shifted to a new quadrant. An ANOVA on escape latency across training blocks indicated main effects of training [F(5,185)=40.53, p<0.0001] and age [F(1,185)=5.19, p<0.05], with a tendency for a treatment effect [F(2,185)=5.19, p=0.07] (Fig. 6A, B). Analysis of escape path length indicated effects of training [F(5, 185)=36.12, p<0.0001] and age [F(1,185)=5.20, p<0.05] (Fig. 6C, D). To localize treatment effects, the data for the last two training blocks were averaged and an ANOVA for treatment effects was run within each age group. An ANOVA for young animals indicated a tendency for a treatment effect [F(2,18)=7.12, p=0.14]; however, post hoc comparisons did not reach significance. Although there was a trend for young SOD1 rats to exhibit an increase in the escape latency relative to young control rats (p=0.05). An ANOVA for aged animals indicated a tendency for a treatment effect [F(2,19)=3.24, p=0.06] and post hoc comparisons indicated that aged SOD1+CAT rats required less time to reach platform compared with aged SOD1 rats (Fig. 6B). Analysis of the mean distance for the last two training blocks also indicated a tendency for a treatment effect in aged rats [F(2,19)=2.67, p=0.09] (Fig. 6C, D) and post hoc comparisons confirmed that aged SOD1+CAT rats had a shorter path length to escape compared with aged SOD1 rats (Fig. 6D).
FIG. 6.
Long-term overexpression of SOD1+CAT improved learning in spatial trials in aged rats. Behavioral measures for young and aged rats during training on the spatial version of the water escape task 4 months after viral injections. Mean latency (A, B) and mean path length (C, D) to escape during spatial discrimination training. The bar graphs represent the mean latency averaged across the last two training blocks. For aged animals, better performance was observed for the SOD1+CAT group relative to the SOD1 group. Asterisk indicates a significant (p<0.05) difference. (To see this illustration in color the reader is referred to the Web version of this article at www.liebertonline.com/ars).
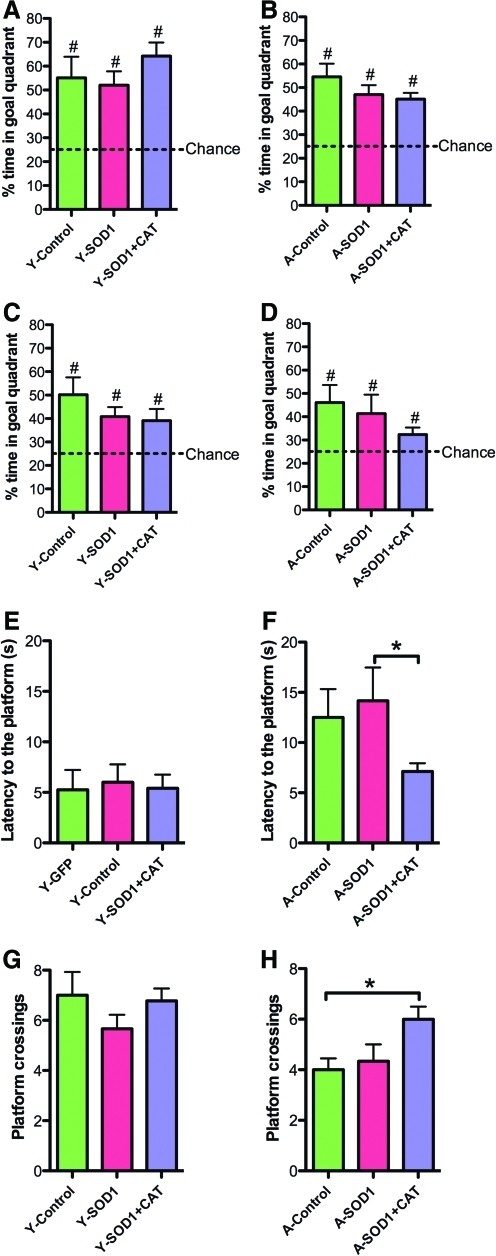
Examination of the first 30 s of the probe trial delivered between blocks 5 and 6 indicated a tendency for young rats to spend more time in the goal quadrant relative to aged rats [F(1,37)=3.33, p=0.07] in the absence of a treatment effect (Fig. 7A, B). The percent time searching the goal quadrant was significantly above chance for all groups, indicating that all groups had acquired a spatial search strategy. A tendency for a treatment effect [F(2, 37)=2.47, p=0.09] in the absence of an age difference was observed for the second half-minute of the probe trial (Fig. 7C, D). Again, all groups spent a significantly longer time in the goal quadrant than expected by chance (Fig. 7C, D). An ANOVA on the latency to the first platform crossing indicated a significant age effect [F(1,37)=12.73, p<0.01], and a tendency for a treatment effect [F(2,37)=2.24, p=0.1]. An ANOVA within each age group indicated a tendency for a treatment effect only in the aged group [F(2,19)=3.29, p=0.05]. Post hoc comparison indicated that aged SOD1+CAT rats required less time for the first platform crossing compared with aged SOD1 rats and there was a tendency (p=0.08) for SOD1+CAT rats to cross quicker than aged controls (Fig. 7E, F). Finally, an ANOVA on total platform crossing indicated an age effect [F(1,37)=12.67, p<0.005] and a tendency for a treatment effect [F(2,37)=2.54, p=0.09]. An ANOVA within each age group indicated a tendency for a treatment effect in aged rats [F(2,19)=3.45, p=0.05] and post hoc comparisons indicated that aged SOD1+CAT rats exhibited significantly more platform crossings than aged controls (Fig. 7G, H). In fact, aged SOD1+CAT rats exhibited performance similar to young animals.
FIG. 7.
Overexpression of SOD1+CAT for 4 months improves spatial learning in probe test. A single probe trial was given between block 5 and block 6 of spatial training. Mean percent of time spent searching the goal quadrant during the first 30 s for (A) young and (B) aged rats. Mean percent of time spent in goal quadrant during the second 30 s of probe trial for (C) young and (D) aged rats. Examination of the latency for first platform crossing for (E) young and (F) aged rats indicated a treatment effect for aged animals due to a superior performance by the SOD1+CAT group compared with the SOD1 group. Examination of the total number of platform crossings for (G) young and (H) aged rats indicated that aged SOD1+CAT rats exhibited significantly more platform crossings compared to aged control. Asterisk indicates group difference (p<0.05). Pound sign (#) indicates a significant (p<0.05) difference from chance. (To see this illustration in color the reader is referred to the Web version of this article at www.liebertonline.com/ars).
Figure 8 shows Western blot data on enzyme expression and lipid peroxidation in the hippocampus for animals that overexpressed viral vectors for ∼5 months. As shown in Figure 8A, the two SOD1 bands (19 and 23 kDa) were observed for SOD1-myc–injected rats. Densitometry confirmed that rats injected with SOD1-myc exhibited approximately threefold increase in total SOD1 [F(1,12)=133.08, p<0.0001) (Fig. 8A, C). Again, the 19 kDa band was not influenced by SOD1-myc expression (data not shown). Injection of SOD1+CAT increased the expression of SOD1 [F(1,12)=31.31, p<0.001] and CAT [F(1,8)=10.40, p<0.05] (Fig. 8B, D). Similar to 2-month overexpression, the level of expression for SOD1 under this condition was reduced relative to injection of SOD1-myc alone (i.e., 2 vs. 2.5-fold).
FIG. 8.
Antioxidant enzymes and oxidative stress markers in hippocampus with 5-month SOD1 and SOD1+CAT overexpression. Western blot analysis of hippocampal lysates from young and aged rats injected with viral vectors to express (A) SOD1 and (B) SOD1+CAT. For SOD1 (A, C), two bands were observed representing endogenous rat SOD1 (rSOD1) and the human myc–tagged SOD1 (hSOD1). CAT level did not change in SOD1 rats but increased in SOD1+CAT rats. Lipid peroxidation measured with anti-HNE antibody was decreased by SOD1 and SOD1+CAT overexpression. The expression of SOD1 did not change the level of GPx1 but the expression of SOD1+CAT in aged rats was associated with a decrease in GPx1 (D). GAPDH was used as a loading control. Asterisk indicates a significant (p<0.05) difference in treatment. ω indicates a significant (p<0.05) difference in age.
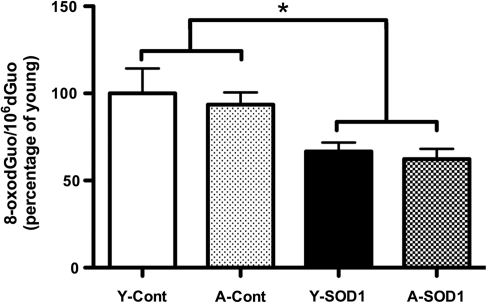
A decrease in HNE-reactive protein was observed for rats that overexpress SOD1 [F(1,12)=24.23, p<0.001] or SOD1+CAT [F(1,12)=7.63, p<0.05] (Fig. 8). For SOD1 rats, there was an age effect on CAT expression [F(1,12)=6.58, p<0.05] in the absence of a treatment effect. Post hoc tests within each treatment group indicated an age-related decrease in expression in animals overexpressing SOD1 (Fig. 8C). Examination of GPx1 indicated no effect of age or treatment for SOD1 animals and SOD1+CAT animals exhibited an effect of age [F(1,16)=15.00, p<0.005] and treatment [F(1,16)=4.27, p<0.05]. Post hoc analyses indicated that GPx1 expression was reduced in aged rats that overexpressed SOD1+CAT [F(1,8)=7.9, p<0.05] compared with aged controls (Fig. 8B, D). Levels of protein carbonyls were measured; however, the results did not reveal any information concerning the effect of SOD1 overexpression, possibly due to the lack of specificity of carbonyl measures (1) or the fact that H2O2 is a poor mediator of stable oxidative damage for protein carbonyls (43). Further, previous research indicates that lipid peroxidation may be more sensitive since SOD overexpression in mice markedly reduces lipid peroxidation in the absence of a significant effect on protein carbonyls (24). Consistent with the lipid peroxidation results, overexpression of SOD1 resulted in a decrease in Oxo8dG [F(1,21)=16.58, p<0.0005] in the absence of an age difference (Fig 9), confirming that SOD1 overexpression decreased oxidative damage.
FIG. 9.
DNA oxidative damage is reduced by overexpression of SOD1. The level of 8-oxo-7,8-dihydro-2-deoxyguanosine (8-oxodGuo) was measured and normalized by young controls (Y-Cont). Bars represent the mean and SEM. Asterisk indicates significant (p<0.05) differences in treatment.
Discussion
While it is clear that oxidative stress is a likely component of aging, it is unclear whether increased production of ROS or the accumulation of oxidative damage is the primary cause of functional decline. Consistent with previous work in tg-mice, overexpression of SOD1, SOD2, and CAT reduced markers of oxidative stress (41). However, decreased oxidative damage was not associated with improved cognitive function. Moreover, SOD1 overexpression impaired spatial learning in aged rats. The impairment was specific to SOD1 and spatial learning in aged animals. The results indicate that accumulation of oxidative damage is not the only mechanism for cognitive decline. Other processes influenced by oxidative stress may be more relevant to age-related cognitive decline, including mitochondrial function, redox signaling, and inflammation.
SOD1 can be toxic to motor neurons (7). However, in the current study, the deficit was limited to aged SOD1 animals, indicating that it was not simply due to SOD1 overexpression. Further, one would expect that toxicity should increase over time. On the contrary, we observed that the spatial learning deficit decreased following 4 months of SOD1 overexpression in our oldest rats. Although there was a tendency for the emergence of impairment as young SOD1 rats approached middle age (Fig. 6). SOD1 catalyzes O2−• into H2O2 and the levels of O2−• and H2O2 in brains of Tg-SOD1 mice are decreased and increased, respectively (35), and tg-SOD1 overexpressing mice exhibit age-dependent effects on cognition and hippocampal synaptic plasticity that are thought to result from elevation of ambient H2O2 levels (26). Thus, the specificity may be due to ambient H2O2 levels. One indication that H2O2 was increased in the current study is that aged SOD1 animals exhibited increased GPx1 expression (Fig. 2A). In other tissues, H2O2 can provoke an increase in GPx activity (39, 42, 45). Finally, aged SOD1+CAT rats did not exhibit cognitive impairments, suggesting improved cognition resulted from better homeostatic regulation of redox signaling due to CAT processing of excess H2O2.
How can SOD1 overexpression reduce oxidative damage if H2O2 levels increase? H2O2 is a relatively mild oxidizing agent. Irreversible oxidative damage results from O2−•; SOD1 removes O2−• and overexpression of SOD1 reduces the level of O2−• in the brain (35). If free iron is available, H2O2 can produce hydroxyl radicals (OH.) through Fenton-type reactions. However, a large body of evidence indicates that H2O2 per se may not induce irreversible oxidative damage (10, 31, 32, 43). In contrast, H2O2 can readily and reversibly influence redox-sensitive signaling cascades. Age-related changes in redox state disrupt calcium homeostasis, increasing the release of calcium from internal stores, decreasing CaMKII activity, and impairing LTP (5, 6, 15). H2O2 application to hippocampal slices mimics age-related changes, promoting calcium release from internal stores (21), decreasing synaptic CaMKII activity (44), and impairing LTP (3, 26, 40). The LTP impairment in tg-SOD1 mice is attenuated by CAT, suggesting the involvement of elevated H2O2 (20). Together the results suggest that SOD1 overexpression could impair learning through increased H2O2 acting on redox signaling pathways involved in memory formation.
Adaptation to altered redox state
The effect of SOD overexpression on memory changes with age (23). SOD1 overexpression impaired or had no effect on learning in young mice and enhanced spatial memory in older mice (25, 27). The authors suggest that aged mice adapt to elevated H2O2. Transcription of genes regulating redox homeostasis increases during middle age (2, 4, 47). Thus, the increase in GPx1 expression in aged SOD1 mice may represent an adaptive response. Other adaptive changes may underlie the improvement in behavior following 4 months of SOD1 overexpression. Finally, aged SOD1+CAT rats exhibited superior water maze performance, indicating that treatment to reduce O2−• and H2O2 may be more beneficial than simply enhancing SOD1. Indeed, administration of a SOD/CAT mimetic improved memory in aging mice (34). Future studies should employ redox proteomics to identify specific changes related to cognitive decline.
In summary, memory function was not related to the level of oxidative damage. Increased SOD1 expression impaired learning in older rats. In contrast, increased expression of SOD1+CAT provided protection from age-related cognitive impairments. The results extend a growing body of evidence indicating the importance of H2O2 and the redox signaling for regulating cognitive function during aging.
Materials and Methods
Animals
All treatments were approved by the Institutional Animal Care and Use Committee and were in accordance with guidelines established by the United States Public Health Service Policy on Human Care and Use of Laboratory Animals. Young tg-SOD1 mice exhibit impaired spatial learning (20); therefore, young rats were included to examine age effects and specificity of gene overexpression. AAV was injected into the hippocampus of young (4 months) and aged (19 months) male Fischer 344/Brown Norway F1 rats (Harlan Laboratories) to express SOD1 (young: n=14; aged: n=13), SOD2 (young: n=9; aged: n=9), CAT (young: n=10; aged: n=9), mix of SOD1+CAT (young: n=11; aged: n=12), or GFP (young: n=8; aged: n=8). We also include young (n=4) and aged (n=4) no-surgery controls. No behavioral difference was observed between GFP and no-surgery controls; therefore, these animals were combined for behavioral analysis.
AAV viral vectors
Virus particles were produced and quantified by dot blot analysis, through the Powell Gene Therapy Center at the University of Florida (Gainesville, FL). Genes were cloned into pseudotyped AAV5 capsid, self-complementary AAV vectors, containing a cytomegalovirus (CMV)/chicken β-actin promoter with a CMV-immediate early (CMV-IE) enhancer. Genes included GFP, human SOD1 with a c-terminal myc tag (myc). The SOD2 and CAT vectors were gifts from Dr. Nicolas Muzyczka.
Rats were anesthetized with ketamine/xylazine (90/10 mg/kg) and virus was stereotactically injected at two sites bilaterally in the hippocampus using glass pipettes. Each injection consisted of 2 μl of GFP (dot blot titer: 1.02×1013 vg/ml), SOD1 (dot blot titer: 1.14×1013 vg/ml), SOD2 (dot blot titer: 6.99×1012 vg/ml), CAT (dot blot titer: 2.53×1013 vg/ml), or 3 μl 2:1 mix of SOD1 and CAT.
Behavior testing
Methods for water maze testing have been published previously (16). Rats were first trained to find a visible platform using 15 trials separated to five blocks. Rats that did not reach the platform within 60 s on all trials during the fifth block were removed from the experiment. Standard water maze testing across several days is insensitive to cognitive decline across this age span in Fischer 344/Brown Norway F1 rats (46). Therefore, the task difficulty was increased by employing a single-day massed training protocol, which is sensitive to age (9). Spatial discrimination testing occurred 3 days later and consisted of 18 trials separated into six blocks. A free swim probe trial was inserted between blocks 5 and 6. For the probe trial, the number of platform crossings was counted and the time spent in both goal and opposite quadrants was recorded.
Western immunoblotting
The hippocampi were frozen in liquid nitrogen, and stored at −80°C. Tissue was prepared in RIPA buffer supplemented with protease inhibitor, phosphatase inhibitor, and EDTA (Thermo Scientific). The lysates were assayed for protein content using BCA kit (Pierce) and then separated on polyacrylamide gels (Bio-Rad Laboratories) and transferred to PVDF membranes (GE Healthcare) for Western blotting. Primary antibodies (SOD1, 1:5000; SOD2, 1:6000; HNE, 1:100; CAT, 1:1000; GPx1, 1:600; GPx4, 1:1000 [Abcam, Inc.]; GAPDH, 1:6000 [EnCor Biotechnology, Inc.]; glutathione, 1:1000 [Arbor Assays]) were diluted in blocking buffer (5% milk in tris-buffered saline Tween-20) and applied to the membrane overnight at 4°C. Membranes were then incubated with horseradish peroxidase–conjugated secondary antibodies directed against the primary antibody (Cell Signaling Technology, Inc.). Membranes were reacted with and enhanced chemiluminescent substrate (Pierce Biotechnology). A medical film processor (SRX-101A; Konica Minolta Medical Imaging U.S.A., Inc.) was used to image the film.
Immunohistochemistry
Brains were postfixed in 4% paraformaldehyde followed by 30% sucrose in PBS (4°C). Sections (10–20 μm) were incubated with primary antibodies (SOD1, 1:1000; MAP2, 1:10,000; CAT, 5 μg/ml; SOD2, 1:500 [Abcam, Inc.]; myc-tag, 1:2000 [Cell Signaling Technology]; NeuN, 1:1000 [Chemicon/Millipore]; Iba1, 1–2 μg/ml [Wako]; GFAP, 1:500 [DakoCytomation]; anti-COX, 1:300 [Invitrogen]) overnight at 4°C. The brain sections were then washed and incubated in corresponding Alexa 488 or 594 secondary antibodies (Molecular Probes) for 1 h at room temperature. Sections were washed and counterstained with 4'-6-diamidino-2-phenylindole solution (0.1 μg/ml in PBS) before mounting. Expression was confirmed using fluorescent microscopy (Zeiss Axioplan 2 upright fluorescent microscope, equipped with a QImaging Retiga 4000R Camera with RGB-HM-5 Color Filter and QImaging QCapture Pro 6.0 software; QImaging Surrey).
SOD activity was measured using the HT SOD assay kit (Trevigen, Inc.) and protein carbonyls were measured using a commercial ELISA (Zentech PC Test, Protein Carbonyl Enzyme Immuno-Assay Kit; Biocell Corp.) according to the manufacturer's instructions. 8-oxo-7,8-dihydro-2-deoxyguanosine (8-oxodGuo) levels were determined according to our previously published methods (12).
Statistical analysis
ANOVAs were used to establish main effects and interactions. Follow-up ANOVAs and/or Fisher's protected least significant difference post hoc comparisons with p<0.05 were employed to determine specific differences. Student's t-tests were used to determine whether quadrant search behavior was different than that expected by chance.
Abbreviations Used
- 8-oxodGuo
8-oxo-7,8-dihydro-2-deoxyguanosine
- AAV
adeno-associated virus
- ANOVAs
analyses of variance
- CAT
catalase
- COX
OxPhos complex IV subunit I
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GFAP
glial fibrillary acidic protein
- GFP
green fluorescence protein
- GPx
glutathione peroxidase
- GSH
glutathione
- HNE
4-hydroxy-2-nonenal
- H2O2
hydrogen peroxide
- LTP
long-term potentiation
- NeuN
neuronal nuclei
- PLSD
protected least significant difference
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- tg-SOD
SOD transgenic mice
Acknowledgments
This work was supported by National Institutes of Aging Grants AG014979, AG037984, and AG036800, and the Evelyn F. McKnight Brain Research Foundation. Special thanks to Katrina Velez, Marvin Servanez, and Christiaan Leeuwenburgh for technical support and advice.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Adams S. Green P. Claxton R. Simcox S. Williams MV. Walsh K. Leeuwenburgh C. Reactive carbonyl formation by oxidative and non-oxidative pathways. Front Biosci. 2001;6:A17–A24. doi: 10.2741/adams. [DOI] [PubMed] [Google Scholar]
- 2.Aenlle KK. Kumar A. Cui L. Jackson TC. Foster TC. Estrogen effects on cognition and hippocampal transcription in middle-aged mice. Neurobiol Aging. 2009;30:932–945. doi: 10.1016/j.neurobiolaging.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auerbach JM. Segal M. Peroxide modulation of slow onset potentiation in rat hippocampus. J Neurosci. 1997;17:8695–8701. doi: 10.1523/JNEUROSCI.17-22-08695.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blalock EM. Chen KC. Sharrow K. Herman JP. Porter NM. Foster TC. Landfield PW. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci. 2003;23:3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodhinathan K. Kumar A. Foster TC. Intracellular redox state alters NMDA receptor response during aging through Ca2+/calmodulin-dependent protein kinase II. J Neurosci. 2010;30:1914–1924. doi: 10.1523/JNEUROSCI.5485-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bodhinathan K. Kumar A. Foster TC. Redox sensitive calcium stores underlie enhanced after hyperpolarization of aged neurons: role for ryanodine receptor mediated calcium signaling. J neurophysiol. 2010;104:2586–2593. doi: 10.1152/jn.00577.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosco DA. Morfini G. Karabacak NM. Song Y. Gros-Louis F. Pasinelli P. Goolsby H. Fontaine BA. Lemay N. McKenna-Yasek D, et al. Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat Neurosci. 2010;13:1396–1403. doi: 10.1038/nn.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butterfield DA. Sultana R. Redox proteomics identification of oxidatively modified brain proteins in Alzheimer's disease and mild cognitive impairment: insights into the progression of this dementing disorder. J Alzheimers Dis. 2007;12:61–72. doi: 10.3233/jad-2007-12107. [DOI] [PubMed] [Google Scholar]
- 9.Carter CS. Leeuwenburgh C. Daniels M. Foster TC. Influence of calorie restriction on measures of age-related cognitive decline: role of increased physical activity. J Gerontol A Biol Sci Med Sci. 2009;64:850–859. doi: 10.1093/gerona/glp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catala A. A synopsis of the process of lipid peroxidation since the discovery of the essential fatty acids. Biochem Biophys Res Commun. 2010;399:318–323. doi: 10.1016/j.bbrc.2010.07.087. [DOI] [PubMed] [Google Scholar]
- 11.Chang LY. Slot JW. Geuze HJ. Crapo JD. Molecular immunocytochemistry of the CuZn superoxide dismutase in rat hepatocytes. J Cell Biol. 1988;107:2169–2179. doi: 10.1083/jcb.107.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui L. Hofer T. Rani A. Leeuwenburgh C. Foster TC. Comparison of lifelong and late life exercise on oxidative stress in the cerebellum. Neurobiol Aging. 2009;30:903–909. doi: 10.1016/j.neurobiolaging.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Haan JB. Cristiano F. Iannello R. Bladier C. Kelner MJ. Kola I. Elevation in the ratio of Cu/Zn-superoxide dismutase to glutathione peroxidase activity induces features of cellular senescence and this effect is mediated by hydrogen peroxide. Hum Mol Genet. 1996;5:283–292. doi: 10.1093/hmg/5.2.283. [DOI] [PubMed] [Google Scholar]
- 14.Forster MJ. Dubey A. Dawson KM. Stutts WA. Lal H. Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci U S A. 1996;93:4765–4769. doi: 10.1073/pnas.93.10.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster TC. Calcium homeostasis and modulation of synaptic plasticity in the aged brain. Aging Cell. 2007;6:319–325. doi: 10.1111/j.1474-9726.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 16.Foster TC. Sharrow KM. Masse JR. Norris CM. Kumar A. Calcineurin links Ca2+dysregulation with brain aging. J Neurosci. 2001;21:4066–4073. doi: 10.1523/JNEUROSCI.21-11-04066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster TC. Rani A. Kumar A. Cui L. Semple-Rowland SL. Viral vector-mediated delivery of estrogen receptor-alpha to the hippocampus improves spatial learning in estrogen receptor-alpha knockout mice. Mol Ther. 2008;16:1587–1593. doi: 10.1038/mt.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fridovich I. Superoxide dismutases: regularities and irregularities. Harvey Lect. 1983;79:51–75. [PubMed] [Google Scholar]
- 19.Fullerton HJ. Ditelberg JS. Chen SF. Sarco DP. Chan PH. Epstein CJ. Ferriero DM. Copper/zinc superoxide dismutase transgenic brain accumulates hydrogen peroxide after perinatal hypoxia ischemia. Ann Neurol. 1998;44:357–364. doi: 10.1002/ana.410440311. [DOI] [PubMed] [Google Scholar]
- 20.Gahtan E. Auerbach JM. Groner Y. Segal M. Reversible impairment of long-term potentiation in transgenic Cu/Zn-SOD mice. Eur J Neurosci. 1998;10:538–544. doi: 10.1046/j.1460-9568.1998.00058.x. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez A. Granados MP. Pariente JA. Salido GM. H2O2 mobilizes Ca2+from agonist- and thapsigargin-sensitive and insensitive intracellular stores and stimulates glutamate secretion in rat hippocampal astrocytes. Neurochem Res. 2006;31:741–750. doi: 10.1007/s11064-006-9078-y. [DOI] [PubMed] [Google Scholar]
- 22.Halliwell B. Reactive oxygen species and the central nervous system. J Neurochem. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- 23.Hu D. Klann E. Thiels E. Superoxide dismutase and hippocampal function: age and isozyme matter. Antioxid Redox Signal. 2007;9:201–210. doi: 10.1089/ars.2007.9.201. [DOI] [PubMed] [Google Scholar]
- 24.Jang YC. Perez VI. Song W. Lustgarten MS. Salmon AB. Mele J. Qi W. Liu Y. Liang H. Chaudhuri A, et al. Overexpression of Mn superoxide dismutase does not increase life span in mice. J Gerontol. 2009;64:1114–1125. doi: 10.1093/gerona/glp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamsler A. Segal M. Paradoxical actions of hydrogen peroxide on long-term potentiation in transgenic superoxide dismutase-1 mice. J Neurosci. 2003;23:10359–10367. doi: 10.1523/JNEUROSCI.23-32-10359.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamsler A. Segal M. Hydrogen peroxide modulation of synaptic plasticity. J Neurosci. 2003;23:269–276. doi: 10.1523/JNEUROSCI.23-01-00269.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamsler A. Avital A. Greenberger V. Segal M. Aged SOD overexpressing mice exhibit enhanced spatial memory while lacking hippocampal neurogenesis. Antioxid Redox Signal. 2007;9:181–189. doi: 10.1089/ars.2007.9.181. [DOI] [PubMed] [Google Scholar]
- 28.Keller GA. Warner TG. Steimer KS. Hallewell RA. Cu,Zn superoxide dismutase is a peroxisomal enzyme in human fibroblasts and hepatoma cells. Proc Natl Acad Sci U S A. 1991;88:7381–7385. doi: 10.1073/pnas.88.16.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelner MJ. Bagnell R. Montoya M. Estes L. Uglik SF. Cerutti P. Transfection with human copper-zinc superoxide dismutase induces bidirectional alterations in other antioxidant enzymes, proteins, growth factor response, and paraquat resistance. Free Radic Biol Med. 1995;18:497–506. doi: 10.1016/0891-5849(94)00167-i. [DOI] [PubMed] [Google Scholar]
- 30.Knoferle J. Koch JC. Ostendorf T. Michel U. Planchamp V. Vutova P. Tonges L. Stadelmann C. Bruck W. Bahr M, et al. Mechanisms of acute axonal degeneration in the optic nerve in vivo. Proc Natl Acad Sci U S A. 2010;107:6064–6069. doi: 10.1073/pnas.0909794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leutner S. Eckert A. Muller WE. ROS generation, lipid peroxidation and antioxidant enzyme activities in the aging brain. J Neural Transm. 2001;108:955–967. doi: 10.1007/s007020170015. [DOI] [PubMed] [Google Scholar]
- 32.Linden A. Gulden M. Martin HJ. Maser E. Seibert H. Peroxide-induced cell death and lipid peroxidation in C6 glioma cells. Toxicol In Vitro. 2008;22:1371–1376. doi: 10.1016/j.tiv.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Liochev SI. Fridovich I. The effects of superoxide dismutase on H2O2 formation. Free Radic Biol Med. 2007;42:1465–1469. doi: 10.1016/j.freeradbiomed.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 34.Liu R. Liu IY. Bi X. Thompson RF. Doctrow SR. Malfroy B. Baudry M. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc Natl Acad Sci U S A. 2003;100:8526–8531. doi: 10.1073/pnas.1332809100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malinska D. Kudin AP. Debska-Vielhaber G. Vielhaber S. Kunz WS. Chapter 23 Quantification of superoxide production by mouse brain and skeletal muscle mitochondria. Methods Enzymol. 2009;456:419–437. doi: 10.1016/S0076-6879(08)04423-6. [DOI] [PubMed] [Google Scholar]
- 36.Massaad CA. Klann E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid Redox Signal. 2011;14:2013–2054. doi: 10.1089/ars.2010.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Navarro A. Sanchez Del Pino MJ. Gomez C. Peralta JL. Boveris A. Behavioral dysfunction, brain oxidative stress, and impaired mitochondrial electron transfer in aging mice. Am J physiol Regul Integr Comp Physiol. 2002;282:R985–R992. doi: 10.1152/ajpregu.00537.2001. [DOI] [PubMed] [Google Scholar]
- 38.Nicolle MM. Gonzalez J. Sugaya K. Baskerville KA. Bryan D. Lund K. Gallagher M. McKinney M. Signatures of hippocampal oxidative stress in aged spatial learning-impaired rodents. Neuroscience. 2001;107:415–431. doi: 10.1016/s0306-4522(01)00374-8. [DOI] [PubMed] [Google Scholar]
- 39.Noack H. Lindenau J. Rothe F. Asayama K. Wolf G. Differential expression of superoxide dismutase isoforms in neuronal and glial compartments in the course of excitotoxically mediated neurodegeneration: relation to oxidative and nitrergic stress. Glia. 1998;23:285–297. doi: 10.1002/(sici)1098-1136(199808)23:4<285::aid-glia1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 40.Pellmar TC. Hollinden GE. Sarvey JM. Free radicals accelerate the decay of long-term potentiation in field CA1 of guinea-pig hippocampus. Neuroscience. 1991;44:353–359. doi: 10.1016/0306-4522(91)90060-2. [DOI] [PubMed] [Google Scholar]
- 41.Perez VI. Bokov A. Van Remmen H. Mele J. Ran Q. Ikeno Y. Richardson A. Is the oxidative stress theory of aging dead? Biochim Biophys Acta. 2009;1790:1005–1014. doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rohrdanz E. Schmuck G. Ohler S. Tran-Thi QH. Kahl R. Changes in antioxidant enzyme expression in response to hydrogen peroxide in rat astroglial cells. Arch Toxicol. 2001;75:150–158. doi: 10.1007/s002040000206. [DOI] [PubMed] [Google Scholar]
- 43.Shacter E. Quantification and significance of protein oxidation in biological samples. Drug Metab Rev. 2000;32:307–326. doi: 10.1081/dmr-100102336. [DOI] [PubMed] [Google Scholar]
- 44.Shetty PK. Huang FL. Huang KP. Ischemia-elicited oxidative modulation of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 2008;283:5389–5401. doi: 10.1074/jbc.M708479200. [DOI] [PubMed] [Google Scholar]
- 45.Wijeratne SS. Cuppett SL. Schlegel V. Hydrogen peroxide induced oxidative stress damage and antioxidant enzyme response in Caco-2 human colon cells. J Agric Food Chem. 2005;53:8768–8774. doi: 10.1021/jf0512003. [DOI] [PubMed] [Google Scholar]
- 46.Wu K. Meyers CA. Guerra NK. King MA. Meyer EM. The effects of rAAV2-mediated NGF gene delivery in adult and aged rats. Mol Ther. 2004;9:262–269. doi: 10.1016/j.ymthe.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 47.Zeier Z. Madorsky I. Xu Y. Ogle WO. Notterpek L. Foster TC. Gene expression in the hippocampus: regionally specific effects of aging and caloric restriction. Mech Ageing Dev. 2011;132:8–19. doi: 10.1016/j.mad.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]