Abstract
Objectives
To test the diagnostic accuracy of myocardial CT perfusion (CTP) imaging using color and gray scale image analysis.
Background
Current myocardial CTP techniques have varying diagnostic accuracy and are prone to artifacts that impair detection. This study evaluated the diagnostic accuracy of color and/or gray-scale CTP and the application of artifact criteria to detect hypoperfusion.
Methods
Fifty-nine prospectively-enrolled patients with abnormal single photon emission computed tomography (SPECT) studies were analyzed. True hypoperfusion was defined if SPECT hypoperfusion corresponded to obstructive coronary stenoses on CT angiography (CTA). CTP applied color and gray scale myocardial perfusion maps to resting CTA images. Criteria for identifying artifacts were also applied during interpretation.
Results
Using combined SPECT plus CTA as the diagnostic standard, abnormal myocardial CTP was present in 33 (56%) patients, 19 suggesting infarction and 14 suggesting ischemia. Patient-level color and gray scale myocardial CTP sensitivity to detect infarction was 90%, with specificity 80%, and negative and positive predictive value of 94% and 68%. To detect ischemia or infarction, CTP specificity and positive predictive value were 92% while sensitivity was 70%. Gray scale myocardial CTP had slightly lower specificity but similar sensitivity. Myocardial CTP artifacts were present in 88% of studies and were identified using our criteria.
Conclusions
Color and gray scale myocardial CTP using resting CTA images identified myocardial infarction with high sensitivity as well as infarction or ischemia with high specificity and positive predictive value without additional testing or radiation. Color and gray scale CTP had slightly better specificity than gray scale alone.
Keywords: Coronary CT Angiography, Myocardial CT perfusion, Cardiac CT, Cardiac CT perfusion
Background
The presence and size of regions of myocardial hypoperfusion have been shown to be independent predictors of poor prognosis.1, 2 Several non-invasive imaging modalities reliably detect myocardial hypoperfusion at rest and stress, suggestive of myocardial infarction, as well as with stress alone, suggestive of myocardial ischemia. Single photon emission computed tomography (SPECT) is commonly used to identify myocardial ischemia or infarction, but can have a relatively high false positive or false negative rate.3 Combining perfusion images with coronary artery anatomic evaluation could improve diagnostic accuracy and clinical care by reducing additional testing and unnecessary treatments.4
Coronary computed tomography angiography (CTA) can evaluate for coronary artery disease (CAD) with high diagnostic accuracy compared to invasive coronary angiography. While the CTA negative predictive value is very high (up to 100%), the positive predictive value is modest (approximately 64% for >50% stenosis), motivating the need for complimentary information to inform the significance of CTA findings.5 Myocardial CT perfusion imaging (CTP) using CTA images has reasonable diagnostic accuracy to detect hypoperfusion using either first pass iodinated contrast myocardial hypo-enhancement or a separate delayed contrast enhancement CT scan. However, prior CTP studies have been limited by use in low prevalence populations6, the need for additional radiation for delayed enhancement scanning7, and inconsistent comparisons to imaging “gold” standards for true myocardial hypoperfusion8. Further, CTP with contemporary 64 slice scanners yields significant artifacts that impair CTP evaluation primarily because of beam hardening and reconstruction artifacts.
The purpose of this study was to assess hypoperfusion by applying criteria to identify CTP artifacts and by testing the diagnostic performance of color and gray scale CTP perfusion maps. Myocardial infarction was identified when a rest and stress SPECT perfusion abnormality occurred in the same region that CTA had evidence of significant stenosis or prior revascularization. Myocardial ischemia was assumed if only stress SPECT hypoperfusion was present and there was a correlating CTA finding consistent with significant stenosis or prior revascularization.
Methods
Sixty-two consecutive subjects referred for CTA because of abnormal SPECT scans were enrolled and underwent CTA scans. All subjects gave informed consent prior to CTA. Demographic and clinical data were gathered from medical records review. This prospective HIPAA-compliant study was approved by each site’s institutional review board.
Subjects were excluded from participation due to known contrast allergy, creatinine > 1.7 mg/dL, irregular heart rhythm, resting heart rate > 100 beats per minute (bpm), and pregnancy, as well as contraindications to beta blocker, calcium channel blocker, or nitroglycerin. Subjects with heart rates >65 beats per minute were given oral or IV beta blockers or calcium channel blockers with a goal HR of <65 bpm. All eligible subjects were scanned regardless of final resting heart rate. Nitroglycerin 0.4 mg sublingual was administered immediately prior to the gated CT acquisition.
All subjects underwent cardiac CT with either prospective or retrospective gating on a 64 slice GE VCT scanner (GE Healthcare, Waukesha, WI) with a collimation of 64 × 0.625 mm and a rotation time of ~0.35 seconds. CT settings, including tube current, voltage and beam on duration, were selected by the supervising physician.
CT Myocardial Perfusion Imaging
Myocardial CTP imaging was performed using the resting CTA images. For inclusion in the analysis, LV chamber contrast density must have been greater than 200 HU. To ensure readers were blinded to the coronary anatomy during CTP evaluation, the myocardium was extracted from the background and left ventricular (LV) cavity using a GE Advantage Workstation (GE AW 4.4, Chalfont St. Giles, UK). The left ventricular images were oriented to short axis, vertical long axis, and four-chamber views. Myocardial images were reconstructed to 3-4 mm slice thickness using average pixel intensity projections.9 Gray scale images for each study were then evaluated using a window of 300 and a level of 100. CTP was evaluated using a color perfusion map based upon a graded myocardial Hounsfield unit (HU) scale (Figure 1).9 The HU scale began at 20 and changed color every 20 HU until >100 HU.10 Levels of enhancement on the CT images were visually scored on a 6 point ordinal scale (0= normal, 5=no enhancement) by 2 readers (K.B., J.B.) using a standard AHA 17-segment model of the LV.11 Both readers were blinded to CTA data. Available diastolic and systolic phases were also scored to assist with artifact identification.12 Wall thickness was also evaluated to support determination of prior myocardial infarction.13 Discrepancies were resolved by consensus. To help identify artifact on CTP, we applied a set of criteria derived from our previous work.14
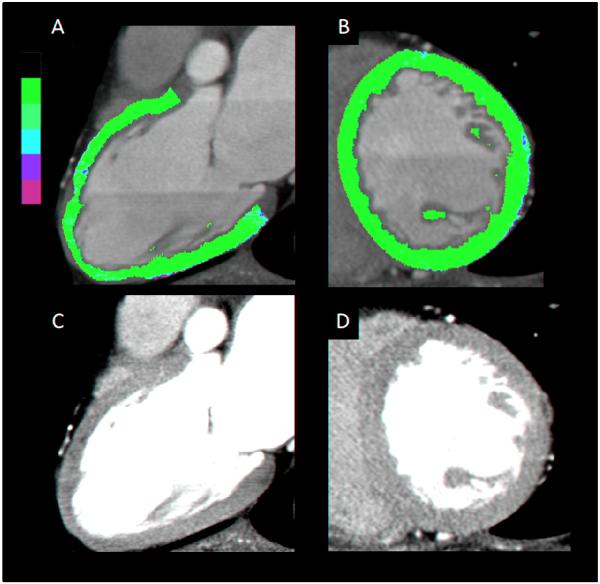
Figure 1.
This is a representative example of a subject with normal CTP images. A – Color scale vertical long axis view. B – Color scale short axis view. C – Gray scale vertical long axis view. D – Gray scale short axis view.
Cardiac CT Angiography
Cardiac CT angiography studies were evaluated by two cardiologists with 3 and 6 years of CTA experience (JB, KB) using axial and multi-planar reconstructions of the coronary arteries (GE AW workstation). Any discrepancies were resolved by consensus. Coronaries were segmented using a modified 19-segment coronary model,15, 16 and visually estimated coronary stenosis was categorized on an ordinal scale (0=0%, 1=1-30%, 2=31-50%, 3=51-70%, 4=71-90%, 5=91-100%). Quantitative CTA tools were used to ensure correct classification of stenosis. All CTA studies were read after CT myocardial perfusion evaluation and reviewers were blinded to patient and SPECT results.
Nuclear SPECT and CTA for Myocardial Hypoperfusion
All subjects underwent exercise or pharmacologic single (99mtechnetium) or dual isotope 201thallum and 99mtechnetium) SPECT stress testing prior to enrollment. Subjects with abnormal SPECT studies were enrolled. SPECT results were abstracted to identify rest, rest/stress, and stress abnormalities and locations on an AHA 17 segment model.17 To create a standard to compare to CTP, we first evaluated only resting SPECT images to define regions of infarction. As SPECT was likely to deliver a high false positive rate, we used a combination of SPECT and CTA results to define true ischemia or infarction for comparison to CTP. By SPECT/CTA criteria, a SPECT perfusion defect was considered to be true if the SPECT region was supplied by either an obstructive (>50%) CTA coronary stenosis or a revascularized coronary segment (stent or a coronary bypass graft proximal to the SPECT segment).18 Given the variability in stenosis evaluations as well as correlation between stenosis and decreased blood flow, we also defined “obstructive” coronary stenosis as >30% stenosis for SPECT/CTA criteria in an exploratory analysis.
Statistics
Diagnostic performance of CTP to detect resting SPECT abnormalities as well as suspected ischemia or infarction based on SPECT plus CTA was calculated at the segmental and patient level. CTP was considered to be correct only if CTP perfusion defects were seen in the same myocardial segments as the SPECT/CTA findings. We calculated sensitivity, specificity, positive predictive value, and negative predictive value for CTP. Receiver operator curves (ROC) and the area under the ROC curves (AUC) were also calculated. We also compared prospective ECG-gated and retrospective ECG-triggered CT diagnostic data by t-test. All statistical analysis was performed using Stata IC software (College Station, PA). A p<0.05 was considered significant.
Results
Sixty-two subjects were enrolled. Three subjects were excluded due to poor contrast enhancement, leaving 59 subjects for final analysis. Of the 30 subjects with rest and stress SPECT abnormalities, 19 had obstructive CAD or revascularization supplying the SPECT defect and were considered true myocardial infarction by our SPECT/CTA criteria. Of the 11 remaining subjects with abnormal rest/stress SPECT and CTA, only one had obstructive CAD on CTA although the coronary disease did not correlate with rest/stress SPECT findings; this subject had proximal LAD stenosis on CTA but inferior wall defect on SPECT. This left 10 patients with abnormal rest/stress SPECT but no coronary artery disease on CTA suggesting false positive SPECT scans.
SPECT defects only on stress testing suggestive of myocardial ischemia were seen in 29 subjects. Of this group, 14 had obstructive CAD supplying the SPECT defect and were classified as having true ischemia. None of the remaining “false positive” ischemia patients had obstructive coronary disease. For final comparison to CTP, true infarction by SPECT/CTA criteria was identified in 19 subjects and true ischemia was identified in 14 subjects.
Table 3 shows the patient-level diagnostic performance data of color and gray scale CTP and gray scale CTP to detect myocardial infarction or ischemia. Using SPECT/CTA criteria, color and gray scale CTP identified 17 of 19 subjects with infarction and 6 of 14 subjects with ischemia (Figure 2). CTP identified infarction with a good sensitivity and modest specificity. When CTP was compared to either infarction or ischemia, sensitivity decreased but specificity improved. Gray scale CTP had similar sensitivity for infarction, but a lower sensitivity. However, specificity was minimally better for infarction or ischemia.
Table 3. Diagnostic Ability of Color or Gray Scale Myocardial CTP to Detect Ischemia or Infarction Compared to Combined SPECT plus Coronary CTA.
| Sensitivity (95% CI) |
Specificity (95% CI) |
PPV (95% CI) |
NPV (95% CI) |
ROC AUC (95% CI) |
|
|---|---|---|---|---|---|
| SPECT/CTA Results | Color and Gray Scale CTP | ||||
| Myocardial Infarction |
90% (67, 99%) |
80% (64, 91%) |
68% (47, 85%) |
94% (80, 99%) |
0.85 (0.75, 0.94) |
| Myocardial Ischemia |
61% (39, 80%) |
69% (52, 84%) |
56% (35, 76%) |
74% (56, 87%) |
0.81 (0.72, 0.91) |
| Myocardial Infarction or Ischemia |
70% (51, 84%) |
92% (75, 99%) |
92% (74, 99%) |
71% (53, 85%) |
0.81 (0.72,0.91) |
| SPECT/CTA Results | Gray Scale CTP | ||||
| Myocardial Infarction |
90% (67%, 99%) |
73% (56%, 85%) |
61% (41%, 79%) |
94% (79%, 99%) |
0.81 (0.71, 0.91) |
| Myocardial Ischemia |
65% (43%, 84%) |
64% (46%, 79%) |
54% (34%, 73%) |
74% (55%, 88%) |
0.64 (0.52, 0/77) |
| Myocardial Infarction or Ischemia |
73% (55%, 87%) |
85% (65%, 96%) |
86% (67%, 96%) |
71% (52%, 86%) |
0.78 (0.68, 0.89) |
PPV – Positive predictive value; NPV- Negative predictive value; ROC AUC- Receiver operating characteristics Area Under the Curve
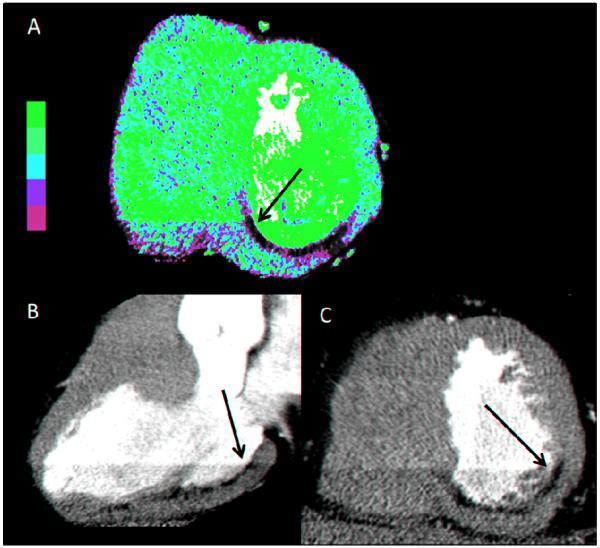
Figure 2.
This is a representative example of a subject with myocardial infarction. The arrow indicates hypo-enhancement crossing a phase delineated boundary which meets criteria for abnormal CTP. A – Color perfusion map in short axis showing inferior hypo-enhancement. B – Gray scale vertical long axis (3 chamber) view showing infero-lateral hypo-enhancement. C- Gray scale short axis view showing inferior and infero-lateral hypo-enhancement.
CTP had two false negative studies for infarction caused by coexisting artifacts,, one due to beam hardening and inferior stair-step artifact19, and one with an anterior wall stair-step artifact. CTP had also had two false positive studies for hypoperfusion. One false positive CTP correlated to an abnormal stress SPECT but had no coronary artery disease. The second subject had discrepant results with SPECT suggesting an inferior infarction, a CTP suggesting an anterior defect and a CTA revealing severe multi-vessel CAD with several stents and bypass grafts. Retrospective ECG-gated CT was used in 33 (55%) of patients and prospective ECG-triggered CT in 26 (45%). There was no difference in diagnostic accuracy between imaging types (p=NS).
To reduce possible patient misclassification for true infarction or ischemia, two exploratory analyses were performed. First, we applied an obstructive CAD cutoff of >30% to the SPECT/CTA criteria and four subjects were identified with CTA stenosis between 30% and 50%. None of these subjects had correlative SPECT defects to suggest either infarction or ischemia and diagnostic ability was unchanged. Second, an exploratory analysis using only resting SPECT as the standard for infarction, CTP had a sensitivity of 59%, specificity of 73%, negative of 65% and positive predictive value of 68%.
Artifacts were common and present on color perfusion images in 52 (88%) patients (Table 4). The most common artifact was an inferior-lateral basal artifact consistent with beam hardening from the spine and/or aorta.20 Axial slice, stair-step artifacts cause a band that corresponds to the width of the CT detector and commonly affects the anterior wall and leads to difficulty in interpretation (Figure 3).19 The two true regions of hypoperfusion missed by CTP were misclassified due to artifacts. Other artifacts were produced by three events: mis-registration caused by respiratory motion or heart rate variability; retrospective gated studies when there is inadequate data for adequate reconstruction the first helical slice; and a lack of homogeneous contrast enhancement as the scan is taken over time.
Table 4. Artifact Data.
| Artifact Locations | N |
| Antero-apical | 7 |
| Antero-apical and inferior | 25 |
| Inferior | 19 |
| Antero-apical, inferior, lateral | 1 |
| Lateral | 0 |
| None | 7 |
| Artifact Types | N |
| Axial slice (stair-step) | 38 |
| Beam Hardening | 40 |
| Axial slice (stair-step) and beam hardening | 27 |
| Mis-registration | 1 |
| Pacer or sternal wire | 2 |
| Subendocardial Sparing | 1 |
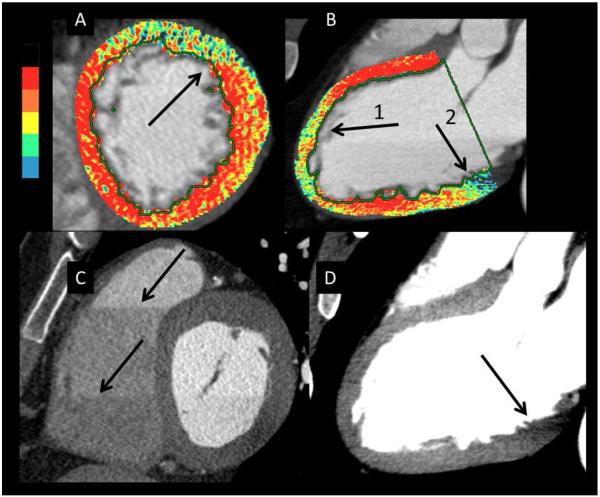
Figure 3.
This figure shows several examples of imaging artifacts seen on color and gray scale images designated by arrows. Panels A and C are two different patients and panels B and D are from the same patient. A – Color perfusion image with an anterior artifact with a linear cut-off typical of a stair-step artifact. B- Anterior artifact caused by phase boundary and beam hardening from sternum (Arrow 1). Artifact due to beam hardening from the aorta or spine (Arrow 2). C –Linear boundaries caused by axial slice/stair-step artifacts. D – Gray scale of the color image in panel B. Artifacts in the apical anterior wall are not as evident as the color image (Arrow 1). However, the beam hardening artifact in the basal inferior wall is still evident (Arrow 2).
Discussion
Identification of myocardial infarction and ischemia are important to inform prognosis and to plan patient treatments. Using resting CTA images, both coronary anatomy and myocardial hypoperfusion evaluation is possible without the need for further studies, or additional radiation or contrast. This study showed that compared to combined SPECT/CTA criteria, resting color and gray scale CTP identifies myocardial infarction with high sensitivity (90%), good specificity (80%) and high negative predictive value (94%). Grayscale CTP has a similar sensitivity but slightly lower specificity (73%). Color and gray scale CTP also identifies myocardial ischemia or infarction with high specificity (92%) but lower sensitivity (70%) compared to infarction alone. Thus, the presence of CTP-detected hypoperfusion suggests either myocardial infarction or ischemia with high predictive power (92%).
Our data are consistent with previous CTP reports that focus on detecting hypoperfusion on resting SPECT, although diagnostic sensitivity and specificity varied widely from 75% to 100% and 57% to 98%, respectively.21-25 Reasons for the range of diagnostic values are likely due to either differences in CTP technique or the comparators to CTP (Table 5). To date, most reported CTP techniques relied on gray scale interpretation of CTP images. In our study, gray scale alone had a sensitivity of 90% but a lower specificity of 73%. Our observations, and the observations of others, suggest that color imaging makes CTP abnormalities easier to see and use of color and gray scale CTP may enhance diagnostic accuracy.10
Table 5. CT Imaging of Myocardial Perfusion.
| Author | Comparators | Num. | Technique | Sensitivity | Specificity |
|---|---|---|---|---|---|
|
Nikolaou, et al
(2005) 21 |
16 slice CT vs MRI | 30 | Gray scale CT vs delayed enhancement on MRI |
91% | 79% |
|
Henneman, et al
(2008) 25 |
64 slice CT vs SPECT |
69 | Gray scale CT vs SPECT |
100% | 57% |
|
Mahnken, et al (2008) 31 |
16 slice CT vs MRI | 15 | 16 slice CT with green/blue weighted color overlay vs delayed enhancement MRI |
87% | 96% |
|
Rubinshtein, et
al (2009) 22 |
64 slice CT vs SPECT |
122 | Dual source CT vs SPECT |
77% | 94% |
|
Kachenoura, et
al (2009) 23 |
64 slice CT vs SPECT |
78 | Novel rescaling of CT vs SPECT |
75% | 72% |
|
Bauer, et al
(2010) 24 |
64 slice CT vs MRI | 36 | Dual energy CT vs delayed enhancement MRI |
77% | 97% |
Previous studies suggest that post-processing techniques, including various types of pixel projections and various slice thickness, may also change diagnostic accuracy.9, 10 We used average pixel intensity projection with 3-4 mm slice thickness based on prior studies. Use of different projections, slice thickness, or windows and levels has been suggested to affect CTP accuracy, but these questions are beyond the scope of this study. Additionally, prior studies have reported other CTP techniques such as dual energy CT 24, 26, or a novel rescaling method using control group data 27. However, to date, none of these techniques have been proven superior and additional formal studies are needed to answer these important questions.
Complicating the diagnostic CTP data are the variety of “gold standard” comparators used in the literature. Bauer, et al used delayed enhancement cardiac magnetic resonance imaging as the standard and showed a CTP specificity of 97% and a sensitivity of 77% to identify of prior myocardial infarction.24 However, most studies used SPECT as the comparator (Table 5). As we observed, this may result in subject misclassification. SPECT has limited spatial resolution and may miss small or sub-endocardial areas of hypoperfusion which would increase the CTP false positive rate (Type 1 error) and decrease specificity. For example, Rubenshtein, et al showed that six of seven “false positive” CTP scans (abnormal CTP with normal resting SPECT) had subendocardial hypoperfusion that corresponded with obstructive coronary disease or revascularized myocardial segments.22 SPECT may also increase CTP false negative results (Type II error) typically because of soft tissue attenuation which would reduce CTP sensitivity. To minimize potential misclassification of patients, we used combined SPECT/CTA criteria as our standard to define myocardial infarction or ischemia. This also reflects the likely clinical use of CTP where CTA and CTP will be used in conjunction. However, since we used CTA in our comparator, we could not evaluate the incremental value of CTA to CTP. This is an important area for future study.
Further complicating CTP interpretation is the fact that resting CTP may identify myocardial ischemia without infarction. Kachenoura, et al23 reported that 8 of 16 subjects diagnosed with ischemia by SPECT perfusion had resting CTP hypoperfusion. Similarly, of the 8 CTP abnormalities identified in our subjects with normal resting SPECT scans, 6 had obstructive CAD corresponding to stress SPECT myocardial hypoperfusion. The reasons resting CTP can detect ischemia are unclear. One hypothesis is that coronary vasodilation from either pre-CT scan nitroglycerin or iodinated contrast28 or the mild stress of having a CT scan may cause myocardial ischemia. Alternatively, the SPECT scan may not be able to detect small areas of prior infarction because of limited spatial resolution. It is possible that the SPECT abnormalities may only become large enough to be detected with stress-induced peri-infarct ischemia. Our data suggest that hypoperfusion on resting CTP commonly reflects myocardial infarction with high specificity, but may detect infarction or ischemia with high sensitivity and positive predictive value. Differentiation of CTP infarction or ischemia using CTP warrants further study, but utilization of delayed enhancement with resting CTP may be one alternative.
One technical challenge for CTP using 64 slice CT was the presence of artifacts which occurred in most (88%) of our subjects. This is a relatively high artifact rate and most likely due accentuation of both abnormal findings and artifacts with color perfusion images. Frequently, imaging artifacts that were not appreciated on gray scale images were prominent on color images. Further, two instances of infarction were missed because CTP studies that had artifacts obscured the regions with hypoperfusion and would have resulted in only 1 missed myocardial infarction by CTP. Further technical advances in CT hardware and processing techniques that eliminate beam hardening artifacts29 and the stair-step artifacts with larger and faster detectors should improve the diagnostic ability of CTP.
This study supports continued technologic improvements in CT hardware as well as efforts in integrating CTP and CTA using rest and/or stress CT imaging.12, 30, 31 For resting CTP, additional CT scanning is not needed and the effective radiation dose is reduced. The combination of CTP and CTA at rest and stress could improve the diagnostic accuracy for identifying hemodynamically significant coronary stenosis and lead to a more accurate, single non-invasive test4. Further CTP studies are needed to explore the incremental benefit of CTA and CTP together, and to build better gold standards.
Limitations
There are several limitations to this study. First, subjects were referred by community physicians and clinical interpretations of SPECT studies were used to assess hypoperfusion. However, incorporation of the CTA and SPECT data likely increased our ability to identify hypoperfusion that resulted from a specific coronary artery lesion. Second, not all CT studies had both systolic and diastolic phases for evaluation, which we and others have used as criteria to define true CTP abnormalities. This likely reflects the real world use of CTA where all phases will not be available due to increasing use of low dose prospective ECG-triggered CT. Third, only first pass, static images for coronary angiography were used. Dynamic imaging of myocardial contrast enhancement over time may better discern hypoperfusion from artifact although radiation dose may become a limiting factor. Finally, the detection accuracy reported in this work is appropriate for the patient population with abnormal SPECT studies and therefore a high prevalence of disease. These values may not be appropriate for the whole population because of this selection bias.
Conclusions
Using resting CTA scans, CTP can identify presumed myocardial infarction with high sensitivity without the need for additional radiation or additional testing. In addition, CTP hypoperfusion may also identify myocardial infarction or ischemia with high specificity and could lead to an evolution in non-invasive risk stratification. Future studies combining CTA and CTP results as well as future techniques to allow differentiation of infarction and ischemia using CTP are warranted.
Table 1. Demographic Data.
| Mean | 95% CI | ||
| Age | 67 | (64,71) | |
| Body Mass Index | (kg/m2) | 26.9 | (25.6,28.3) |
|
N N=59 |
Percent (%) | ||
| Gender | Male | 41 | (59) |
| Race: | Caucasian | 40 | (68) |
| African American | 2 | (3.4) | |
| Hispanic | 9 | (15.3) | |
| Asian | 3 | (5.1) | |
| Other/Unknown | 5 | (8.5) | |
| Prior myocardial infarction | 21 | (35.6 %) | |
| Risk Factors | High Cholesterol | 35 | (59.3) |
| Diabetes | 14 | (23.9) | |
| Hypertension | 34 | (57.6) | |
| Smoker | 14 | (23.7) | |
| Family History | 33 | (55.9) |
Table 2. Criteria for Artifact on CT-MPI.
| Not in the distribution of a coronary artery |
| Not consistent through all available cardiac phases |
| Does not cross a phase determined boundary |
| Occurs at commonly reported location: postero-basal, anteriorly at LVOT |
Acknowledgments
Relationship with Industry: Drs. Branch and Budoff are on the Speaker’s Bureau for GE Healthcare. Drs. Shuman, Alessio and Caldwell have received research funding from GE Healthcare.
Abbreviations List
- SPECT
single photon emission computed tomography
- CAD
coronary artery disease
- CTA
computed tomography angiography
- CTP
computed tomography perfusion imaging
- RCA
right coronary artery
- LAD
left anterior descending
- LV
left ventricle
- HU
Hounsfield units
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Orn S, Manhenke C, Anand IS, Squire I, Nagel E, Edvardsen T, Dickstein K. Effect of left ventricular scar size, location, and transmurality on left ventricular remodeling with healed myocardial infarction. Am J Cardiol. 2007;99:1109–1114. doi: 10.1016/j.amjcard.2006.11.059. [DOI] [PubMed] [Google Scholar]
- 2.Choi KM, Kim RJ, Gubernikoff G, Vargas JD, Parker M, Judd RM. Transmural extent of acute myocardial infarction predicts long-term improvement in contractile function. Circulation. 2001;104:1101–1107. doi: 10.1161/hc3501.096798. [DOI] [PubMed] [Google Scholar]
- 3.Hendel RC, Berman DS, Cullom SJ, Follansbee W, Heller GV, Kiat H, Groch MW, Mahmarian JJ. Multicenter clinical trial to evaluate the efficacy of correction for photon attenuation and scatter in spect myocardial perfusion imaging. Circulation. 1999;99:2742–2749. doi: 10.1161/01.cir.99.21.2742. [DOI] [PubMed] [Google Scholar]
- 4.Kajander S, Joutsiniemi E, Saraste M, Pietila M, Ukkonen H, Saraste A, Sipila HT, Teras M, Maki M, Airaksinen J, Hartiala J, Knuuti J. Cardiac positron emission tomography/computed tomography imaging accurately detects anatomically and functionally significant coronary artery disease. Circulation. 2010;122:603–613. doi: 10.1161/CIRCULATIONAHA.109.915009. [DOI] [PubMed] [Google Scholar]
- 5.Budoff M, Dowe D, Jollis J, Gitter M, Sutherland J, Halamert E, Scherer M, Bellinger R, Martin A, Benton R, Delago A, Min J. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: Results from the prospective multicenter accuracy (assessment by coronary computed tomographic angiography of individuals undergoing invasive coronary angiography) trial. J Am Coll Cardiol. 2008;52:1724–1732. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 6.Nicol ED, Stirrup J, Reyes E, Roughton M, Padley SPG, Rubens MB, Underwood SR. Comparison of 64-slice cardiac computed tomography with myocardial perfusion scintigraphy for assessment of global and regional myocardial function and infarction in patients with low to intermediate likelihood of coronary artery disease. Journal of Nuclear Cardiology. 15:497–502. doi: 10.1016/j.nuclcard.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Mahnken AH, Koos R, Katoh M, Wildberger JE, Spuentrup E, Buecker A, Günther RW, Kühl HP. Assessment of myocardial viability in reperfused acute myocardial infarction using 16-slice computed tomography in comparison to magnetic resonance imaging. Journal of the American College of Cardiology. 2005;45:2042–2047. doi: 10.1016/j.jacc.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 8.Francone M, Carbone I, Napoli A, Algeri E, Grazhdani H, Lezoche R, Mirabelli F, Gaudio C, Calabrese FA, Catalano C, Passariello R. Imaging of myocardial infarction using a 64-slice mdct scanner: Correlation between infarcted region and status of territory-dependent coronary artery. Radiol Med. 2007;112:1100–1116. doi: 10.1007/s11547-007-0209-6. [DOI] [PubMed] [Google Scholar]
- 9.Rogers IS, Cury RC, Blankstein R, Shapiro MD, Nieman K, Hoffmann U, Brady TJ, Abbara S. Comparison of postprocessing techniques for the detection of perfusion defects by cardiac computed tomography in patients presenting with acute st-segment elevation myocardial infarction. J Cardiovasc Comput Tomogr. 2010;4:258–266. doi: 10.1016/j.jcct.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagao M, Matsuoka H, Kawakami H, Higashino H, Mochizuki T, Ohshita A, Kohno T, Shigemi S. Detection of myocardial ischemia using 64-slice mdct. Circ J. 2009;73:905–911. doi: 10.1253/circj.cj-08-0940. [DOI] [PubMed] [Google Scholar]
- 11.Klocke F, Baird M, Lorell B, Bateman T, Messer J, Berman D, O’Gara P, Carabello B, Russell RJ, Cerqueira M, St John Sutton M, DeMaria A, Udelson J, Kennedy J, Verani M, Williams K, Antman E, Smith SJ, Alpert J, Gregoratos G, Anderson J, Hiratzka L, Faxon D, Hunt S, Fuster V, Jacobs A, Gibbons R, Russell R. Acc/aha/asnc guidelines for the clinical use of cardiac radionuclide imaging--executive summary: A report of the american college of cardiology/american heart association task force on practice guidelines (acc/aha/asnc committee to revise the 1995 guidelines for the clinical use of cardiac radionuclide imaging) Circulation. 2003;108:1404–1418. doi: 10.1161/01.CIR.0000080946.42225.4D. [DOI] [PubMed] [Google Scholar]
- 12.Blankstein R, Shturman LD, Rogers IS, Rocha-Filho JA, Okada DR, Sarwar A, Soni AV, Bezerra H, Ghoshhajra BB, Petranovic M, Loureiro R, Feuchtner G, Gewirtz H, Hoffmann U, Mamuya WS, Brady TJ, Cury RC. Adenosine-induced stress myocardial perfusion imaging using dual-source cardiac computed tomography. Journal of the American College of Cardiology. 2009;54:1072–1084. doi: 10.1016/j.jacc.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Nieman K, Cury RC, Ferencik M, Nomura CH, Abbara S, Hoffmann U, Gold HK, Jang I-K, Brady TJ. Differentiation of recent and chronic myocardial infarction by cardiac computed tomography. The American Journal of Cardiology. 2006;98:303–308. doi: 10.1016/j.amjcard.2006.01.101. [DOI] [PubMed] [Google Scholar]
- 14.Shuman WP, Branch KR, May JM, Soine LA, Mitsumori LM, Caldwell JH. Myocardial iodine distribution in rest ecg-gated ct coronary angiography: Prevalence of artifactual focal defects which simulate infarction; Presented at: Radiological Society of North America 2010 Scientific Assembly and Annual Meeting; Chicago IL. 2010; November 2028-December 2013. [Google Scholar]
- 15.Raff GL, Chair, Abidov A, Achenbach S, Berman DS, Boxt LM, Budoff MJ, Cheng V, DeFrance T, Hellinger JC, Karlsberg RP. Scct guidelines for the interpretation and reporting of coronary computed tomographic angiography. Journal of Cardiovascular Computed Tomography. 3:122–136. doi: 10.1016/j.jcct.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, McGoon DC, Murphy ML, Roe BB. A reporting system on patients evaluated for coronary artery disease. Report of the ad hoc committee for grading of coronary artery disease, council on cardiovascular surgery, american heart association. Circulation. 1975;51:5–40. doi: 10.1161/01.cir.51.4.5. [DOI] [PubMed] [Google Scholar]
- 17.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the american heart association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 18.Pereztol-Valdés O, Candell-Riera J, Santana-Boado C, Angel J, Aguadé-Bruix S, Castell-Conesa J, Garcia EV, Soler-Soler J. Correspondence between left ventricular 17 myocardial segments and coronary arteries. European Heart Journal. 2005;26:2637–2643. doi: 10.1093/eurheartj/ehi496. [DOI] [PubMed] [Google Scholar]
- 19.Kroft LJM, de Roos A, Geleijns J. Artifacts in ecg-synchronized mdct coronary angiography. Am. J. Roentgenol. 2007;189:581–591. doi: 10.2214/AJR.07.2138. [DOI] [PubMed] [Google Scholar]
- 20.Rodríguez-Granillo G, Rosales M, Degrossi E, Rodriguez A. Signal density of left ventricular myocardial segments and impact of beam hardening artifact: Implications for myocardial perfusion assessment by multidetector ct coronary angiography. The International Journal of Cardiovascular Imaging (formerly Cardiac Imaging) 2010;26:345–354. doi: 10.1007/s10554-009-9531-5. [DOI] [PubMed] [Google Scholar]
- 21.Nikolaou K, Sanz J, Poon M, Wintersperger BJ, Ohnesorge B, Rius T, Fayad ZA, Reiser MF, Becker CR. Assessment of myocardial perfusion and viability from routine contrast-enhanced 16-detector-row computed tomography of the heart: Preliminary results. European Radiology. 2005;15:864–871. doi: 10.1007/s00330-005-2672-6. [DOI] [PubMed] [Google Scholar]
- 22.Rubinshtein R, Miller TD, Williamson EE, Kirsch J, Gibbons RJ, Primak AN, McCollough CH, Araoz PA. Detection of myocardial infarction by dual-source coronary computed tomography angiography using quantitated myocardial scintigraphy as the reference standard. Heart. 2009;95:1419–1422. doi: 10.1136/hrt.2008.158618. [DOI] [PubMed] [Google Scholar]
- 23.Kachenoura N, Lodato J, Gaspar T, Bardo D, Newby B, Gips S, Peled N, Lang R, Mor-Avi V. Value of multidetector computed tomography evaluation of myocardial perfusion in the assessment of ischemic heart disease: Comparison with nuclear perfusion imaging. European Radiology. 2009;19:1897–1905. doi: 10.1007/s00330-009-1365-y. [DOI] [PubMed] [Google Scholar]
- 24.Bauer RW, Kerl JM, Fischer N, Burkhard T, Larson MC, Ackermann H, Vogl TJ. Dual-energy ct for the assessment of chronic myocardial infarction in patients with chronic coronary artery disease: Comparison with 3-t mri. Am. J. Roentgenol. 2010;195:639–646. doi: 10.2214/AJR.09.3849. [DOI] [PubMed] [Google Scholar]
- 25.Henneman MM, Schuijf JD, Dibbets-Schneider P, Stokkel MP, van der Geest RJ, van der Wall EE, Bax JJ. Comparison of multislice computed tomography to gated single-photon emission computed tomography for imaging of healed myocardial infarcts. The American Journal of Cardiology. 2008;101:144–148. doi: 10.1016/j.amjcard.2007.07.084. [DOI] [PubMed] [Google Scholar]
- 26.Nagao M, Kido T, Watanabe K, Saeki H, Okayama H, Kurata A, Hosokawa K, Higashino H, Mochizuki T. Functional assessment of coronary artery flow using adenosine stress dual-energy ct: A preliminary study. Int J Cardiovasc Imaging. 2010 doi: 10.1007/s10554-010-9676-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kachenoura N, Gaspar T, Lodato JA, Bardo DM, Newby B, Gips S, Peled N, Lang RM, Mor-Avi V. Combined assessment of coronary anatomy and myocardial perfusion using multidetector computed tomography for the evaluation of coronary artery disease. Am J Cardiol. 2009;103:1487–1494. doi: 10.1016/j.amjcard.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Limbruno U, Petronio AS, Amoroso G, Baglini R, Paterni G, Merelli A, Mariotti R, Raffaele De Caterina Mariani M. The impact of coronary artery disease on the coronary vasomotor response to nonionic contrast media. Circulation. 2000;101:491–497. doi: 10.1161/01.cir.101.5.491. [DOI] [PubMed] [Google Scholar]
- 29.So A, et al. Beam hardening correction in ct myocardial perfusion measurement. Physics in Medicine and Biology. 2009;54:3031. doi: 10.1088/0031-9155/54/10/005. [DOI] [PubMed] [Google Scholar]
- 30.Tamarappoo BK, Dey D, Nakazato R, Shmilovich H, Smith T, Cheng VY, Thomson LEJ, Hayes SW, Friedman JD, Germano G, Slomka PJ, Berman DS. Comparison of the extent and severity of myocardial perfusion defects measured by ct coronary angiography and spect myocardial perfusion imaging. JACC: Cardiovascular Imaging. 2010;3:1010–1019. doi: 10.1016/j.jcmg.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Cury RC, Magalhaes TA, Borges AC, Shiozaki AA, Lemos PA, Junior JS, Meneghetti JC, Rochitte CE. Dipyridamole stress and rest myocardial perfusion by 64-detector row computed tomography in patients with suspected coronary artery disease. Am j cardiol. 2010:310–315. doi: 10.1016/j.amjcard.2010.03.025. United States: 2010 Elsevier Inc. [DOI] [PubMed] [Google Scholar]