Abstract
We investigated whether the combined treatment of 17-(allylamino)-17-demethoxygeldanamycin (17-AAG), an inhibitor of heat-shock protein 90 (hsp90), and celecoxib, an inhibitor of cyclooxygenase-2, can cooperatively enhance the radiosensitivity of various human cancer cells. Combined treatment with 17-AAG and celecoxib, at clinically relevant concentrations, cooperatively induced radiosensitization in all tested cancer cells, but not in normal cells. Cooperative radiosensitization by the drug combination was also shown in a human tumor xenograft system. We found that ataxia-telangiectasia and rad3-related (ATR) and ataxia-telangiectasia mutated (ATM) are novel client proteins of hsp90. Combined treatment with 17-AAG and celecoxib cooperatively induced downregulation of ATR and ATM. In conclusion, combined treatment with 17-AAG and celecoxib at clinically relevant concentrations may significantly enhance the therapeutic efficacy of ionizing radiation.
Introduction
Radiotherapy is one of the three major cancer treatment modalities, the others being surgery and chemotherapy. Enhancing the radiosensitivity of cancer cells while preserving the health of normal cells is one of the most important tasks in clinical radiobiology, but no single drug able to accomplish this task has been introduced into routine clinical practice despite the development of a number of radiosensitizing agents. In chemotherapy, single chemotherapeutic agents alone are rarely administered to treat cancer patients, but are rather typically used in combination chemotherapy with two or more drugs. Combination chemotherapy enhances the anticancer effects of the individual treatments and decreases their toxicities by requiring lower concentrations of each drug, compared to those when either drug is administered alone. Similarly, single agents may not be able to effectively radiosensitize all the types of diverse-origin human cancers because different types of cancer frequently show very different radiosensitivities, and because tumors use complicated and diverse mechanisms to induce radioresistance (Chistiakov et al., 2008).
Based on the limitations when using a single agent to radiosensitize cancer cells, we hypothesized that simultaneous administration of two or more radiosensitivity-targeted agents may be more effective and efficient in the enhancement of ionizing radiation (IR) effect compared with using a single agent. We previously showed that the combined administration of celecoxib and gefitinib can induce synergistic cytotoxicity and radiosensitization in several cancer cell lines (Park et al., 2006). Therefore, simultaneous administration of several targeted agents may be a useful strategy to develop widely effective and practical radiosensitization tools.
Various exogenously or endogenously derived stresses cause denaturation, aberrant expression, aggregation, or breakdown of cellular proteins. Accumulation of aberrant proteins is associated with a number of human diseases, including cancer (McClellan et al., 2007; Powers et al., 2008). Stress-exposed cells activate several protective modalities to maintain cellular homeostasis or genomic integrity (Stravopodis et al., 2007). The molecular chaperone system, which regulates protein folding, stability, and activity, is a representative of these protective modalities. Heat-shock protein (hsp) 90 is a central component of this complex chaperone system (Powers et al., 2008). Hsp90 is an evolutionarily conserved protein that is expressed in most cells. It is also an abundant protein, making up about 1%–2% of total proteins in cells (Tse et al., 2009). Hsp90 does not act to fold non-native proteins, in contrast to hsp70, but binds to near-native proteins, and regulates cell cycle, meiosis, and cytokinesis when cells are facing environmental stresses (Zhao et al., 2005; Powers et al., 2007).
Recent studies have shown that hsp90 is frequently overexpressed in cancer cells, and that it interacts with many oncogenic client proteins (Tsutsumi et al., 2008; Yano et al., 2008). These client proteins are involved in various signal transduction pathways, cell cycle regulation, growth control, angiogenesis, metastasis, and apoptosis. Hsp90 inhibition can simultaneously block these functions of multiple client proteins. Therefore, hsp90 is an important target for cancer therapy (Powers et al., 2006; Neckers, 2007).
17-(Allylamino)-17-demethoxygeldanamycin, a derivative of geldanamycin (17-AAG), has entered phase II clinical trials as a hsp90 inhibitor. 17-AAG shows more than a 100-fold higher affinity for hsp90 in cancer cells than in normal cells (Powers et al., 2007; Sauvageot et al., 2009). Several studies also showed that 17-AAG induces synergistic anticancer effects when combined with several chemotherapeutic agents or IR (Enmon et al., 2003; Kobayashi et al., 2005).
Cyclooxygenase-2 (COX-2) is an enzyme that mediates biosynthesis of various prostanoids, including prostaglandins from arachidonic acid. COX-2 is rapidly induced by stimuli such as inflammation, cytokines, and growth factors in contrast to cyclooxygenase-1 (COX-1), which is constitutively expressed in cells. COX-2 is frequently overexpressed in malignant tumors in a variety of tissues (Park et al., 2006). COX-2 is also known to be involved in carcinogenic processes through various pathways (Kim et al., 2009). Therefore, COX-2 is also regarded as an important target molecule for cancer treatment as we previously reviewed (Jun et al., 2009; Kim et al., 2009).
COX-2 inhibitors are well known for their anticancer effects. Celecoxib is a COX-2-specific inhibitor that is currently used in clinical settings. Celecoxib is known to exert anticancer and chemo- or radiosensitizing effects by inhibiting various signaling pathways in both COX-2-dependent and -independent manners (Park et al., 2006; Dittmann et al., 2008). In addition, celecoxib interacts synergistically with other targeted agents (Shin et al., 2005; Dittmann et al., 2008).
Interaction between COX-2 and hsp90 has been rarely researched. A few studies showed that hsp90 inhibitors induce hsp70 expression, whereas chronic COX-2 inhibition reduces hsp70 expression (Neuhofer et al., 2004; Powers et al., 2008). These results suggest that hsp90 and COX-2 may be related to each other via hsp70. In addition, since hsp90 has many client proteins, many of which are related to tumor growth, and COX-2 also interacts with many critical tumor-related proteins in cells, these two molecules may share one or more critical proteins as their target. Taken together with the observations that both celecoxib and 17-AAG have radiosensitizing effects, these findings suggest the possibility that, when administered concurrently, 17-AAG and celecoxib may cooperatively interact to show synergistic anticancer effects or radiosensitization. In the present study, we investigated whether concurrent treatment of 17-AAG and celecoxib induces cooperative radiosensitizing effects in human cancer cells.
Materials and Methods
Materials
Celecoxib was provided from Pfizer, Inc. 17-AAG and geldanamycin (GA) were purchased from Stressgen. Cycloheximide was purchased from Sigma.
Cell culture
A549, NCI-H460 lung cancer cell lines, BEAS-2B, an immortalized bronchial epithelial cell line, and HCT-116 colon cancer cells were purchased from American Type Culture Collection (ATCC). MOR-P lung cancer cells were kindly provided by Dr. Y.M. Zhu (The University of Sheffield, UK).
in vitro clonogenic assay
To measure cytotoxicity, the cells were exposed to a vehicle (0.1% DMSO) or to graded doses of 17-AAG, celecoxib, or IR for 72 h at 37°C. To determine radiosensitizing effects, the cells were preincubated with 17-AAG or celecoxib at indicated concentrations for 4 h, exposed to graded doses of IR, and then further incubated for 68 h at 37°C. Thereafter, processes were followed as described previously (Park et al., 2006).
Radiation enhancement ratio and synergy analysis
Radiation enhancement ratio (RER) for 17-AAG, celecoxib, or the combination of both drugs was determined at surviving fraction (SF)0.1 and SF0.25 from clonogenic survival assays as described previously (Park et al., 2006). Synergisms for three agent combinations were analyzed using two different methods, median-drug effect analysis (Chou, 2006) and drug-independent action model analysis (Berenbaum, 1978; Park et al., 2006). These are representative two models for synergy analysis and can also substitute for isobolographic analysis when used concurrently because these two models are basis of mode I and II lines of envelope of additivity in isobologram (Steel and Peckham, 1979). In addition, the isobolographic analysis becomes complicated to express when more than two agents are concurrently treated. Combination index (CI) for median drug effect analysis was evaluated as described previously (Berenbaum, 1978; Chou, 2006). CI is indicated that CI <1 is synergistic, CI=1 is additive, and CI >1 is antagonistic. In independent action model analysis, observed SF (OSF) was obtained from the results of clonogenic assays after treatment with 17-AAG and celecoxib with IR, and expected SF (ESF) was calculated using independent action model, and then OSF and ESF were statistically compared as previously described (Park et al., 2006).
Western blot analysis
Western blot was performed as described previously (Kim et al., 2009). The antibodies used for western blot were as follows: ataxia telangiectasia and rad3-related (ATR; Santa Cruz Biotechnology), ataxia-telangiectasia mutated (ATM; Novus), β-actin (Sigma), and phospho-Tyr15-Cdk1 and Cdk1 (Cell Signaling Technology). All experiments were performed at least in triplicate. The levels of ATR, ATM, and phospho-Tyr15-Cdk1 proteins were quantified using Multi Gauge V3.0 program (FUJIFILM) and normalized by β-actin.
Reverse transcription–polymerase chain reaction
Total cellular RNA extraction and reverse transcription were as described previously (Kim et al., 2009). We conducted polymerase chain reaction (PCR) using primers for ATR (forward primer: 5′ AAACTGACTCTCAGCCAACCTC 3′ and reverse primer: 5′ GCATACTCATCAACTGCAAAGG 3′) and ATM (forward primer: 5′ GAGGTGCAAAAAAAGTCTTTTG 3′ and reverse primer: 5′ CTGAGATTTCGTTTGCATTCT 3′) in a PCR machine (GeneAmp PCR System 9700; Applied Biosystems). The mRNA levels of ATR and ATM were also quantified using Multi Gauge V3.0 program and normalized by GAPDH.
Establishment of human tumor xenograft and tumor growth delay assay in vivo
All animal studies were carried out following protocols approved by Laboratory Animal Research Center in Samsung Medical Center. The nude mice (BALBC nu/nu) aged 7–8 weeks (Orient) were housed in sterile filter-capped cages, fed, and watered. All handling and implantation procedures before IR were conducted in a laminar-flow biosafety hood.
The mice were divided into eight groups with similar average weight (five mice per group) and 4×106 NCI-H460 cells suspended in 50 μL of RPMI were injected into the subcutaneous tissue of right hind leg of each mouse. Ten days after implantation, tumor-bearing mice were intraperitoneally injected with vehicle (equal volume DMSO), celecoxib (15 mg/kg body weight), 17-AAG (40 mg/kg body weight), or drug combination (15 mg/kg celecoxib+40 mg/kg 17-AAG) for 7 consecutive days. From 2nd day of drug treatment, mice in IR-combined groups were irradiated with 2 Gy on the tumor-bearing right-hind leg for 5 consecutive days (total dose was 10 Gy). Changes in tumor size and body weight were measured every other day. Tumor volume was determined by the formula V=LW2/2, where V is volume of tumor, L is longest length of tumor, and W is width (perpendicular length to the L) of the tumor. Enhancement factor (EF) for 17-AAG, celecoxib, or the combination of both drugs was determined as previously described (Pyo et al., 2001): EF=(GDdrug+xIR–GDdrug)/GDxIR. In this formula, “drug+xIR” represents combined treatment of 17-AAG+IR, celecoxib+IR, or 17-AAG+celecoxib+IR; “drug” represents 17-AAG or celecoxib alone treatment, or combination of both drugs; and “xIR” represents radiation plus vehicle treatment. Growth delay (GD) was calculated as the time for treated tumors to reach an average volume of 0.6 or 0.8 cm3 minus the time for control tumors to reach 0.6 or 0.8 cm3.
Statistical analysis
Data from the clonogenic assay were also calculated as±SD or SE via the pooling of the results of three independent experiments. The data were analyzed by Student's t-test to compare the two groups. Data from animal experiments were expressed as mean±SE.
Results
Lung cancer cells are more susceptible to 17-AAG and celecoxib than is a normal cell line
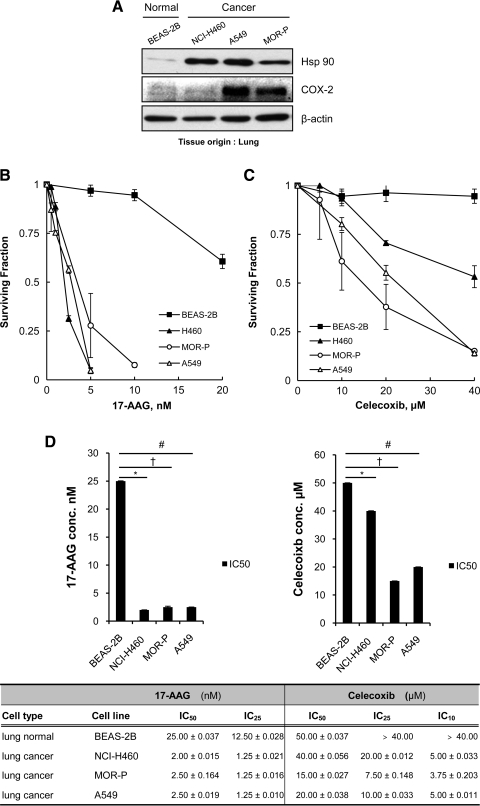
We compared expression levels of hsp90 and COX-2 in a normal cell line and cancer cells. Hsp90 overexpression was observed in all lung cancer cells (NCI-H460, A549, and MOR-P) in contrast to a normal cell line (BEAS-2B). COX-2 overexpression was found in A549 and MOR-P cells but not in NCI-H460 and BEAS-2B cells (Fig. 1A).
FIG. 1.
Lung cancer cells are more susceptible to 17-(allylamino)-17-demethoxygeldanamycin (17-AAG) and celecoxib than a normal cell line. (A) Basal heat-shock protein 90 (hsp90) and cyclooxygenase-2 (COX-2) expression levels were compared between a normal bronchial cell line (BEAS-2B) and lung cancer cells (NCI-H460, A549, and MOR-P) by western blot analysis. (B, C) Cells were treated with 17-AAG or celecoxib at indicated concentrations for 72 h, and then cell survival was monitored by clonogenic assay. (D) Inhibitory concentrations (IC) of each drug were determined from the data with clonogenic assays. Each IC50 value of 17-AAG and celecoxib was statistically compared between normal and cancer cells. 17-AAG: *p=0.010; †p=0.031; #p=0.006. Celecoxib: *p=0.049; †p=0.027; #p=0.045. All experiments were performed at least in triplicate and error bars represent±SE.
To determine the effect of each inhibitor in tested normal and cancer cells, cells were treated with 17-AAG or celecoxib at various concentrations for 72 h, and SFs were obtained by clonogenic assay. 17-AAG and celecoxib showed greater cytotoxic effects in lung cancer cells compared to a normal cell line (Fig. 1B, C).
Based on the data from clonogenic survival analyses, we determined the inhibitory concentration (IC) for each drug (Fig. 1D). IC50 values of 17-AAG in vitro were below plasma concentration (≤10 μM) (Modi et al., 2007) in all tested normal or cancer cells. IC50 values of 17-AAG in lung cancer cells were 10–12.5 times lower than values for the drug in a normal cell line (2.00–2.50 nM vs. 25.00 nM). In contrast, IC50 values of celecoxib in vitro were determined to be above plasma concentration (≥10.00 μM) (Davies et al., 2000) in all tested cells. IC10 values of celecoxib were below plasma concentration in all cancer cells, but not in BEAS-2B cells (3.75–5.00 μM vs. >40.00 μM). These results suggest that lung cancer cells may be more susceptible to 17-AAG and celecoxib compared to normal bronchial epithelial cells.
Combined treatment of 17-AAG and celecoxib increases cytotoxicity cooperatively in cancer cells but not in a normal cell line
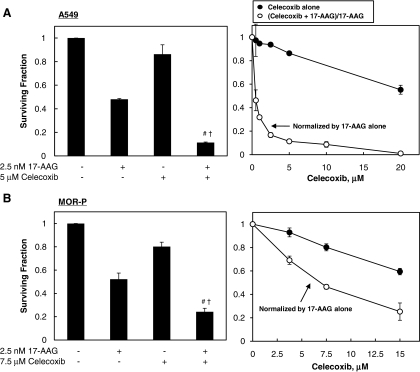
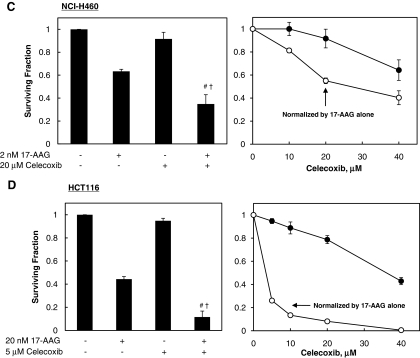
We measured the cytotoxic effect of combination treatment with 17-AAG and celecoxib. Cells were treated with either 17-AAG at IC50 or celecoxib at the indicated concentrations, or with combinations of both drugs for 72 h, and then SFs were determined by clonogenic assay. The SFs of the combined treatment groups were normalized by the SF from cells treated with 17-AAG alone to show the degree of cooperative cytotoxic effect between the drugs (Fig. 2A–E). The combined treatments of 17-AAG and celecoxib enhanced cell death in all tested cancer cells probably in a more than additive manner, but only in an additive manner in a normal cell line (Fig. 2).
FIG. 2.
The combined treatment of 17-AAG and celecoxib increases cytotoxicity cooperatively in cancer cells but not in a normal cell line. (A–E) Cells were treated with 17-AAG at IC50 for cancer cells (A549, MOR-P, NCI-H460, or HCT-116) and at 2.5 nM (the highest concentration among IC50 of tested cancer cell lines) for the normal BEAS-2B cell line, and celecoxib at indicated concentrations for 72 h and then surviving fraction (SF) was determined by clonogenic assay. The SFs of combined treatment groups with 17-AAG and celecoxib were normalized by that of 17-AAG alone. (F) Cooperatively cytotoxic effects for 17-AAG and celecoxib combination were analyzed by comparing clonogenic death fractions by combined treatment (C) and sum of death fractions by each drug treatment (A+B). All experiments were performed at least in triplicate and error bars represent±SE. “#” and “†,” indicate statistically significant (p<0.05) cooperative effects by combined treatment of 17-AAG and celecoxib when compared to each 17-AAG and celecoxib alone, respectively. ns, nonspecific effect.
To quantitatively assess the cooperative cytotoxic effect of two drugs, we further analyzed clonogenic assay data using three different analytic methods: (1) analysis using an independent action model, (2) analysis using the median-drug effect method (CI value), and (3) statistical comparison of SFs from combined drug treatment group with sum of SFs from either drug alone treatment. The results of analysis by independent action model and statistical comparison between SF(drug combination) and sum of SF(either agent alone)s showed that the combined treatments of 17-AAG and celecoxib could cooperatively induce cell death (ESF/OSF >1 in Table 1 and C >A+B in Fig. 2F) in all cancer cell lines tested (A549, MOR-P, H460, and HCT-116), but not in a normal cell line (BEAS-2B). The analysis results by median-drug effect method also showed that 17-AAG+celecoxib synergistically enhances cell death in three cancer cell lines (CIs were <1 in A549, MOR-P, and HCT-116) (Table 1). However, CI values for NCI-H460 cells were >1 (antagonistic), and for BEAS-2B cells were 0.57 (synergistic) after combined drug treatment (Table 1). In summary, three out of four cancer cell lines showed synergistic cell death by 17-AAG+celecoxib combination treatment according to all three analytical methods. At the same time, a normal cell line showed additive cell death by the same drug combination according to two analytical methods, but the median-drug effect method showed synergistic cell death in these cells.
Table 1.
Synergism Analyses for Triple Combined Treatment with 17-AAG and Celecoxib with Radiation According to the Independent Action Model and Median-Drug Effect Method in Four Cancer Cell Lines and One Normal Cell Line
| |
Independent action model |
Median-drug effect method |
|||||
|---|---|---|---|---|---|---|---|
| Treatment group | ESF | OSF | Ratio (ESF/OSF) | p-Value | Synergism | CI | Synergism |
| BEAS-2B cells | |||||||
| 2.5 nM 17-AAG+20 μM Celx | 0.809±0.037 | 0.769±0.083 | 1.05 | 0.426 | No | 0.57±0.108 | Yes |
| 2.5 nM 17-AAG+5 μM Celx+2 Gy IR | 0.455±0.062 | 0.515±0.026 | 0.88 | 0.340 | No | 1.43±0.119 | Antagonism |
| 2.5 nM 17-AAG+20 μM Celx+2 Gy IR | 0.276±0.016 | 0.266±0.012 | 1.04 | 0.240 | No | 1.00±0.011 | Additive |
| A549 cells | |||||||
| 2.5 nM 17-AAG+5 μM Celx | 0.468±0.007 | 0.115±0.008 | 4.07 | 0.001 | Yes | 0.62±0.031 | Yes |
| 2.5 nM 17-AAG+1 μM Celx+2 Gy IR | 0.250±0.015 | 0.095±0.010 | 2.62 | 0.100 | Marginally yes | 0.97±0.011 | Yes |
| 2.5 nM 17-AAG+2.5 μM Celx+2 Gy IR | 0.245±0.004 | 0.048±0.007 | 5.12 | 0.010 | Yes | 0.91±0.009 | Yes |
| 2.5 nM 17-AAG+20 μM Celx+2 Gy IR | 0.166±0.001 | 0.008±0.003 | 21.96 | 0.010 | Yes | 0.93±0.014 | Yes |
| MOR-P cells | |||||||
| 2.5 nM 17-AAG+7.5 μM Celx | 0.419±0.025 | 0.242±0.021 | 1.73 | 0.050 | Yes | 0.64±0.005 | Yes |
| 2.5 nM 17-AAG+3.75 μM Celx+2 Gy IR | 0.198±0.011 | 0.059±0.004 | 3.35 | 0.030 | Yes | 0.71±0.005 | Yes |
| 2.5 nM 17-AAG+7.5 μM Celx+2 Gy IR | 0.160±0.003 | 0.025±0.0003 | 6.28 | 0.010 | Yes | 0.70±0.026 | Yes |
| 2.5 nM 17-AAG+20 μM Celx+2 Gy IR | 0.073±0.001 | 0.007±0.003 | 10.33 | 0.010 | Yes | 0.78±0.009 | Yes |
| NCI-H460 cells | |||||||
| 2.0 nM 17-AAG+20 μM Celx | 0.530±0.001 | 0.348±0.035 | 1.52 | 0.019 | Yes | 1.11±0.043 | Antagonism |
| 2.0 nM 17-AAG+5 μM Celx+2 Gy IR | 0.241±0.002 | 0.056±0.006 | 4.31 | 0.010 | Yes | 0.85±0.001 | Yes |
| 2.0 nM 17-AAG+20 μM Celx+2 Gy IR | 0.206±0.009 | 0.020±0.001 | 10.31 | 0.030 | Yes | 0.81±0.017 | Yes |
| HCT-116 cells | |||||||
| 20.0 nM 17-AAG+5 μM Celx | 0.447±0.030 | 0.146±0.006 | 3.07 | 0.027 | Yes | 0.56±0.005 | Yes |
| 20.0 nM 17-AAG+5 μM Celx+2 Gy IR | 0.205±0.002 | 0.020±0.001 | 10.48 | 0.003 | Yes | 0.81±0.003 | Yes |
| 20.0 nM 17-AAG+20 μM Celx+2 Gy IR | 0.183±0.003 | 0.008±0.003 | 21.87 | 0.002 | Yes | 0.93±0.043 | Yes |
Mean±SE, 17-AAG; IC50, 10 μM≤celecoxib; within plasma concentration, 20 μM celecoxib; over plasma concentration. The p-value was statistically compared between ESF and OSF.
17-AAG, 17-(allylamino)-17-demethoxygeldanamycin; CI; combination index; IR, ionizing radiation; ESF, expected surviving fraction; OSF, observed surviving fraction.
A mathematical model that can be universally applied to all biological cases has not been developed to determine synergy between agents. Synergy (or additivity) is a rather conceptual term and is hard to determine in complicated biological systems (Greco et al., 1995; Peterson and Novick, 2007). Not surprisingly, different calculation models frequently result in conflicting outcomes. Our data also showed slight discrepancies in results of drug–drug cooperation calculated from the two models, and we suggest that the independent action model seemed to fit our data better according to the shape of the graphical data (Fig. 2C, E). Synergistic-looking data may be additive in reality. However, additive-looking data (Fig. 2E) do not seem to be synergistic.
The combined treatment of 17-AAG and celecoxib shows cooperative radiosensitizing effects in cancer cells
We examined whether combined treatments with 17-AAG and celecoxib at clinically achievable concentrations induce cooperative radiosensitizing effects. Cells were incubated with celecoxib at levels below plasma concentration (<5.00–10.00 μM) and/or 17-AAG at IC50 (2.00–2.50 nM) for 4 h, exposed to graded doses of γ-radiation, and then used in a clonogenic assay to determine SF. The SFs of 17-AAG+celecoxib+IR treatment groups were normalized by the SF of the 17-AAG+celecoxib treatment group.
Treatment with either drug alone did not show radiosensitizing effects at these low concentrations for all tested cell lines. However, combined treatments with 17-AAG and celecoxib showed significant radiosensitizing effects in all cancer cells tested (Fig. 3A–D). In contrast, the combined drug treatment did not radiosensitize the normal cell line even when celecoxib was used at an above-plasma concentration (20.00 μM) (Fig. 3E). These results suggest that the combined treatment with 17-AAG and celecoxib can induce cooperative radiosensitization in various types of cancer cells but not in normal cells at below clinically available concentrations, whereas treatment with either drug alone at these low concentrations does not exert a radiosensitizing effect.
FIG. 3.
The combined treatment of 17-AAG and celecoxib shows synergistic radiosensitizing effects in cancer cells but not in a normal cell line. Cells were preincubated with 17-AAG at IC50 or celecoxib at indicated concentrations, or the combination of both drugs for 4 h, exposed to graded doses of γ-radiation, and then SF was determined by clonogenic assay. The SFs of combined groups were normalized by that of drug alone or drug combination. Error bars represent±SE, which was calculated after the pooling of the results of three independent experiments. (A) A549, (B) MOR-P, (C) NCI-H460, (D) HCT116, (E) BEAS-2B.
Next, to further evaluate the cooperative radiosensitizing effect of the two-drug combination treatment, RERs were measured for 17-AAG, celecoxib, or the combination of both drugs at SF0.1 or SF0.25 determined from the clonogenic assays shown in Figure 3. The RERs for the combined treatment of 17-AAG and celecoxib were between 1.3 and 1.6 at SF0.1 and between 1.5 and 1.9 at SF0.25 in the tested cancer cell lines, whereas treatment with either drug alone showed RERs between 0.97 and 1.1 (Table 2). In contrast, RERs for the combined drug treatment in the normal cell line were between 1.06 and 1.15 (Table 2). These results show that the combined treatment of 17-AAG and celecoxib can induce more than moderate-degrees of radiosensitization in the tested cancer cells at very low concentrations of each drug.
Table 2.
Radiation Enhancement Ratio for 17-AAG, Celecoxib, or Combined Drug Treatment at SF0.1 and SF0.25 in Cancer Cells and Normal Bronchial Epithelial BEAS-2B Cells
| Cell line | 17-AAG (IC50) | Celecoxib (≤IC10) | Combined | |
|---|---|---|---|---|
| SF0.1 | A549 | 1.05±0.04 | 1.02±0.08 | 1.44±0.04 |
| MOR-P | 1.00±0.01 | 1.00±0.00a | 1.42±0.04 | |
| NCI-H460 | 1.06±0.11 | 1.02±0.01 | 1.59±0.06 | |
| HCT-116 | 1.07±0.03a | 1.00±0.00 | 1.32±0.01 | |
| BEAS-2B | 1.04±0.02 | 1.01±0.01 | 1.06±0.02 | |
| SF0.25 | A549 | 1.11±0.06 | 1.03±0.10 | 1.72±0.08 |
| MOR-P | 1.00±0.00 | 0.97±0.02a | 1.68±0.01 | |
| NCI-H460 | 1.11±0.13 | 1.03±0.02 | 1.88±0.04 | |
| HCT-116 | 1.02±0.01a | 1.00±0.00 | 1.53±0.02 | |
| BEAS-2B | 1.07±0.01 | 1.00±0.00 | 1.15±0.03 |
IC25, normal BEAS-2B cells were treated with 2.5 nM 17-AAG or 20 μM celecoxib, the highest concentrations among IC50 or IC25 values of each drug for cancer cells.
Potentiation of tumor cell killing effect by triple combined treatment with 17-AAG, celecoxib, and IR
To investigate how much the triple-combination treatment (17-AAG+celecoxib+IR) enhances the cytotoxic effect compared to the additive summation of the effects of each treatment alone, we compared the OSF with the ESF after the triple combination treatment using independent action model (Table 1). In addition, we calculated the CI values using median-drug effect analysis (Table 1). The triple-combination treatment in a normal cell line showed no cooperative effects, which the OSF was almost the same as the ESF, with the ESF/OSF ratio in the range of 0.88–1.04 and CIs also were ≥1. In contrast, cooperative cytotoxic effects were shown in all tested cancer cells, and the highest ESF/OSF ratios at physiologically achievable concentrations in each cancer cell line were between 4.31 and 10.48, and were between 10.31 and 21.96 with higher concentrations of celecoxib. In addition, CIs of all tested cancer cell lines were <1 after triple-combination (Table 1). Independent action model and median-drug effect method showed concordant results after triple-combination treatment. These results suggest that the triple-combined treatment with 17-AAG, celecoxib, and IR may induce cooperatively enhanced cell death in cancer cells but not in normal cells.
Taken together, combined treatment with 17-AAG and celecoxib showed cooperative radiosensitization and enhanced cell death with IR up to 4 to 10 times greater than expected additive values at clinically achievable, very low concentrations of each drug.
ATR and ATM are novel client proteins of hsp90
We recently showed that celecoxib downregulated ATR and that this may be an underlying mechanism for radiosensitization by this drug (Jun et al., 2009). In addition, hsp90 has been shown to have checkpoint kinase 1 (chk1) as a client protein. Chk1 is an important downstream molecule of ATR in the G2 checkpoint to regulate G2 arrest in IR-exposed cells. Recent studies have shown that inhibition or abrogation of the G2/M checkpoint sensitizes cancer cells to chemotherapy or IR (Nomura et al., 2005; Tse et al., 2007). We were curious whether ATR or ATM might be client proteins for hsp90. If so, then it could be hypothesized that cooperative downregulation of ATR and/or ATM by 17-AAG and celecoxib might be able to induce cooperative radiosensitization, and that this may be an underlying mechanism for the synergistic radiosensitization by both drugs in combination.
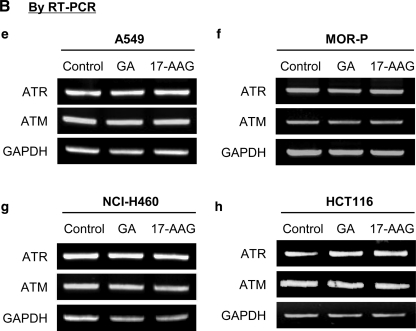
Therefore, we examined whether ATR, ATM, or both could be client proteins of hsp90 using the methods that were previously described (Nomura et al., 2005). Four cancer cell lines were treated with 50 nM GA or 17-AAG for the indicated times (4 or 8 h), and then protein and mRNA levels of ATR and ATM were analyzed by western blot (Fig. 4A) and semiquantitative reverse transcription–PCR (Fig. 4B). The protein levels of ATR and ATM were both significantly decreased by GA and 17-AAG in all cancer cells (Fig. 4A), but mRNA levels were not changed (Fig. 4B), indicating that these molecules could be client proteins of hsp90 (Nomura et al., 2005).
FIG. 4.
The combined treatment 17-AAG and celecoxib cooperatively downregulates protein levels of ataxia-telangiectasia and rad3-related (ATR) and ataxia-telangiectasia mutated (ATM) but not of mRNA. Four cancer cells a, e, and k, A549, b and f, MOR-P, c, g, i, and j. NCI-H460, d and h. HCT116, l, BEAS-2B were incubated with 50 nM geldanamycin (GA) or 17-AAG for indicated times (0, 4, or 8 h) at 37°C. The protein or mRNA levels of ATR or ATM were measured by western blot (A) and by reverse transcription–polymerase chain reaction (RT-PCR) (B), respectively. (C) NCI-H460 cells were incubated with 30 μg/mL cycloheximide with or without 50 nM 17-AAG for 0, 0.5, 1, 2, 4, and 6 h. (D) A549 and BEAS-2B cells were incubated with 2.5 nM 17-AAG, 5 μM celecoxib, or 17-AAG+celecoxib for 24 h at 37°C. The protein levels of ATR, ATM, or β-actin were detected by western blot and quantified using Multi Gauge V3.0 program. All experiments and measurements were done at least in triplicate; 0 h; 0.1% DMSO alone treated.
To further verify whether ATR and ATM are novel client proteins of hsp90, NCI-H460 cells were treated with cycloheximide with or without 17-AAG. The protein levels of ATR and ATM were decreased more rapidly in cells treated with 17-AAG and cycloheximide than in cycloheximide alone-treated cells (Fig. 4C). HCT-116 and MOR-P cells also showed similar patterns to NCI-H460 cells when they were treated with cycloheximide alone or cycloheximide+17-AAG (Supplementary Fig. S1A, B; Supplementary Data are available online at www.liebertonline.com/dna). These results indicate that ATR and ATM may be novel client proteins of hsp90.
Cooperative downregulation of ATR and ATM by 17-AAG and celecoxib
To investigate whether ATR and ATM were cooperatively downregulated by 17-AAG and celecoxib, A549 and BEAS-2B cells were incubated with 2.5 nM 17-AAG, 5 μM celecoxib, or a combination of both drugs for 24 h, and then the levels of ATR and ATM were analyzed by western blot (Fig. 4D). Treatment with either drug alone at these low concentrations did not affect the levels of ATR and ATM proteins on both A549 and BEAS-2B cells, but the combined treatment of both drugs downregulated protein levels of ATR and ATM on A549 cells (Fig. 4D–k) but not BEAS-2B cells (Fig. 4D–l). We also compared endogenous protein levels of ATR and ATM in four cancer cell lines that originated from different organs (HCT-116, MOR-P, H460, and MCF-7) and in a normal cell line (BEAS-2B) (Supplementary Fig. S2). ATR was highly expressed in four cancer cell lines compared to normal cells, but ATM levels were not higher than normal cells in all cancer cells. In addition, it has been shown that 17-AAG has more than a 100-fold higher affinity for hsp90 in cancer cells than in normal cells (Powers et al., 2007; Sauvageot et al., 2009). These suggest that the synergistic radiosensitizing effects may be induced by cooperative downregulation of ATR and ATM proteins in cancer cells but not in normal cells after the combined treatment of 17-AAG and celecoxib.
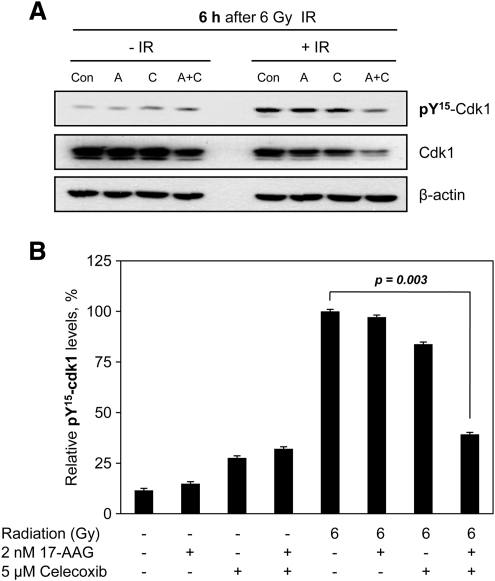
The combined treatment of 17-AAG and celecoxib cooperatively decreases level of pTyr15-Cdk1 after IR treatment
We further investigated whether combined treatment of 17-AAG and celecoxib induces cytotoxicity or radiosensitization via changing cell cycle after downregulating ATR and ATM. The level of pTyr15-Cdk1, a marker for G2 phase, at 6 h after IR exposure was decreased by combined treatment of both drugs, whereas either drug alone-treatment with IR did not change the level of pTyr15-Cdk1, when compared to IR alone (Fig. 5). This result suggests that the combined treatment of 17-AAG and celecoxib may attenuate the IR-induced G2 checkpoint activation, and thus supports our observations of cooperatively downregulating ATR and ATM by combined drug treatment.
FIG. 5.
The combined treatment of 17-AAG and celecoxib cooperatively decreased level of pTyr15-Cdk1 after ionizing radiation (IR) treatment. NCI-H460 lung cancer cells were pretreated with 2 nM 17-AAG, 5 μM celecoxib, or combination of both drugs for 4 h and then exposed or not to 6 Gy IR. The cells were recovered for 6 h and levels of pTyr15–Cdk1 were determined by western blot analysis (A). Densitometric analysis of pTyr15–Cdk1 level (B). Con, 0.1% DMSO control; A, 2 nM 17-AAG, C, 5 μM celecoxib; A+C, 2 nM 17-AAG+5 μM celecoxib.
The combined treatment of 17-AAG and celecoxib cooperatively enhances in vivo tumor GD after IR exposure
To investigate whether the cooperative radiosensitizing effects by 17-AAG plus celecoxib can be shown in vivo system, we performed tumor GD assay using human tumor xenograft in BALB/C nude mice. NCI-H460 human lung cancer cells were injected into the subcutaneous tissue of right hind leg and the tumors were grown in vivo for 10 days. The tumor-bearing mice were treated with celecoxib (15 mg/kg), 17-AAG (40 mg/kg), or combination of both drugs for 7 consecutive days, with or without IR exposure (2 Gy ×5 times).
EF was calculated as described in Materials and Methods. Combined administration of both drugs exerted cooperative enhancement of tumor GD after irradiation compared with either drug treatment alone plus radiation (Fig. 6A). The EFs were >2.0 after combination drug treatment (Fig. 6B).
FIG. 6.
The combined treatment of 17-AAG and celecoxib effectively delayed tumor growth in BALB/C nude mice via enhancing radiosensitivity. (A) NCI-H460 lung cancer cells (4×106 cells/50 μL) were injected into the subcutaneous tissue of the right hind leg as described in Materials and Methods. Tumor-bearing mice were given i.p. with celecoxib (15 mg/kg), 17-AAG (40 mg/kg), or drug combination (celecoxib+17-AAG) for 7 consecutive days after 10 days postimplantation, with or without irradiation on tumor (2 Gy×five times) starting from the next day after drug administration. The mice were monitored every 2–3 days for changes in tumor growth, body weight, and health status. Control groups were given i.p. with equal volume of DMSO. (B) The enhancement factor (EF) ratio was determined at tumor volume 0.6 and 0.8 cm3 as described in Materials and Methods. Error bars represent±SE. The symbols are p<0.05 and represented as follows: * and #—IR alone versus 17-AAG+IR and IR alone versus celecoxib+17-AAG+IR at tumor volume 0.6 cm3; ‡—IR alone versus celecoxib+17-AAG+IR at tumor volume 0.8 cm3.
We monitored common toxicities such as weight loss, change in skin texture, nausea or vomiting, diarrhea, fatigueness, poor feeding, and shortness of breath (Fracasso et al., 2011) until mice were sacrified. However, we could not observe any toxicities described above, including weight loss (Supplementary Fig. S3), in mice in all groups.
These results indicate that 17-AAG and celecoxib also cooperatively enhance radiation effect without increasing side effects in vivo, and good EFs (>2.0) could be acquired after combination of mild- to moderate-degree radiosensitizers.
Discussion
In this study, we investigated whether the combined treatment of 17-AAG and celecoxib can enhance the radiosensitivity of various human lung and colon cancer cells. We found that a combined treatment with 17-AAG and celecoxib could result in cooperative enhancement of cytotoxic and radiosensitizing effects in the tested cancer cell lines, but not in a normal cell line (Fig. 3). These effects were achieved at very low (∼2.00–2.50 nM) and clinically relevant concentrations (∼10 μM) of 17-AAG and celecoxib, respectively. At these low concentrations, combined treatment with 17-AAG and celecoxib showed at least a moderate degree of radiosensitizing effects (RERs of 1.5–1.9 at SF0.25) in all tested cancer cells, whereas neither drug alone caused any radiosensitization at these concentrations (Table 2).
One major issue for this kind of experiment is about synergism among agents (drug, radiation, etc.) for a targeted effect. Many mathematical models have developed to assess the closest additive value for certain agent combinations, because additive value is a conceptually difficult issue to determine. There have been two representative mathematical models to explain additivity and synergism. One is the Loewe additivity model (median-drug effect is based on this model) (Berenbaum, 1978; Chou, 2006) and the other one is the Bliss independence model (Park et al., 2006). However, the status of synergy analysis methodology is that there is no perfect model to be universally applied to all biological cases (Greco et al., 1995; Peterson and Novick, 2007).
In this study, we used these two different model-based calculation methods to quantitatively evaluate the cooperative cytotoxic and radiosensitizing effects after drug combination, but not to determine the real synergism itself between agents, which is rather a complicated and still unsolved scientific issue. The results from the independent action model showed that the total antitumor effect could be enhanced 4 to 10 times that of the expected additive values after triple-combination (17-AAG+celecoxib+IR) treatments (Table 1). The results by the median-drug effect method were generally concordant with the ones from the independent action model. Both calculation methods were well matched in the triple-combination cases.
We also found that 17-AAG and celecoxib could cooperatively enhance the tumor GD by radiotherapy in an in vivo xenograft system (Fig. 6). Good EF (2.4) could be acquired after combination of mild- to moderate-degree radiosensitizers. Taken together, the data indicate that combined treatment of 17-AAG and celecoxib can radiosensitize cancer cells in a cooperative manner in in vivo as well as in vitro systems.
Interestingly, cooperative cytotoxic effects between 17-AAG and celecoxib were strong in all tested cancer cells, but not in a normal cell line (Fig. 2). Hsp90 has many client proteins, including various oncoproteins, and celecoxib is known to have many COX-2-dependent and -independent target molecules. Therefore, our observations may indicate that 17-AAG and celecoxib interact intimately in cells and may share one or more cancer-related target proteins, leading to cooperative cytotoxic effects. Research to identify these common target molecules will not be easy. However, we focused on molecules involved in the G2 checkpoint, especially ATR and ATM, because we previously found that COX-2 profoundly prolongs IR-induced G2 arrest and it is caused by upregulation of ATR by COX-2 (Shin et al., 2005; Kim et al., 2009). In addition, hsp90 also has many client proteins involved in cell cycle regulation, including Chk1, which is directly downstream of ATR. Therefore, we examined whether ATR and ATM are client proteins of hsp90, and identified both ATR and ATM as client proteins of hsp90. Further, we found that the combined treatment with both drugs induced significant downregulation of ATR and ATM at the low concentrations, which were used in the clonogenic assays in the current study, whereas neither drug alone showed any significant change in ATR or ATM level at these concentrations (Fig. 4D–k).
Recently, On et al. (2011) reported that inhibition of ATR/ATM-Chk1/Chk2 pathway by anti-cancer agents can trigger premature mitotic entry and result in mitotic catastrophe that defined as cell death associated with inappropriate entry into mitosis. Prise et al. (2009) also described that inhibition of ATR and ATM increases radiosensitivity in directly irradiated cells. Celecoxib could also downregulate ATR expression at transcription level (Kim et al., 2009). These suggest that, in combined treatment of 17-AAG and celecoxib, 17-AAG degrades ATR/ATM proteins and celecoxib concurrently inhibits transcription of ATR, and it results in cooperative downregulation of endogenous ATR/ATM levels.
We also found that the level of pTyr15-Cdk1, a marker for G2 phase, at 6 h after IR exposure was decreased by combined treatment of both drugs, whereas either drug alone-treatment with IR did not change the level of pTyr15-Cdk1, when compared to IR alone (Fig. 5). This result indicates that the combined treatment of 17-AAG and celecoxib attenuated the IR-induced G2 checkpoint activation and thus supports our observations of synergistically downregulating ATR and ATM by combined drug treatment.
Base on these results, we suggest that synergistic downregulation of ATR and/or ATM by 17-AAG and celecoxib may be related to the underlying mechanism for the synergistic cytotoxic or radiosensitizing effects of these drug combinations.
In summary, we found that combined treatment with 17-AAG and celecoxib at clinically relevant concentrations significantly enhanced the therapeutic efficacy of IR in in vitro/in vivo systems. We also found that cooperative downregulation of ATR and ATM, principal G2/M checkpoint molecules, may be related to the underlying mechanisms for this synergistic radiation-enhancing effect. Our results suggest that the combined-targeted radiosensitization strategy may be an effective and clinically applicable therapeutic tool.
Supplementary Material
Acknowledgments
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (KRF-2009-0074782) and by Samsung Biomedical Research Institute grant, #SBRI C-B1-134-1.
Disclosure Statement
No competing financial interests exist.
References
- Berenbaum M.C. A method for testing for synergy with any number of agents. J Infect Dis. 1978;137:122–130. doi: 10.1093/infdis/137.2.122. [DOI] [PubMed] [Google Scholar]
- Chistiakov D.A. Voronova N.V. Chistiakov P.A. Genetic variations in DNA repair genes, radiosensitivity to cancer and susceptibility to acute tissue reactions in radiotherapy-treated cancer patients. Acta Oncol. 2008;47:809–824. doi: 10.1080/02841860801885969. [DOI] [PubMed] [Google Scholar]
- Chou T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- Davies N.M. McLachlan A.J. Day R.O. Williams K.M. Clinical pharmacokinetics and pharmacodynamics of celecoxib: a selective cyclo-oxygenase-2 inhibitor. Clin Pharmacokinet. 2000;38:225–242. doi: 10.2165/00003088-200038030-00003. [DOI] [PubMed] [Google Scholar]
- Dittmann K.H. Mayer C. Ohneseit P.A. Raju U. Andratschke N.H. Milas L. Rodemann H.P. Celecoxib induced tumor cell radiosensitization by inhibiting radiation induced nuclear EGFR transport and DNA-repair: a COX-2 independent mechanism. Int J Radiat Oncol Biol Phys. 2008;70:203–212. doi: 10.1016/j.ijrobp.2007.08.065. [DOI] [PubMed] [Google Scholar]
- Enmon R. Yang W.H. Ballangrud A.M. Solit D.B. Heller G. Rosen N. Scher H.I. Sgouros G. Combination treatment with 17-N-allylamino-17-demethoxy geldanamycin and acute irradiation produces supra-additive growth suppression in human prostate carcinoma spheroids. Cancer Res. 2003;63:8393–8399. [PubMed] [Google Scholar]
- Fracasso P.M., et al. A phase 1 study of UCN-01 in combination with irinotecan in patients with resistant solid tumor malignancies. Cancer Chemother Pharmacol. 2011;67:1225–1237. doi: 10.1007/s00280-010-1410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco W.R. Bravo G. Parsons J.C. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev. 1995;47:331–385. [PubMed] [Google Scholar]
- Jun H.J. Kim Y.M. Park S.Y. Park J.S. Lee E.J. Choi S.A. Pyo H. Modulation of ionizing radiation-induced G2 arrest by cyclooxygenase-2 and its inhibitor, Celecoxib. Int J Radiat Oncol Biol Phys. 2009;75:225–234. doi: 10.1016/j.ijrobp.2009.04.086. [DOI] [PubMed] [Google Scholar]
- Kim Y.M. Lee E.J. Park S.Y. Cho K.H. Kim J.Y. Pyo H. Cyclooxygenase-2 up-regulates ataxia telangiectasia and Rad3 related through extracellular signal-regulated kinase activation. Mol Cancer Res. 2009;7:1158–1168. doi: 10.1158/1541-7786.MCR-08-0493. [DOI] [PubMed] [Google Scholar]
- Kobayashi S. Nantz R. Kitamura T. Higashikubo R. Horikoshi N. Combined inhibition of extracellular signal-regulated kinases and HSP90 sensitizes human colon carcinoma cells to ionizing radiation. Oncogene. 2005;24:3011–3019. doi: 10.1038/sj.onc.1208508. [DOI] [PubMed] [Google Scholar]
- McClellan A.J. Xia Y. Deutschbauer A.M. Davis R.W. Gerstein M. Frydman J. Diverse cellular functions of the Hsp90 molecular chaperone uncovered using systems approaches. Cell. 2007;131:121–135. doi: 10.1016/j.cell.2007.07.036. [DOI] [PubMed] [Google Scholar]
- Modi S., et al. Combination of trastuzumab and tanespimycin (17-AAG, KOS-953) is safe and active in trastuzumab-refractory HER-2 overexpressing breast cancer: a phase I dose-escalation study. J Clin Oncol. 2007;25:5410–5417. doi: 10.1200/JCO.2007.11.7960. [DOI] [PubMed] [Google Scholar]
- Neckers L. Heat shock protein 90: the cancer chaperone. J Biosci. 2007;32:517–530. doi: 10.1007/s12038-007-0051-y. [DOI] [PubMed] [Google Scholar]
- Neuhofer W. Holzapfel K. Fraek M.L. Ouyang N. Lutz J. Beck F.X. Chronic COX-2 inhibition reduces medullary HSP70 expression and induces papillary apoptosis in dehydrated rats. Kidney Int. 2004;65:431–441. doi: 10.1111/j.1523-1755.2004.00387.x. [DOI] [PubMed] [Google Scholar]
- Nomura M. Nomura N. Yamashita J. Geldanamycin-induced degradation of Chk1 is mediated by proteasome. Biochem Biophys Res Commun. 2005;335:900–905. doi: 10.1016/j.bbrc.2005.07.160. [DOI] [PubMed] [Google Scholar]
- On K.F. Chen Y. Ma H.T. Chow J.P.H. Poon R.Y.C. Determinants of mitotic catastrophe upon abrogation of the G2 DNA damage checkpoint by UCN-01. Mol Cancer Ther. 2011;10:784–794. doi: 10.1158/1535-7163.MCT-10-0809. [DOI] [PubMed] [Google Scholar]
- Park J.S. Jun H.J. Cho M.J. Cho K.H. Lee J.S. Zo J.I. Pyo H. Radiosensitivity enhancement by combined treatment of celecoxib and gefitinib on human lung cancer cells. Clin Cancer Res. 2006;12:4989–4999. doi: 10.1158/1078-0432.CCR-05-2259. [DOI] [PubMed] [Google Scholar]
- Peterson J.J. Novick S.J. Nonlinear blending: a useful general concept for the assessment of combination drug synergy. J Recept Signal Transduct Res. 2007;27:125–146. doi: 10.1080/10799890701417576. [DOI] [PubMed] [Google Scholar]
- Powers M.V. Clarke P.A. Workman P. Dual targeting of HSC70 and HSP72 inhibits HSP90 function and induces tumor-specific apoptosis. Cancer Cell. 2008;14:250–262. doi: 10.1016/j.ccr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Powers M.V. Workman P. Targeting of multiple signalling pathways by heat shock protein 90 molecular chaperone inhibitors. Endocr Relat Cancer. 2006;13(Suppl 1):S125–S135. doi: 10.1677/erc.1.01324. [DOI] [PubMed] [Google Scholar]
- Powers M.V. Workman P. Inhibitors of the heat shock response: biology and pharmacology. FEBS Lett. 2007;581:3758–3769. doi: 10.1016/j.febslet.2007.05.040. [DOI] [PubMed] [Google Scholar]
- Prise K.-M. O'Sullivan J.-M. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer. 2009;9:351–360. doi: 10.1038/nrc2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyo H. Choy H. Amorino G.P. Kim J. Cao Q. Hercules S.K. DuBois R.N. A selective cyclooxygenase-2 inhibitor, NS-398, enhances the effect of radiation in vitro and in vivo preferentially on the cells that express cyclooxygenase-2. Clin Cancer Res. 2001;7:2998–3005. [PubMed] [Google Scholar]
- Sauvageot C.M., et al. Efficacy of the HSP90 inhibitor 17-AAG in human glioma cell lines and tumorigenic glioma stem cells. Neuro Oncol. 2009;11:109–121. doi: 10.1215/15228517-2008-060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y.K. Park J.S. Kim H.S. Jun H.J. Kim G.E. Suh C.O. Yun Y.S. Pyo H. Radiosensitivity enhancement by celecoxib, a cyclooxygenase (COX)-2 selective inhibitor, via COX-2-dependent cell cycle regulation on human cancer cells expressing differential COX-2 levels. Cancer Res. 2005;65:9501–9509. doi: 10.1158/0008-5472.CAN-05-0220. [DOI] [PubMed] [Google Scholar]
- Stravopodis D.J. Margaritis L.H. Voutsinas G.E. Drug-mediated targeted disruption of multiple protein activities through functional inhibition of the Hsp90 chaperone complex. Curr Med Chem. 2007;14:3122–3138. doi: 10.2174/092986707782793925. [DOI] [PubMed] [Google Scholar]
- Steel G.G. Peckham M.J. Exploitable mechanisms in combined radiotherapy-chemotherapy: the concept of additivity. Int J Radiat Oncol Biol Phys. 1979;5:85–91. doi: 10.1016/0360-3016(79)90044-0. [DOI] [PubMed] [Google Scholar]
- Tse A.N. Carvajal R. Schwartz G.K. Targeting checkpoint kinase 1 in cancer therapeutics. Clin Cancer Res. 2007;13:1955–1960. doi: 10.1158/1078-0432.CCR-06-2793. [DOI] [PubMed] [Google Scholar]
- Tse A.N. Sheikh T.N. Alan H. Chou T.C. Schwartz G.K. 90-kDa heat shock protein inhibition abrogates the topoisomerase I poison-induced G2/M checkpoint in p53-null tumor cells by depleting Chk1 and Wee1. Mol Pharmacol. 2009;75:124–133. doi: 10.1124/mol.108.050807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi S. Scroggins B. Koga F. Lee M.J. Trepel J. Felts S. Carreras C. Neckers L. A small molecule cell-impermeant Hsp90 antagonist inhibits tumor cell motility and invasion. Oncogene. 2008;27:2478–2487. doi: 10.1038/sj.onc.1210897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano A. Tsutsumi S. Soga S. Lee M.J. Trepel J. Osada H. Neckers L. Inhibition of Hsp90 activates osteoclast c-Src signaling and promotes growth of prostate carcinoma cells in bone. Proc Natl Acad Sci U S A. 2008;105:15541–15546. doi: 10.1073/pnas.0805354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R., et al. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell. 2005;120:715–727. doi: 10.1016/j.cell.2004.12.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.