Abstract
In order to better identify the role of the Salmonella pathogenicity island 2 (SPI-2) type III secretion system (T3SS) in chickens, we used the well-known gentamicin protection assay with activated HD11 cells. HD11 cells are a macrophage-like chicken cell line that can be stimulated with phorbol 12-myristate 13-acetate (PMA) to exhibit more macrophage-like morphology and greater production of reactive oxygen species (ROS). Activated HD11 cells were infected with a wild-type Salmonella enterica subspecies enterica serovar Typhimurium (S. Typhimurium) strain, a SPI-2 mutant S. Typhimurium strain, a wild-type Salmonella enterica subspecies enterica serovar Enteritidis (S. Enteritidis) strain, a SPI-2 mutant S. Enteritidis strain, or a non-pathogenic Escherichia coli (E. coli) strain. SPI-2 mutant strains were found to survive as well as their parent strain at all time points post-uptake (PU) by the HD11 cells, up to 24 h PU, while the E. coli strain was no longer recoverable by 3 h PU. We can conclude from these observations that the SPI-2 T3SS of S. Typhimurium and S. Enteritidis is not important for survival of Salmonella in the activated macrophage-like HD11 cell line, and that Salmonella must employ other mechanisms for survival in this environment, as E. coli is effectively eliminated.
Introduction
Infections by Salmonella enterica subspecies enterica are one of the leading causes of food borne gastroenteritis in humans [1]. Among those serovars responsible for food poisoning in humans, serovars Typhimurium (S. Typhimurium) and Enteritidis (S. Enteritidis) are most commonly isolated serovars from both humans and animals in many regions. In North America, S. Typhimurium is the primary serovar isolated from both humans and animals, while S. Enteritidis is the second most common serovar isolated from humans. The opposite is true for most of the European Union, with S. Enteritidis being the number one isolate from both humans and animals and S. Typhimurium being number two [2]. Both S. Typhimurium and S. Enteritidis are capable of causing systemic disease in humans, although this is not the normal course of infection and only occurs in very young, very old, and immunocompromised individuals [3].
Salmonella uses two specialized type III secretion systems (T3SS) that facilitate invasion and survival within the host cell. These two T3SSs are encoded within Salmonella pathogenicity islands 1 and 2 (SPI-1 and SPI-2) and secrete effectors into the host cell, triggering a number of events in the infected cell. These events ultimately lead to the symptoms of disease. It is the current view that the SPI-1 T3SS is mainly involved in invasion of the host cell, while the SPI-2 T3SS plays a role in survival within the host cell and maintenance of the Salmonella containing vacuole (SCV) [4], [5], [6]. SPI-2 is a region of approximately 40 kb and has been reported to be necessary for systemic infection, intracellular proliferation and survival, and maintenance of the SCV. However, the majority of these studies have been performed in mice, where S. Typhimurium and S. Enteritidis produce a typhoid-like infection rather than gastroenteritis, and therefore may not be indicative of the normal course of infection in healthy adult humans and chickens [7], [8], [9], [10].
The preferred site of invasion for Salmonella is through microfold (M) cells of the intestine. M cells reside within the follicular associated epithelium that overlays the Peyer's patches, have a less pronounced brush boarder, and are associated with mucous in less abundance than other intestinal epithelial cells. Once through the epithelial barrier, Salmonella are taken up by resident or recruited macrophages and dendritic cells [11], [12], [13], [14], [15]. An effective innate immune response is necessary to clear Salmonella and prevent systemic spread; recruited macrophages, natural killer cells and dendritic cells are paramount in this process, but in some cases Salmonella is able to manipulate and evade the host immune response and spread systemically [3], [12], [16], [17]. Within phagocytic cells, SPI-2 effectors are secreted across the SCV membrane and stop the fusion of lysosomes with the SCV, thereby avoiding bacterial killing by defensins, cathelicidins, lysozymes, lipases, proteases [18], [19], [20], [21]. This action is also thought to prevent the ability of reactive oxygen and nitrogen species (ROS and RNS) to form [18], [21]. T3SS effectors facilitate the maturation of the SCV, and can act as pro- or anti-inflammatory factors [22], [23].
Previously, our group found that while S. Enteritidis SPI-2 mutants were slower to colonize the spleens and livers of chickens, the levels of the mutant and wild-type were similar by day 4 post-challenge [24]. A major mode of transport for Salmonella to systemic sites like the liver and spleen is likely within macrophages [25]. There is a vast array of conflicting evidence in the literature about the importance of the SPI-2 T3SS to the survival of S. Typhimurium and S. Enteritidis within macrophages. In this study, we demonstrate that in activated HD11 chicken macrophage-like cells, the SPI-2 T3SS does not contribute to survival of S. Typhimurium and S. Enteritidis.
Methods
Cloning and production of Salmonella pathogenicity island 2 mutants
Construction of the SPI-2 mutants used in this study have been described previously [24].
Bacterial strains and growth conditions
Bacterial strains used in this study are described in Table 1. Standard growth procedures were followed using Luria-Bertani (LB) broth and agar at 37°C.
Table 1. List of bacterial strains used in this study.
| Bacterial strain | Short name | Properties | Source |
| E. coli DH5α | DH5α | F-, ϕ80dlacZΔM15, Δ(lacZYA-argF)U169, deoR, recA1, endA1, hsdR17(rK −, mK +), phoA, supE44, λ−, thi-1, gyrA96, relA1 | Invitrogen |
| S. Typhimurium SL1344 | SL1344 | A wild-type S. Typhimurium strain, streptomycin resistant | Dr. B. Finlaya |
| S. Typhimurium SL1344 ΔssaR | ΔssaR | SL1344 missing the ssaR gene from SPI-2 | Dr. B. Finlaya |
| S. Enteritidis Sal18 | Sal18 | Virulent for birds, invades liver and spleen, and colonizes gut | Dr. C. Poppeb |
| S. Enteritidis Sal18 ΔSPI-2::cat | ΔSPI-2::cat | Sal18 with the whole SPI-2 region deleted and replaced by a chloramphenicol resistance gene using the λRed system. | [24] |
| S. Enteritidis Sal18 ΔSPI-2 | ΔSPI-2 | Derivative of Sal18 ΔSPI-2::cat with the chloramphenicol resistance gene deleted using the λRed system. | [24] |
Dr. B. Finlay, University of British Columbia, Vancouver, British Colombia.
Dr. C. Poppe, Laboratory for Foodborne Zoonoses, Health Canada, Guelph, Ontario.
HD11 cell line and growth conditions
HD11 cells were kindly provided to VIDO by Dr. Kirk C. Klasing (currently Department of Animal Science, University of California – Davis, Davis, CA, USA). HD11 cells are a macrophage-like immortalized cell line derived from chicken bone marrow and transformed with the avian myelocytomatosis type MC29 virus [26]. HD11 cells were maintained at 42°C, in a humidified incubator (5% CO2), in RPMI 1640 media (Gibco) supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine (Gibco), and 10 mM HEPES. For all assays involving bacteria the medium was changed to RPMI 1640 containing 10% heat-inactivated FBS, 2 mM L-glutamine, and 10 mM HEPES, before cells were seeded into 24-well cell-bind plates (Corning). HD11 cells were used for all assays between passages 15 and 25.
Gentamicin protection assay
Approximately 12 hours before infection, HD11 cells were placed in RPMI 1640 (10% heat-inactivated FBS, 2 mM L-glutamine and 10 mM HEPES) containing 100 ng/ml phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich), and seeded into 24-well cell-bind plates (Corning) at a concentration of 5×105 cells per well. At this time growth of bacterial strains were started. 12 hours post-activation, HD11 cells were checked to ensure that differentiation was induced by PMA (Figure 1) and bacterial overnight cultures were sub-cultured and grown to an OD600 corresponding to 1×108 CFU/ml. The number of viable HD11 cells was determined from three wells via trypan blue exclusion in order to calculate the MOI. Media was removed from the cells, and bacteria (in pre-warmed RPMI 1640) were added to each well at a multiplicity of infection (MOI) of 25 (time 0 h). Serial dilutions of the bacteria were made and plated in order to confirm that each strain was added to the HD11 cells at an MOI of approximately 25. Plates containing HD11 cells and bacteria were subject to centrifugation at 200 x g in a Sorvall benchtop centrifuge for 5 minutes at room temperature, and then placed at 42°C. After 0.5 h, media containing bacteria was carefully removed from the HD11 cells, and the cells were washed once with PBS containing 500 µg/ml gentamicin. RPMI 1640 containing 250 µg/ml gentamicin was then added to each well, and the plates were placed back at 42°C. At each time point (0.5, 3, 6, 12 and 24 hours post-uptake [PU]), media was removed from three wells per bacterial strain and centrifuged at 20,800 x g in an Eppendorf benchtop microfuge for 10 minutes at 4°C. Sediments from this media fraction were resuspended in 0.5 ml 1% Triton X-100 (Sigma), and 100 µl portions from each sample were plated on LB-agar using 3 mm borosilicate glass beads. The cell monolayer from three wells, per bacterial strain, were washed once with PBS, and lysed in 0.5 ml 1% Triton X-100 in PBS. Dilution series of the cell monolayer fractions were made and 100 µl of each dilution (10−4, 10−3, 10−2, 10−1, 100) was plated on LB agar using borosilicate glass beads. In order to confirm the effectiveness of the gentamicin, at the 0 h time point bacteria were also added to one 14 ml culture tube containing 5 ml of RPMI and one containing 5 ml of RPMI with 250 µg/ml gentamicin added. 100 µl from each tube was plated on LB agar at each time point. This experiment was repeated three times, and data pooled for statistical analysis.
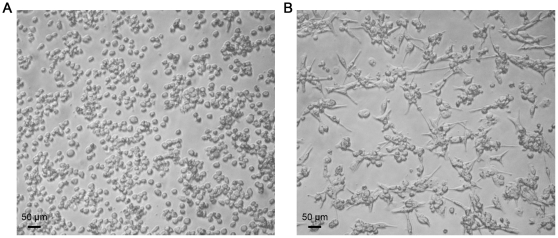
Figure 1. HD11 chicken macrophage-like cells.
Panel A shows HD11 cells that have not been stimulated with PMA, while Panel B shows HD11 cells 12 hours after stimulation with PMA. Photographs were taken under 10X magnification.
Location of Salmonella in the media fraction
It was important to determine if the bacteria in the media fraction were free, or contained within detached HD11 cells or cell fragments. A portion (100 µl) of the media fraction from HD11 cells infected with S. Typhimurium strain SL1344 from the 3 h PU time point was plated on LB agar to see if the bacteria will still viable in the media fraction, which contained gentamicin. The media fraction was then divided into two 5 ml portions in 14 ml culture tubes. To one tube, 1% Triton X-100 was added in order to lyse any eukaryotic cell membranes, and the tubes were placed in a shaker at 37°C for 3 hours, following which a further 100 µl from each tube was plated on LB agar. A portion of the initial media fraction was also subject to staining with Giemsa or PKH26 (Sigma-Aldrich) and viewed using a fluorescence microscope (Zeiss Axiovert 200M). PKH26 is a red fluorescent dye linked to long aliphatic tails that can insert into lipid regions of cell membranes. PKH26 and Giemsa staining were carried out as indicated by the manufacturer.
Immunofluorescence
Survival assays were performed as above, but in 8 well chamber slides instead of 24-well plates, and bacterial numbers were not enumerated by plating. Instead, at each time point (0, 0.5, 3, 6, 12, and 24 hours PU), the media fraction was collected, sedimented, and resuspended in 100 µl 0.1% EDTA in PBS. The samples from the media fraction were then placed on slides using a CytoSpin 4 centrifuge (Thermo Scientific). Briefly, slides were placed in the CytoSpin centrifuge, and 100 µl of FBS was added and centrifuged onto the slides at 400 x g for 3 minutes, followed by the addition of 100 µl of sample under the same conditions. Slides were allowed to dry overnight, and fixed in ice-cold acetone for 10 minutes. After fixation, slides were washed 3 times with PBS, and then incubated for 0.5 h with fluorescein-conjugated mouse anti-Salmonella IgG or fluorescein-conjugated rabbit anti-E. coli IgG (1/50 in PBS). Samples were washed as before, and then treated with rhodamine-conjugated goat anti-mouse IgG or rhodamine-conjugated goat anti-rabbit IgG (1/50 in PBS). Samples were washed, and then treated with 0.5% Triton X-100 for 5 minutes to permeabilize HD11 cell membranes. Following an additional wash, goat anti-Salmonella IgG conjugated with fluorescein or rabbit anti-E. coli IgG conjugated with fluorescein (1/50 in PBS) were added to the samples. All antibodies were purchased from AbD Serotec. Samples were washed, and then stained with DAPI (10 µg/ml) (Sigma-Aldrich) for 15 minutes at room temperature. Coverslips were added to the slides using FluorSave™ mounting medium (EMD chemicals INC), and all samples were viewed using the Zeiss Axiovert 200M microscope with a mercury vapour short-arc lamp for fluorescence. Photographs were taken using the Zeiss Axiocam and were processed using Adobe© Photoshop© CS 5 for Mac OS X. Manipulations included cropping for space and level adjustment to reduce background noise.
Superoxide assay
The assay to measure superoxide production via luminometry was adapted from Thrasher et al. [27]. Briefly, 12 hours before the experiment, 2×107 HD11 cells were seeded into two 75 cm2 tissue culture flasks. PMA was added to one of the flasks at a concentration of 100 ng/ml. The next day cells were washed once with PBS and harvested by the addition of 0.5% trypsin. Cells were counted, and amounts corresponding to 5.0×105 cells were added to individual eppendorf tubes prior to being centrifuged at 2700 x g for 5 min at 4°C, and resuspended in 100 µl Hanks' buffered saline (HBS) with calcium and magnesium (137 mM NaCl, 5.4 mM KCl, 358 mM NaHCO3, 0.44 mM KH2PO4, 0.34 mM Na2HPO4, 0.5 mM CaCl2, 1 mM MgCl2). Directly before reading luminescence, 100 µl of 10 µM luminol (Sigma-Aldrich) in HBS and 10 U of horseradish peroxidase (HRP) (Sigma-Aldrich) in 50 µl HBS were added to the cells. Samples were read for 5 seconds at 3–5 minute intervals using a GloMax 20/20 luminometer (Promega Biosciences).
Statistical analysis
Data from each survival assay were pooled and ranked using Microsoft® Excel™ for Mac OS X. All statistical analyses were performed using GraphPad Prism® 5.0 for Mac OS X. One-way ANOVAs were performed on each time point. If significance between groups was found, the data was further analyzed using the post-hoc Tukey test. p-values≤0.05 were considered significant. Data from two superoxide assays were ranked and a two-way ANOVA was performed, followed by the post-hoc Bonferroni multiple comparisons test.
Results
Activation of HD11 cells
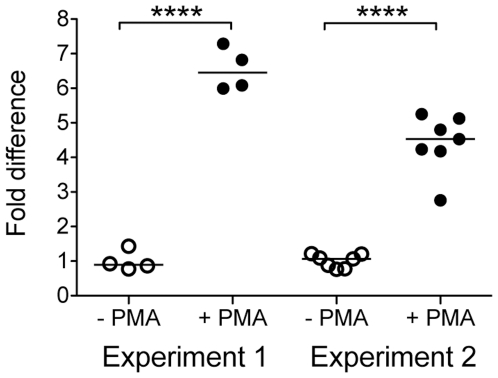
HD11 cells are normally loosely adherent to plastic and have an ovoid shape; but can be activated to become more macrophage-like after stimulation with PMA (Figure 1, panel A). Twelve hours after exposure to 100 ng/ml PMA, the HD11 cells become more adherent to plastic, and their morphology becomes further macrophage-like, with a spindle shape (Figure 1, panel B). In addition, stimulated cells were found to produce more reactive oxygen species than unstimulated cells, as measured by production of hydrogen peroxide (p-value<0.0001) (Figure 2).
Figure 2. Hydrogen peroxide production by activated HD11 cells.
Fold difference in hydrogen peroxide production in HD11 cells 12 hours after stimulation with PMA compared to that of unstimulated cells, as measured by luminescence produced by the reaction between hydrogen peroxide, luminal, and HRP.
Survival of Salmonella pathogenicity island 2 mutants within activated HD11 cells
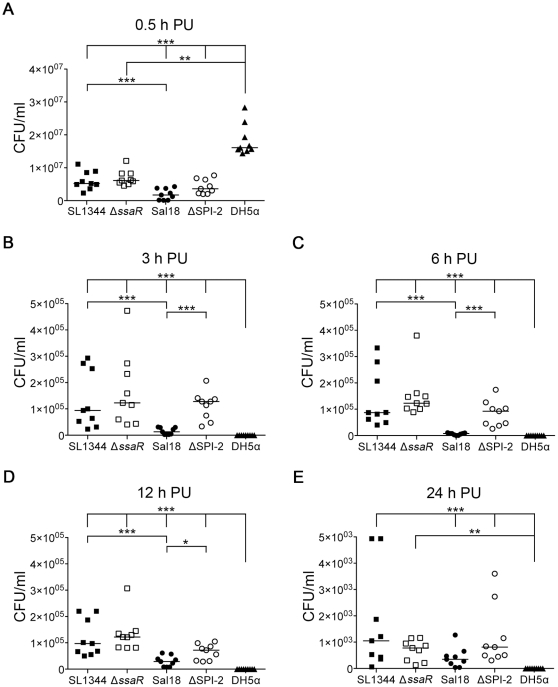
At 0.5 hours PU, the non-pathogenic E. coli DH5α strain was recovered from the cell monolayer fraction at much higher levels than were any of the Salmonella strains (SL1344, Sal18, ΔSPI-2 p-value<0.001, and ΔssaR p-value<0.01) (Figure 3, panel A). At 3, 6, 12, and 24 h PU, viable E. coli DH5α were no longer recoverable from the cell monolayer fraction (all p-value<0.001, except ΔssaR at 24 h PU: p-value<0.01) (Figure 3, panels B–E). This observation indicates that even though more viable E. coli DH5α bacteria were recovered 0.5 h PU compared to the Salmonella strains, it is clear that the HD11 cells are able to effectively kill the E. coli strain after the initial infection process. Non-viable (or non-recoverable) E. coli DH5α are still visible by immunofluorescence at all time points, although in less abundant amounts than the Salmonella strains (data not shown). When the Salmonella strains were examined it was found that more of the S. Typhimurium wild-type strain (SL1344) was recovered from the cell monolayer fraction than the wild-type S. Enteritidis strain (Sal18) at all time points, with statistical significance observed at 0.5, 3, 6 and 12 h PU (p-value<0.001) (Figure 3). There was no difference in recovery between the wild-type and SPI-2 mutant (ΔssaR) S. Typhimurium strains, while greater amounts of the S. Enteritidis SPI-2 (ΔSPI-2) mutant were recovered than the wild-type S. Enteritidis strain at 3, 6 (p-values<0.001), and 12 h PU (p-value<0.05) (Figure 3).
Figure 3. Recovery of Salmonella from the cell monolayer fraction of HD11 cells over time.
The ability of SPI-2 mutants (S. Typhimurium ΔssaR and S. Enteritidis ΔSPI-2) to survive in the chicken macrophage HD11 cell line was compared to their parent wild-type strains (S. Typhimurium SL1344 and S. Enteritidis Sal18) as well as to the non-pathogenic E. coli strain DH5α. Panel A shows the recovered CFU/ml from the cell monolayer fraction at 0.5 h post-uptake (PU), prior to addition of gentamicin. Panels B, C, D, and E show the recovered CFU/ml from the cell monolayer fraction after the addition of gentamicin at 3, 6, 12, and 24 h PU respectively. *, p-value<0.05; **, p-value<0.01; ***, pvalue<0.001. Note that the scale of the Y-axis is linear, and differs between time points.
Bacteria in the media fraction
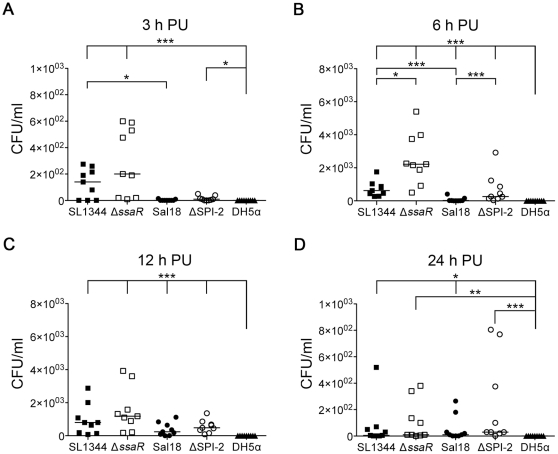
At 3, 6, 12, and 24 h PU (after the addition of gentamicin), Salmonella could be recovered from the media taken off the cell monolayer. When portions of the media fraction were added to either a tube containing RPMI and a tube containing RPMI with 1% Triton X-100, and grown for 3 hours, bacteria was only recoverable from the media fraction to which no Triton X-100 had been added. As 1% Triton X-100 is capable of disrupting eukaryotic, but not bacterial, cell membranes, this indicates that the bacteria are contained within eukaryotic cell membranes. Furthermore, PKH and Giemsa staining of the media fraction showed both whole cell and smaller membrane fragments in the media fraction (data not shown). At 3 and 6 h PU, more of the wild-type S. Typhimurium strain SL1344 was recovered from the media fraction than the wild-type S. Enteritidis strain Sal18 (p-values<0.05 and <0.001 respectively) (Figure 4, panels A and B). At 6 h PU, both mutant strains (ΔssaR and ΔSPI-2) were recovered in greater amounts than their respective parent strains (SL1344 and Sal18) (Figure 4, panel B). Immunofluorescence at 0.5, 3, 6, 12, and 24 h PU showed that most Salmonella were associated with whole HD11 cells that have detached from the monolayer (Figure 5, panels A, B, and C), or fragmented cells (Figure 5, panels D and E). Salmonella was found to be sensitive to gentamicin, except when in the media fraction, until the addition of 1% Triton X-100. This, along with the aforementioned microscopy, indicates that the bacteria found in the media fraction are contained within whole and fragmented cells, where they are protected from the action of gentamicin. Finally, E. coli strain DH5α was not recoverable from the media fraction at any time point (Figure 4).
Figure 4. Recovery of Salmonella from the media fraction of HD11 cells over time.
The ability of SPI-2 mutants (S. Typhimurium ΔssaR and S. Enteritidis ΔSPI-2) to survive in the chicken macrophage-like HD11 cell line was compared to their parent wild-type strains (S. Typhimurium SL1344 and S. Enteritidis Sal18) as well as to the non-pathogenic lab E. coli strain DH5α. Surprisingly, Salmonella, but not E. coli, was recovered from the media at all time points PU following the addition of gentamicin. Panel A shows the CFU/ml recovered from the media fraction at 3 h post-uptake (PU) while Panels B, C, and D show the CFU/ml recovered from the media fraction at 6, 12, and 24 h PU respectively. *, p-value<0.05; **, p-value<0.01; ***, p-value<0.001. Note that the scale of the Y-axis is linear, and differs between time points.
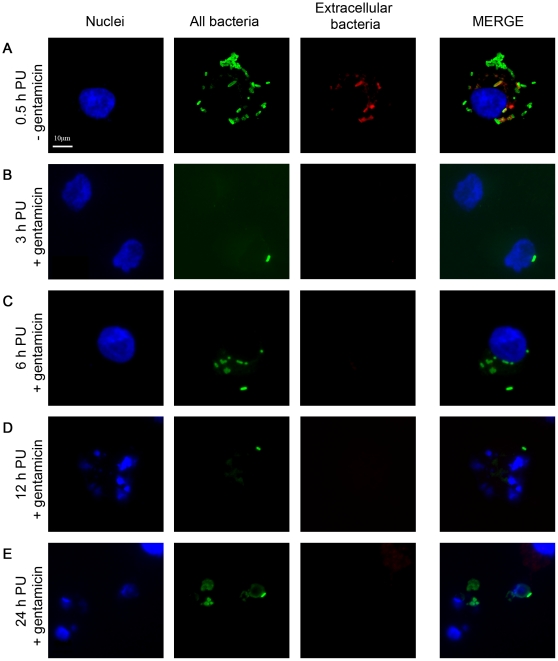
Figure 5. Wild-type S. Typhimurium strain SL1344 in the media fraction over time.
HD11 cell nuclei are stained blue, both intra- and extracellular bacteria are green, and extracellular bacteria appear red or yellow. Panel A shows an HD11 cell loaded with wild-type S. Typhimurium strain SL1344 at 0.5 h PU, just prior to addition of gentamicin. Panels B and C show whole HD11 cells containing SL1344 at 3 and 6 h PU, respectively, after addition of gentamicin. Panels D and E show fragmented HD11 cells containing SL1344 at 12 and 24 h PU, respectively, after the addition of gentamicin. Whole and fragmented cells containing SL1344 were visible at all time points in the media fraction.
Discussion
Chicken macrophage-like HD11 cells can be induced to be more macrophage-like by stimulation with PMA, as evidenced by morphology and production of reactive oxygen species (Figures 1 and 2, respectively). At 0.5 h PU, prior to addition of gentamicin, more E. coli DH5α were recovered from the cell monolayer fraction than the Salmonella strains. It is unclear why this may be, as similar numbers of intracellular and extracellular bacteria were present in all samples when visualized by immunofluorescence microscopy (data not shown). However, this microscopy does not differentiate viable from killed bacteria and it is possible that more live E. coli DH5α bacteria are initially phagocytosed by the macrophages, or that more E. coli DH5α remain associated with (but not phagocytosed) by the cells after the washing process. Following the addition of gentamicin, activated HD11 cells effectively killed E. coli DH5α, as viable E. coli DH5α were not recoverable from either the cell monolayer fraction or media fraction at any time point past 0.5 h. In comparison, all Salmonella strains were recoverable up to 24 h PU from HD11 cells. At most time points, the S. Typhimurium strain appeared to survive better within the HD11 cells than the S. Enteritidis strain. Importantly, at no time did the wild-type S. Typhimurium or S. Enteritidis strains survive better than their respective SPI-2 mutant strains, and, at 3, 6, and 12 h PU, the S. Enteritidis SPI-2 mutant (ΔSPI-2) out-performed the wild-type strain. All strains, including non-recoverable E. coli DH5α, were visible within macrophages at all time points PU by immunofluorescence, but it is likely that the immunofluorescence was detecting killed but intact, as well as viable, bacteria (data not shown). Typically only one or two bacteria were seen within an individual cell, although a few instances of large bacterial load were also observed. After the addition of gentamicin, fewer macrophages containing E. coli were visible, when compared to those infected with Salmonella strains.
In a mouse model of infection, multiple groups have shown that various SPI-2 mutants are highly attenuated in virulence (measured by LD50) [6], [28], [29], [30]. Initially, Hensel et al. showed that S. Typhimurium SPI-2 mutants replicated at similar levels to wild-type S. Typhimurium in the mouse macrophage-like RAW264.7 cell line [31]. However, later work by the same group (and others) indicated that SPI-2 mutants failed to replicate as well in mouse macrophages than wild-type strains if they were first grown to stationary phase and then opsonized before infection to enhance uptake of bacteria by macrophages [6], [28], [29]. In our experiments, bacteria were grown to mid-log phase before infection, and were not opsonized, because these conditions do not mimic the initial stages of infection by Salmonella. Many groups have recently published results in accordance with our findings. Forest et al. [32] determined that the absence of a functional SPI-2 T3SS in serovar Typhi (S. Typhi) did not affect survival in human macrophages. Aussel et al. [33] demonstrated that S. Typhimurium containing a non-functional SPI-2 or SPI-1 T3SS was able to survive similarly to wild-type S. Typhimurium in both mouse bone marrow-derived macrophages and RAW264.7 macrophages, but that the same mutants did not replicate to similar levels of the wild-type strain in vivo. A study by Helaine et al. [34] determined that while SPI-2 is important for replication of S. Typhimurium in macrophages, it does not affect the survival of phagocytosed bacteria within the SCV. Furthermore, it was shown in the study by Helaine et al. that most wild-type S. Typhimurium that are taken up by macrophages do not undergo replication at all, but rather enter a dormant state within the SCV. The number of bacteria in stasis did not differ between wild-type macrophages, phox −/− macrophages, or macrophages stimulated with IFNγ. Thus, Salmonella may be able to survive within macrophages without replicating and disseminate to systemic sites, regardless of the presence of SPI-2. In fact, it has been shown that SPI-2 mutants are able to reach the livers and spleens of mice and that similar numbers of spleen cells are infected, but mice infected with SPI-2 mutants have a reduced overall bacterial load in these organs [30], [34]. Previous work by our group showed that although S. Enteritidis SPI-1 and SPI-2 mutants were recovered in the livers and spleens of infected chickens in less abundance than the wild-type S. Enteritidis strain initially, the mutant strains reached comparable levels to the wild-type strain by day 4 PC [24], [35].
It is well known that the production of reactive oxygen species (ROS) by phagocytes is important for control of intracellular pathogens. Humans, or animals, with mutations in NADPH oxidase (Phox) are prone to severe recurrent infections by fungi and intracellular bacteria, including Salmonella [21], [36]. Phox assembles on phagosomes that contain intracellular pathogens, and is responsible for the production of superoxide (O2 −). Superoxide is not readily able to cross the membranes of bacteria, but can spontaneously dismutate, or be dismutated by superoxide dismutases, into hydrogen peroxide (H2O2). Hydrogen peroxide can easily diffuse across bacterial membranes and can form highly reactive hydroxyl (HO) radicals in the presence of iron (Fe2+) that damage bacterial DNA, proteins, and lipids [21], [33]. Salmonella has developed multiple defenses to this process. Salmonella has two periplasmic superoxide dismutases (SodCI and SodCII) that combat exogenous superoxide. It also expresses three known cytosolic catalases (KatG, KatE, and KatN) and three cytosolic peroxidases (SodA, SodB, and Tpx) that degrade hydrogen peroxide within the bacterial cytoplasm. SodCI and SodCII, along with the cytoplasmic peroxidase Tpx, have been shown to be important for survival of S. Typhimurium in mouse macrophages [33], [36], [37]. It has also been previously shown that SPI-2 is important for vesicular trafficking and the association of Phox with the SCV in human and mouse macrophages; this observation led researchers to propose that the SPI-2 T3SS was essential in avoiding the oxidative burst [36], [38], [39]. However, recent work by Aussel et al. [33] indicates that SodCI, SodCII, and Tpx are sufficient for Salmonella to overcome ROS, and that S. Typhimurium SPI-2 mutants perform similarly to wild-type S. Typhimurium in vitro. They found that, in vivo, the wild-type S. Typhimurium had increased replication in relation to the SPI-2 mutant in both wild-type and phox −/− mice (although both strains reached higher levels in the phox −/− mice), indicating that while SPI-2 is important for replication, it does not play a major role in evasion of ROS. While we showed that HD11 macrophages activated with PMA produced greater levels of hydrogen peroxide than non-activated macrophages (Figure 2), we did not see a major difference in survival of SPI-2 mutants compared to wild-type strains in these activated cells. This indicates that any avoidance of ROS in this case was independent of a functional SPI-2 T3SS. Slauch et al. [40] state that ROS in the SCV of infected cells may not be diminished by wild-type Salmonella as previously reported, so the role of SPI-2 in avoidance of ROS, as well as the importance of ROS in control of Salmonella infection, remains unclear.
SPI-2 has been shown to change cytokine and chemokine production by macrophages, including the HD11 cell line [16], [41], [42], [43]. In HD11 cells, PipB has been shown to be important in the down regulation of pro-inflammatory cytokines, indicating a role for SPI-2 in repression of the host's innate immune response [42]. SPI-2 has also been shown to be important in limiting the antigen presenting abilities of macrophages and dendritic cells to T cells [44], [45]. It may be that SPI-2 is less important for survival of Salmonella within macrophages, but more important in modulation of macrophage stimulation of other immune cells via cytokine/chemokine production and antigen presentation. This would better explain some of the differences seen between in vitro SPI-2 Salmonella mutant survival and in vivo SPI-2 Salmonella mutant vulnerability.
Surprisingly, in our study, Salmonella was recoverable in the media fraction at each time point after the addition of gentamicin. These bacteria were found to be associated with both whole detached cells and closed cell fragments. If the bacteria within the cell fragments are viable, this would be a novel way for Salmonella to avoid the host immune system between host cell death and uptake by other phagocytic cells.
Taken together, these results indicate that survival in activated chicken HD11 macrophage-like cells is likely SPI-2 independent. Further research into the role of the SPI-2 T3SS in systemic spread in chickens is needed. Specific experiments involving challenge of chickens with labeled wild-type and Salmonella SPI-2 mutants would allow real-time observation of how the strains disseminate throughout the chicken. This would also allow for the surveillance of the specific cell types that the two strains are residing in within the chickens. Further, after isolation of infected phagocytic cells from challenged chickens, specific cytokine and chemokine profiles of these cells could be undertaken to see how SPI-2 effects immune cell function, as well as study of ROS and RNS production. Rather than using the available immortalized cell lines, isolation of a primary chicken macrophage cell line is desirable. Observations on how the cytokine and chemokine profiles of these cells change over time, when infected with wild-type Salmonella compared to the SPI-2 mutant, as well as whether the lack of SPI-2 affects replication of the bacteria within these cells would be helpful. Characterization of how Salmonella induces cell death in primary chicken macrophages, and the role SPI-2 plays, would be very useful and further study of the media fraction in the gentamicin protection assay would be interesting to see if Salmonella is truly contained in small membrane vesicles that are able to be taken up by naïve macrophages, and continue the infection process.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC), Saskatchewan Health Research Foundation (SHRF). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization (WHO) World Health Organization: Media Centre; 2007. Food safety and foodborne illness. [Google Scholar]
- 2.Vieira A. 2009. WHO Global Foodborne Infection Network Country Databank - A resource to link human and non-human sources of Salmonella; 2009; Durban.
- 3.Srikanth CV, Cherayil BJ. Intestinal innate immunity and the pathogenesis of Salmonella enteritis. Immunologic Research. 2007;37:61–78. doi: 10.1007/BF02686090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown NF, Vallance BA, Coombes BK, Valdez Y, Coburn BA, et al. Salmonella Pathogenicity Island 2 Is Expressed Prior to Penetrating the Intestine. PLoS Pathogens. 2005;1:e32. doi: 10.1371/journal.ppat.0010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakravortty D, Rohde M, Jager L, Deiwick J, Hensel M. Formation of a novel surface structure encoded by Salmonella Pathogenicity Island 2. The EMBO Journal. 2005;24:2043–2052. doi: 10.1038/sj.emboj.7600676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ochman H, Soncini FC, Solomon F, Groisman EA. Identification of a pathogenicity island required for Salmonella survival in host cells. Proceedings of the National Acadamy of Sciences. 1996;93:7800–7804. doi: 10.1073/pnas.93.15.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galán JE. Salmonella interactions with host cells: type III secretion at work. Annual Review of Cell and Developmental Biology. 2001;17:53–86. doi: 10.1146/annurev.cellbio.17.1.53. [DOI] [PubMed] [Google Scholar]
- 8.Olekhnovich IN, Kadner RJ. Crucial Roles of Both Flanking Sequences in Silencing of the hilA promoter in Salmonella enterica. Journal of Molecular Biology 357: 2006;373 - 386 doi: 10.1016/j.jmb.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Thompson A, Rowley G, Alston M, Danino V, Hinton JC. Salmonella transcriptomics: relating regulons, stimulons and regulatory networks to the process of infection. Current Opinion in Microbiology. 2006;9:109–116. doi: 10.1016/j.mib.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Winstanley C, Hart CA. Type III secretion systems and pathogenicity islands. Journal of Medical Microbiology. 2001;50:116–126. doi: 10.1099/0022-1317-50-2-116. [DOI] [PubMed] [Google Scholar]
- 11.Fournier B, Williams IR, Gewirtz AT, Neish AS. Toll-like receptor 5-dependent regulation of inflammation in systemic Salmonella enterica serovar Typhimurium infection. Infection and Immunity. 2009;77:4121–4129. doi: 10.1128/IAI.00656-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uematsu S, Akira S. Immune responses of TLR5(+) lamina propria dendritic cells in enterobacterial infection. Journal of Gastroenterology. 2009;44:803–811. doi: 10.1007/s00535-009-0094-y. [DOI] [PubMed] [Google Scholar]
- 13.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nature Reviews Immunology. 2008;8:411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 14.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nature Reviews Immunology. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srinivasan A, McSorley SJ. Activation of Salmonella-specific immune responses in the intestinal mucosa. Archivum Immunologiae et Therapia Experimentalis. 2006;54:25–31. doi: 10.1007/s00005-006-0003-5. [DOI] [PubMed] [Google Scholar]
- 16.Ciraci C, Tuggle CK, Wannemuehler MJ, Nettleton D, Lamont SJ. Unique genome-wide transcriptome profiles of chicken macrophages exposed to Salmonella-derived endotoxin. BMC Genomics. 2010;11:545. doi: 10.1186/1471-2164-11-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashkar AA, Reid S, Verdu EF, Zhang K, Coombes BK. Interleukin-15 and NK1.1+ cells provide innate protection against acute Salmonella enterica serovar Typhimurium infection in the gut and in systemic tissues. Infection and Immunity. 2009;77:214–222. doi: 10.1128/IAI.01066-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Babu US, Gaines DW, Lillehoj H, Raybourne RB. Differential reactive oxygen and nitrogen production and clearance of Salmonella serovars by chicken and mouse macrophages. Developmental and Comparitive Immunology. 2006;30:942–953. doi: 10.1016/j.dci.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Coombes BK, Finlay BB. Insertion of the bacterial type III translocon: not your average needle stick. Trends in Microbiology. 2005;13:92–95. doi: 10.1016/j.tim.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Kuhle V, Hensel M. Cellular microbiology of intracellular Salmonella enterica: functions of the type III secretion system encoded by Salmonella pathogenicity island 2. Cellular and Molecular Life Sciences. 2004;61:2812–2826. doi: 10.1007/s00018-004-4248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flannagan RS, Cosio G, Grinstein S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nature Reviews Microbiology. 2009;7:355–366. doi: 10.1038/nrmicro2128. [DOI] [PubMed] [Google Scholar]
- 22.Schlumberger MC, Hardt WD. Salmonella type III secretion effectors: pulling the host cell's strings. Current Opinion in Microbiology. 2006;9:46–54. doi: 10.1016/j.mib.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 23.McGhie EJ, Brawn LC, Hume PJ, Humphreys D, Koronakis V. Salmonella takes control: effector-driven manipulation of the host. Current Opinion in Microbiology. 2009;12:117–124. doi: 10.1016/j.mib.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wisner AL, Desin TS, Koch B, Lam PK, Berberov EM, et al. Salmonella enterica subspecies enterica serovar Enteritidis SPI-2 T3SS: Role in Intestinal Colonization of Chickens and Systemic Spread. Microbiology. 2010;156:2770–2781. doi: 10.1099/mic.0.038018-0. [DOI] [PubMed] [Google Scholar]
- 25.Okamura M, Lillehoj HS, Raybourne RB, Babu US, Heckert RA, et al. Differential responses of macrophages to Salmonella enterica serovars Enteritidis and Typhimurium. Veterinary Immunology and Immunopathology. 2005;107:327–335. doi: 10.1016/j.vetimm.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Beug H, von Kirchbach A, Doderlein G, Conscience JF, Graf T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell. 1979;18:375–390. doi: 10.1016/0092-8674(79)90057-6. [DOI] [PubMed] [Google Scholar]
- 27.Thrasher A, Chetty M, Casimir C, Segal AW. Restoration of superoxide generation to a chronic granulomatous disease-derived B-cell line by retrovirus mediated gene transfer. Blood. 1992;80:1125–1129. [PubMed] [Google Scholar]
- 28.Hensel M, Shea JE, Waterman SR, Mundy R, Nikolaus T, et al. Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Molecular Microbiology. 1998;30:163–174. doi: 10.1046/j.1365-2958.1998.01047.x. [DOI] [PubMed] [Google Scholar]
- 29.Cirillo DM, Valdivia RH, Monack DM, Falkow S. Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Molecular Microbiology. 1998;30:175–188. doi: 10.1046/j.1365-2958.1998.01048.x. [DOI] [PubMed] [Google Scholar]
- 30.Shea JE, Beuzon CR, Gleeson C, Mundy R, Holden DW. Influence of the Salmonella typhimurium pathogenicity island 2 type III secretion system on bacterial growth in the mouse. Infection and immunity. 1999;67:213–219. doi: 10.1128/iai.67.1.213-219.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hensel M, Shea JE, Raupach B, Monack D, Falkow S, et al. Functional analysis of ssaJ and the ssaK/U operon, 13 genes encoding components of the type III secretion apparatus of Salmonella Pathogenicity Island 2. Molecular Microbiology. 1997;24:155–167. doi: 10.1046/j.1365-2958.1997.3271699.x. [DOI] [PubMed] [Google Scholar]
- 32.Forest CG, Ferraro E, Sabbagh SC, Daigle F. Intracellular survival of Salmonella enterica serovar Typhi in human macrophages is independent of Salmonella pathogenicity island (SPI)-2. Microbiology. 2010;156:3689–3698. doi: 10.1099/mic.0.041624-0. [DOI] [PubMed] [Google Scholar]
- 33.Aussel L, Zhao W, Hebrard M, Guilhon AA, Viala JP, et al. Salmonella detoxifying enzymes are sufficient to cope with the host oxidative burst. Molecular Microbiology. 2011;80:628–640. doi: 10.1111/j.1365-2958.2011.07611.x. [DOI] [PubMed] [Google Scholar]
- 34.Helaine S, Thompson JA, Watson KG, Liu M, Boyle C, et al. Dynamics of intracellular bacterial replication at the single cell level. Proceedings of the National Acadamy of Sciences. 2010;107:3746–3751. doi: 10.1073/pnas.1000041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desin TS, Lam PK, Koch B, Mickael C, Berberov E, et al. Salmonella enterica serovar Enteritidis pathogenicity island 1 is not essential for but facilitates rapid systemic spread in chickens. Infection and Immunity. 2009;77:2866–2875. doi: 10.1128/IAI.00039-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vazquez-Torres A, Fang FC. Salmonella evasion of the NADPH phagocyte oxidase. Microbes and Infection. 2001;3:1313–1320. doi: 10.1016/s1286-4579(01)01492-7. [DOI] [PubMed] [Google Scholar]
- 37.Horst SA, Jaeger T, Denkel LA, Rouf SF, Rhen M, et al. Thiol peroxidase protects Salmonella enterica from hydrogen peroxide stress in vitro and facilitates intracellular growth. Journal of Bacteriology. 2010;192:2929–2932. doi: 10.1128/JB.01652-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallois A, Klein JR, Allen LA, Jones BD, Nauseef WM. Salmonella pathogenicity island 2-encoded type III secretion system mediates exclusion of NADPH oxidase assembly from the phagosomal membrane. Journal of Immunology. 2001;166:5741–5748. doi: 10.4049/jimmunol.166.9.5741. [DOI] [PubMed] [Google Scholar]
- 39.Vazquez-Torres A, Fantuzzi G, Edwards CK, 3rd, Dinarello CA, Fang FC. Defective localization of the NADPH phagocyte oxidase to Salmonella-containing phagosomes in tumor necrosis factor p55 receptor-deficient macrophages. Proceedings of the National Acadamy of Sciences. 2001;98:2561–2565. doi: 10.1073/pnas.041618998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slauch JM. How does the oxidative burst of macrophages kill bacteria? Still an open question. Molecular Microbiology. 2011;80:580–583. doi: 10.1111/j.1365-2958.2011.07612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uchiya K, Nikai T. Salmonella enterica serovar Typhimurium infection induces cyclooxygenase 2 expression in macrophages: involvement of Salmonella pathogenicity island 2. Infection and Immunity. 2004;72:6860–6869. doi: 10.1128/IAI.72.12.6860-6869.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang S, Lillehoj HS, Kim CH, Keeler CL, Jr, Babu U, et al. Transcriptional response of chicken macrophages to Salmonella enterica serovar Enteritidis infection. Developments in Biologicals. 2008;132:141–151. doi: 10.1159/000317154. [DOI] [PubMed] [Google Scholar]
- 43.Uchiya K, Groisman EA, Nikai T. Involvement of Salmonella pathogenicity island 2 in the up-regulation of interleukin-10 expression in macrophages: role of protein kinase A signal pathway. Infection and immunity. 2004;72:1964–1973. doi: 10.1128/IAI.72.4.1964-1973.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Halici S, Zenk SF, Jantsch J, Hensel M. Functional analysis of the Salmonella pathogenicity island 2-mediated inhibition of antigen presentation in dendritic cells. Infection and immunity. 2008;76:4924–4933. doi: 10.1128/IAI.00531-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Albaghdadi H, Robinson N, Finlay B, Krishnan L, Sad S. Selectively reduced intracellular proliferation of Salmonella enterica serovar typhimurium within APCs limits antigen presentation and development of a rapid CD8 T cell response. Journal of Immunology. 2009;183:3778–3787. doi: 10.4049/jimmunol.0900843. [DOI] [PMC free article] [PubMed] [Google Scholar]