Abstract
Background
Most studies reporting cognitive deficits in chronic alcoholics have relied on treatment samples (predominantly men) from inpatient or outpatient treatment facilities. However, the majority of chronic alcoholics have never been in treatment, and there is increasing evidence that treated and non-treatment seeking alcoholic samples come from different populations with regard to alcohol use and other factors related to the severity of disease. Accordingly, in the present study, we assessed a broad range of cognitive functions in 55 treatment-naïve alcohol dependent (TNAD) individuals and 55 non-alcoholic controls (NAC) matched for age and education. In addition, a goal of the present study was to assess potential differential effects of alcohol dependence on cognitive performance in TNAD men and women.
Methods
Comprehensive neuropsychological assessment was conducted on TNAD and NAC. The following nine performance domains, each consisting of multiple measures, were examined: attention, auditory working memory, verbal processing, abstraction/cognitive flexibility, psychomotor function, immediate memory, delayed memory, reaction time, and spatial processing.
Results
Analysis revealed no cognitive deficits in TNAD, relative to NAC, in any of the nine cognitive domains. TNAD performed better than NAC in the attention domain. In addition, while men performed better than women in the spatial domain, there were no TNAD vs. NAC group by gender interactions for any domain.
Conclusions
Our results extend findings that TNAD show minimal behavioral effects of chronic heavy alcohol use, and are consistent with the contention that TNAD are relatively cognitively intact. Differences between our findings and those often reported for alcoholics recruited from treatment settings may be understood in terms of differences in alcohol use, along with genetic, psychiatric, and nutritional factors. In addition, the lack of differential effects of alcohol dependence on male and female cognitive performance in our study suggests that TNAD men and women do not differ in the severity of cerebral consequences of alcohol dependence.
Keywords: alcoholism, active alcoholics, cognition, neuropsychology, treatment-naive
Introduction
Numerous studies have identified a pattern of cognitive deficits that can occur as a result of chronic alcoholism. The most severe effects are reported to include frontal system functions—problem solving, abstraction, working memory, susceptibility to interference, behavioral inhibition (Fein et al., 1990; Oscar-Berman, 2000; Sullivan et al., 2000), right hemisphere functions—visuospatial abilities, perceptual-motor skills, attention (Parsons et al., 1993), and impaired memory function associated with hippocampal and temporal lobe atrophy (Sullivan et al., 2000). Disruption of fronto-cerebellar circuitry, affecting problem solving, contextual memory, and learning and execution of procedures, has also been documented (Sullivan, 2003; Sullivan et al., 2005). However, most of this research has relied on convenience samples (predominantly men) from inpatient or outpatient treatment settings, and the degree to which conclusions from these studies can be generalized to the population of individuals with chronic alcohol dependence has recently been called into question (Fein et al., 2005; Gazdzinski et al., 2008).
Alcoholics in treatment constitute a small proportion of alcoholics in the general population. Findings from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) (Cohen et al., 2007) indicate that, of the estimated 27 million Americans exhibiting alcohol use disorders at some time during their lives, only about 15% have ever received any treatment. Among those with a prior-to-past year alcohol dependence (AD), NESARC results indicate that 25.5% have received treatment (Dawson et al., 2005); thus, about 75% of AD are not represented by samples drawn from treatment settings.
Bias in treated samples can be associated with a number of factors that may contribute to the decision to seek treatment. Indeed, findings from recent studies reveal that AD individuals in treatment, compared to treatment-naïve alcohol dependent individuals (TNAD), have a higher incidence of psychiatric co-morbidity, including externalizing (primarily antisocial) disorders, mood disorders, and anxiety disorders (Di Sclafani et al., 2008; Fein et al., 2002; Moss et al., 2007), as well as greater dysfunction in intimate relationship and vocational functioning (Tucker et al., 2004). In addition, AD individuals in treatment have reported more severe dependence, more emotional problems, and less engagement in everyday activities than TNAD individuals (Kaskutas et al., 1997; Lloyd et al., 2004; Lukassen et al., 2005; Tucker et al., 2004). Perhaps the most telling evidence of bias in treated AD samples comes from Fein and Landman (2005), who demonstrated differences in alcohol use trajectories between treated AD and TNAD individuals in the period after meeting “heavy drinking” criteria. Treated alcoholics had higher average and peak alcohol doses than did TNAD. In fact, the treated alcoholics had alcohol doses over 50% higher than treatment naïve alcoholics in the years just after they met criteria for heavy alcohol use.
In sum, the foregoing findings provide strong evidence that treated alcoholics and TNAD come from different populations with regard to alcohol use, and do not simply represent different stages in the progression of alcohol use and dependence. Consequently, results from most studies of cognitive functioning in alcoholics in treatment or post-treatment, may not be directly generalizable to the majority of alcoholics.
Several recent studies by Fein and colleagues have focused on the effects of chronic alcohol dependence in treatment-naïve active alcoholics. For example, in a study by Fein and colleagues (2002), structural MRI revealed no significant differences between TNAD and non-alcoholic controls in overall or regional gray matter volumes. Moreover, white matter and temporal cortex, which usually show reduced volume in AD samples drawn from treatment, did not differ between treatment-naïve alcoholics and control subjects. However, age by group interactions reflected an age-related reduction of whole brain, prefrontal, and parietal gray matter volumes in TNAD individuals that was greater than the age-related decline in these volumes in control subjects. A second study, with a larger sample that included both men and women, (Fein, 2010 IN PRESS) replicated the above finding of no significant overall or regional reductions in TNAD, supporting the hypothesis that the neurological effects on non-treatment seeking alcohol dependent individuals are less severe than in those who seek treatment. The age by alcoholism interactions for gray matter volumes were also replicated in both TNAD men and women. Partial correlation analysis showed that the effect was a function of a greater age-related volume reduction in TNAD, and not a function of lifetime alcohol burden. In another line of research, a study of decision-making (Fein et al., 2006a) showed normal performance in TNAD individuals on a simulated gambling task (SGT) that has revealed disadvantageous decision-making in treated alcoholics (Bechara et al., 2002; Fein et al., 2004b; Mazas et al., 2000). A related study (Fein et al., 2008) examined EEG recordings from treatment-naïve alcoholics while performing the Balloon Analogue Risk Task, which measures risk-taking propensity. Reduced feedback error-related negativity (F-ERN) amplitude, suggesting a lower emotional valence attached to feedback regarding the negative consequences of behavior, was associated with a greater family history density of alcohol problems within the TNAD group.
The above results are consistent with the idea that treated alcoholics and TNAD individuals come from different populations. However, they also show that TNAD exhibit more subtle effects of alcohol use, such as steeper age-related declines in brain volumes than NAC, and smaller F-ERNs as a function of family history density of alcohol problems than NAC. In the present study we examined cognitive function in treatment-naïve active alcoholics compared to non-alcoholic control subjects.
As noted earlier, most assessments of cognitive performance in alcoholics have relied on samples that were not only drawn from treatment settings, but were also predominantly men. Two studies (Bergman, 1987; Jacobson, 1986) of structural brain changes in alcoholic men and women drawn from clinical samples concluded that women suffer more severe cerebral consequences of long-term alcohol dependence than do men. This reported difference is consistent with the hypothesis (Glenn et al., 1988) that cognitive processes in women are especially vulnerable to the toxic effects of alcohol. However, the reported difference in cerebral consequences may be the result of an inherent bias in studying clinical populations that is greater for women than for men. That is, given evidence that women are less likely than men to receive treatment for alcohol dependence (Dawson, 1996; Schober et al., 1996), they may have a higher threshold for alcohol related problems before receiving treatment. Indeed, Dawson (1996) found that, at the most common levels of alcohol dependence severity (5 to 10 symptoms), men were about 50% more likely than women to have received treatment, while at the most severely dependent (20 or more symptoms), they were equally likely. This gender by severity interaction may lead to over-representation of the most severely affected women in treatment samples. In contrast, samples of men and women drawn from treatment-naïve alcoholic populations may not have this bias and, hence, may be more likely to have comparable preservation/impairment of cognitive function across gender. In fact, after controlling for lifetime alcohol consumption, Pfefferbaum and colleagues (2001) did not find gender differences in alcoholics in cortical gray and white matter, and found less abnormality in cortical sulcal volumes for alcoholic women than for alcoholic men. Moreover, Yonker and colleagues (2005) reported that moderate to heavy drinking women performed somewhat better on episodic memory and spatial visualization tasks than did non/light drinking women, a pattern not found in men. Finally, Sullivan and colleagues (2002) have found that, while cognitive deficits in abstinent alcoholic men are most often reported in visuospatial and executive domains, the cognitive functions most severely affected in abstinent alcoholic women were visuospatial abilities and verbal and nonverbal working memory processes, with relative sparing of executive functions. Taken together, the above results underscore the need for investigations using treatment-naïve AD samples to better understand potential sex differences in effects of alcohol on cognitive function. In the present study TNAD individuals were matched for age and gender with non-alcoholic control subjects to allow for unbiased comparisons of cognitive profiles in men and women.
Methods
Subjects
Participants consisted of TNAD (33 men and 25 women) matched for age and gender with non-alcoholic controls (NAC: 33 men and 25 women), both groups ranging in age from 19.7 to 50.4 years (M = 31.5). All alcohol-use and gender groups had comparable years of education (See Table 1). Participants were recruited from the community through postings, mailings, newspaper ads, ads on an internet site, and referrals from other participants. All TNAD participants met American Psychiatric Association (2000) criteria for current alcohol dependence, were currently drinking, and had never sought treatment for alcoholism. All NAC had a lifetime drinking average of less than 30 drinks per month, no periods of drinking more than 60 drinks per month, and a negative lifetime history for alcohol abuse/dependence. Exclusion criteria for both groups were: 1) lifetime or current diagnosis of schizophrenia or schizophreniform disorder (c-DIS) (Robins et al., 1998), 2) history of, or current drug abuse or dependence (other than nicotine or caffeine), 3) significant history of head trauma or cranial surgery, 4) history of significant neurological disease, 5) history of diabetes, stroke, or hypertension that required medical intervention, 6) laboratory evidence of hepatic disease, or 7) clinical evidence of Wernicke-Korsakoff syndrome.
Table 1.
Demographics and Neuropsychological Measures – Treatment-Naïve Alcoholics vs. Non-Alcoholic Controls
| Variables | Treatment-Naïve | Non-Alcoholic Controls | % Effect Size (partial eta square) | ||||
|---|---|---|---|---|---|---|---|
| Men (n=33) | Women (n=25) | Men (n=33) | Women (n=25) | Group | Gender | Group × Gender | |
| Demographics | |||||||
| Age (years) | 32.32 ± 8.6 | 30.40 ± 8.5 | 32.25 ± 8.32 | 30.45 ± 8.51 | 0.0 | 1.2 | 0.0 |
| Years Education | 16.36 ± 1.60 | 16.00 ± 1.76 | 16.53 ± 1.96 | 16.58 ± 1.50 | 1.2 | 0.2 | 0.4 |
| Proportion 1st degree Relative Problem | |||||||
| Drinkers a | 0.14 ± 0.20 | 0.25 ± 0.31 | 0.12 ± 0.18 | 0.14 ± 0.26 | 2.1 | 2.1 | |
| AMNART | 1.30 ± 0.34 | 1.16 ± 0.37 | 1.21 ± 0.42 | 1.11 ± 0.45 | 0.8 | 2.2 | 0.1 |
|
| |||||||
| Neuropsychological Measures | |||||||
| Average z-score | 0.25 ± 0.34 | 0.27 ± 031 | 0.17 ± 0.51 | 0.17 ± 0.35 | 1.4 | 0.0 | 0.0 |
| Attention | 0.20 ± 0.40 | 0.17 ± 0.45 | -0.24 ± 0.62 | -0.08 ± 0.45 | 11.3** | 0.4 | 0.9 |
| Auditory Working Memory | 0.12 ± 0.76 | 0.17 ± 0.45 | 0.11 ± 0.88 | -0.29 ± 0.97 | 1.8 | 1.0 | 1.6 |
| Verbal | 0.69 ± 1.00 | 0.80 ± 0.86 | 0.61 ± 1.08 | 0.58 ± 1.22 | 0.3 | 0.2 | 0.0 |
| Abstraction/Cognitive Flexibility | 0.13 ± 0.55 | 0.12 ± 0.48 | 0.12 ± 0.62 | -0.01 ± 0.49 | 0.4 | 0.4 | 0.3 |
| Psychomotor | -0.22 ± 0.69 | 0.18 ± 0.62 | -0.03 ± 0.94 | -0.09 ± 0.85 | 0.1 | 1.2 | 2.1 |
| Immediate Memory | 0.70 ± 0.44 | 0.70 ± 0.33 | 0.61 ± 0.65 | 0.61 ± 0.67 | 0.3 | 0.1 | 0.1 |
| Delayed Memory | 0.55 ± 0.70 | 0.35 ± 0.73 | 0.26 ± 0.87 | 0.58 ± 0.72 | 0.0 | 0.2 | 2.8 |
| Reaction Time | -0.13 ± 0.51 | -0.39 ± 0.70 | -0.05 ± 0.63 | -0.17 ± 0.63 | 1.5 | 2.4 | 0.3 |
| Spatial Processing | 0.28 ± 0.52 | 0.15 ± 0.46 | 0.30 ± 0.57 | 0.01 ± 0.58 | 0.3 | 3.7* | 0.5 |
|
| |||||||
| p-value | |||||||
| Global Clinical Impairment Score | 0.36 ± 0.70 | 0.36 ± 0.76 | 0.91 ± 1.40 | 0.71 ± 1.23 | .042 | .55 | n/a |
Measures are reported mean ± standard deviation. Effect is significant:
p≤0.05,
p≤0.01
n = 32 for Male Treatment-Naïve Alcoholics
Procedures
Participant screening was initially conducted by a phone interview assessing alcohol use/dependence, use/dependence of other drugs, medical history, and mental health history. All participants were fully informed of the study’s procedures and aims and signed a consent form prior to their participation. Participation consisted of four sessions that lasted between 1.5 to 3 hours and included clinical, neuropsychological, electrophysiological, and neuroimaging assessments. All of the cognitive measures were administered during one session and were completed by all subjects in our sample. Over 95% of subjects completed all four sessions, most within two to three weeks. In rare cases, where scheduling was especially difficult, sessions were spread out up to six weeks. NAC subjects were asked to abstain from consuming alcohol for at least 24 hours prior to any lab visit. A Breathalyzer (Intoximeters, Inc., St. Louis, MO) test was administered to all subjects. A 0.000 alcohol concentration was required of all participants in all sessions. Subjects were compensated for time and travel expenses upon completion of each session. Subjects who completed the entire study were also given a completion bonus.
All participants completed the following general assessments: 1) psychiatric status was assessed using the c-DIS (Robins et al., 1998), 2) subjects were interviewed on their drug and alcohol use using the lifetime drinking history methodology (Skinner et al., 1982a; 1982b; Sobell et al., 1990; 1988), 3) medical histories were reviewed, 4) blood was drawn to test liver functions, and 5) the Family Drinking Questionnaire was administered based on the methodology of Mann and colleagues (1985; Stoltenberg et al., 1998).
Neuropsychological Assessment
All subjects were individually administered a comprehensive neuropsychological assessment, beginning with the MicroCog (MC) Assessment of Cognitive Functioning standard version (Powell et al., 1993). The MC is a computerized battery consisting of 18 subtests designed to be sensitive to impairment across a wide range of cognitive functions. It provides age- and education-level adjusted norms. Following a 15 minute break, subjects were administered the following individual tests: Rey-Osterrieth Complex Figure (copy, immediate, and 20 minute delayed) (Osterrieth, 1944), Trail Making Test A and B (Reitan et al., 1985), Controlled Oral Word Association Test (COWAT) (Benton et al., 1983), Symbol Digit Modalities Test (written administration only) (Smith, 1968), Short Category Test (booklet format) (Wetzel et al., 1987), Paced Auditory Serial Addition Test (PASAT) (Gronwall, 1977), Block Design (WAIS-R) (Wechsler, 1981), Stroop Color and Word Test (Golden, 1978) and the American version of the Nelson Adult Reading Test (AMNART), as an estimate of premorbid IQ (Grober et al., 1991). The following 9 domains, and their component tests, were included in the final analyses for neuropsychological performance: (1) Attention (Stroop Color, MC Numbers Forward, MC Numbers Reversed, MC Alphabet, MC Word List 1), (2) Verbal Processing (COWAT), (3) Abstraction/Cognitive Flexibility (Short Categories, Stroop interference score, Trail Making Test B, MC Analogies, MC Object Match A), (4) Psychomotor (Trails A, Symbol Digit), (5) Immediate Memory (MC Story immediate recall, Rey immediate recall, MC Word List 2), (6) Delayed Memory (MC Story delayed recall, Rey delayed recall), (7) Reaction Time (MC Timers simple and cued), (8) Spatial Processing (MC Tic Tac, MC Clocks, Block Design), and (9) Auditory Working Memory (PASAT at delays of 2.4, 2.0, 1.6, and 1.2 seconds). Normative scores derived from a nationally representative sample of adults are available for each test, either from the creators or distributors of the tests.
For each of the neuropsychological domains, composite Z-scores were computed by averaging Z-scores of the individual measures within each domain. Z-scores for the measures were computed based on standardized norms adjusted for age [Stroop (Golden, 1978), Short Categories (Wetzel et al., 1987), PASAT (Stuss et al., 1988), Block Design (Wechsler, 1997), and Rey (Denman, 1987)], age and years of education Symbol Digit Modalities [(Smith, 1982), MicroCog (Powell et al., 1993)] and age, gender, and years of education [(Trails A and B (Heaton et al., 1991), COWAT (Ruff et al., 1996)]. The Stroop, Symbol Digit Modalities, and the MicroCog test norms are not specific to gender, since gender did not significantly affect scores in the normative samples (Golden, 1978; Powell et al., 1993; Smith, 1982). The AMNART, which is used as a measure of premorbid intelligence (Grober et al., 1991), was adjusted for years of education (Schwartz et al., 1987), but did not have age norms because the test was designed to be resistant to the effects of normal aging. Grober and colleagues (1991) have reported that gender does not influence AMNART scores.
For the NP measures, a multivariate analysis of variance (MANOVA) was carried out, with Group and Gender as main effects, and average Z-scores for the 9 domains as dependent variables. In addition, a Group × Gender ANOVA was performed for the average Z-score across all domains. Finally, for each subject, a global clinical impairment score was generated as follows: 1) Each domain Z-score was converted to a percentile score. Next, a clinical impairment score of 0, or no impairment, was assigned to domain Z-scores falling above the 15th percentile, a score of 1, or moderately impaired, was assigned to domain Z-scores falling at or below the 15th and above the 5th percentile, and a score of 2, or severely impaired, was assigned to domain Z-scores falling at or below the 5th percentile. The domain clinical impairment scores (0, 1, or 2) were then summed across domains to yield the GCIS, with greater GCIS scores indicating more severe impairment. The cutoff points for the clinical impairment scores were designed to make the GCIS sensitive to clinically relevant impairment. A nonparametric Mann-Whitney U test was performed in order to determine whether NAC and TNAD differed on the GCIS.
The data were also analyzed by constructing age- and education-adjusted Z-scores based on our own control group. First, within the control group, regression analysis on raw scores for each cognitive measure (including both the MicroCog and supplementary measures) was carried out to determine coefficients to correct for age and education. These coefficients were then applied to all subjects’ data (both control and TNAD) to compute predicted scores and residuals based on the association of age and education with the measure. Next, for all subjects, the residuals were added to the NAC raw score mean of the measure to obtain scores adjusted for the association of age and education (in controls) with the measure. Finally, Z-scores for both TNAD and NAC were computed using means and standard deviations of these adjusted scores from NAC. Using the adjusted Z-scores based on controls, multivariate analysis of variance (MANOVA) was carried out, with Group and Gender as main effects, and average Z-scores for the 9 domains as dependent variables, and a Group by Gender ANOVA was performed for the average Z-score (based on controls, age- and education-adjusted) across all domains.
Results
Table 1 presents demographic and family history variables for all participants, along with results for neuropsychological performance (NP) in the nine domains (Z-scores based on standardized norms). Analyses of variance with Group (Treatment-Naïve vs. Control) and Gender as main effects yielded no significant differences in age, years of education, proportion of 1st degree relative problem drinkers, nor AMNART Z-scores (an estimate of pre-morbid cognitive functioning).
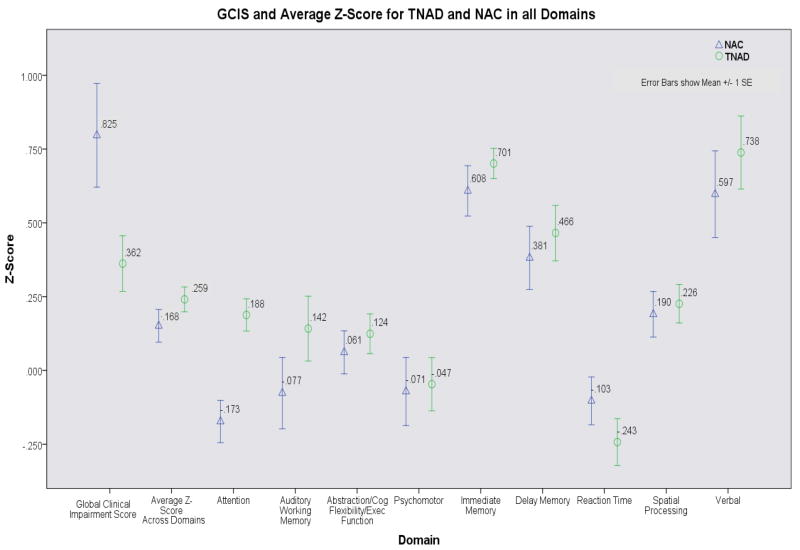
Analyses using the Z-scores computed based on standardized norms yielded the following: There were no significant Group, Gender, or Group by Gender effects for the average Z-score across the nine domains. The MANOVA on NP domain Z-scores yielded a significant main effect for Group (F (9, 104) = 2.368, p < .019), a trend toward a significant main effect for Gender (F (9, 104) = 1.753, p < .087) and no Group × Gender interaction (p > .11). In light of the significant Group main effect, we examined individual domain scores (See Figure 1). For the Attention domain, TNAD performed better than NAC (F (1, 112) = 14.215, p < .001). Men (across both groups) performed better than women in the Spatial Processing domain (F (1, 112) = 4.298, p < .041), but there were no significant Group × Gender interactions for any domain. Finally, NAC had higher GCIS scores (reflecting worse performance) than TNAD (p ≤ .04). It should be noted that no TNAD had a GCIS higher than 3 while three NACs had a GCIS of more than 4 (5, 5, 6). A second Mann-Whitney U test with these NAC outliers removed yielded no significant difference between Groups (p = 0.123). In order to determine whether the four outlying GCIS accounted for the Group difference in the Attention domain (reported above) we repeated the Attention domain analysis without these subjects. The Group difference was still significant (p < .001).
Figure 1.
Average Z-Scores (based on standardized norms) for TNAD and NAC in all neuropsychological domains.
Using Z-scores based on our control group (adjusted for age and education based on our NAC), the Group difference in average Z-score across all domains reached significance (F (3, 110) = 5.263, p < .025, partial eta2 = 4.6), and multivariate analysis of variance of domain scores revealed a Group effect (F (9, 102) = 2.252, p < .025, partial eta2 = 1.66) reflecting better performance of TNAD compared to NAC. Analysis of individual domains yielded a significant (F (1, 110) = 10.64, p < .002, partial eta2 = .088) Group effect for the Attention domain, with TNAC performing better than NAC, and a significant (F (1, 110) = 4.41, p = .038, partial eta2 = .039) overall advantage of men over women in the spatial processing domain, consistent with the earlier analysis reported above. As with the earlier analysis, TNAD still showed better performance than NAC in the Attention domain after the three GCIS outliers were removed (p < .007).
Table 2 shows mean raw scores (scaled raw scores for MicroCog) and standard deviations for each NP measure in each NP domain. Reported significance levels for Group, Gender, and Group by Gender effects are based upon raw score comparisons within multivariate analysis of variance. Examination of individual measures indicates that better performance of TNAD in the Attention domain, compared to TNAD, was due to higher scores on MicroCog Numbers Forward (p < .001, partial eta2 = .116) and Numbers Reversed (p < .001, partial eta2 = .116), along with a trend toward better scores in the Stroop Color condition (p = .10). In the Immediate and Delayed Memory domains, TNAD scored higher than NAC on MicroCog Wordlist 2 (p < .05, partial eta2 = 4.0), while there were trends toward better performance of NAC, compared to TNAD, on the Rey-Immediate (p = .06) and -Delayed (p = .08) Recall measures. In the Abstraction/Cognitive Flexibility domain, women scored higher than men on the Stroop-Interference (p < .05, partial eta2 = .046), and MicroCog Analogies (trend - p = .052). In addition, for the Spatial Processing domain, TNAD scored higher than NAC on MicroCog Clocks (p < .01), and men scored better than women on Block Design (p < .01, partial eta2 = 5.9), consistent with the finding (reported above) of an overall advantage for men, compared to women, in the spatial processing domain. However, caution should be taken in interpreting the individual measure results due to increased Type I error associated with multiple comparisons.
Table 2.
Neuropsychological Tests Raw Scores for Treatment-Naïve Alcoholics and Controls
| Variable | Treatment-Naive | Control | Effect Size (%) | ||||
|---|---|---|---|---|---|---|---|
| Men (n=33) | Women (n=25) | Men (n=33) | Women (n=25) | Group | Gender | Group by Gender | |
| Abstraction/Cognitive Flexibility | |||||||
| MicroCog Analogies | 11.36 ± 3.47 | 10.24 ± 2.65 | 11.77 ± 3.19 | 10.20 ± 3.51 | 0.4 | 3.4.052 | 1.6 |
| MicroCog Object Match A | 10.58 ± 2.65 | 10.36 ± 3.15 | 9.27 ± 3.42 | 9.80 ± 3.19 | .7 | 0.0 | 1.2 |
| Short Categories | 24.45 ± 11.09 | 29.56 ± 15.06 | 24.20 ± 14.33 | 25.48 ± 14.24 | 0.6 | 1.4 | 0.5 |
| Stroop-Interference | 45.7 0 ± 10.14 | 52.44 ± 9.554 | 44.47 ± 10.18 | 46.56 ± 10.76 | 3.0.07 | 4.6* | 1.3 |
| Trails B | 56.82 ± 19.23 | 45.12 ± 10.73 | 49.40 ± 15.16 | 53.44 ± 13.12 | 0.0 | 1.6 | 6.4** |
| Attention | |||||||
| MicroCog Alphabet | 10.79 ± .56 | 10.4 ± .91 | 10.17 ± 1.91 | 10.08 ± 2.18 | 0.9 | 0.5 | 0.3 |
| MicroCog Numbers Forward | 11.00 ± 3.11 | 11.36 ± 2.50 | 9.07 ± 3.28 | 8.96 ± 2.41 | 11.6*** | 0.1 | 0.7 |
| MicroCog Numbers Reversed | 11.79 ± 2.20 | 10.32 ± 2.58 | 8.70 ± 3.68 | 9.48 ± 2.28 | 11.6*** | 0.1 | 2.8.08 |
| MicroCog Wordlist 1 | 9.97 ± 3.19 | 9.88 ± 3.38 | 10.13 ± 2.93 | 10.76 ± 2.40 | 0.8 | 0.0 | 0.2 |
| Stroop-Color | 76.88 ± 12.62 | 82.52 ± 12.88 | 74.87 ± 11.44 | 76.88 ± 11.82 | 2.5.10 | 2.5.10 | 1.3 |
| Auditory Working Memory | |||||||
| PASAT 2.4 (seconds delay) | 45.79 ± 9.76 | 48.24 ± 10.44 | 47.60 ± 8.71 | 41.92 ± 11.80 | 1.2 | 0.6 | 3.9* |
| PASAT 2.0 (seconds delay) | 42.06 ± 9.26 | 43.52 ± 12.64 | 42.73 ± 9.87 | 37.68 ± 13.19 | 1.4 | 0.7 | 2.1 |
| PASAT 1.6 (seconds delay) | 37.27 ± 9.61 | 38.00 ± 12.79 | 38.63 ± 9.64 | 34.08 ± 13.37 | 0.3 | 0.7 | 1.4 |
| PASAT 1.2 (seconds delay) | 27.55 ± 10.15 | 26.12 ± 8.76 | 28.83 ± 9.96 | 21.20 ± 9.82 | 0.9 | 5.2* | 2.5.10 |
| Immediate Memory | |||||||
| MicroCog Story Recall | 10.30 ± 2.72 | 9.52 ± 3.29 | 9.97 ± 3.39 | 9.76 ± 3.17 | 0.0 | 0.5 | 0.0 |
| MicroCog Wordlist 2 | 11.18 ± 1.40 | 11.20 ± 1.61 | 10.17 ± 2.49 | 10.40 ± 2.93 | 4.0* | 0.0 | 0.1 |
| Rey-Immediate Recall | 48.24 ± 13.81 | 47.84 ± 11.88 | 52.20 ± 10.98 | 52.76 ± 11.83 | 3.2.06 | 0.0 | 0.0 |
| Delayed Memory | |||||||
| MicroCog Story-Delayed Recall | 12.00 ± 2.83 | 11.00 ± 2.50 | 10.23 ± 3.45 | 11.20 ± 3.21 | 0.8 | 0.1 | 1.0 |
| Rey-Delayed Recall | 48.58 ± 13.49 | 47.80 ± 12.19 | 51.87 ± 11.67 | 52.84 ± 11.47 | 2.8.08 | 0.0 | 0.1 |
| Psychomotor | |||||||
| Symbol Digit Modalities | 57.85 ± 9.76 | 63.20 ± 7.81 | 59.73± 10.33 | 58.56 ± 7.58 | 0.6 | 1.3 | 3.2.06 |
| Trails A | 26.52 ± 7.97 | 24.00 ± 5.10 | 24.33 ± 9.08 | 25.28 ± 8.51 | 0.1 | 0.3 | 1.2 |
| Reaction Time | |||||||
| MicroCog Cued Timers | 10.00 ± 2.45 | 9.56 ± 2.57 | 10.10 ± 2.70 | 10.04 ± 2.30 | 1.1 | 0.3 | 0.1 |
| MicroCog Simple Timers | 9.30 ± 2.42 | 8.16 ± 3.09 | 9.80 ± 3.00 | 9.20 ± 2.55 | 2.5.09 | 1.0 | 0.0 |
| Spatial Processing | |||||||
| Block Design | 51.82 ± 10.13 | 47.24 ± 10.33 | 53.30 ± 10.38 | 47.76 ± 10.15 | 0.2 | 5.9** | 0.1 |
| MicroCog Clocks | 11.27 ± 1.48 | 11.68 ± 1.03 | 10.33 ± 2.04 | 10.76 ± 1.90 | 6.2** | 0.8 | 0.1 |
| MicroCog Tic Tac | 8.09 ± 2.17 | 7.84 ± 2.08 | 8.73 ± 2.00 | 7.36 ± 1.89 | 0.0 | 2.3 | 3.0.07 |
| Verbal | |||||||
| COWAT | 45.03 ± 12.48 | 44.96 ± 10.35 | 45.00 ± 12.08 | 44.48 ± 12.46 | 0.0 | 0.0 | 0.0 |
Measures are reported mean ± standard deviation.
Effect is significant:
= p≤0.05,
= p≤0.01,
= p≤0.001
P values for trends are reported as superscripts.
Discussion
Our results are consistent with the contention that TNAD are relatively cognitively intact. Our investigation assessed a broad range of cognitive domains, including those that are most susceptible to disruption from chronic heavy alcohol use. The TNAD in our study did not display the pattern of cognitive deficits typically found in clinical samples. This finding is consistent with results from another recent study (Chao et al., 2003) which, along with event-related potentials (ERPs) and regional brain volumes, assessed cognitive performance in treatment-naïve active alcoholics on three measures that are considered to be highly sensitive to frontal lobe dysfunction—the Wisconsin Card Sorting Test (WCST), Stroop color-word interference, and the Trail Making Test B. Their TNAD sample was somewhat older (mean age = 43) than ours (mean = 31.5), so their sample should be more likely to display cognitive deficits. However, although TNAD individuals in Chao and colleagues’ (2003) study differed from control subjects on categories completed, non-perseverative errors on the WCST, and word reading for the Stroop, they did not differ on those measures which are most commonly associated with frontal lobe function (perseverative errors on the WCST, color-word interference on the Stroop, and Trails B). It should be noted that some studies (Drewe, 1974; Grafman et al., 1986) have found that the number of categories completed on the WCST was reduced in patients with frontal lobe damage, although others (Janowsky et al., 1989; Stuss et al., 1983) have found that frontal patients did not differ from control subjects on this measure. Chao and colleagues’ (2003) TNAD sample did exhibit reduced late contingent negative variation (CNV) ERPs (which are linked to response preparation) over midline and parietal electrode sites. Although there was no significant difference in frontal lobe volume between TNAD and controls, in TNAD reduced late CNV amplitudes were associated with decreased frontal lobe gray matter volume.
Our results provide further evidence that TNAD show minimal behavioral effects of chronic heavy alcohol use. It is unlikely that our finding of no cognitive deficits in TNAD is due to a lack of sensitivity in our cognitive measures. Many of the measures we employed (e.g. Stroop interference, Trails B, Short Categories, PASAT, Rey Immediate and Delayed recall, Symbol-digit modalities, Block Design, and COWAT) have reliably demonstrated sensitivity (Lezak et al., 2004) to damage in those brain regions most frequently compromised by heavy alcohol consumption. Moreover, the Spatial domain measures we used were sensitive enough to show processing deficits in long-term abstinent alcoholics (Fein et al., 2006b), as well as better spatial processing by men vs. women in the current study. The difference between TNAD and treated alcoholics may be understood in terms of several factors. First, as reported earlier (Fein et al., 2005) TNAD are likely to have lower average and peak alcohol doses than treated alcoholics. Direct comparison of alcohol use histories in samples of treated and non-treated alcoholics (Fein et al., 2005) showed that treated alcoholics had alcohol doses more than 50% higher than TNAD in the years immediately after meeting criteria for heavy alcohol use. In addition, genetic, psychiatric, and nutritional factors likely play a role in behavioral differences between treated and treatment-naïve alcoholic samples. Indeed, a study of 3,572 alcohol-dependent men and women (Raimo et al., 1999) reported a progression, from never treated to outpatient or AA only to inpatient treatment, in increased unemployment, marital instability, additional drug dependencies and psychiatric disorders, and more alcohol-related adverse events. While treated alcoholic samples have consistently shown higher family history density of alcohol problems than NAC, our TNAD sample did not differ from NAC in terms of proportion of 1st degree relative problem drinkers (See Table 1), and had a lower family history density of alcohol problems than a comparison group of middle-aged long-term abstinent alcoholics (Di Sclafani et al., 2008). A positive family history of addiction problems is associated with smaller regional brain volumes (Benegal et al., 2007), electrophysiological abnormalities (Begleiter et al., 1984; Porjesz et al., 1998), and disinhibitory personality traits associated with an externalizing diathesis (Benegal et al., 2007; Krueger et al., 2009).
Treated alcoholics also have a higher incidence of psychiatric co-morbidity (Berglund et al., 2006; Di Sclafani et al., 2008; Raimo et al., 1999) than TNAD. Di Sclafani and colleagues (2008) examined a TNAD sample of which the current sample is a subset and found that, in contrast to a long-term abstinent alcohol dependent sample, TNAD did not differ from NAC in psychiatric diagnosis rates. TNAD did have more sub (diagnostic) threshold psychiatric problems than NAC (more symptoms and more abnormal measures of the psychological substrate of psychiatric problems). Moreover, more social and emotional problems, and less engagement in everyday activities are reported for treated samples than TNAD (Lloyd et al., 2004). In addition, large sample studies (Berglund et al., 2006; Raimo et al., 1999) found that alcoholic individuals with no prior treatment history were also more often cohabitating and employed than individuals with treatment histories. Not surprisingly, inadequate diet and nutritional deficiencies are commonly reported in clinical samples of alcoholics. Thiamine deficiency is increasingly identified as an important contributor to alcohol-related brain damage of all kinds (See Martin et al. (2003) for a review), especially disruption of fronto-cerebellar and cerebellothalamocortical circuitry affecting abstract problem solving, visuospatial processing, verbal memory and perceptual motor skills (Sullivan, 2003; Sullivan et al., 2005). Given that treated alcoholics are less likely than TNAD to attend to everyday activities, or to be cohabitating or employed, it is reasonable to hypothesize that TNAD individuals are more likely to attend adequately to their diet and, hence, are less likely to suffer the effects of thiamine deficiency, especially given that TNAD also have lower alcohol doses after meeting criteria for heavy alcohol use (Fein et al., 2005). Future research may focus on comparing nutritional histories of TNAD and treated alcoholics.
An additional question for future investigation involves the extent to which older treatment-naïve alcoholics are able to maintain normal cognitive processes. Our TNAD sample was relatively young (mean age = 31.5). There is mounting evidence, reviewed by Fein and Di Sclafani (2004a), that cerebral reserve capacity (which erodes as a function of age) plays an important role in determining the onset and progression of clinical manifestations of neurodegenerative diseases (including those of chronic abuse of substances). Since normal aging results in a loss of reserve capacity, aging may unmask cognitive deficits in TNAD that are compensated for in younger middle-aged samples.
An unanticipated finding from our study was the better performance of TNAD, compared to NAC, in the Attention domain. A possible explanation for this may involve compensatory attentional strategies in heavy drinking functional alcoholics. TNAD in our study were comparable to NAC on employment status, with school and/or employment adding up to full-time occupation. In order to maintain occupational standards while continuing to drink heavily, TNAD may recruit more attentional resources (e.g., be more focused, concentrate harder) than their non-alcoholic counterparts. If so, this could offer an additional partial explanation (along with relatively small cerebral consequences of alcohol dependence) for what appears to be normal cognitive performance in TNAD. In TNAD there may be subtle effects of chronic drinking on cognitive function that individuals are able to compensate for. More challenging tests, with competing demands, may be necessary to unmask such effects, if they exist.
Finally, our results show the advantage for men over women in spatial processing, consistent with the literature (Halpern, 1997; Yonker et al., 2005), an advantage that did not differ between TNAD and NAC. Yonker and colleagues (2005) found that the spatial processing advantage for men over women decreased as chronic alcohol dose increased from moderate to heavy, while our sample of TNAD showed no such effect. It should be noted that Yonker and colleagues’ (2005) sample of heavy drinking men had a mean age of 56.3, likely associated with a greater lifetime alcohol burden and less reserve capacity than our TNAD men who had a mean age of 32.3. In addition, our results revealed no significant Group × Gender interactions in NP domain performance, failing to show differential effects of alcohol dependence on cognitive performance in men and women. This lack of cognitive differences suggests that, in treatment-naïve samples at least, women do not suffer more severe cerebral consequences of alcohol dependence than do men.
Acknowledgments
This work was supported by National Institutes for Health, NIH Grants #AA013659, and #AA011311.
References
- American Psychiatric Association. DSM-IV-TR: Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Publishing; Washington, DC: 2000. [Google Scholar]
- Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40:1675–1689. doi: 10.1016/s0028-3932(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225:1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Benegal V, Antony G, Venkatasubramanian G, Jayakumar PN. Gray matter volume abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addict Biol. 2007;12:122–132. doi: 10.1111/j.1369-1600.2006.00043.x. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher K. Multilingual aphasia examination. AJA Associates; Iowa City: 1983. [Google Scholar]
- Berglund K, Fahlke C, Berggren U, Eriksson M, Balldin J. Individuals with excessive alcohol intake recruited by advertisement: demographic and clinical characteristics. Alcohol Alcohol. 2006;41:200–204. doi: 10.1093/alcalc/agh244. [DOI] [PubMed] [Google Scholar]
- Bergman H. In: Brain dysfunction related to alcoholism: Some results from the KARTAD project, in Neuropsychology of Alcoholism: Implications for Diagnosis and Treatment. Parsons OA, Butters N, Nathan P, editors. Guilford Press; New York: 1987. pp. 21–45. [Google Scholar]
- Chao LL, Meyerhoff DJ, Cardenas VA, Rothlind JC, Weiner MW. Abnormal CNV in chronic heavy drinkers. Clin Neurophysiol. 2003;114:2081–2095. doi: 10.1016/s1388-2457(03)00230-x. [DOI] [PubMed] [Google Scholar]
- Cohen E, Feinn R, Arias A, Kranzler HR. Alcohol treatment utilization: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2007;86:214–221. doi: 10.1016/j.drugalcdep.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Dawson D. Gender differences in the risk of alcohol dependence: United States, 1992. Addiction. 1996;91:1831–1842. [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Stinson FS, Chou PS, Huang B, Ruan WJ. Recovery from DSM-IV alcohol dependence: United States, 2001-2002. Addiction. 2005;100:281–292. doi: 10.1111/j.1360-0443.2004.00964.x. [DOI] [PubMed] [Google Scholar]
- Denman SB. Denman Neuropsychology Memory Scale. SB Denman; Charleston, SC: 1987. [Google Scholar]
- Di Sclafani V, Finn P, Fein G. Treatment-naive Active Alcoholics Have Greater Psychiatric Comorbidity Than Normal Controls but less than Treated Abstinent Alcoholics. Drug Alcohol Depend. 2008;98:115–122. doi: 10.1016/j.drugalcdep.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewe EA. The effect of type and area of brain lesion on Wisconsin card sorting test performance. Cortex. 1974;10:159–170. doi: 10.1016/s0010-9452(74)80006-7. [DOI] [PubMed] [Google Scholar]
- Fein G, Bachman L, Fisher S, Davenport L. Cognitive impairments in abstinent alcoholics. West J Med. 1990;152:531–537. [PMC free article] [PubMed] [Google Scholar]
- Fein G, Chang M. Smaller feedback ERN amplitudes during the BART are associated with a greater family history density of alcohol problems in treatment-naive alcoholics. Drug Alcohol Depend. 2008;92:141–148. doi: 10.1016/j.drugalcdep.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V. Cerebral reserve capacity: implications for alcohol and drug abuse. Alcohol. 2004a;32:63–67. doi: 10.1016/j.alcohol.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical gray matter loss in treatment-naive alcohol dependent individuals. Alcohol Clin Exp Res. 2002;26:558–564. [PMC free article] [PubMed] [Google Scholar]
- Fein G, Klein L, Finn P. Impairment on a simulated gambling task in long-term abstinent alcoholics. Alcohol Clin Exp Res. 2004b;28:1487–1491. doi: 10.1097/01.alc.0000141642.39065.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Landman B. Treated and treatment-naive alcoholics come from different populations. Alcohol. 2005;35:19–26. doi: 10.1016/j.alcohol.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Fein G, McGillivray S, Finn P. Normal performance on a simulated gambling task in treatment-naive alcohol-dependent individuals. Alcohol Clin Exp Res. 2006a;30:959–966. doi: 10.1111/j.1530-0277.2006.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Shimotsu R, Barakos J. Age-related gray matter shrinkage in a treatment naive actively drinking alcohol dependent sample. Alcohol Clin Exp Res. 2010 doi: 10.1111/j.1530-0277.2009.01079.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Torres J, Price LJ, Di Sclafani V. Cognitive performance in long-term abstinent alcoholic individuals. Alcohol Clin Exp Res. 2006b;30:1538–1544. doi: 10.1111/j.1530-0277.2006.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Weiner MW, Meyerhoff DJ. Are treated alcholics representative of the entire population with alcohol use disorders? A magnetic resonance study of brain injury. Alcohol and Tobacco: From Basic Science to Clinical Practice. 2008;42:67–76. doi: 10.1016/j.alcohol.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn SW, Parsons OA, Sinha R, Stevens L. The effects of repeated withdrawals from alcohol on the memory of male and female alcoholics. Alcohol Alcohol. 1988;23:337–342. doi: 10.1093/oxfordjournals.alcalc.a044826. [DOI] [PubMed] [Google Scholar]
- Golden CJ. Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Western Psychological Services; Los Angeles, CA: 1978. [Google Scholar]
- Grafman J, Vance SC, Weingartner H, Salazar AM, Amin D. The effects of lateralized frontal lesions on mood regulation. Brain. 1986;109:1127–1148. doi: 10.1093/brain/109.6.1127. [DOI] [PubMed] [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuropsychol. 1991;13:933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Gronwall DM. Paced auditory serial-addition task: a measure of recovery from concussion. Perceptual and Motor Skills. 1977;44:367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Halpern DF. Sex differences in intelligence. Implications for education. Am Psychol. 1997;52:1091–1102. doi: 10.1037//0003-066x.52.10.1091. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Matthews CG. Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographic Corrections, Research Findings, and Clinical Applications. Psychological Assessment Resources, Inc.; Odessa, FL: 1991. [Google Scholar]
- Jacobson R. The contributions of sex and drinking history to the CT brain scan changes in alcoholics. Psychol Med. 1986;16:547–559. doi: 10.1017/s003329170001031x. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Shimamura AP, Kritchevsky M, Squire LR. Cognitive impairment following frontal lobe damage and its relevance to human amnesia. Behav Neurosci. 1989;103:548–560. doi: 10.1037//0735-7044.103.3.548. [DOI] [PubMed] [Google Scholar]
- Kaskutas LA, Weisner C, Caetano R. Predictors of help seeking among a longitudinal sample of the general population, 1984-1992. J Stud Alcohol. 1997;58:155–161. doi: 10.15288/jsa.1997.58.155. [DOI] [PubMed] [Google Scholar]
- Krueger RF, South SC. Externalizing disorders: cluster 5 of the proposed meta-structure for DSM-V and ICD-11. Psychol Med. 2009;39:2061–2070. doi: 10.1017/S0033291709990328. [DOI] [PubMed] [Google Scholar]
- Lezak M, Howieson D, Loring D. Neuropsychological assessment. 4. Oxford University Press; New York: 2004. [Google Scholar]
- Lloyd JJ, Chen CY, Storr CL, Anthony JC. Clinical features associated with receipt of alcohol treatment. J Stud Alcohol. 2004;65:750–757. doi: 10.15288/jsa.2004.65.750. [DOI] [PubMed] [Google Scholar]
- Lukassen J, Beaudet MP. Alcohol dependence and depression among heavy drinkers in Canada. Soc Sci Med. 2005;61:1658–1667. doi: 10.1016/j.socscimed.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Mann RE, Sobell LC, Sobell MB, Pavan D. Reliability of a family tree questionnaire for assessing family history of alcohol problems. Drug Alcohol Depend. 1985;15:61–67. doi: 10.1016/0376-8716(85)90030-4. [DOI] [PubMed] [Google Scholar]
- Martin PR, Singleton CK, Hiller-Sturmhofel S. The role of thiamine deficiency in alcoholic brain disease. Alcohol Res Health. 2003;27:134–142. [PMC free article] [PubMed] [Google Scholar]
- Mazas CA, Finn PR, Steinmetz JE. Decision-making biases, antisocial personality, and early-onset alcoholism. Alcohol Clin Exp Res. 2000;24:1036–1040. [PubMed] [Google Scholar]
- Moss HB, Chen CM, Yi HY. Subtypes of alcohol dependence in a nationally representative sample. Drug Alcohol Depend. 2007;91:149–158. doi: 10.1016/j.drugalcdep.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M. In: Neuropsychological vulnerabilities in chronic alcoholism, in Review of NIAAA’s Neuroscience and Behavioral Research Portfolio, vol NIAAA Research Monograph No 34. Noronha A, Eckardt M, Warren K, editors. NIAAA Publications; Bethesda: 2000. pp. 437–471. [Google Scholar]
- Osterrieth PA. Le test de copie d’une figure complex. Archives de Psychologie. 1944;30:206–356. [Google Scholar]
- Parsons OA, Nixon SJ. Neurobehavioral sequelae of alcoholism. Neurol Clin. 1993;11:205–218. [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Deshmukh A, Sullivan E. Sex differences in the effects of alcohol on brain structure. Am J Psychiatry. 2001;158:188–197. doi: 10.1176/appi.ajp.158.2.188. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Reich T, Van Eerdewegh P, Edenberg HJ, Foroud T, Goate A, Litke A, Chorlian DB, Stimus A, Rice J, Blangero J, Almasy L, Sorbell J, Bauer LO, Kuperman S, O’Connor SJ, Rohrbaugh J. Amplitude of visual P3 event-related potential as a phenotypic marker for a predisposition to alcoholism: preliminary results from the COGA Project. Collaborative Study on the Genetics of Alcoholism. Alcohol Clin Exp Res. 1998;22:1317–1323. [PubMed] [Google Scholar]
- Powell DH, Kaplan EF, Whitla D, Weinstraub S, Catlin R, Funkenstein HH. MicroCog Assessment of Cognitive Functioning. The Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- Raimo EB, Daeppen JB, Smith TL, Danko GP, Schuckit MA. Clinical characteristics of alcoholism in alcohol-dependent subjects with and without a history of alcohol treatment. Alcohol Clin Exp Res. 1999;23:1605–1613. [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery. Neuropsychology Press; Tucson: 1985. [Google Scholar]
- Robins LN, Cottler L, Buckholz K, Compton W. The Diagnostic Interview Schedule for DSM-IV. Washington University School of Medicine; St Louis, MO: 1998. [Google Scholar]
- Ruff RM, Light RH, Parker SB, Levin HS. Benton Controlled Oral Word Association Test: reliability and updated norms. Arch Clin Neuropsychol. 1996;11:329–338. [PubMed] [Google Scholar]
- Schober R, Annis HM. Barriers to help-seeking for change in drinking: a gender-focused review of the literature. Addict Behav. 1996;21:81–92. doi: 10.1016/0306-4603(95)00041-0. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Saffran E. The American-NART: Replication and extension of the British findings on the persistence of the word pronunciation skills in patients with dementia. Philadelphia, PA: 1987. [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. Abnormal Psychology. 1982a;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J Stud Alcohol. 1982b;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Smith A. In: The symbol digit modalities test: A neuropsychological test of learning and other cerebral disorders, Learning Disorders. Helmuth J, editor. Special Child Publications; Seattle, WA: 1968. pp. 83–91. [Google Scholar]
- Smith A. Symbol Digit Modalities Test. Western Psychological Services; Los Angeles, CA: 1982. [Google Scholar]
- Sobell LC, Sobell MB. Self-reports issues in alcohol abuse: State of the art and future directions. Behav Assess. 1990;12:77–90. [Google Scholar]
- Sobell LC, Sobell MB, Riley DM, Schuller R, Pavan DS, Cancilla A, Klajner F, Leo GI. The reliability of alcohol abusers’ self-reports of drinking and life events that occurred in the distant past. Stud Alcohol. 1988;49:225–232. doi: 10.15288/jsa.1988.49.225. [DOI] [PubMed] [Google Scholar]
- Stoltenberg SF, Mudd SA, Blow FC, Hill EM. Evaluating measures of family history of alcoholism: density versus dichotomy. Addiction. 1998;93:1511–1520. doi: 10.1046/j.1360-0443.1998.931015117.x. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Benson DF, Kaplan EF, Weir WS, Naeser MA, Lieberman I, Ferrill D. The involvement of orbitofrontal cerebrum in cognitive tasks. Neuropsychologia. 1983;21:235–248. doi: 10.1016/0028-3932(83)90040-4. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Stethem LL, Pelchat G. Three tests of attention and rapid information processing: an extension. The Clinical Neuropsychologist. 1988;2:246–250. [Google Scholar]
- Sullivan EV. Compromised pontocerebellar and cerebellothalamocortical systems: speculations on their contributions to cognitive and motor impairment in nonamnesic alcoholism. Alcohol Clin Exp Res. 2003;27:1409–1419. doi: 10.1097/01.ALC.0000085586.91726.46. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Fama R, Rosenbloom MJ, Pfefferbaum A. A profile of neuropsychological deficits in alcoholic women. Neuropsychology. 2002;16:74–83. doi: 10.1037//0894-4105.16.1.74. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res. 2000;24:611–621. [PubMed] [Google Scholar]
- Tucker JA, Vuchinich RE, Rippens PD. Different variables are associated with help-seeking patterns and long-term outcomes among problem drinkers. Addict Behav. 2004;29:433–439. doi: 10.1016/j.addbeh.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised. The Psychological Corporation; New York: 1981. [Google Scholar]
- Wechsler D. Administration and Scoring Manual. Third Edition. The Psychological Corporation; San Antonio, TX: 1997. Wechsler Adult Intelligence Scale. [Google Scholar]
- Wetzel L, Boll T. Short Category Test, Booklet Format. Western Psychological Services; Los Angeles, CA: 1987. [Google Scholar]
- Yonker JE, Nilsson LG, Herlitz A, Anthenelli RM. Sex differences in spatial visualization and episodic memory as a function of alcohol consumption. Alcohol Alcohol. 2005;40:201–207. doi: 10.1093/alcalc/agh141. [DOI] [PubMed] [Google Scholar]