Abstract
Background
Disorders of the Ras/MAPK pathway have an overlapping skeletal phenotype (eg. scoliosis, osteopenia). The Ras proteins regulate cell proliferation and differentiation and NF1 individuals have osteoclast hyperactivity and increased bone resorption as measured by urine pyridinium crosslinks [pyridinoline (Pyd) and deoxypyridinoline (Dpd)]. Pyd and Dpd are hydroxylysine derived cross-links of collagen found in bone and cartilage and excreted in the urine. Dpd is most abundant in bone. The aim of this study was to evaluate if other syndromes of the Ras/MAPK pathway have increased bone resorption, which may impact the skeletal phenotype.
Methods and Results
Participants: [Noonan syndrome (n=14), Costello syndrome (n=21), and cardiofaciocutaneous (CFC) syndrome (n=14)]. Pyridinium cross-links from two consecutive first morning urines were extracted after acid hydrolysis and analyzed by High Performance Liquid Chromotography. Three separate analyses of covariance (ANCOVA) were performed to compare Pyd, Dpd, and Dpd/Pyd ratio of each group to controls after controlling for age. Data were compared to 99 healthy controls.
Conclusions
The Dpd and the Dpd/Pyd ratio were elevated (p<0.0001) in all 3 conditions compared to controls suggesting that collagen degradation was predominantly from bone. The data suggest that the Ras/MAPK signal transduction pathway is important in bone homeostasis.
Keywords: bone, cardiofaciocutaneous syndrome, Costello syndrome, Noonan syndrome, pyridinium
INTRODUCTION
Mutations in a variety of Ras/mitogen-activated protein kinase (MAPK) pathway associated genes are causative for several distinct but clinically overlapping disorders. Neurofibromatosis type 1 (NF1) is due to loss of function mutations in NF1. Noonan syndrome is a heterogeneous condition and most have mutations in either PTPN11, SOS1, KRAS, RAF1, NRAS, or SHOC2 (1–6). Cardiofaciocutaneous (CFC) syndrome is due to mutations in BRAF, MAP2K1, MAP2K2, and KRAS (6–8). Costello syndrome is exclusively due to mutations in HRAS (9). These genes play a role in the Ras/MAPK signal transduction pathway, and Ras proteins are expressed in osteoprogenitor cells (10–13). The musculoskeletal system is frequently observed in several of the Ras/MAPK pathway syndromes suggesting that activation of the Ras/MAPK pathway impacts cells regulating bone development and homeostasis.
Skeletal abnormalities including osteoporosis are seen in NF1 (14–29). The skeletal findings of Noonan and CFC syndrome are not as well delineated but include short stature, scoliosis, and chest wall deformities (30–34). The skeletal findings of Costello syndrome have been better characterized in small cohorts and include scoliosis, hip dysplasia, chest wall deformities, short stature, and osteopenia (35–36). White et al. reported on bone mineral density (BMD) of 8 individuals with Costello syndrome; all had osteopenia and 3 of the 8 individuals were symptomatic suggesting that decreased BMD in Costello syndrome is common and clinically significant (35).
Based on the musculoskeletal phenotypes, one infers that activation of the Ras pathway could impact bone cellular processes. Evaluation of markers of bone resorption provides a good correlation with bone mass and insight into bone homeostasis (37–40). Collagen molecules are bound together by “crosslinks” which provide strength to bone. As bone is resorbed by osteoclastic activity, the degraded crosslinks of the mature collagen, termed pyridinium crosslinks, are excreted in the urine. The urinary pyridinium crosslinks, pyridinoline (Pyd) and deoxypyridinoline (Dpd), provides an excellent inference of bone resorption (38–46). Pyd and Dpd are found in bone and cartilage, but bone has the highest content of Dpd (42, 43, 46). We previously reported that individuals with NF1 have increased markers of bone resorption (47), and osteoclasts have been shown to be hyperactive in NF1 (10, 48). Therefore, we hypothesized that other syndromes of the Ras/MAPK pathway have increased bone resorption.
MATERIALS AND METHODS
Subjects
Physical examinations and medical histories were obtained on individuals with Noonan, Costello, and CFC syndromes. Individuals were examined by a medical geneticist (DS) and the diagnosis confirmed clinically by phenotype with supportive genotypic information when available. Individuals with recent radiographically confirmed fractures were not included. Families completed questionnaires on calcium intake estimates (49), and metabolic cost (MET) per week based on past-year physical activity levels (50). Pyridinium crosslink data from a local regional cohort of healthy children (N=99) were used for comparison (ages 1–17 years). Given the age range of the controls, and to minimize potential effects related to age, only individuals ≤31 years were included in the syndromic groups.
Written informed consent was obtained and the study was approved by the Institutional Review Board at the University of Utah.
Measurements
Urine from two first morning voids, when possible, was obtained for the extraction and analysis of total, free and the peptide-bound pyridinium crosslinks, Pyd and Dpd, by High Performance Liquid Chromatography (HPLC) according to established procedures (44, 47). In brief, urine was hydrolyzed and pyridinium crosslinks were isolated using a cellulose column and analyzed by reverse-phase HPLC. Pyridinium crosslink concentration was calculated using a 4-levels calibration curve obtained with an external standard. Urinary pyridinium crosslink concentration was normalized to urinary creatinine, measured by an Olympus AU 400 and calculated as umol/mol creatinine. The average of the two first-morning urine samples was used to minimize the effect of the day-to-day variation in the excretion of the crosslinks.
Mutation Analysis
DNA was extracted either from peripheral blood or buccal cheek cells through standard protocols at the University of Utah Center for Clinical and Translational Science Core Facility using a commercial kit manufactured by QIAGEN (Valencia, CA).
PTPN11, SOS1, HRAS, BRAF, MEK1, MEK2 Sequencing
Sanger sequencing of PCR products amplified from genomic DNA was performed using standard techniques on exons 3, 8, and 13 of PTPN11, all exons of SOS1, exon 2 of HRAS, exons 6, 11, 12, 14 and 15 of BRAF, and exons 2 and 3 of MEK1 (MAP2K1) and MEK2 (MAP2K2). Conditions and primer sequences are available upon request.
Data Analysis
Standard univariate descriptive statistics were used to summarize the distributions of the pyridinium crosslink measurements for each syndrome, and scatter plots with regression curves were used to examine the joint relationships of the crosslink measurements with age and syndrome.
Three separate analyses of covariance (ANCOVA) models were performed to compare mean levels of Pyd, Dpd, and the Dpd/Pyd ratio between each of the Costello, CFC, and Noonan syndrome groups to the control group after adjusting for age. In sensitivity analyses, expanded models with quadratic terms for age and interactions between age and syndrome group were examined to confirm the robustness of the age-adjusted comparisions to potential violations of the basic ANCOVA model. Adjusted means of pyridinium crosslink measurements were calculated to provide estimates of the mean crosslink measurements given the average age of 10.4 years for the full cohort. All hypothesis tests were performed using a 2-sided level of 0.05, without adjustment for multiple comparisons. All statistical analyses were performed using SAS 9.2 (SAS Institute Inc., NC).
RESULTS
The cohort consisted of 148 participants: Noonan syndrome (n=14), CFC syndrome (n=14), Costello syndrome (n=21), controls (n=99) (Table I).
Table I.
Characteristics of Patients(age)and the Unadjusted Average of Pyridinium Crosslink Measurements for Syndromic Groups
| Controls (N=99) | Noonan Syndrome(N=14) | CFC Syndrome (N=14) | Costello Syndrome (N=21) | P value* | |
|---|---|---|---|---|---|
| Age (yrs) (mean ±SD) | 9.06 ± 4.35 | 12.86 ± 7.89 | 10.73 ± 6.49 | 14.57 ± 7.21 | 0.0002 |
| Pyd(umol/molcrt)(mean ±SD) | 212.24 ± 95.37 | 253.50 ± 156.52 | 227.81 ± 109.20 | 194.34 ±88.61 | 0.38 |
| Dpd(umol/molcrt)(mean ±SD) | 46.78 ± 20.85 | 70.23 ± 40.33 | 69.87 ± 38.50 | 56.25 ±27.68 | 0.0008 |
| Dpd/Pyd ratio (mean ±SD) | 0.22 ± 0.04 | 0.28 ± 0.03 | 0.30 ± 0.07 | 0.29 ±0.05 | <0.0001 |
Overall comparison of means among syndromic groups using Analysis of Variance (ANOVA) F-Test.
Pyd=pyridinoline; Dpd=deoxypyridinoline; crt=creatinine
Mean ages differed significantly among the four groups (p<0.001) (Table I). Two separate consecutive first morning urine samples were available in the majority of individuals.
The mean past-year physical activity estimation of those who completed questionnaires for the syndromic groups was 29.2 MET/week (range 2.6 – 121; median 16.5; n=27) [Costello syndrome: mean 38.4, range 6.1–121, median 17.5, n=11; CFC syndrome: mean 19.3, range 4.1–82.2, median 14.6, n=9; Noonan syndrome: mean 27.4, range 2.6–59.7, median 26.3, n=7]. Two individuals with Costello syndrome utilized a wheelchair, and two individuals with CFC syndrome used “walkers”. Activity estimation levels were not available in controls, however, mean levels for a cohort of 241 healthy children have been reported previously to be 38.1 MET/week (range 0.6–316.8; median 25.6) (23). Mean calcium intake estimations for the syndromic groups were 1379 mg/day (range 157–3161, median 1193, n=35) [Costello syndrome: mean 1449, range 423–3161, median 1293, n=14; CFC syndrome: mean 1114, range 157–2875, median 1019, n=13; Noonan syndrome: mean 1686, range 493–2692, median 1558, n=8]. Calcium intake estimations were not available in controls, but average ranges based on age and sex for healthy individuals <30 years in one report were 918–1296 mg/day (52).
Dual energy x-ray absorptiometry (DXA) measurements performed previously on a clinical or research basis were available on 9 individuals (Table II).
Table II.
Dual Energy X-ray Absorptiometry Bone Mineral Density (BMD) Scores
| Study Participant
|
Body region imaged
|
Derived scores for BMD
|
|---|---|---|
| Costello #8 | whole body | z-score (−4.6) |
| lumbar spine | z-score (−4) | |
| distal femur | z-score (−6.8) | |
| Costello #10 | whole body | z-score (−1.4) |
| lumbar spine | z-score (−2.4) | |
| Costello #15 | lumbar spine | t-score (−4.1) |
| femoral neck | t-score (−2.3) | |
| radius | t-score (−5.6) | |
| Costello #17 | total hip | z-score (−2.8) |
| Costello #19 | lumbar spine | t-score (−3.7) |
| femoral neck | t-score (−2.0) | |
| radius | t-score (−5.2) | |
| Costello #24 | lumbar spine | z-score (−2.4) |
| femoral neck | z-score (−1.9) | |
| Costello #25 | total hip | z-score (−2.7) |
| forearm | z-score (−3.6) | |
| CFC #16 | whole body | z-score (−1.3) |
| lumbar spine | z-score (−1.3) | |
| femoral neck | z-score (−1.7) | |
| Noonan #16 | lumbar spine | t-score (−1.6) |
| femoral neck | t-score (−1.2) |
Mutations in HRAS were identified in all individuals with Costello syndrome in which adequate DNA was available: p.G12S (n=17), p.G13C (n=2), p.G13D (n=1). In one individual in which adequate DNA was not available, an HRAS mutations leading to the following predicted amino acid change (p.A146V) was verbally reported by parents.
Upon sequencing of PTPN11 exons 3, 8, and 13, mutations were identified in 6 individuals with Noonan syndrome: p.N308D (n=2), p.D106A (n=1), p.N58D (n=1), p.N58H (n=1), p.Y63C (n=1). A mutation in SOS1 (p.T266K) was identified in one individual with Noonan syndrome. In 2 individuals, mutations were identified in a clinical laboratory but not confirmed in our laboratory: RAF1 (p.P261A), PTPN11 (p.F285S). No causative mutations were identified in 5 individuals after sequencing of SOS1 and PTPN11 (exons 3, 8, 13), reviewing available medical records, and assessing parental report of possible mutations.
Upon sequencing of BRAF exons 6, 11, 12, 14 and 15, mutations were identified in 5 individuals with CFC syndrome: p.S467A (n=1), p.F468S (n=1), p.G469E (n=1), p.T470del (n=1), p.L597V (n=1). A mutation in MEK1 was identified in one individual (p.K59del), and a mutation in MEK2 was identified in another (p.Y134C). In the remaining individuals with CFC syndrome in which adequate quality DNA was not available or genes or exons not selected for sequencing, mutations in the following genes were reported by parents: BRAF (n=2), MEK2 (n=2), KRAS (n=1), unknown (n=2).
After adjustment for age, Pyd was elevated in each syndromic group compared to controls, with statistical significance reached for the Noonan and Costello syndrome groups [adjusted mean differences ± SE vs. controls were 85.1 ± 23.8 (p=0.0005) for Noonan syndrome, 45.3 ± 20.8 (p=0.0311) for Costello syndrome, 34.8 ± 23.4 (p=0.1397) for CFC syndrome (Fig. 1, Table III)].
Figure 1. Comparisons of Pyridinoline (Pyd) Among Syndromic Groups.
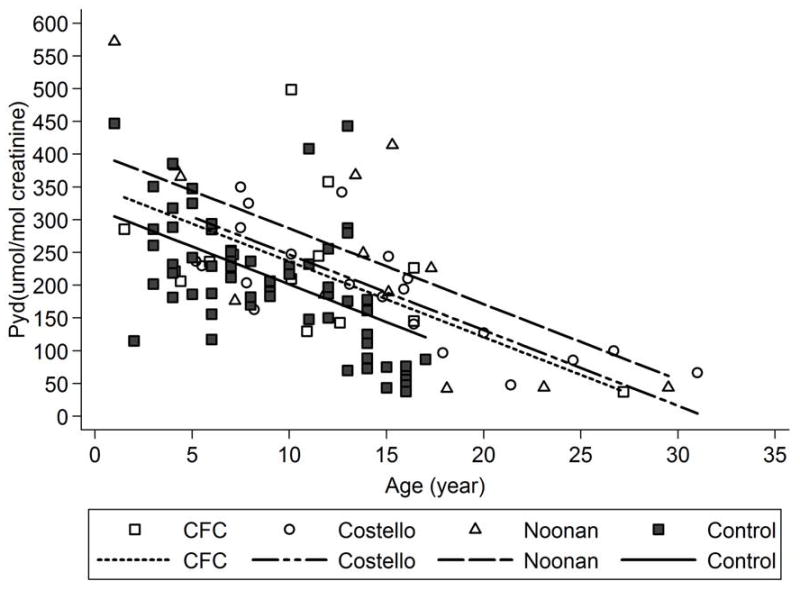
Pyd (umol/mol creatinine) in each syndromic group (Noonan syndrome, Costello syndrome and cardiofaciocutaneous (CFC) syndrome) was compared to controls using age-adjusted analyses of covariance (ANCOVA) adjusted for age shown graphically.
Table III.
The Comparisons of Adjusted Means in Syndromic Groups with Controls
| Pyridinium Crosslink | Control
|
Noonan Syndrome
|
CFC Syndrome
|
Costello Syndrome
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LS Mean3 ± SE3 | LS Mean3 ± SE3 | Diff.3 ±SE | p-value1 | LS Mean3± SE3 | Diff.3 ±SE | p-value1 | LS Mean3 ± SE3 | Diff.3 ±SE | p-value1 | |
| Pyd2 (umol/molcrt) | 197.30 ± 8.38 | 282.42 ± 22.08 | 85.12 ± 23.83 | 0.0005 | 232.12 ± 21.86 | 34.82 ±23.44 | 0.1397 | 242.62 ± 18.60 | 45.32 ± 20.82 | 0.0311 |
| Dpd2 (umol/molcrt) | 42.84 ± 2.8 | 77.86 ± 5.48 | 35.03 ± 5.92 | <0.0001 | 71.01 ± 5.43 | 28.17 ±5.82 | <0.0001 | 68.99 ± 4.62 | 26.16 ± 5.17 | <0.0001 |
| Dpd/Pyd ratio | 0.22 ± 0.004 | 0.28 ± 0.01 | 0.057 ± 0.013 | <0.0001 | 0.30 ± 0.01 | 0.080 ± 0.013 | <0.0001 | 0.29 ± 0.01 | 0.070 ±0.011 | <0.0001 |
Note:
Comparison with control
Pyd=pyridinoline; Dpd=deoxypyridinoline; crt=creatinine
LS=least square, SE=standard error of LS mean, Diff. =difference
Age-adjusted Dpd levels were significantly elevated in all 3 syndromic groups compared to controls [adjusted mean differences ± SE vs. controls were 35.0 ± 5.9 (p<0.0001) for Noonan syndrome, 28.2 ± 5.8 (p<0.0001) for Costello syndrome, 28.2 ± 5.8 (p<0.0001) for CFC syndrome (Fig. 2, Table III)]. The age adjusted differences among the four groups were larger relative to the variability in the data for Dpd (increase in R2 attributable to group differences = 24%) than for Pyd (increase in R2 = 21%), and age-adjusted Dpd/Pyd ratios were also significantly elevated in all three syndromic groups vs. controls (adjusted mean differences vs. controls were 0.06 ± 0.013 (p<0.0001) for Noonan syndrome, 0.07 ± 0.011 (p<0.0001) for Costello syndrome, 0.08 ± 0.013 (p<0.0001) for CFC syndrome (Fig. 3, Table III). While formal adjustments for multiple comparisons were not included in the original analysis plan, the age adjusted comparisons of Dpd and of Dpd/Pyd between each syndrome group and controls remained statistically significant at the 0.001 level after post-hoc Bonferroni adjustment for 9 separate comparisons (3 syndromic groups vs. control comparisons for each of three outcomes).
Figure 2. Comparisons of Deoxypyridinoline (Dpd) Among Syndromic Groups.
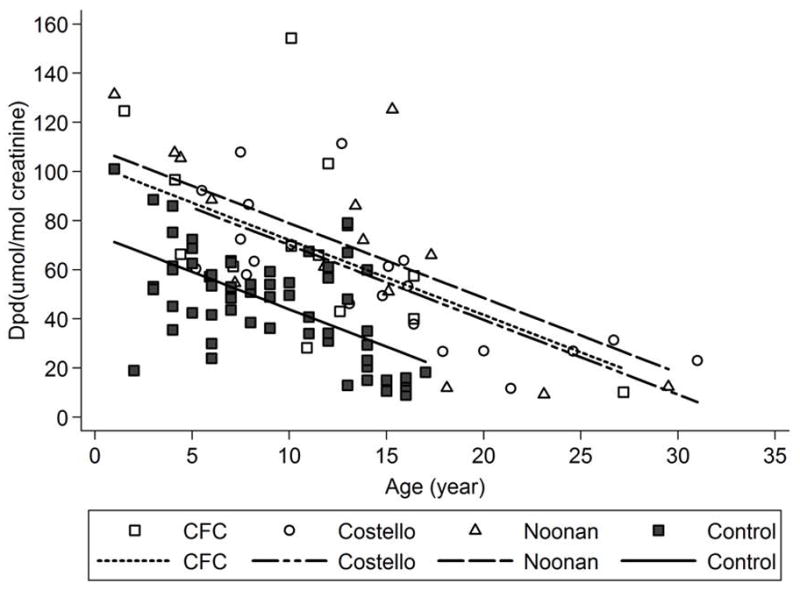
Dpd (umol/mol creatinine) in each syndromic group (Noonan syndrome, Costello syndrome and cardiofaciocutaneous (CFC) syndrome) was compared to controls using age-adjusted analyses of covariance (ANCOVA) and shown graphically.
Figure 3. Comparisons of Deoxypyridinoline/Pyridinoline (Dpd/Pyd) Ratio Among Syndromic Groups.
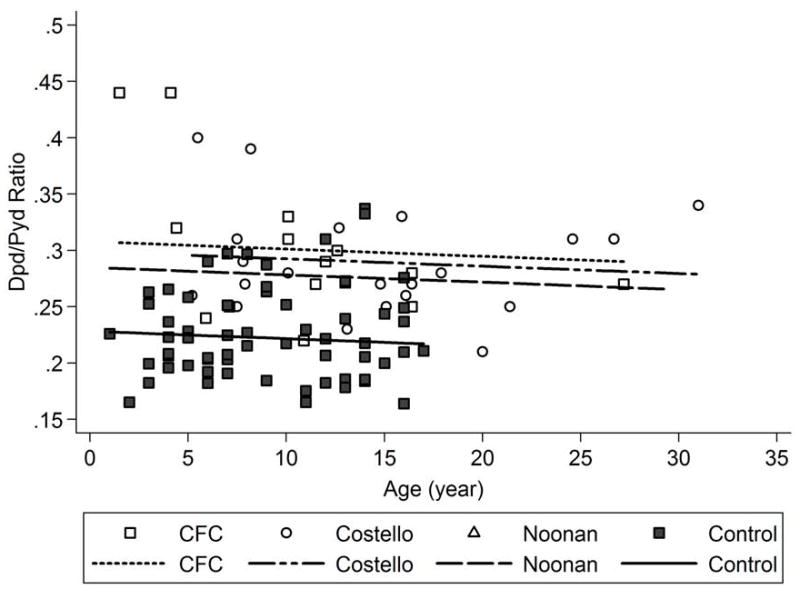
Dpd/Pyd ratio in each syndromic group (Noonan syndrome, Costello syndrome and cardiofaciocutaneous (CFC) syndrome) was compared to controls using age-adjusted analyses of covariance (ANCOVA) and shown graphically.
Age-adjusted elevations in Dpd and the Dpd/Pyd ratio vs. controls were similar between the Noonan, CFC, and Costello syndrome groups, and no statistically significant differences (p<0.05) were observed between the syndrome groups for any of the pyridinium crosslink measurements.
The Dpd/Pyd ratio was not significantly correlated with physical activity level estimation (MET/wk) in the 27 participants with physical activity level data (Pearson partial R = 0.22, p=0.29).
One individual with Costello syndrome was receiving pamidronate. Two individuals with Noonan syndrome, 2 individuals with Costello syndrome and 1 individual with CFC syndrome were receiving growth hormone. Given the potential effect of these therapeutic agents on bone resorption the data were re-analyzed excluding these individuals and the comparisons of each syndrome group with controls and between syndrome groups were similar.
DISCUSSION
Individuals with selected syndromes of the Ras/MAPK pathway (i.e. Noonan syndrome, CFC syndrome, and Costello syndrome) have increases in urinary pyridinium crosslinks similar to what we have previously reported in NF1 (least squares mean for individuals with NF1 with a skeletal dysplasia: Pyd = 233 umol/mol creatinine, Dpd = 71 umol/mol creatinine, Dpd/Pyd ratio = 0.31) (47). Dpd and the Dpd/Pyd ratio were significantly elevated in all syndromes suggesting that the increase in pyridinium crosslink excretion is primarily from bone resorption.
The exact etiology of the increase in the excretion of bone resorption markers is not know and likely multifactorial. Our data from individuals with Noonan, CFC, and Costello syndromes support the hypothesis that bone resorption is increased in syndromes associated with increased Ras signaling. It is likely that additional factors contribute to the increased bone resorption markers such as inactivity, hypotonia, and poor motor function. However, based on results from the Nf1+/− mouse model, it is known that the myeloid and mesenchymal progenitor cells function abnormally (10–13), and in vitro studies on NF1 human osteoclasts have increased lytic activity suggesting that increased signaling through the Ras/MAPK pathway impacts bone remodeling (10, 48). Functional assays of osteoclasts from individuals with Noonan, CFC and Costello syndromes will help determine the direct relationship of increased Ras signaling on osteoclast cellular functions.
Decreased BMD has been reported in Costello syndrome and NF1 (19–29, 35), and was also observed clinically from DXA scans in small numbers of individuals with Costello, CFC, and Noonan syndromes. It is possible that the decreased BMD is appropriate for size given the association of short stature with these syndromes, yet several of the previously reported individuals with Costello syndrome had significant fractures associated with osteoporosis (35). Even if generalized skeletal abnormalities are common, there are discrepancies in the focal skeletal phenotype of the Ras/MAPK pathway syndromes (eg. sphenoid wing dysplasia and tibial dysplasia only observed in NF1). This is likely secondary to somatic events (53, 54), modifier genes, expression patterns of the various causative genes, and activation of other effector pathways.
The clinical consequences of the increased bone resorption are still not well known and there may be compensatory effects by the mesenchymal lineages. Although bone resorption is increased, many of the musculoskeletal findings such as pectus abnormalities and hand anomalies are likely not related to osteoclast functions suggesting that the Ras/MAPK signal transduction pathway impacts other cell lineages important in musculoskeletal development.
A number of limitations make interpretation of our results difficult. The rarity of the syndromes, particularly CFC syndrome and Costello syndrome, results in a small cohort of individuals. In addition, co-morbidities may contribute to bone resorption. For example, one individual with Costello syndrome was receiving pamidronate infusions, which interferes with osteoclast function likely decreasing the bone resorption markers. Also, 5 individuals were receiving growth hormone injections which potentially could increase bone resorption markers (55). Any factors decreasing bone loading could also increase bone resorption markers. The mean MET/week for the combined syndromic groups was lower compared to previous reports of healthy children (23) and decreased physical activity from a number of co-morbidities such as hypotonia, scoliosis, seizures, etc. may contribute to increased bone resorption. However, there was no statistically significant correlation between the Dpd/Pyd ratio and physical activity level estimation (MET/wk). Interestingly the mean MET/week for the Costello syndrome group was similar to previous reports of healthy children, but the effectiveness of performing activities that put forces upon bone may be impaired.
Although bone resorption was increased in the syndromic groups compared to controls, there were no significant differences between the phenotypically categorized Noonan, CFC, and Costello syndrome groups. Assessment of bone resorption based on the various genotypes was difficult to assess give small number of individual genotypes. Future studies with larger numbers of participants will be needed to examine correlations of genotypes with bone resorption.
Acknowledgments
We thank the study participants for their help. We thank the leaders and members of the Noonan Syndrome Support Group, CFC International, and the International Costello Syndrome Support Group for their support. We thank Lisa Smith, Kyle Berg, and Austin Stevens for their help in research coordination and sample processing. We thank Dr. Judith Allanson, Dr. Jacques D’Astous, Dr. Karen Gripp, Dr. Bronwyn Kerr, Dr. Angela Lin, Dr. Nicola Longo, Dr. Laurie Moyer-Mileur, Dr. Mary Murray, Dr. Katherine Rauen, Hillarie Slater, and Dr. Susan White for their insights and guidance, as well as statistical support from Study Design and Biostatistics Center at the School of Medicine at the University of Utah.
Support was provided by a Public Health Services research grant numbers #UL1-RR025764 and C06-RR11234 from the National Center for Research Resources, research grant K23 NS052500 from the National Institute of Neurological Disorders and Stroke, Doris Duke Charitable Foundation Clinical Scientist Development Award, Shriners Hospitals for Children Research Foundation, the Primary Children’s Medical Center Research Foundation, and the Children’s Health Research Center and Clinical Genetics Research Program at the University of Utah.
Footnotes
Conflicts of Interest: None
References
- 1.Tartaglia M, Pennacchio LA, Zhao C, et al. Gain-of-function SOS1 mutations cause a distinctive form of Noonan syndrome. Nat Genet. 2007;39:75–79. doi: 10.1038/ng1939. [DOI] [PubMed] [Google Scholar]
- 2.Tartaglia M, Mehler EL, Goldberg R, et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 2001;29:465–468. doi: 10.1038/ng772. [DOI] [PubMed] [Google Scholar]
- 3.Pandit B, Sarkozy A, Pennacchio LA, et al. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat Genet. 2007;39:1007–1012. doi: 10.1038/ng2073. [DOI] [PubMed] [Google Scholar]
- 4.Cirstea IC, Kutsche K, Dvorsky R, et al. A restricted spectrum of NRAS mutations causes Noonan syndrome. Nat Genet. 2010;42:27–29. doi: 10.1038/ng.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cordeddu V, Di Schiavi E, Pennacchio LA, et al. Mutation of SHOC2 promotes aberrant protein N-myristoylation and causes Noonan-like syndrome with loose anagen hair. Nat Genet. 2009;41:1022–1026. doi: 10.1038/ng.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schubbert S, Zenker M, Rowe SL, et al. Germline KRAS mutations cause Noonan syndrome. Nat Genet. 2006;38:331–336. doi: 10.1038/ng1748. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Viciana P, Tetsu O, Tidyman WE, et al. Germline mutations in genes within the MAPK pathway cause cardio-facio-cutaneous syndrome. Science. 2006;311:1287–1290. doi: 10.1126/science.1124642. [DOI] [PubMed] [Google Scholar]
- 8.Niihori T, Aoki y, Narumi Y, et al. Germline KRAS and BRAF mutations in cardio-facio-cutaneous syndrome. Nat Genet. 2006;38:294–296. doi: 10.1038/ng1749. [DOI] [PubMed] [Google Scholar]
- 9.Aoki Y, Niihori T, Kawame H, et al. Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat Genet. 2005;37:1038–1040. doi: 10.1038/ng1641. [DOI] [PubMed] [Google Scholar]
- 10.Yang FC, Chen S, Robling AG, et al. Hyperactivation of p21ras and PI3K cooperate to alter murine and human neurofibromatosis type 1 – haploinsufficient osteoclast functions. J Clin Invest. 2006;116:2880–2891. doi: 10.1172/JCI29092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu X, Chen S, Potter OL, et al. Neurofibromin and its inactivation of Ras are prerequisites for osteoblast functioning. Bone. 2005;36:793–802. doi: 10.1016/j.bone.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Wu X, Estwick SA, Chen S, et al. Neurofibromin plays a critical role in modulating osteoblast differentiation of mesenchymal stem/progenitor cells. Hum Mol Genet. 2006;15:2837–2845. doi: 10.1093/hmg/ddl208. [DOI] [PubMed] [Google Scholar]
- 13.Elefteriou F, Benson MD, Sowa H, et al. ATF4 mediation of NF1 functions in osteoblast reveals a nutritional basis for congenital skeletal dysplasiae. Cell Metab. 2006;4:441–451. doi: 10.1016/j.cmet.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutmann DH, Aylsworth A, Carey JC, et al. The diagnostic evaluation and multidisciplinary management of neurofibromatosis 1 and neurofibromatosis 2. JAMA. 1997;278:51–57. [PubMed] [Google Scholar]
- 15.Crawford AH, Schorry EK. Neurofibromatosis in children; the role of the orthopaedist. J Am Academy of Orthopaedic Surgeons. 1999;7:217–230. doi: 10.5435/00124635-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Friedman JM, Birch PH. Type 1 Neurofibromatosis: A descriptive analysis of the disorder in 1728 patients. Am J Med Genet. 1997;70:138–143. doi: 10.1002/(sici)1096-8628(19970516)70:2<138::aid-ajmg7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 17.Stevenson DA, Birch PH, Friedman JM, et al. Descriptive analysis of tibial pseudarthrosis in patients with neurofibromatosis 1. Am J Med Genet. 1999;84:413–419. doi: 10.1002/(sici)1096-8628(19990611)84:5<413::aid-ajmg5>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 18.Vitale MG, Guha A, Skaggs DL. Orthopaedic manifestations of neurofibromatosis in children: an update. Clin Orthop Relat Res. 2002;401:107–118. doi: 10.1097/00003086-200208000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Illes T, Halmai V, de Jonge T, et al. Decreased bone mineral density in neurofibromatosis-1 patients with spinal deformities. Osteoporos Int. 2001;12:823–827. doi: 10.1007/s001980170032. [DOI] [PubMed] [Google Scholar]
- 20.Yilmaz K, Ozmen M, Bora Goksan S, et al. Bone mineral density in children with neurofibromatosis 1. Acta Paediatr. 2007;96:1220–1222. doi: 10.1111/j.1651-2227.2007.00401.x. [DOI] [PubMed] [Google Scholar]
- 21.Kuorilehto T, Pöyhönen M, Bloigu R, et al. Decreased bone mineral density and content in neurofibromatosis type 1: lowest local values are located in the load-carrying parts of the body. Osteoporos Int. 2005;16:928–396. doi: 10.1007/s00198-004-1801-4. [DOI] [PubMed] [Google Scholar]
- 22.Lammert M, Kappler M, Mautner VF, et al. Decreased bone mineral density in patients with neurofibromatosis 1. Osteoporos Int. 2005;16:1161–1166. doi: 10.1007/s00198-005-1940-2. [DOI] [PubMed] [Google Scholar]
- 23.Stevenson DA, Moyer-Mileur LJ, Murray M, et al. Bone mineral density in children and adolescents with neurofibromatosis type 1. J Pediatr. 2007;150:83–88. doi: 10.1016/j.jpeds.2006.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dulai S, Briody J, Schindeler A, et al. Decreased bone mineral density in neurofibromatosis type 1: results from a pediatric cohort. J Pediatr Orthop. 2007;27:472–475. doi: 10.1097/01.bpb.0000271310.87997.ae. [DOI] [PubMed] [Google Scholar]
- 25.Stevenson DA, Moyer-Mileur LJ, Carey JC, et al. Case-control study of the muscular compartments and osseous strength in neurofibromatosis type 1 using peripheral quantitative computed tomography. J Musculoskel Neuron Interact. 2005;5:145–149. [PubMed] [Google Scholar]
- 26.Caffarelli C, Gonnelli S, Tanzilli L, et al. Quantitative ultrasound and dual energy x-ray absorptiometry in children and adolescents with neurofibromatosis of type 1. J Clin Densitom. 2010;13:77–83. doi: 10.1016/j.jocd.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Tucker T, Schnabel C, Hartmann M, et al. Bone health and fracture rate in individuals with neurofibromatosis 1 (NF1) J Med Genet. 2009;46:259–265. doi: 10.1136/jmg.2008.061895. [DOI] [PubMed] [Google Scholar]
- 28.Seitz S, Schnabel C, Busse B, et al. High bone turnover and accumulation of osteoid in patients with neurofibromatosis 1. Osteoporos Int. 2010;21:119–127. doi: 10.1007/s00198-009-0933-y. [DOI] [PubMed] [Google Scholar]
- 29.Brunetti-Pierri N, Doty SB, Hicks J, et al. Generalized metabolic bone disease in Neurofibromatosis type 1. Mol Genet Metab. 2008;94:105–111. doi: 10.1016/j.ymgme.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharland M, Burch M, McKenna WM, et al. A clinical study of Noonan syndrome. Arch Dis Child. 1992;67:178–183. doi: 10.1136/adc.67.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nora JJ, Nora AH, Sinha AK, et al. The Ullich-Noonan syndrome (Turner phenotype) Am J Dis Child. 1974;127:48–55. doi: 10.1001/archpedi.1974.02110200050007. [DOI] [PubMed] [Google Scholar]
- 32.Allanson JE. Noonan syndrome. J Med Genet. 1987;24:9–13. doi: 10.1136/jmg.24.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee CK, Chang BS, Hong YM, et al. Spinal deformities in Noonan syndrome: a clinical review of sixty cases. J Bone Joint Surg Am. 2001;83-A:1495–1502. [PubMed] [Google Scholar]
- 34.Armour CM, Allanson JE. Further delineation of cardio-facio-cutaneous syndrome: clinical features of 38 individuals with proven mutations. J Med Genet. 2008;45:249–254. doi: 10.1136/jmg.2007.054460. [DOI] [PubMed] [Google Scholar]
- 35.White SM, Graham JM, Jr, Kerr B, et al. The adult phenotype in Costello syndrome. Am J Med Genet A. 2005;136:128–135. doi: 10.1002/ajmg.a.30747. [DOI] [PubMed] [Google Scholar]
- 36.Yassir WK, Grottkau BE, Goldberg MJ. Costello syndrome: orthopaedic manifestations and functional health. J Pediatr Orthop. 2003;23:94–98. [PubMed] [Google Scholar]
- 37.Eastell R, Blumsohn A. The value of biochemical markers of bone turnover in osteoporosis. J Rheum. 1997;24:1215–1217. [PubMed] [Google Scholar]
- 38.McLaren AM, Hordon LD, Bird HA, et al. Urinary excretion of pyridinium crosslinks of collagen in patients with osteoporosis and the effects of bone fracture. Ann Rheum Dis. 1992;51:648–651. doi: 10.1136/ard.51.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosen HN, Dresner-Pollak R, Moses AC, et al. Specificity of urinary excretion of cross-linked N-telopeptides of type I collagen as a marker of bone turnover. Calcif Tissue Int. 1994;54:26–29. doi: 10.1007/BF00316285. [DOI] [PubMed] [Google Scholar]
- 40.Delmas PD, Schlemmer A, Gineyts E, et al. Urinary excretion of pyridinoline crosslinks correlates with bone turnover measured on iliac crest biopsy in patients with vertebral osteoporosis. J Bone Miner Res. 1991;6:639–644. doi: 10.1002/jbmr.5650060615. [DOI] [PubMed] [Google Scholar]
- 41.Robins SP, Black D, Paterson CR, et al. Evaluation of urinary hydroxypyridinium crosslink measurements as resorption markers in metabolic bone diseases. Eur J Clin Invest. 1991;21:310–315. doi: 10.1111/j.1365-2362.1991.tb01375.x. [DOI] [PubMed] [Google Scholar]
- 42.Pasquali M, Still MJ, Vales T, et al. Abnormal formation of collagen cross-links in skin fibroblasts cultured from patients with Ehlers-Danlos Syndrome Type VI. Proc Assoc Am Physicians. 1997;109:33–41. [PubMed] [Google Scholar]
- 43.Pasquali M, Still MJ, Dembure PP, et al. Pyridinium cross-links in heritable disorders of collagen. Am J Hum Genet. 1995;57:1508–1510. [PMC free article] [PubMed] [Google Scholar]
- 44.Uebelhart D, Gineyts E, Chapuy MC, et al. Urinary excretion of pyridinium crosslinks: a new marker of bone resporption in metabolic bone disease. Bone Miner. 1990;8:87–96. doi: 10.1016/0169-6009(91)90143-n. [DOI] [PubMed] [Google Scholar]
- 45.Eyre DR, Koob TJ, Van Ness KP. Quantification of hydroxypyridinium crosslinks in collagen by high-performance liquid chromatography. Anal Biochem. 1984;137:380–388. doi: 10.1016/0003-2697(84)90101-5. [DOI] [PubMed] [Google Scholar]
- 46.Bettica P, Moro L, Robins SP, et al. Bone-resorption markers galactosyl hydroxylysine, pyridinium crosslinks, and hydroxyproline compared. Clin Chem. 1992;38:2131–2318. [PubMed] [Google Scholar]
- 47.Stevenson DA, Schwarz EL, Viskochil DH, et al. Evidence of increased bone resorption in neurofibromatosis type 1 using urinary pyridinium crosslink analysis. Pediatr Res. 2008;63:697–701. doi: 10.1203/PDR.0b013e31816fee45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heervä E, Alanne MH, Peltonen S, et al. Osteoclasts in neurofibromatosis type 1 display enhanced resorption capacity, aberrant morphology, and resistance to serum deprivation. Bone. 2010 doi: 10.1016/j.bone.2010.06.001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 49.Rockett HR, Breitenbach M, Frazier AL, et al. Validation of a youth/adolescent food frequency questionnaire. Prev Med. 1997;26:808–16. doi: 10.1006/pmed.1997.0200. [DOI] [PubMed] [Google Scholar]
- 50.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy cost of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 51.Tartaglia M, Kalidas K, Shaw A, et al. PTPN11 mutations in Noonan syndrome: molecular spectrum, genotype-phenotype correlation, and phenotypic heterogeneity. Am J Hum Genet. 2002;70:1555–63. doi: 10.1086/340847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bailey RL, Dodd KW, Goldman JA, et al. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr. 2010;140:817–22. doi: 10.3945/jn.109.118539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stevenson DA, Zhou H, Ashrafi S, et al. Double inactivation of NF1 in tibial pseudarthrosis. Am J Hum Genet. 2006;79:143–148. doi: 10.1086/504441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kolanczyk M, Kossler N, Kühnisch J, et al. Multiple roles for neurofibromin in skeletal development and growth. Hum Mol Genet. 2007;16:874–886. doi: 10.1093/hmg/ddm032. [DOI] [PubMed] [Google Scholar]
- 55.Branca F, Spagnoli A, Cianfarani S, et al. Urinary excretion of pyridinium crosslinks in short children treated with growth hormone. J Pediatr Endocrinol Metab. 2002;15:27–34. doi: 10.1515/jpem.2002.15.1.27. [DOI] [PubMed] [Google Scholar]


