Abstract
Aims: To evaluate mechanisms underlying modulation of inflammatory chemokines in primary human keratinocytes (normal human epidermal keratinocytes) and repair-related processes in wound models by plant polyphenols (PPs) with antioxidant and superoxide scavenging properties (verbascoside [Vb], resveratrol [Rv], polydatin [Pd], quercetin [Qr], and rutin). Results: Epidermal growth factor receptor (EGFR)-controlled chemokines CXCL8/interleukin 8 (IL-8), CCL2/monocyte chemotactic protein-1 (MCP-1), and CXCL10/interferon gamma-produced protein of 10 kDa (IP-10) were modulated by transforming growth factor alpha (TGF-α) and by the tumor necrosis factor alpha/interferon gamma combination (T/I). EGFR phosphorylation, nuclear translocation, and downstream cytoplasmic signaling pathways (extracellular regulation kinase [ERK]1/2, p38, STAT3, and PI-3K) were studied. All PPs did not affect TGF-α-induced STAT3 phosphorylation, whereas they suppressed T/I-activated NFkappaB and constitutive and T/I-induced but not TGF-α-induced ERK1/2 phosphorylation. Vb and Qr suppressed total EGFR phosphorylation, but they synergized with TGF-α to enhance nuclear accumulation of phosphorylated EGFR. Vb strongly inhibited TGF-α-induced p38 phosphorylation and T/I-induced NFkappaB and activator protein-1 (AP-1) binding to DNA. Vb was an effective inhibitor of T/I-stimulated chemokine synthesis, and it accelerated scratch wound healing in vitro. Anti-inflammatory and wound healing activities of Vb were confirmed in vivo in the full-thickness excision wound. Although Pd and Rv did not affect EGFR activation/translocation, they and Qr synergized with TGF-α and T/I in the induction of IL-8 transcription/synthesis while opposing enhanced MCP-1 and IP-10 transcription/synthesis connected with pharmacologically impaired EGFR functioning. Innovation: PPs perturb the EGFR system in human keratinocytes, and this effect may be implicated in the regulation of inflammatory and repair-related processes in the skin. Conclusion: Anti-inflammatory and wound healing effects of PPs depend on their interaction with EGFR-controlled cytoplasmic and nuclear pathways rather than on their direct redox properties. Antioxid. Redox Signal. 16, 314–328.
Introduction
Keratinocytes are not only primary sensors of stressful conditions but also major players of the extremely complex response in the skin conducting an orchestrated recruitment and functions of the immune cells, fibroblasts, and vascular cells involved in the inflammatory responses and wound healing (30, 38). Epidermal growth factor receptor (EGFR) located on the cellular membrane of keratinocytes is widely recognized as a key regulator of numerous essential processes underlying skin development, homeostasis, and repair (30, 35, 38, 39). EGFR belongs to a group of membrane bound receptor tyrosine kinases with extracellular ligand-binding domain and cytoplasmic domain possessing intrinsic protein kinase activity (1, 25, 26, 31). EGFR is expressed through all layers of human epidermis with the strongest presence in the basal layer of epidermal keratinocytes (2, 10, 24–27).
In keratinocytes, EGFR can be activated by disparate mechanisms under physiological or pathological conditions by specific pre-existing ligands, such as epidermal growth factor (EGF), transforming growth factor alpha (TGF-α), etc., and via matrix metalloproteinase (MMP)-mediated cleavage of mature ligands from their membrane-bound precursors (25, 30). The autocrine formation of EGFR ligands may be stimulated by the proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and interferon gamma (IFN-γ) (24, 26, 27, 29). Many of the proinflammatory cytokines that are produced upon stimulation with TNF-α/IFN-γ depend on the activation of transcription factors nuclear factor kappa B (NFκB) and activator protein-1 (AP-1) by redox mechanisms (4, 11, 29). Upon activation, the cytoplasmic domain of EGFR dimer undergoes auto- or trans-phosphorylation of distinct tyrosine residues that serve as docking sites for cytoplasmic signal transduction proteins (1, 24, 28, 30, 43). Several lines of evidence suggest the existence of two modes of EGFR signaling. Traditional cytoplasmic EGFR route involves transduction of mitogenic signals through activation of numerous signaling cascades, such as phospholipase C-gamma-protein kinase C, Ras-Raf-mitogen-activated protein kinases (MAPKs), phosphatidylinositol-3-kinase (PI3K)-protein kinase B (Akt), and signal transducer and activator of transcription (STATs) (1, 5, 22, 24, 25). In the nuclear pathway, activated EGFR undergoes fast nuclear translocation, where it physically or functionally interacts with other transcription factors possessing DNA-binding activity and STAT3, that leads to upregulation of distinct genes controlling cell proliferation and DNA repair (20, 24, 44). Both EGFR pathways can be activated in a ligand-free manner, for example, by UV and ionizing radiation, by heat, cisplatin, and H2O2 (8, 22, 24, 25, 30, 43, 44). It seems that cytoplasmic EGFR mechanism mainly controls adaptive inflammatory/stress responses in keratinocytes, whereas the nuclear pathway is involved in keratinocyte proliferation and motility and in their resistance to oxidative stress and heat shock (8, 24, 25). A number of publications from our group (26, 27, 29) has shown that EGFR controls expression of inflammatory chemokines (CXCL8/interleukin 8 [IL-8], CCL2/monocyte chemotactic protein-1 [MCP-1], CXCL10/interferon gamma-induced protein of 10 kDa [IP-10], CCL5/regulated upon activation, normal T-cell expressed [RANTES]) and granulocyte-macrophage growth factor in normal human keratinocytes, and EGFR impairment accounts for overexpression of distinct chemokines in the skin affected by chronic inflammation. It has also been suggested that adverse skin reactions (inflammation and hyperkeratosis) to anti-EGFR antitumor therapies may be a consequence of the impaired control of suppressed EGFR over keratinocyte proliferation and their inflammatory responses (10, 25, 29, 30, 46). Although EGFR involvement in cancer cell proliferation and their chemo-resistance attracts major attention (24), there is substantial evidence that EGFR system is essential to guide keratinocyte proliferation, migration, and survival to maintain normal skin homeostasis or restore epidermal integrity after wounding. Indeed, skin wounding releases EGFR ligands (21) and they potentiate keratinocyte migration (2). The application of EGFR ligands accelerates wound healing (39), whereas pharmacological (28) or genetic (35) EGFR impairment leads to retardation of wound closure/epithelization.
Innovation.
Traditionally, anti-inflammatory activity of plant polyphenols (PPs) is ascribed to their direct antioxidant and free radical scavenging activity. Their wound healing effect is often seen as a consequence of suppression of acute inflammation. The in vitro experiments are usually carried out on immortalized or tumor cell lines, which bear significant molecular peculiarities as compared with normal cells. Here, we used primary human keratinocytes for mechanistic studies. We showed for the first time that PPs, depending on the chemical structure of polyphenolic core and the presence of sugar moieties, time-dependently up- and downregulated proinflammatory and repair-regulating chemokines and accelerated in vitro and in vivo wound healing through their interaction with different cytoplasmic and nuclear components of epidermal growth factor receptor (EGFR) system. No correlation with PPs antioxidant/superoxide scavenging properties was found. Very first experimental evidence was obtained on the involvement of EGFR nuclear translocation and retention as possible molecular targets for the skin inflammation/repair modulating PPs.
There is a continuously growing interest in beneficial effects of plant polyphenols (PPs) toward human skin: anti-inflammatory, wound healing, cancer preventing, antiage, etc. The skin benefits of PPs have been traditionally attributed to their chain-breaking antioxidant or free radical scavenging activities (6, 14). However, evidence from numerous in vitro skin cell studies suggests that PPs can influence cellular functions by multiple other mechanisms, such as interaction with several receptors, modulation of signal transduction and transcription of a number of genes, post-translational modulation of enzymatic activities (9, 12, 16, 18, 19, 31, 33, 36, 40), and epigenetic regulation of gene expression (42). Although most of these functions are redox-dependent, their modulation does not compulsory depend on direct antioxidant/free radical scavenging/metal chelating properties of PPs (15, 18).
Here, we focused on the elucidation of EGFR-related mechanisms underlying modulation of inflammatory responses and skin repair processes by selected PPs-antioxidants belonging to three major groups: flavonoids, stilbenes, and phenylpropanoids. The pairs of glycosylated and nonglycosylated flavonoids (rutin [Rt] and quercetin [Qr], respectively), glycosylated and nonglycosylated forms of stilbenoids (polydatin [Pd] and resveratrol [Rv], respectively), and glycosylated phenylpropanoid verbascoside (Vb) (Fig. 1) were studied. Primary cultures of human keratinocytes were used for mechanistic studies and the in vitro data were confirmed in the animal model of excision wound. We found that PPs could differentially affect cytoplasmic and nuclear parts of EGFR system. These effects did not correspond to antioxidant or superoxide scavenging capacities of PPs.
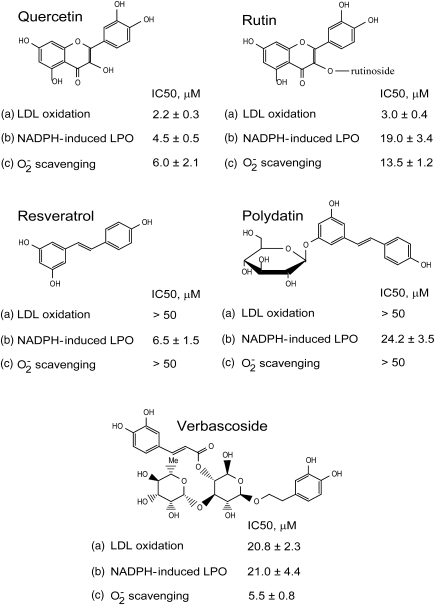
FIG. 1.
Chemical structures and antioxidant and superoxide scavenging properties of plant polyphenols (PPs) studied. Concentrations of 50% inhibition (IC50, μM) of the reactions: (a) human low-density lipoprotein (LDL) oxidation by (myeloperoxidase [MPO]+H2O2) and nitrite; (b) NADPH-induced lipid peroxidation (LPO) in rat liver microsomes; (c) superoxide production in photo-oxidation of riboflavin assessed by nitroblue tetrazolium reduction.
Results
Antioxidant and superoxide scavenging properties of selected PPs
Antioxidant properties of PPs were closely tested in two in vitro systems resembling free radical-driven oxidation under physiological conditions, namely, low-density lipoprotein (LDL) oxidation by myeloperoxidase (MPO)+H2O2 in the presence of nitrite and NADPH-induced lipid peroxidation (LPO) in microsomes. Two flavonoids, Rt and its aglycon Qr were the most effective inhibitors of LDL oxidation, whereas Vb was 10 times less effective, and both stilbenes Rv and Pd did not inhibit the reaction up to 50 μM concentration (Fig. 1). On contrast, two aglycons, Qr and Rv inhibited LPO at low micromolar concentrations, and three glycosylated PPs, Rt, Pd, and Vb were less effective. To avoid the interference of PPs with xanthine oxidase routinely applied for the in vitro superoxide generation, we used the reaction of photo-oxidation of riboflavin as a source of O2−, and the scavenging action changed in the range: Qr=Vb > Rt > Rv=Pd.
PPs modulate constitutive EGFR-extracellular regulation kinase signal transduction and chemokine expression
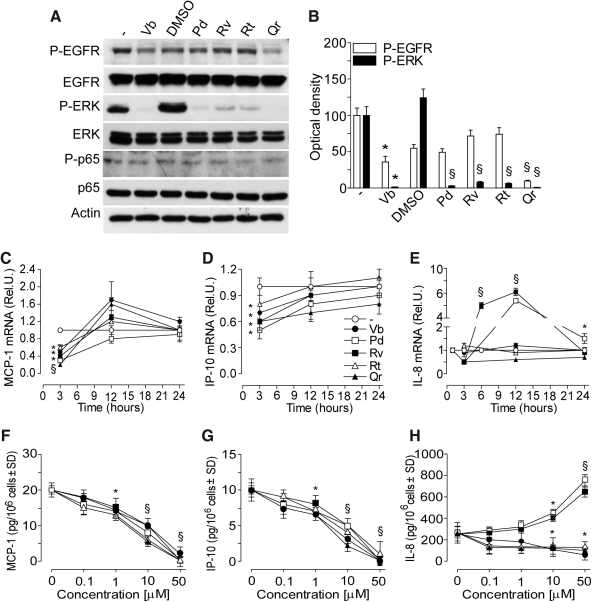
At 15 min, only 50 μM Vb and Qr significantly reduced EGFR phosphorylation, whereas all PPs abrogated extracellular regulation kinase (ERK) phosphorylation (Fig. 2A, B), and did not affect low background level of p65 phosphorylation (Fig. 2A). At 3 h incubation, the transcripts of both MCP-1 and IP-10 were significantly and transiently downregulated by all the PPs (50 μM), then, at 12 and 24 h time-points the constitutive levels were restored (Fig. 2C, D). The expression of IL-8 was dramatically upregulated by Rv at 6 and 12 h, whereas Pd significantly increased IL-8 transcripts at 12 and 24 h (Fig. 2E). All the PPs significantly and dose-dependently reduced the content of MCP-1 and IP-10 in the 24 h supernatant (Fig. 2F, G). Vb, Rt, and Qr dose-dependently reduced, whereas both Pd and Rv (10 or 50 μM) significantly enhanced IL-8 release by normal human epidermal keratinocytes (NHEK) (Fig. 2H).
FIG. 2.
PP effects on spontaneous epidermal growth factor receptor (EGFR)/extracellular regulation kinase (ERK)/p65 phosphorylation, chemokine gene expression, and protein synthesis in normal human epidermal keratinocytes (NHEK). (A) Western blots of EGFR and its phosphorylation form (P-EGFR), of ERK and its phosphorylation form (P-ERK) of p65 and its phosphorylation form (P-p65) after 15 min NHEK incubation with or without 50 μM PPs. (B) Quantification of Western blots by densitometry. *p<0.01 versus untreated control, §p<0.01 versus 0.25% dimethyl sulfoxide (DMSO)-treated conditions. (C–E) Time-dependent spontaneous chemokine gene expression (mRNA, fold induction vs. untreated cultures) affected by incubation of NHEK with 50 μM verbascoside (Vb), resveratrol (Rv), polydatin (Pd), rutin (Rt), or quercetin (Qr). Results are expressed as the mean±SD. *p<0.05 and §p<0.01 versus control. (F–H) Concentration-dependent effects of PPs on spontaneous monocyte chemotactic protein-1 (MCP-1) (F), interferon gamma-produced protein of 10 kDa (IP-10) (G), and interleukin 8 (IL-8) (H) release from NHEK. Measurements were performed 24 h after PPs addition to NHEK. *p<0.05 and §p<0.01 versus control.
PPs differently affect TGF-α-driven EGFR-dependent intracellular signaling and TGF-α-associated chemokine expression
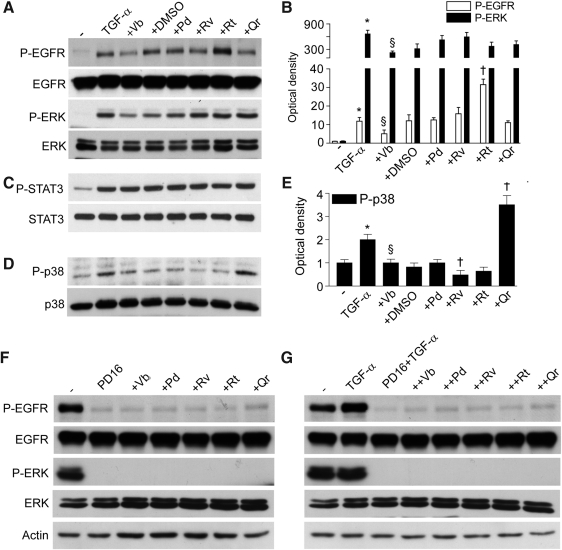
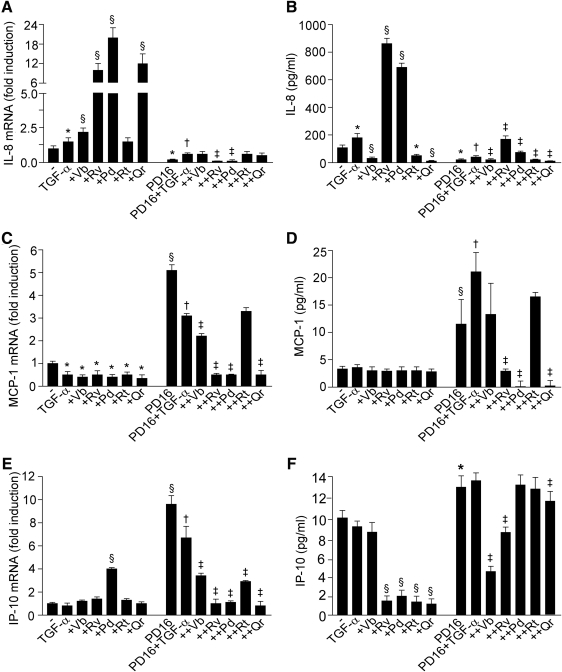
Being a prototypic EGFR ligand, TGF-α (50 ng/ml, 15 min) stimulated phosphorylation of EGFR and ERK (Fig. 3A, B). This TGF-α-induced phosphorylation was not influenced by PPs, except for Vb inhibiting, and Rt further enhancing EGFR activation. As expected, TGF-α also induced STAT3 and p38 phosphorylation. While none of the PPs studied (50 μM, 1 h preincubation) affected STAT3 phosphorylation (Fig. 3C), Vb and Rv inhibited, and Qr further enhanced p38 phosphorylation (Fig. 3D, E). NHEK incubation with PD168393 (2 μM, 30 min preincubation), a specific inhibitor of EGFR tyrosine kinase, resulted in abrogation of spontaneous and TGF-α-induced EGFR and ERK phosphorylation, and PPs did not reverse this dramatic effect (Fig. 3F, G). Of note, PD168393 effectively opposed both IL-8 gene overexpression associated to TGF-α, TGF-α+Vb, TGF-α+Rv, TGF-α+Pd, and TGF-α+Qr (Fig. 4A) and also the enhanced IL-8 protein release induced by TGF-α, TGF-α+Rv, and TGF-α+Pd (Fig. 4B). We also observed that PD168393 strongly upregulated both constitutive and TGF-α-associated MCP-1 (Fig. 4C) and IP-10 (Fig. 4E) gene expression. A significant PD168393-induced increase of these two chemokines was also detected at the protein level (Fig. 4D, F). Notably, Rv, Pd, and Qr strongly inhibited the effect of PD168393 on MCP-1 expression (Fig. 4C, D), and also on IP-10 transcript (Fig. 4E). By contrast, Vb and Rv were the most effective inhibitors of IP-10 protein release (Fig. 4F).
FIG. 3.
PP effects on transforming growth factor-α (TGF-α)-stimulated cytoplasmic pathways in keratinocytes. (A) Phosphorylation of ERK1/2 and EGFR induced by coincubation with TGF-α or its combination with PPs (50 μM) for 15 min and visualized by Western blot. (B) Quantification of Western blots by densitometry. §p<0.01 versus untreated controls, †p<0.01 versus TGF-α, and ‡p<0.01 versus 0.25% DMSO in culture medium. (C) Western blots of STAT3 phosphorylation (P-STAT3) induced by TGF-α alone or its combination with 50 μM PPs for 15 min. (D) Western blots of p38 phosphorylation (P-p38) induced by TGF-α alone or its combination with 50 μM PPs for 15 min. (E) Quantification of Western blots for P-p38 by densitometry. *p<0.05 versus untreated control, §p<0.05 versus TGF-α, †p<0.05 versus 0.25% DMSO+TGF-α. (F) Spontaneous and (G) TGF-α-induced EGFR and ERK1/2 phosphorylation in the presence of the specific EGFR tyrosine kinase inhibitor P168393 (PD16, 2 μM), and PPs (50 μM) effects.
FIG. 4.
PP effects of TGF-α-connected chemokine expression. TGF-α-connected IL-8 (A), MCP-1 (C), and IP-10 (E) gene expression (mRNA, fold induction vs. untreated cultures) affected by 3 h incubation of NHEK with 10 ng/ml TGF-α plus 50 μM Vb, Rv, Pd, Rt, or Qr in the absence or presence of P168393 (2 μM). Results are expressed as the mean±SD. *p<0.05 and §p<0.01 versus control. PPs effects on TGF-α-or TGF-α+P168393-connected IL-8 (B), MCP-1 (D), and IP-10 (F) release (24 h) in the conditioned medium expressed in pg/ml protein. For all the panels, *p<0.05 and §p<0.01 versus control, †p<0.05 versus PD16-treated condition and ‡p<0.05 versus PD16+TGF-α-treated condition.
PPs differently affect TNF-α+IFN-γ-induced chemokine expression and intracellular signaling
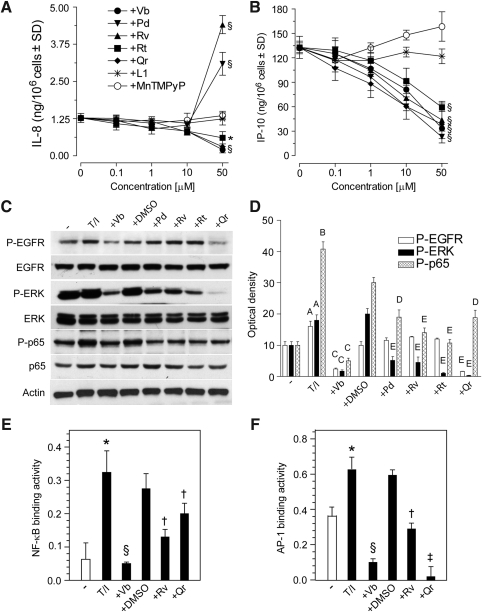
Analysis of dose-dependent effects of PPs on IL-8, and IP-10 release from NHEK exposed to TNF-α+IFN-γ (T/I) showed that Pd and Rv (50 μM) maintained their ability to enhance, whereas Vb > Qr=Rt inhibited IL-8 release (Fig. 5A). In contrast, all the PPs dose-dependently inhibited IP-10 (Fig. 5B) and MCP-1 (data not shown) release. Cell penetrable compounds, SOD-mimicking MnTMPyP, and iron chelator L1, did not exert any effect (Fig. 5A, B). As expected, NHEK treatment with T/I induced the phosphorylation of EGFR, ERK, and p65. All the PPs significantly inhibited ERK and p65 phosphorylation, whereas only Vb and Qr effectively opposed EGFR phosphorylation (Fig. 5C, D). Vb, Rv, and Qr were all effective inhibitors of both T/I-induced NFκB and AP-1 binding activity, with Vb strongly acting against both transcription factors (Fig. 5E, F).
FIG. 5.
PP effects on (tumor necrosis factor alpha [TNF-α] plus IFN-γ)-induced EGFR, ERK, and p65 phosphorylation, chemokine production, and nuclear factor κB (NFκB) and activator protein 1 (AP-1) DNA binding. (A) Concentration-dependent effects of PPs, SOD-mimicking compound (MnTMPyP), and iron chelator (L1) on IL-8 and (B) IP-10 release by NHEK stimulated with TNF-α (100 ng/ml) and IFN-γ (100 U/ml) (T/I) for 24 h. Before T/I addition, NHEK underwent 1 h pretreatment with polyphenols or antioxidants. *p<0.05 and §p<0.01 versus. keratinocytes treated with T/I. (C) Effects of PPs (50 μM, preincubation for 1 h) on EGFR, ERK, and p65 phosphorylation triggered by 15 min stimulation with T/I, and (D) quantification of Western blot bands by densitometry. Ap<0.05 and Bp<0.01 versus untreated controls; Cp<0.01 versus T/I-treated conditions; Dp<0.05 and Ep<0.01 versus T/I+0.25% DMSO-treated conditions. Effects of PPs (50 μM, 1 h preincubation) on T/I-induced (E) NFκB-DNA binding and (F) AP-1-DNA binding. *p<0.01 versus untreated keratinocytes, §p<0.01 versus keratinocytes treated with T/I, †p<0.05 and ‡p<0.01 versus keratinocytes treated with 0.25% DMSO+T/I.
PPs differently affect EGFR translocation or/and retention in the nucleus
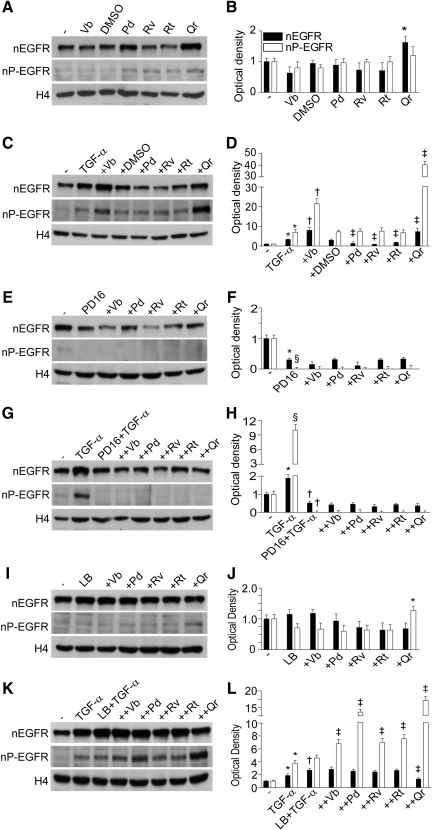
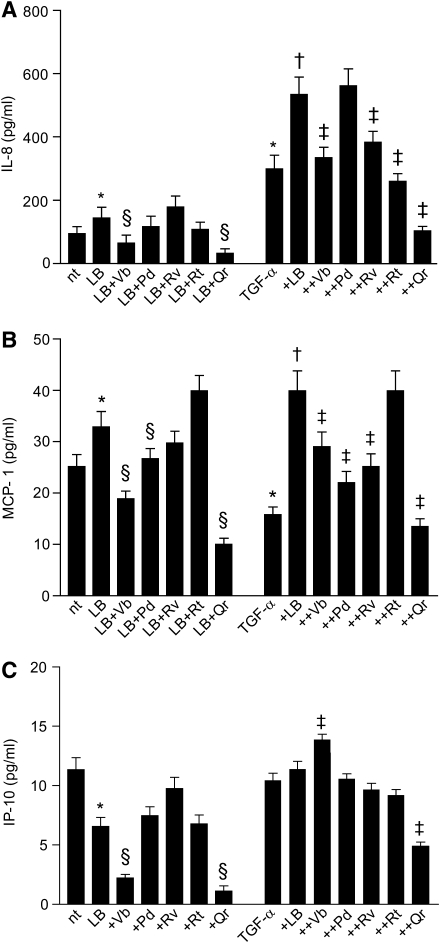
We observed that Qr exclusively enhanced background nuclear levels of EGFR (nEGFR) with no perturbation of the nuclear levels of P-EGFR (nP-EGFR) (Fig. 6A, B). NHEK treatment with TGF-α led to an early (15 min) increase in both nEGFR and nP-EGFR. In this condition, both Vb and Qr further increased nEGFR and nP-EGFR, whereas Pd, Rv, and Rt inhibited nEGFR only (Fig. 6C, D). The presence of PD168393 dramatically reduced both nEGFR and nP-EGFR in nonstimulated (Fig. 6E, F) and TGF-α-stimulated (Fig. 6G, H) NHEK, no effects of PPs were observed. By contrast, the combination Qr+LB increased nP-EGFR levels (Fig. 6I, J). In TGF-α-stimulated NHEK, leptomycin B (LB) further selectively enhanced nEGFR (Fig. 6K, L). The TGF-α+LB+PP combination induced a significant increase of nP-EGFR (Qr>Pd>Rv=Vb=Rt) and TGF-α+LB+Qr led also to significant nEGFR reduction (Fig. 6K, L). LB substantially increased background release of IL-8 and MCP-1 from NHEK, and effect toward IL-8 was significantly impaired by Vb and Qr (Fig. 7A), and toward MCP-1 by Pd (Fig. 7B). By contrast, IP-10 release was downregulated by LB and Vb and Qr further reduced the IP-10 levels (Fig. 7C). In TGF-α-stimulated NHEK, LB worked by further increasing IL-8 release, and, apart from Pd, all the PPs opposed LB-associated increase of IL-8 (Fig. 7A). On the other hand, LB contrasted TGF-α-associated suppression of MCP-1 and strongly enhanced the release of this chemokine, whereas Vb, Rv, Rt, and Qr significantly reduced LB activity (Fig. 7B).
FIG. 6.
PP effects on spontaneous levels of EGFR in the nucleus and TGF-α-induced nuclear translocation of the receptor. Western blots of nonphosphorylated (nuclear levels of EGFR [nEGFR]) and phosphorylated EGFR (nuclear levels of P-EGFR [nP-EGFR]) in the nuclei of NHEK (A) and densitometry quantification of the bands (B) in the presence of 50 μM PPs (15 min). *p<0.05 versus 0.25% DMSO. The same (E, F) in the presence of P168393 (2 μM) and 50 μM PPs. *p<0.05 and §p<0.01 versus untreated control. TGF-α-induced (10 ng/ml, 15 min) and PPs-modulated nuclear translocation of nonphosphorylated and phosphorylated forms of EGFR (C) and quantification of Western blots (D). *p<0.05 versus untreated control, §p<0.05 versus TGF-α or TGF-α+0.25% DMSO, †p<0.01 versus TGF-α+0.25% DMSO. The same (G, H) in the presence of P168393 (2 μM). *p<0.05 versus untreated control, †p<0.05 and ‡p<0.01 versus TGF-α. Effect of leptomycin B (LB) on the nuclear level of nEGFR and nP-EGFR (I, J). *p<0.05 versus untreated control. Effect of LB on TGF-α–stimulated NHEK (K, L). *p<0.05 versus untreated control, †p<0.05 versus TGF-α, ‡p<0.05 versus TGF-α+0.25% DMSO. Histone 4 (H4) was used as a loading control.
FIG. 7.
Effects of LB, an inhibitor of EGFR export from the nucleus, on spontaneous and TGF-α-induced chemokine production by NHEK. IL-8 (A), MCP-1 (B), and IP-10 (C) expression upon action of LB or its combination with 50 μM PPs or its combination with TGF-α (10 ng/ml) or its combination with TGF-α and PPs. *p<0.05 versus untreated control, §p<0.05 versus LB alone, †p<0.05 versus TGF-α alone, and ‡p<0.01 versus LB+TGF-α.
Vb accelerates in vitro scratch wound healing and exerts anti-inflammatory and wound healing effects in vivo
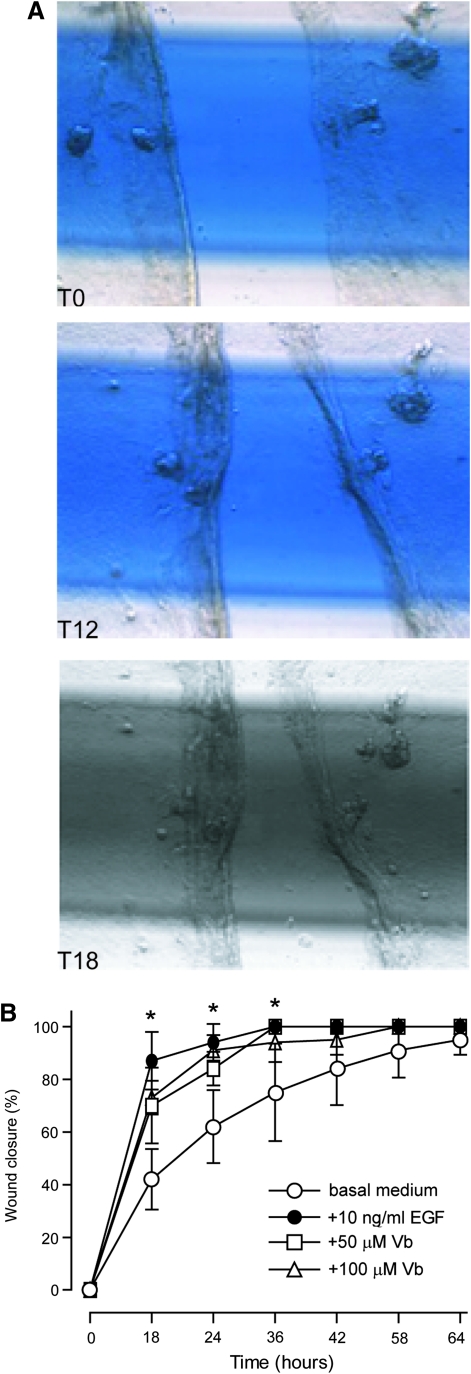
In the in vitro model of scratch wound healing (Fig. 8A), Vb significantly accelerated restoration of damaged NHEK layer at 18 and 24 h time points, with an effect comparable to the healing effect of EGF (Fig. 8B).
FIG. 8.
Vb effects on scratch wound healing in vitro. (A) Microphotographs of keratinocyte sheet scratch immediately after wounding (T0), 12 h (T12), and 18 h (T18) afterward. (B) Time-dependent wound closure in the presence of 10 ng/ml epidermal growth factor (EGF), or 50 or 100 μg/ml Vb expressed in% as the mean±SD. *p<0.05 versus untreated control.
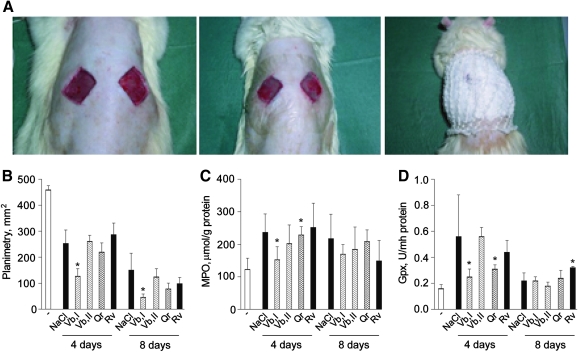
In the animal model of full-thickness excision wound (Fig. 9A), 50 μM Vb applied topically substantially accelerated wound closure (Fig. 9B) and inhibited both MPO and glutathione peroxidase (Gpx) activities in the skin biopsies closely taken to wound area (Fig. 9C, D) at the 4th postwounding day. On contrast, 100 μM Vb and 50 μM Rv or Qr did not have any of the above effects.
FIG. 9.
Anti-inflammatory and wound healing properties of PPs in the full-thickness excision wound model. Full-thickness excision wounds (A) were covered by plastic film Tegaderm™ to maintain moisture and were treated daily by 100 μL of 0.9% NaCl or 50 μM solutions of Vb (Vb I), Rv, or Qr or 100 μM solution of Vb (Vb II). Each group of treatment was composed of 10 rats. (B) Planimetry of wound size (C) MPO activity in skin biopsies taken from experimental animals before wounding and at days 4 and 8 after. (D) Glutathione peroxidase (Gpx) activity in skin biopsies taken from experimental animals before wounding and at days 4 and 8 after. *p<0.05 versus application of physiologic solution (NaCl).
Discussion
Being a ubiquitously expressed, cell surface transmembrane receptor with intrinsic protein-tyrosine kinase activity (1, 24, 25, 30, 43), EGFR is highly expressed in human keratinocytes in vivo and in vitro (26, 27, 29, 43, 44). Growing evidence suggests that EGFR is a key functional regulator of keratinocyte responses to a vast array of extracellular stimuli including specific EGFR ligands, ligands for other cell surface receptors such as G-protein-coupled and cytokine receptors, and a number of nonligand agents such as H2O2, UV, heat, radiation, and some chemotherapeutics (8, 24, 25, 30). Disparate mechanisms of EGFR activation in skin cells under physiological and pathological conditions suggest that it could mediate numerous cellular functions, for example, changes of cell shape, proliferation, migration, adhesion, and inflammatory responses during wound healing (28, 30, 34, 45). The experimental data demonstrate that the EGFR system plays a relevant role in the epidermal cell reaction to wounding, first by mounting a robust, but transient inflammation (7, 26, 27, 30). Taking into account that EGFR is a redox-regulated receptor (1, 4, 18, 43), here, we attempted to elucidate the modulation of EGFR-dependent chemokine production and repair-related processes in NHEK by redox-active PPs (14). In nature, many PPs exist in glycosylated forms with sugar moieties substituting hydrogen in one of the polyphenolic hydroxyl groups. Since both number and position of OH-groups in the phenolic core determine free radical scavenging and metal chelating properties of PPs, glycosylated forms are often less effective antioxidants than corresponding aglycons (6). The antioxidant potency of PPs depends also on the oxidation model used (Fig. 1). Antioxidant properties of the selected PPs were confirmed in two physiologically relevant models of LDL oxidation by MPO+H2O2+nitrite and NADPH-induced LPO in liver microsomes. Our data on superoxide scavenging action of Qr, Rt, Rv, and Vb in xanthine oxidase-free O2- -producing system well correspond to literature data (6, 9, 15). Exclusively Vb and Qr, which were the strongest superoxide scavengers (Fig. 1), effectively inhibited constitutive (Fig. 2A) TGF-α-induced (Fig. 3A, B), and T/I-induced (Fig. 5C, D) EGFR phosphorylation. Auto-phosphorylation of EGFR tyrosine residues 1068 and 1173 important for the functional activity of the receptor is counter-balanced by receptor-type protein tyrosine phosphatase (RT-PTP) that directly de-phosphorylates it to maintain low levels of active P-EGFR (43, 44). Several publications indicate that reversible oxidative inactivation of RT-PTP takes place as a consequence of reactive oxygen species generated in response to EGF and cytokine receptor activation (1, 12, 43). We suggest that Vb and Qr could invariably inhibit EGFR phosphorylation via redox regulation of RT-PTP. Another possibility of direct inhibition of EGFR tyrosine kinase activity by Vb and Qr is less probable due to serious differences between the action of classical inhibitor of the activity P168393 and the PPs: (i) P168393 completely abrogated both EGFR and downstream ERK phosphorylation induced by TGF-α whereas PPs did not affect ERK (Fig. 3A) and (ii) P168393 and Vb/Qr effects on TGF-α-connected chemokine expression were opposite (Fig. 4). Although P168393 inhibited EGFR-dependent IL-8 gene expression, the PPs synergized with TGF-α in its overexpression. At the same time, P168393 induced overexpression of MCP-1 and IP-10, whereas Vb/Qr did not affect the chemokine gene expression and MCP-1 protein. Only Qr inhibited IP-10 synthesis.
We observed that all PPs independent of their antioxidant capacity inhibited T/I activated p65 phosphorylation (Fig. 5C, D) although the process is regarded as redox-dependent (11). In accord with our previous observation (31), Vb profoundly inhibited inflammatory responses in NHEK significantly downregulating both NFκB and AP-1 DNA binding whereas Qr mainly inhibited AP-1, and Rv less potently affected DNA binding of both transcription factors (Fig. 5E, F). Here, Vb was an effective inhibitor of T/I-induced phosphorylation of the three factors ERK, NFκB, and EGFR; it profoundly suppressed both NFκB and AP-1 DNA binding, and downregulated chemokine production (Fig. 5A, B). The PPs effects toward chemokine expression in NHEK did not depend on their superoxide scavenging and iron chelating action since SOD-mimicking compound and iron chelator exhibited no effect (Fig. 5A, B). Surprisingly, Rv and Pd at a concentration of 50 μM remarkably activated IL-8 production by nonstimulated and T/I-challenged NHEK (Figs. 2E, 2H, and 5) notwithstanding their significant inhibition of ERK at early time points. In addition, although TGF-α alone slightly induced IL-8, its combination with the stilbenes, significantly upregulated IL-8 both at the mRNA and protein levels (Fig. 4). The discrepancy between the chemokine transcript levels measured at an early time point (3 h) and the protein levels at 24 h in the supernatants in the presence of Vb (Fig. 4) suggests that the polyphenol might exert a complex, time-dependent regulation of chemokine gene expression, or that it might differentially affect the two processes of gene transcription and subsequent transcript translation. Notably, none of the PPs inhibited TGF-α-induced ERK or STAT3 phosphorylation, whereas Vb inhibited and Qr further stimulated p38 phosphorylation (Fig. 3). We have recently verified that Rv-induced IL-8 upregulation was not associated to RNA stabilization in T/I-stimulated NHEK, and that Rv-induced aryl hydrocarbon receptor significantly contributes, but not fully explains, IL-8 upregulation (33). On these grounds, we hypothesize that the intracellular mechanisms underlying stilbene-associated IL-8 upregulation cannot be detected in early time points, and that a delayed, strong activation of signal transduction should appear after hours-long contact of NHEK with these PPs.
In keeping with our previous results (26, 27, 29), we observed that the specific EGFR tyrosine kinase inhibitor PD168393 critically perturbed TGF-α-associated expression of chemokines in NHEK, with strong suppression of IL-8 and significant upregulation of MCP-1 and IP-10 (Fig. 4). Importantly, PPs opposed PD168393 inhibitory effects on the release of IL-8 (stilbenes), the mRNA synthesis and protein release of MCP-1, and the transcript of IP-10 (stilbenes and Qr). Altogether, these data indicate that the PPs can be potent modulators of the proinflammatory behavior of keratinocytes in a compound-specific way, despite the quantitative suppression of EGFR and ERK due to PD168393 (Fig. 3F, G). We also investigated the impact of the PPs on the nuclear accumulation of EGFR (44), a process upregulated by TGF-α, suppressed by PD168383, and relevantly perturbed by LB. Although LB alone did not affect the background levels of both nEGFR and nP-EGFR (Fig. 6I, J), Qr increased nP-EGFR in LB-treated cells. In the presence of TGF-α+Qr or TGF-+Vb both EGFR and P-EGFR accumulated in the nucleus (Fig. 6C, D), thus showing a contrasting effect as compared with their own significant downregulation of EGFR phosphorylation in the whole cells (Fig. 5C, D). Also, Vb and Qr were the most effective PPs in downregulating IL-8, MCP-1, and IP-10, either upregulated (IL-8 and MCP-1) or downregulated (IP-10) by LB (Fig. 7), suggesting that these two PPs specifically interfere with LB-driven inhibition of EGFR nuclear-cytoplasm export (44). LB strongly upregulated both TGF-α-associated IL-8 and MCP-1, suggesting that enhanced retention of nEGFR/nP-EGFR in the nucleus might facilitate the expression of these chemokine genes (Fig. 7). PPs exerted strong but disparate effects on (LB+TGF-α)-induced chemokine expression. Collectively, these results suggest that the differential perturbation of EGFR phosphorylation and intracellular trafficking by PPs heavily contribute to their impact on the proinflammatory potential of NHEK.
Nuclear EGFR has been strongly correlated to enhanced proliferation activity (20, 24). During the epidermal response to wounding, epidermal keratinocytes initiate a program of de novo synthesis of growth factors and autocrine production of EGFR ligands (30). Our observation that wound closure was accelerated by Vb both in vitro (Fig. 8) and in vivo (Fig. 9), could be attributed to its potentiation of the nuclear EGFR accumulation in the presence of EGFR ligands. In agreement with its capacity to inhibit EGFR-dependent IL-8, a chemoattractant for granulocytes, Vb suppressed granulocyte recruitment to the wound site at the early inflammatory stage of wound healing (MPO test) thus exhibiting anti-inflammatory properties assessed by Gpx test.
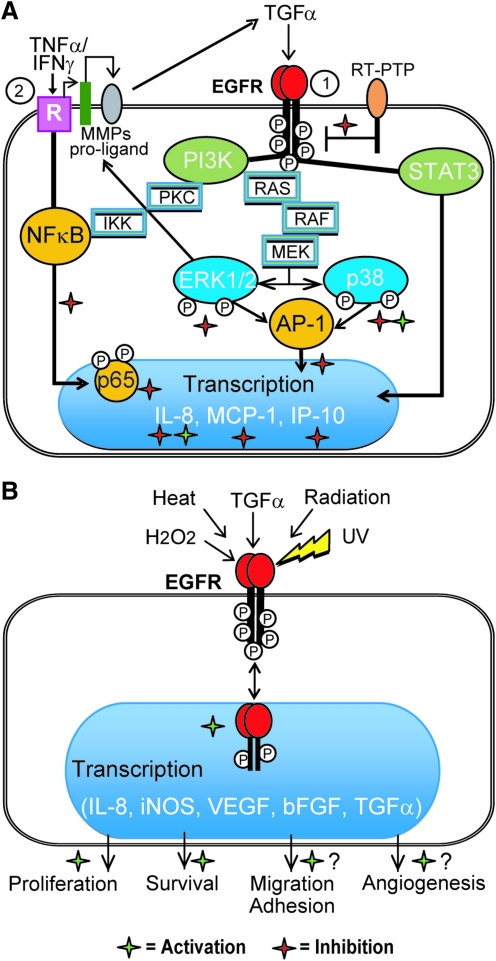
Hypothetical mechanisms of PPs effects on EGFR-driven proinflammatory and repair processes in keratinocytes are proposed in Figure 10.
FIG. 10.
PP-associated modulation of inflammatory chemokine production and repair-related processes in keratinocytes through EGFR system. (A) Cytoplasmic EGFR-regulated pathways. Pathway 1 connected with direct activation of EGFR by ligands (e.g., EGF and TGF-α), exogenous or formed from proligands by matrix metalloproteinase (MMPs) cleavage is inhibited by distinct PPs (red asterisks) at the levels of EGFR and ERK phosphorylation, which may result in the enhancement of EGFR-regulated inflammatory responses (green asterisks). Normally, reactive oxygen species (ROS)-mediated inhibition of RT-PTP maintains EGFR in an activated state. ROS-scavenging PPs could shift the balance of EGFR phosphorylation-de-phosphorylation in favor of the former, thus, limiting P-EGFR formation (red asterisk). Cytoplasmic TGF-α-activated STAT3 pathway is insensitive to PPs. Cytoplasmic EGFR-regulated p38-connected pathway could be either activated or inhibited by PPs (red and green asterisks). Pathway 2 connected with membrane receptors (R) for proinflammatory cytokines TNF-α and IFN-γ converges with Pathway 1 at the levels of EGFR proligand cleavage by MMPs. Stimulation of R by the combination of TNF-α/IFN-γ activates NFκB, which target genes coding proinflammatory cytokines and enzymes. PPs may inhibit either NFκB or AP-1 or both transcription factors (red asterisks). Since maximal activation of several chemokine genes needs cooperation of both transcription factors, PPs inhibiting NFκB and AP-1 may be considered as efficient anti-inflammatory agents. (B) Nuclear pathway depends on direct translocation of stimulated (by ligands or in a nonligand fashion) and phosphorylated EGFR from cellular membrane into nucleus, where it connects with DNA-binding transcription factors, thus inducing gene transcription followed by adaptive response of keratinocytes. As a result, cells escape apoptosis and their proliferation, migration, and adhesion increase. Growth factors produced by keratinocytes promote angiogenesis. PPs-enhancers of P-EGFR accumulation in the nucleus may promote all these processes, thus, facilitating wound healing. Red and green asterisks correspond to PPs-associated inhibition or promotion of inflammatory response, respectively. PI3K, phosphoinositol-3-kinase; RT-PTP, receptor-type protein tyrosine phosphatase.
Materials and Methods
Animals and excision wound model
Adult Wistar rats (300–400 g, 10 animals per group) were anesthetized with 10 mg/kg ketamine, the dorsal fur shaved, and two 21.5×21.5 mm squares were excised down to muscles (0.2 cm depth) (Fig. 9A). The wounds were daily treated with 100 μl of tested compounds or 0.9% NaCl, then, dressed to maintain moisture. Biopsies were taken in a close vicinity to wounds at days 4 and 8. Tissue samples were homogenized, centrifuged, and enzyme activities were measured in supernatants (16). Animal experiments were approved by the Local Board. The measurements of the wound areas were performed on the 4th and 8th day by planimetry.
Cell cultures and treatments
Primary cultures of NHEK were obtained from skin biopsies of healthy volunteers (n=5) after their informed consent (29). Keratinocytes were grown up to 60%–80% of confluence in serum-free medium (KGM-Gold; Lonza, Walkersville, MD). In the 24 h preceding the experiments, NHEK cultures were switched to EGF-depleted medium.
In the experiments with the in vitro scratch wounds, NHEK were grown in 6×multiwell plates. Bottom of each plate was marked with three horizontal lines. At 80% of confluence, cultures were disrupted by plastic tip drawing three vertical lines (Fig. 9) and the time-dependence of the gap between cell sheet margins at 9 cross points was digitally measured. As positive control, 10 ng/ml EGF (R&D Systems, Abingdon, UK) was used.
Quercetin dihydrate (Qr) and Rt were from Sigma-Aldrich (Milan, Italy), Rv was from Biomol (Research Lab, Plymouth, MA), Pd (95% purity; HPLC grade) was a gift from Prof. Ravagnan (University of Venice, Italy), and Vb (97% purity; HPLC grade) was a gift from Dr. Dal Toso (IRB, AltavillaVicentina, Italy). Chemical structures of tested PPs are given in Figure 1. PPs were added to cell cultures 30 min or 1 h before exposure to proinflammatory triggers. Equal volumes of dimethyl sulfoxide (a vehicle for Rv, Pd, Qr, and Rt) were added to the control cultures.
TGF-α (50 ng/ml; R&D Systems) was added to cell cultures 1 h after preincubation with PPs (50 μM) and protein phosphorylation was measured after 15 min, gene expression after 3, 12, and 24 h, whereas chemokine levels in NHEK supernatant were determined after 24 h.
In the experiments with cytokine-induced chemokine expression, NHEK were preincubated with escalating concentrations of PPs for 1 h, then, the mixture of TNF-α (100 ng/ml) and IFN-γ (100 U/ml) both from R&D Systems was added. Tetrakis(1-methyl-4-pyridyl)porphyrin pentachloride (MnTMPyP), a cell permeable superoxide dismutase mimetic and peroxynitrite scavenger (32) was a kind gift of Prof. Mollace (Pharmacology Department, Catanzaro University, Italy). Cell permeable iron chelator 1,2-dimethyl-3-hydroxypyrid-4-one (L1) (13) was a gift from Lipomed Co. (Zurich, Switzerland).
In the experiments with pharmacological impairment of EGFR, NHEK were preincubated with 2 μM P168393, a specific inhibitor of EGFR tyrosine kinase (Calbiochem, La Jolla, CA) for 30 min before treatment with PPs. Then, PPs alone (50 μM) were added for additional 30 min followed by 50 ng/ml TGF-α. The same experimental design was used with LB (Sigma-Aldrich), an inhibitor of EGFR export from the nucleus (44)
Preparation of cell extracts and immunoblotting
Total nonphosphorylated and phosphorylated EGFR, ERK1/2, and phosphorylated p65 subunit of NFκB were investigated in total cell lysates as previously described (31). Anti-EGFR antibodies were from Santa Cruz, whereas anti-ERK, and anti-p65 NFκB antibodies were from Cell Signaling Technology (Beverly, MA).
NHEK were differentially lysed to obtain nucleic extracts according to (37).
DNA binding activity of transcription factors
NFκB and AP-1 specific DNA binding activity was detected in cell nuclear lysates and quantified using the transcription factor-specific TransAM kits (Active Motif, Florence, Italy).
Assessment of antioxidant and free radical scavenging properties of PPs
Antioxidant effects of PPs were studied in LDL oxidation by MPO plus nitrite as previously described (17). Peroxidation products (LOOH) were quantified using the PeroxiDetect Kit (Sigma-Aldrich).
PPs-related inhibition of NADPH-induced LPO was evaluated in rat liver microsomes (1.2–1.3 mg protein/ml), which were incubated with 0.3 mM NADPH, 20 mM NaCl, and 10 μM FeSO4 dissolved in 0.05 M phosphate buffer, pH 7.4, at 37°C for 5 min. The content of thiobarbituric acid reactive substances was determined (41). Superoxide scavenging activity of PPs was assessed in a photochemical system reducing riboflavin and yielding superoxide as a by-product. Superoxide-dependent reduction of nitroblue tetrazolium was spectrophotometrically monitored (3). Results were expressed as concentrations of 50% inhibition (I50, μM).
Quantitative real-time-PCR assay
Total RNA was isolated using the GenElute Mammalian Total RNA Kit (Sigma-Aldrich) and was reverse transcribed using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). cDNA was amplified with IQ SYBR green Supermix (Bio-Rad) using the MiniOpticon Real-Time PCR Detection System (Bio-Rad). Two housekeeping genes, ribosomal 18S and beta-actin were chosen as reference and fold changes were calculated (23). The primer sets were synthesized by Eurofins MWG Operon (Ebersberg, Germany): β-actin fwd:5′-AATCTGGCACCACACCTTCTAC-3′; β-actin rev:5′-ATAGCACAGCCTGGATAGCAAC-3′; 18S rRNA fwd:5'- TCCCCCAACTTCTTAGAGG-3'; 18S rRNA rev:5'- GCTTATGACCCGCACTTAC-3'; MCP1 frw:5'-AAGCAGAAGTGGGTTCAGGA−3'; MCP1 rev:5'-TAAAACAGGGTGTCTGGGGA-3'; IL-8 frw:5'-GTCCTTGTTCCACTGTGCCT-3'; IL-8 rev:5'-GCTTCCACATGTCCTCACAA-3'; IP-10 frw:5′-GGGAGCAAAATCGATGCAGTGCT-3′; IP-10 rev:5′-GCAGCCTCTGTGTGGTCCATCC-3′.
Assays for inflammatory chemokine production by NHEK
The proinflammatory chemokines IP-10, MCP-1, and IL-8 were measured in cell supernatants following 24 h stimulation with PPs alone or in combination with TGF-α or (TNF-α+IFN-γ), using BD OptEIA Elisa kits from BD Biosciences (San Diego, CA) (33).
Statistics
All measurements were done in triplicate, and data of at least three independent experiments were statistically evaluated. Statistical evaluation was carried out with the software package for Windows XP. Results were expressed as the mean±SD. To evaluate the difference between experimental groups, two-tailed Student's t-test was applied and p-values<0.05 were considered to be significant.
Abbreviations Used
- Akt1
protein kinase B
- AP-1
activator protein-1
- DMSO
dimethyl sulfoxide
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- ERK
extracellular regulation kinase
- Gpx
glutathione peroxidase
- IFN-γ
interferon gamma
- IL-8
interleukin 8
- IP-10
interferon gamma-produced protein of 10 kDa
- LB
leptomycin B
- LDL
low-density lipoprotein
- LPO
lipid peroxidation
- MAPK
mitogen-activated protein kinase
- MCP-1
monocyte chemotactic protein-1
- MPO
myeloperoxidase
- nEGFR
nuclear levels of EGFR
- NFκB
nuclear factor κB
- NHEK
normal human epidermal keratinocytes
- nP-EGFR
nuclear levels of P-EGFR
- Nrf2
nuclear factor erythroid-derived 2 (NFE2)-related factor
- P-
phosphorylated form of protein
- PI3K
phosphoinositol-3-kinase
- Pd
polydatin
- PKC
protein kinase C
- PPs
plant polyphenols
- Qr
quercetin
- RANTES
regulated upon activation, normal T-cell expressed
- ROS
reactive oxygen species
- Rt
rutin
- RT-PTP
receptor-type protein tyrosine phosphatase
- Rv
resveratrol
- TGF-α
transforming growth factor alpha
- TNF-α
tumor necrosis factor alpha
- Vb
verbascoside
Acknowledgments
This work was supported by The Italian Ministry for Health (Grant IDI IRCCS-2010). The skillful technical assistance of Dr. Giovanni Primavera and Dr. Virginia Barone in keratinocyte culturing and Dr. Michail Anurov and Dr. Svetlana Titkova in the excision wound model is gratefully acknowledged.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bae Y. Kang S. Seo M. Baines I. Tekle E. Chock P. Rhee S. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role of EGF receptor-mediated tyrosine phosphorylation. J Biol Chem. 1997;272:217–221. [PubMed] [Google Scholar]
- 2.Barrandon Y. Green H. Cell migration is essential for sustained growth of keratinocyte colonies: the role of transforming growth factor-alpha and epidermal growth factor. Cell. 1987;50:1131–1137. doi: 10.1016/0092-8674(87)90179-6. [DOI] [PubMed] [Google Scholar]
- 3.Beauchamp C. Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 4.Brigelius-Flohè R. Flohè L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxid Redox Signal. 2011;15:2335. doi: 10.1089/ars.2010.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cattaneo F. Iaccio A. Guerra G. Montagnani S. Ammendola R. NADPH-oxidase-dependent reactive oxygen species mediate EGFR transactivation by FPRL1 in WKYMVm-stimulated human lung cancer cells. Free Radic Biol Med. 2011;51:1126–1136. doi: 10.1016/j.freeradbiomed.2011.05.040. [DOI] [PubMed] [Google Scholar]
- 6.Denisov E. Afanas'ev I. Oxidation and Antioxidants in Organic Chemistry and Biology. Boca Raton-London-NewYork-Singapore: CBC Taylor & Francis Group; 2005. pp. 844–849. [Google Scholar]
- 7.Eming SA. Kreig T. Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 8.Fritsche E. Schafer C. Calles C. Bernsmann T. Bernshausen T. Wurm M. Hubenthal U. Cline JE. Hajimiragha H. Schroeder P. Klotz LO. Rannug A. Furst P. Hanenberg H. Abel J. Krutmann J. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmic target for ultraviolet B radiation. Proc Nat Acad Sci U S A. 2007;104:8851–8856. doi: 10.1073/pnas.0701764104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukui M. Choi HJ. Zhu BT. Mechanism for the protective effect of resveratrol against oxidative stress-induced neuronal death. Free Radic Biol Med. 2010;49:800–813. doi: 10.1016/j.freeradbiomed.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han SS. Lee M. Park GH. Bang SH. Kang YK. Kim TW. Lee JL. Chang HM. Ryu MH. Investigation of papulopustular eruptions caused by cetuximab treatment shows altered differentiation markers and increases in inflammatory cytokines. Br J Dermatol. 2010;162:371–379. doi: 10.1111/j.1365-2133.2009.09536.x. [DOI] [PubMed] [Google Scholar]
- 11.Kamata H. Honda S. Maeda N. Chang I. Hirai H. Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 12.Kluth D. Banning A. Paur I. Blomhoff R. Brigelius-Flohe R. Modulation of pregnane X receptor- and electrophile responsive element-mediated gene expression by dietary polyphenolic compounds. Free Radic Biol Med. 2007;42:315–325. doi: 10.1016/j.freeradbiomed.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 13.Kontoghiorghes GJ. Prospects for introducing deferiprone as potent pharmaceutical antioxidant. Front Biosci. 2009;1:161–178. doi: 10.2741/E16. [DOI] [PubMed] [Google Scholar]
- 14.Korkina LG. Phenylpropanoids as naturally occurring antioxidants: from plant defense to human health. Cell Mol Biol. 2007;53:15–25. [PubMed] [Google Scholar]
- 15.Korkina LG. Mikhalçhik EV. Suprun MV. Pastore S. Dal Toso R. Molecular mechanisms underlying wound healing and anti-inflammatory properties of naturally occurring biotechnologically produced phenylpropanoid glycosydes. Cell Mol Biol. 2007;53:78–83. [PubMed] [Google Scholar]
- 16.Korkina LG. Pastore S. De Luca C. Kostyuk VA. Metabolism of plant polyphenols in the skin: beneficial versus deleterious effects. Curr Drug Metab. 2009;9:10–29. doi: 10.2174/138920008786049267. [DOI] [PubMed] [Google Scholar]
- 17.Kostyuk VA. Kraemer T. Sies H. Schewe T. Myeloperoxidase/nitrite-mediated lipid peroxidation of low-density lipoprotein is modulated by flavonoids. FEBS Lett. 2003;537:146–150. doi: 10.1016/s0014-5793(03)00113-3. [DOI] [PubMed] [Google Scholar]
- 18.Lee EJ. Jeon MS. Kim BD. Kim JH. Kwon YG. Lee H. Lee YS. Yang JH. Kim TY. Capsiate inhibits ultraviolet B-induced skin inflammation by inhibiting Src family kinases and epidermal growth factor receptor signaling. Free Radic Biol Med. 2010;48:1133–1143. doi: 10.1016/j.freeradbiomed.2010.01.034. [DOI] [PubMed] [Google Scholar]
- 19.Li Y. Ying C. Zuo X. Yi H. Yi W. Meng Y. Ikeda K. Ye X. Yamori Y. Sun X. Green tea polyphenols down-regulate caveolin-1 expression via ERK1/2 and p38MAPK in endothelial cells. J Nutr Biochem. 2009;20:1021–1027. doi: 10.1016/j.jnutbio.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Lin SY. Makino K. Matin A. Wen Y. Kwong KY. Bourguignon L. Hung MC. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001;3:802–808. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- 21.Liou A. Elias PM. Grunfeld C. Feingold KR. Wood LC. Amphigerulin and nerve growth factor expression are regulated by barrier status in murine epidermis. J Invest Dermatol. 1997;108:73–77. doi: 10.1111/1523-1747.ep12285638. [DOI] [PubMed] [Google Scholar]
- 22.Liu W. Akhand AA. Kato M. Yokoyama I. Miyata T. Kurokawa K. Uchida K. Nakashima I. 4-hydroxynonenal triggers an epidermal growth factor-linked signal pathway for growth inhibition. J Cell Sci. 1999;112:2409–2417. doi: 10.1242/jcs.112.14.2409. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ. Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Lo HW. Hung MC. Nuclear EGFR signalling network in cancers: linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. Br J Cancer. 2006;94:184–188. doi: 10.1038/sj.bjc.6602941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez-Carpio PA. Trelles MA. Cutaneous epidermal growth factor receptor system following ultraviolet irradiation: exploring the role of molecular mechanisms. Photodermatol Photoimmunol Photomed. 2010;26:250–256. doi: 10.1111/j.1600-0781.2010.00534.x. [DOI] [PubMed] [Google Scholar]
- 26.Mascia F. Catalsson C. Lee TC. Threadgill D. Mariani V. Almerio P. Chandrasekhara C. Adeva GS. Girolomoni G. Yuspa SH. Pastore S. EGFR regulates the expression of keratinocyte-derived granulocyte/macrophage colony-stimulating factor in vitro and in vivo. J Invest Dermatol. 2010;130:682–693. doi: 10.1038/jid.2009.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mascia F. Mariani V. Girolomoni G. Pastore S. Blockade of EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol. 2003;163:303–312. doi: 10.1016/S0002-9440(10)63654-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura Y. Sotozono C. Kinoshita S. The epidermal growth factor receptor (EGFR): role in corneal wound healing and homeostasis. Exp Eye Res. 2001;72:511–517. doi: 10.1006/exer.2000.0979. [DOI] [PubMed] [Google Scholar]
- 29.Pastore S. Mascia F. Mariotti F. Dattilo C. Mariani V. Girolomoni G. ERK1/2 regulates epidermal chemokine expression and skin inflammation. J Immunol. 2005;174:5047–5056. doi: 10.4049/jimmunol.174.8.5047. [DOI] [PubMed] [Google Scholar]
- 30.Pastore S. Mascia F. Mariani V. Girolomoni G. The epidermal growth factor receptor system in skin repair and inflammation. J Invest Dermatol. 2008;128:1365–1374. doi: 10.1038/sj.jid.5701184. [DOI] [PubMed] [Google Scholar]
- 31.Pastore S. Potapovich A. Kostyuk V. Mariani V. Lulli D. De Luca C. Korkina L. Plant polyphenols effectively protect HaCaT cells from ultraviolet C-triggered necrosis and suppress inflammatory chemokine expression. Ann N Y Acad Sci. 2009;1171:305–311. doi: 10.1111/j.1749-6632.2009.04684.x. [DOI] [PubMed] [Google Scholar]
- 32.Pfeiffer S. Schrammel A. Koesling D. Schmidt K. Mayer B. Molecular actions of a Mn(III)porphyrin superoxide dismutase mimetic and peroxynitrite scavenger: reaction with nitric oxide and direct inhibition of NO synthase and soluble guanylyl cyclase. Mol Pharmacol. 1998;53:795–800. doi: 10.1124/mol.53.4.795. [DOI] [PubMed] [Google Scholar]
- 33.Potapovich AI. Lulli D. Fidanza P. Kostyuk VA. De Luca C. Pastore S. Korkina LG. Plant polyphenols differentially modulate inflammatory responses of human keratinocytes by interfering with activation of transcription factors NFkB and AhR and EGFR-ERK pathway. Toxicol Appl Pharmacol. 2011;255:138–149. doi: 10.1016/j.taap.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Pullar CE. Isseroff SS. The b2-adrenergic receptor activates pro-migratory and pro-proliferative pathways in dermal fibroblasts via divergent mechanisms. J Cell Sci. 2006;119:592–602. doi: 10.1242/jcs.02772. [DOI] [PubMed] [Google Scholar]
- 35.Repertinger SK. Campagnaro E. Fuhrman J. Al-Abaseri T. Yuspa SH. Hansen LA. EGFR enhances early healing after cutaneous incisional wound. J Invest Dermatol. 2004;123:982–989. doi: 10.1111/j.0022-202X.2004.23478.x. [DOI] [PubMed] [Google Scholar]
- 36.Sarkar FH. Li Y. Wang Z. Kong D. Cellular signaling perturbation by natural products. Cell Signal. 2009;21:1541–1547. doi: 10.1016/j.cellsig.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schreiber E. Matthias P. Muller MM. Schaffner W. Rapid detection of octamer binding proteins with mini-extracts, prepared from a small number of cells. Nucl Acid Res. 1989;17:6419–6424. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sutter CH. Yin H. Li Y. Mammen JS. Bodreddigari S. Stevens G. Cole JA. Sutter TR. EGF receptor signaling blocks aryl hydrocarbon receptor-mediated transcription and cell differentiation in human epidermal keratinocytes. Proc Natl Acad Sci U S A. 2009;106:4266–4271. doi: 10.1073/pnas.0900874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tokumaru S. Higashiyama S. Endo T. Nakagawa Y. Miyagawa J. Yamamori K. Hanakawa Y. Ohmoto H. Yoshiro K. Shirakata Y. Matsuzawa Y. Hashimoto K. Taniguchi N. Ectodomain shedding of epidermal growth factor receptor ligands is required for keratinocyte migration in cutaneous wound healing. J Cell Biol. 2000;151:209–219. doi: 10.1083/jcb.151.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tunon MJ. Garcia-Mediavilla MV. Sanchez-Campos S. Gonzalez-Gallego J. Potential of flavonoids as anti-inflammatory agents: modulation of pro-inflammatory gene expression and signal transduction pathways. Curr Drug Metab. 2009;10:256–271. doi: 10.2174/138920009787846369. [DOI] [PubMed] [Google Scholar]
- 41.Uchiyama M. Mihara M. Determination of malonyldialdehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 42.Vanden Berghe W. Haegeman G. Epigenetic remedies by dietary phytochemicals against inflammatory skin disorders: myth or reality? Curr Drug Metab. 2010;11:436–450. doi: 10.2174/138920010791526079. [DOI] [PubMed] [Google Scholar]
- 43.Xu Y. Shao Y. Voorhees JJ. Fisher GJ. Oxidative inhibition of receptor-type protein-tyrosine phosphatase k by ultraviolet irradiation activates epidermal growth factor receptor in human keratinocytes. J Biol Chem. 2006;281:27389–27397. doi: 10.1074/jbc.M602355200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu Y. Shao Y. Zhou J. Voorhees JJ. Fisher GJ. Ultraviolet irradiation-induced epidermal growth factor receptor (EGFR) nuclear translocation in human keratinocytes. J Cell Biochem. 2009;107:873–880. doi: 10.1002/jcb.22195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yahata Y. Shirakata Y. Tokumaru S. Yang L. Dai X. Tohyama M. Tsuda T. Sayama K. Iwai M. Horiuchi M. Hashimoto K. A novel function of angiotensin II in skin wound healing: induction of fibroblast and keratinocyte migration by angiotensin II via heparin-binding epidermal growth factor (EGF)-like growth factor-mediated EGF receptor transactivation. J Biol Chem. 2006;281:13209–13216. doi: 10.1074/jbc.M509771200. [DOI] [PubMed] [Google Scholar]
- 46.Yamaki M. Sugiura K. Muro Y. Shimoyama Y. Tomita Y. Epidermal growth factor receptor tyrosine kinase inhibitors induce CCL2 and CCL5 via reduction in IL-1R2 in keratinocytes. Exp Dermatol. 2010;19:730–735. doi: 10.1111/j.1600-0625.2010.01108.x. [DOI] [PubMed] [Google Scholar]