Abstract
Objective
Low 25-hydroxyvitamin D (25OHD) concentrations have been associated with tumors and osteopenia/fractures in adults with neurofibromatosis type 1 (NF1). We report 25OHD concentrations in 109 children with NF1 and 218 age, sex, geographic location, and time-of-year matched controls.
Methods
Children with NF1 were recruited (n=109; 2–17yr), and clinical data and dual energy x-ray absorptiometry measurements obtained. 25OHD concentrations were measured in subjects and controls.
Results
More NF1 individuals (50%) were in the 25OHD insufficient/deficient range (<30ng/ml) compared to controls (36%) (p=0.0129). 25OHD concentrations were higher in individuals with neurofibromas after controlling for age (p=0.0393), and negatively associated with whole body subtotal bone mineral density (BMD) z-scores (p=0.0385).
Conclusions
More children with NF1 had 25OHD concentrations <30ng/ml, potentially due to increased pigmentation and/or decreased sunlight exposure. In contrast to adults, decreased 25OHD concentrations were not associated with neurofibromas, and there was not a positive association of 25OHD with BMD.
Keywords: neurofibromatosis, vitamin D, neurofibromas, bone dysplasia, bone health
Introduction
Neurofibromatosis type 1 (NF1) is a relatively common genetic disorder presenting in infancy and childhood with an incidence of approximately one in 3000.1 NF1 is a tumor suppressor gene,2 and the NF1 protein product, neurofibromin, is expressed in multiple tissue types resulting in clinical manifestations in numerous organ systems. Pigmentary findings such as café au lait macules and intertriginous freckling are typically the first clinical manifestation of NF1 observed. Tumors such as neurofibromas, optic gliomas, and malignant peripheral nerve sheath tumors are common and can develop during the pediatric period.3–6 Cutaneous neurofibromas are eventually observed in ≈90% of individuals with NF1 and can cause significant morbidity including pain and disfigurement.6 Although some pigmentary findings and tumors are almost universally observed in NF1, skeletal abnormalities are also common and include osteoporosis, increased fracture rates, scoliosis, long bone dysplasia and pseudarthrosis, and sphenoid wing dysplasia.7–21
Vitamin D status contributes significantly to bone homeostasis and deficient concentrations have been linked to cancer predisposition in the general population,22–24 both of which are abnormal in NF1. Low serum 25-hydroxyvitamin D (25OHD; a reliable measure of an individual’s vitamin D status) concentrations have been reported primarily in adults with NF1.21,25–27 One study by Lammert et al. reported that decreases in 25OHD concentrations were associated with an increased number of neurofibromas in NF1 adults in a patient population in Germany.26
Osteopenia and osteoporosis have been reported in cohorts of both adults and children with NF1.13–21,26 Vitamin D and calcium supplementation had conflicting results on bone mineral density improvement in two studies.25,27
We report serum 25OHD concentrations in a cohort of 109 children with NF1 and 218 seasonal age- and gender-matched controls, and correlate 25OHD concentrations in NF1 children with whole body subtotal bone mineral density (BMD) and the presence or absence of neurofibromas.
Subjects and Methods
Subjects
NF1 children were recruited from the University of Utah NF1 Clinic. Physical examinations and medical histories were obtained and children fulfilled the clinical diagnostic criteria for NF1.3,4 NF1 individuals were examined by one investigator (DS). Phenotypic information was recorded in a standardized fashion using an NF1 clinical data form. The presence of cutaneous neurofibromas was documented upon time of examination. Given that some individuals with Legius syndrome fulfill the diagnostic criteria for NF1 based on pigmentary findings, children were screened for SPRED1 mutations as previously reported,28 and individuals with SPRED1 mutations consistent with Legius syndrome were excluded.
Two healthy controls were selected for each individual with NF1 and were matched by age, sex, geographic location, and time of year. Samples for controls <7 years-of-age were collected at the time of elective surgical procedures. After review of the child’s medical chart to ensure they were healthy and not taking medications, parental permission was obtained. Blood was collected through an intravenous line which was inserted after induction of anesthesia. Samples were collected from healthy 7–17 year-old children who volunteered to provide blood for a research study after obtaining parental permission and assent from the child. A physical examination and medical history were also performed to ensure the child was healthy and not taking medications. Measurements of serum 25OHD were made using a Liaison analyzer (DiaSorin, Stillwater, MN) with DiaSorin reagents according to the manufacturer’s instructions. Serum 25OHD concentrations were categorized into 3 ranges: optimal range (≥30ng/ml), insufficient range (20–29ng/ml), and deficient range (<20ng/ml). For NF1 individuals, concurrent measurements of parathyroid hormone (PTH) and calcium were obtained at convenient times for subjects.
Dual energy x-ray absorptiometry (DXA) (Hologic QDR-4500A, Waltham, MA) scans were obtained on a subset of the NF1 individuals (N=77) in which 25OHD measurements were available under well-established standard protocols as previously described.17 Whole body scans were obtained with the patient supine, toes adducted (≈45°) and fixed with tape, and with arms placed at one’s sides with palms down. Subtotal body measurements were obtained by subtracting the head region from the whole body. Imaging was performed by a densitometry technologist certified by the International Society for Clinical Densitometry. Whole body subtotal BMD z-scores were generated on NF1 individuals by utilizing age- and sex-matched pediatric DXA reference data from a cohort of 293 healthy children living in the same geographic region available within the Center for Pediatric Nutrition Research at the University of Utah.
All studies with human subjects were approved by the Institutional Review Board of the University of Utah.
Data Analysis
Two separate pair-sample t-tests were performed to compare 25OHD concentrations between individuals with NF1 and their matched controls, and then a random effect model was fit by accounting for dependence within each matched NF1, control #1, and control #2 triad. Similarly, two separate McNemar’s tests for paired samples were used to compare percentages of vitamin D insufficient or deficient concentrations between NF1 individuals and the two matched controls. A conditional logistic regression model was used to test the low vitamin concentrations between NF1 individuals and the two matched controls.
General linear models were fitted to compare 25OHD concentrations in NF1 individuals with and without presence of cutaneous neurofibromas and other tumors, after controlling for any significant covariates (eg. sex, age) where applicable. A general linear model was also used to find association between 25OHD concentrations and whole body subtotal BMD z-scores.
Results
A total of 109 NF1 individuals (50 F; 59 M) were enrolled (mean age 9.3 years, SD 3.7, range 2–17 years). 25OHD concentrations, data from 2 time-of-year-, age-, and gender-matched controls for each NF1 individual were obtained (N=218) (Fig. 1). Mean 25OHD concentrations are shown in Table I.
Figure 1.
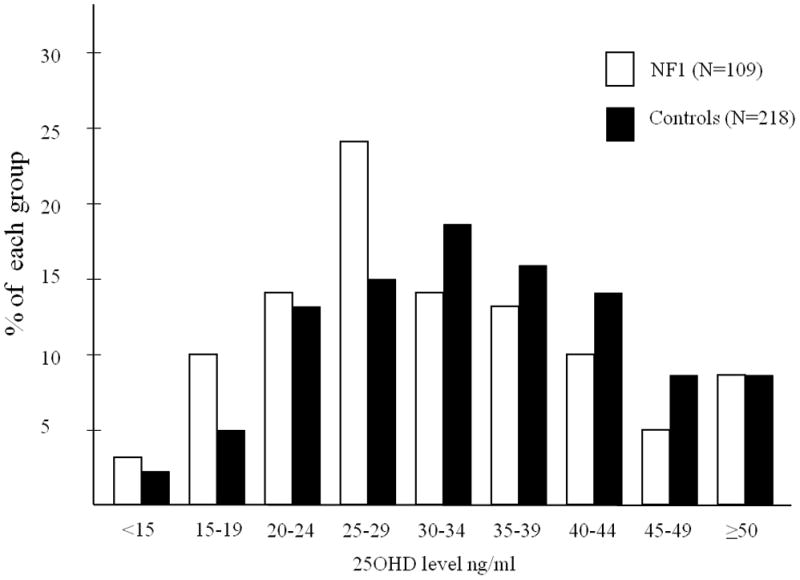
Bar graph of the percent of individuals with specific levels (intervals of 4 ng/ml) of 25OHD concentrations within each neurofibromatosis type 1 (NF1) and control group. Black bars represent control individuals and white bars represent individuals with NF1.
Table I.
25OHD Levels
| Group | Mean 25OHD (ng/ml) | SD | Range 25OHD (ng/ml) | N |
|---|---|---|---|---|
| NF1 | 31.76 | 11.07 | 8–64 | 109 |
| Control #1 | 34.65 | 12.21 | 10–92 | 109 |
| Control #2 | 32.94 | 10.76 | 8–76 | 109 |
| All Controls | 33.79 | 11.52 | 8–92 | 218 |
(NF1=neurofibromatosis type 1, 25OHD=25-hydroxyvitamin D, SD=standard deviation).
There was a moderate correlation of 0.2778 (p=0.0034) for 25OHD concentrations between the two control measures, but the NF1 measure did not correlate with either of the two control measures. Using two separate pair-sample t-tests, we demonstrated that mean differences between the two sets of controls and individuals with NF1 were 2.89 ng/ml (SE=1.60, 95% CI:−0.27 to 6.05, p=0.0729) and 1.17 ng/ml (SE=1.38, 95% CI: −1.56 to 3.91), respectively. Using a random effect model accounting for dependence within each matched NF1-control #1-control #2 triad, we noted that the mean difference between controls and individuals with NF1 was 2.03 ng/ml (SE=1.34, 95% CI: −0.61 to 4.68, p=0.131).
Concentrations of 25OHD within each respective range were as follows: optimal range (≥30ng/ml) [NF1 = 54/109 (49.5%), controls = 140/218 (64.2%)]; insufficient range (20−29ng/ml) [NF1 = 42/109 (38.5%), controls = 61/218 (30%)]; and deficient range (<20ng/ml) [NF1 = 13/109 (11.9%), controls = 17/218 (7.8%)] (Fig. 2). More NF1 individuals were in the insufficient or deficient range (50%) compared to controls (36%). Upon dichotomization of 25OHD concentrations into two groups (<30 and ≥30 ng/ml), there was a significant association between individuals with NF1 and lower 25OHD concentrations. Using a conditional logistic regression model, the children with NF1 were more likely to have 25OHD concentrations <30ng/ml (p=0.0129).
Figure 2.
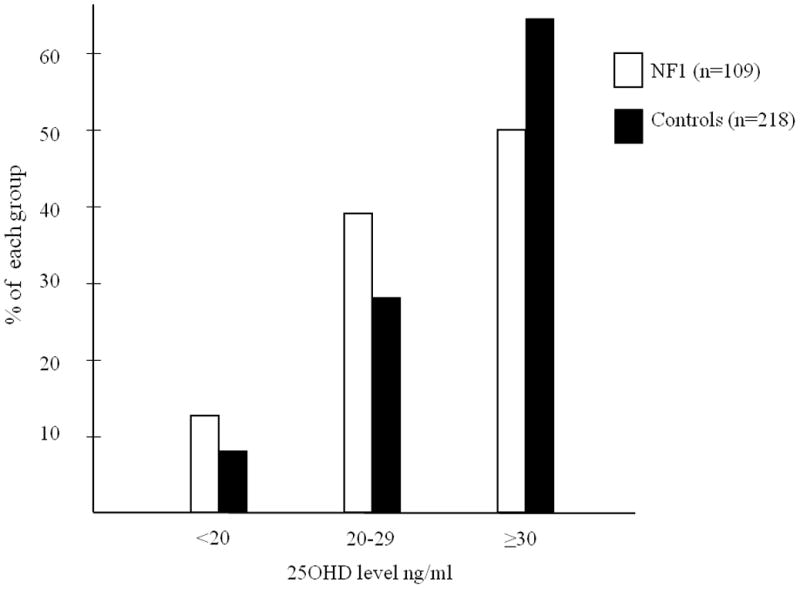
Bar graph of the percent of individuals with 25OHD concentrations within the deficient range (<20ng/ml), insufficient range (20–29ng/ml), and optimal range (≥30ng/ml) for each neurofibromatosis type 1 (NF1) and control group. Black bars represent control individuals and white bars represent individuals with NF1.
The mean serum PTH concentration for individuals with NF1 was 27.4pg/ml (range 7–80pg/ml) and only one individual had a concentration above the assay reference interval (15–75pg/ml). Mean serum calcium concentrations were 9.6mg/dl (range 8.4–10.8mg/dl) and only 4 individuals with NF1 had a concentration below the assay reference interval (8.8–10.1mg/dl).
Whole body subtotal BMD z-scores were decreased in NF1 individuals (mean z-score -1) consistent with the decreased BMD values previously reported in this overlapping cohort.17 General linear model analysis of whole body subtotal BMD z-scores with the covariates 25OHD, sex, age, height, weight and Tanner stage, showed a moderately significant association with 25OHD (p=0.0385), after adjusting for strongly significant associations with age (p<0.0001), and weight (p<0.0001) (Table II; Fig. 3).
Table II.
25OHD and Whole Body Subtotal BMD in Children with NF1
| Parameter | Estimate | Standard Error | t value | p-value |
|---|---|---|---|---|
| 25OHD | −0.0226 | 0.0107 | −2.11 | 0.0385 |
| Age | −0.3153 | 0.0624 | −5.05 | <0.0001 |
| Weight | 0.0702 | 0.0141 | 4.97 | <0.0001 |
R-square 0.311; (NF1=neurofibromatosis type 1, 25OHD=25-hydroxyvitamin D)
Figure 3.
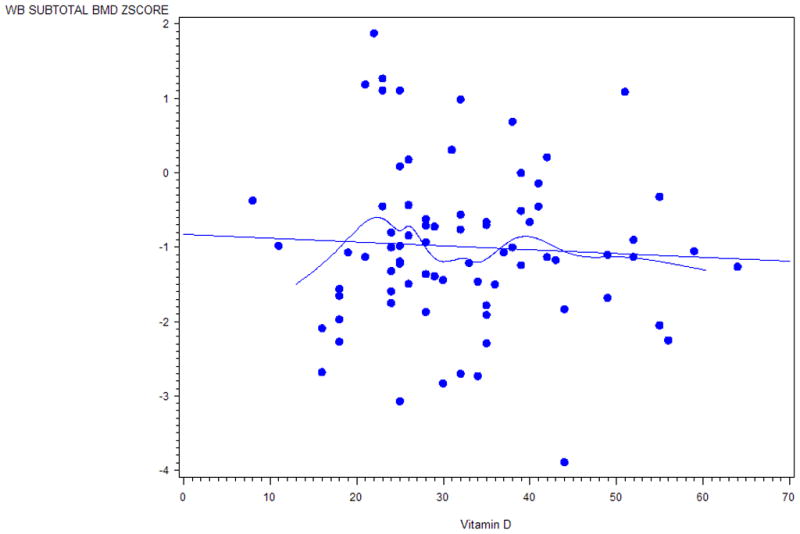
Scatter plot with a fitted line and a smooth cubic spline that describes a general trend of the association of 25-hydroxyvitamin D (25OHD) concentrations and whole body subtotal bone mineral density (BMD) z-scores in individuals with neurofibromatosis type 1 (NF1). Plot is based on a univariate analysis and not adjusted for other covariates.
Concentrations of 25OHD were slightly higher in NF1 children with cutaneous neurofibromas (mean 25OHD = 34.72ng/ml; 95% CI 31.48–37.96) compared to NF1 children without cutaneous neurofibromas (mean 25OHD = 29.88ng/ml; 95% CI 26.86-32.91) after controlling for age (p=0.0393). However, mean 25OHD concentrations were within the optimal range for both groups. Optic gliomas were not associated with 25OHD concentrations (p=0.6700).
Discussion
Children and adolescents with NF1 (50%) from the Intermountain west region of the United States of America (USA) more often have 25OHD concentrations <30ng/ml than do children and adolescents from the same region without NF1 (36%) when matched for time of year sample collection, age, and sex. To our knowledge 4 other studies have specifically assessed 25OHD concentrations in NF1 individuals, but 3 of the 4 studies were performed primarily in adults with NF1 in the German population (latitude of approximately 53o north),21,25,26 while one study was performed in a small cohort in Texas, USA27 (latitude of approximately 29o north). Concentrations of 25OHD in the adult cohorts in Germany were significantly lower in NF1 individuals [Lammert et al.: NF1 (mean 25OHD = 15.7ng/ml; N=55), controls (mean 25OHD = 35.5ng/ml; N=58);26 Seitz et al.: (range 5–23ng/ml; N=14) compared to controls (range 13–46ng/ml);25 Tucker et al.: NF1 (56% with low 25OHD concentrations; N=72)21]. Vitamin D deficiency is common in Germany and fortification of food items with vitamin D is largely prohibited.29,30 The 25OHD concentrations of the adults with NF1 in the German population are much lower than the mean levels (32ng/ml) measured in our pediatric population in the Intermountain region of the USA. We postulate that differences in dietary supplementation within food sources in Germany compared to the US contribute to the observed differences. In addition, neurofibromas are age-related in NF1 and hence adults have a higher number of cutaneous tumors. The increasing amount of neurofibromas may impact clothing choice and decrease outside activity levels, subsequently decreasing sunlight exposure. However, Brunetti-Pierri et al.27 measured 25OHD concentrations 16 individuals with NF1 from the state of Texas, USA (ages 6–38 years) and found a mean 25OHD concentration of 20.6±4.5ng/ml, and approximately a third had 25OHD concentrations >25ng/ml. The 25OHD concentrations were only measured in NF1 individuals who had lumbar BMD z-scores <−2.1, and therefore represent a selected small subset of NF1 individuals, which potentially explains the differences in 25OHD concentrations compared to our cohort. As reported by Brunetti-Pierri et al.,27 the mean 25OHD concentration in the cohort with osteopenia was still higher than the NF1 adults reported in Germany providing further support of the effects of dietary supplementation within food sources on 25OHD concentrations in NF1.
It is interesting to note that even though a greater proportion of children with NF1 had 25OHD concentrations <30ng/ml, a large number of controls (36%) also had 25OHD concentrations <30ng/ml raising the controversial question of how one determines appropriate ranges for 25OHD concentrations.31 We elected to utilize categorical ranges described as optimal (≥30ng/ml), insufficient (20–29ng/ml), and deficient (<20ng/ml) based upon several factors. One reason was that our laboratory used these reference intervals at the time the majority of individuals with NF1 had the 25OHD concentrations measured. Additionally, Priemel et al. showed that osteomalacia was present in ≈25% of iliac crest biopsies from 675 individuals, but no pathologic accumulation of osteoid was found in any individual with a 25OHD concentration >30ng/ml, suggesting that such a concentration is optimal to prevent osteomalacia.30 Although the definition of optimal 25OHD concentrations is controversial, many experts suggest that concentrations greater than 30ng/ml are optimal.30,32,33
Decreased BMD in children and adults and increased fracture rates in adults have been reported in NF1,13–21 but the underlying cause is not well understood and likely multifactorial. Mineralization defects have been shown in iliac crest biopsies in NF1 adults in Germany25 and markers of bone resorption have been reported to be increased in NF1 individuals.21,34 In Nf1+/− mice, osteoclastic activity is increased, which was also observed in a few individuals with NF1.25 It is possible that increased bone resorption and decreased BMD in individuals with NF1 is largely due to osteoclastic hyperactivity from activation of the Ras-MAPK pathway, and that 25OHD plays only a minimal role until 25OHD concentrations become severely deficient. There are a multitude of other factors that contribute to BMD in humans including sex steroid levels, and the forces placed upon bone from physical activity. Therefore, teasing out the effect of a single variable is often difficult.
The effects of 25OHD on BMD in NF1 individuals are still controversial. Of the 16 individuals reported by Brunetti-Pierri et al.,27 8 had PTH concentrations that were increased compared to age-matched controls and 6 of the 8 individuals had 25OHD concentrations between 5–20ng/ml. These 8 individuals were treated with 400 IU per day of vitamin D and 1000mg of elemental calcium, but 25OHD concentrations and BMD did not significantly improve after 24 months of therapy. Seitz et al.25 performed iliac crest biopsies on 14 adults with NF1 and showed increased osteoid compared to controls from autopsy specimens. Seitz et al.25 then treated 4 individuals with NF1 who had BMD T-scores −3 SD at the spine and −2 SD at the hip with 1000 IU of cholecalciferol and 1000mg calcium for 1 year and showed normalization of 25OHD levels and statistically significant improvement in BMD in the spine but not the hip. In the pediatric cohort in this report, there was not a positive association of 25OHD concentrations with subtotal body BMD z-scores. However, based on review of the smooth cubic spline on the scatter plot (Fig.3), there is a general trend of a positive association of 25-hydroxyvitamin D (25OHD) concentrations with whole body subtotal bone mineral density (BMD) z-scores in the small subset of individuals with 25OHD concentrations within the deficient range. It is possible that vitamin D deficiency over a prolonged time is needed in the NF1 population before effects on BMD are observed. In addition, 25OHD concentrations may be decreased in NF1 children, but not to the degree necessary to develop measurable and clinically significant BMD differences. A larger number of NF1 individuals, especially those individuals with 25OHD concentrations in the deficient range (<20ng/ml), may be needed to show significant differences.
Lammert et al.26 also showed that there was a significant inverse correlation between 25OHD concentrations and the number of dermal neurofibromas [# of neurofibromas/25OHD geometric mean respectively: 10–99 neurofibromas/19.5ng/ml; 100–999 neurofibromas/13.1ng/ml; ≥1000 neurofibromas/8.2ng/ml]. Given that neurofibroma development is age related, it is difficult to assess the effects of 25OHD on neurofibroma number in a pediatric cohort. Therefore, we elected to assess the presence or absence of cutaneous neurofibromas and control for age. Surprisingly, in this cohort, 25OHD concentrations were higher in children with cutaneous neurofibromas, but the mean 25OHD concentrations in both groups were essentially in the optimal range, and we question the clinical significance of the difference between the 25OHD means in the two groups.
Conclusion
A greater proportion of children with NF1 have vitamin D deficiency or insufficiency (<30ng/ml) than in the general population. Potential explanations for the increased number of children with NF1 who have 25OHD concentrations <30ng/ml could include abnormalities in calcium absorption, or decreased sun exposure from increased pigmentation from café au lait macules, differences in clothing selection due to cutaneous manifestations, or decreases in outdoor activities from associatied comorbidities of NF1 (e.g. scoliosis, visual impairment, pseudarthrosis, poor coordination). In contrast to other studies in adults with NF1, in this pediatric cohort lower 25OHD concentrations were not observed in those with neurofibromas as compared to those without neurofibromas after adjusting for age, and there was not a positive association of 25OHD with whole body subtotal BMD. We suggest that the degree of 25OHD deficiency observed in previous studies of adults with NF1 compared to this pediatric NF1 cohort are likely due to environmental factors (e.g. geographic location, governmental regulation of dietary supplementation) and secondary effects of the age-related manifestations of the cutaneous lesions (e.g. decreased sunlight exposure from clothing choice and outdoor activities due to increasing tumor development) rather than a direct effect of increased Ras-MAPK signaling caused by NF1 mutations. The proapoptotic effects of vitamin D are attractive targets for a cancer predisposition syndrome such as NF1, and topical vitamin D3 analogues have been reported to lighten café au lait macules in NF1 and inhibit cell growth from neurofibromas.35–37 Further studies are needed to assess the clinical consequences of specific 25OHD concentrations in NF1, and to better understand the differences observed in the pediatric and adult NF1 population.
Acknowledgments
We thank the study participants for their time and involvement. We thank Jeanne Siebert, Janice Davis, Susan Geyer, and Hillarie Slater for technical assistance and coordination. We thank Dr. Zulf Mughal, Dr. Linlea Armstrong, Dr. Elizabeth Schorry, and Dr. Jan Friedman for their discussion and insight.
The study was supported in part by the following: The Shriners Research Foundation; grant #M01-RR00064 from the National Center for Research Resources; research grants #K23 NS052500 from the National Institute of Neurological Disorders and Stroke; Doris Duke Charitable Foundation; the Children’s Health Research Center, Clinical Genetics Research Program, and Center for Pediatric Nutrition Research at the University of Utah, and the Primary Children’s Research Foundation
References
- 1.Rasmussen SA, Friedman JM. NF1 gene and neurofibromatosis 1. Am J Epidemiol. 2000;151:33–40. doi: 10.1093/oxfordjournals.aje.a010118. [DOI] [PubMed] [Google Scholar]
- 2.Viskochil D. Genetics of neurofibromatosis 1 and the NF1 gene. J Child Neurol. 2002;17:562–570. doi: 10.1177/088307380201700804. [DOI] [PubMed] [Google Scholar]
- 3.Stumpf DA, Alksne JF, Annegers JF, Brown SS, Conneally PM, Housman D, Leppert MF, Miller JP, Moss ML, Pileggi AJ, Rapin I, Strohman RC, Swanson LW, Zimmerman A. Neurofibromatosis. Conference statement. National Institutes of Health consensus development conference. Arch Neurol. 1988;45:575–578. [PubMed] [Google Scholar]
- 4.Gutmann DH, Aylsworth A, Carey JC, Korf B, Marks J, Pyeritz RE, Rubenstein A, Viskochil D. The diagnostic evaluation and multidisciplinary management of neurofibromatosis 1 and neurofibromatosis 2. JAMA. 1997;278:51–57. [PubMed] [Google Scholar]
- 5.Friedman JM, Birch PH. Type 1 Neurofibromatosis: A descriptive analysis of the disorder in 1728 patients. Am J Med Genet. 1997;70:138–143. doi: 10.1002/(sici)1096-8628(19970516)70:2<138::aid-ajmg7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 6.Rosser T, Packer RJ. Neurofibromas in children with neurofibromatosis 1. J Child Neurol. 2002;17:585–91. doi: 10.1177/088307380201700808. [DOI] [PubMed] [Google Scholar]
- 7.Crawford AH, Schorry EK. Neurofibromatosis in children; the role of the orthopaedist. J Am Academy of Orthopaedic Surgeons. 1999;7:217–230. doi: 10.5435/00124635-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Stevenson DA, Birch PH, Friedman JM, Viskochil DH, Balestrazzi P, Buske A, Korf BR, Niimura M, Pivnick E, Schorry E, Short P, Tenconi R, Tonsgard J, Carey JC. Descriptive analysis of tibial pseudarthrosis in patients with neurofibromatosis 1. Am J Med Genet. 1999;84:413–419. doi: 10.1002/(sici)1096-8628(19990611)84:5<413::aid-ajmg5>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert A, Brockman R. Congenital pseudarthrosis of the tibia. Long-term followup of 29 cases treated by microvascular bone transfer. Clin Orthop Relat Res. 1995;314:37–44. [PubMed] [Google Scholar]
- 10.Morrissy RT, Riseborough EJ, Hall JE. Congenital pseudarthrosis of the tibia. J Bone Joint Surg Br. 1981;63-B:367–375. doi: 10.1302/0301-620X.63B3.6790551. [DOI] [PubMed] [Google Scholar]
- 11.Sofield HA. Congenital pseudarthrosis of the tibia. Clin Orthop Relat Res. 1971;76:33–42. doi: 10.1097/00003086-197105000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Vitale MG, Guha A, Skaggs DL. Orthopaedic manifestations of neurofibromatosis in children: an update. Clin Orthop Relat Res. 2002;401:107–118. doi: 10.1097/00003086-200208000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Illes T, Halmai V, de Jonge T, Dubousset J. Decreased bone mineral density in neurofibromatosis-1 patients with spinal deformities. Osteoporos Int. 2001;12:823–827. doi: 10.1007/s001980170032. [DOI] [PubMed] [Google Scholar]
- 14.Yilmaz K, Ozmen M, Bora Goksan S, Eskiyurt N. Bone mineral density in children with neurofibromatosis 1. Acta Paediatr. 2007;96:1220–1222. doi: 10.1111/j.1651-2227.2007.00401.x. [DOI] [PubMed] [Google Scholar]
- 15.Kuorilehto T, Pöyhönen M, Bloigu R, Heikkinen J, Väänänen K, Peltonen J. Decreased bone mineral density and content in neurofibromatosis type 1: lowest local values are located in the load-carrying parts of the body. Osteoporos Int. 2005;16:928–936. doi: 10.1007/s00198-004-1801-4. [DOI] [PubMed] [Google Scholar]
- 16.Lammert M, Kappler M, Mautner VF, Lammert K, Störkel S, Friedman JM, Atkins D. Decreased bone mineral density in patients with neurofibromatosis 1. Osteoporos Int. 2005;16:1161–1166. doi: 10.1007/s00198-005-1940-2. [DOI] [PubMed] [Google Scholar]
- 17.Stevenson DA, Moyer-Mileur LJ, Murray M, Slater H, Sheng X, Carey JC, Dube B, Viskochil DH. Bone mineral density in children and adolescents with neurofibromatosis type 1. J Pediatr. 2007;150:83–88. doi: 10.1016/j.jpeds.2006.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dulai S, Briody J, Schindeler A, North KN, Cowell CT, Little DG. Decreased bone mineral density in neurofibromatosis type 1: results from a pediatric cohort. J Pediatr Orthop. 2007;27:472–475. doi: 10.1097/01.bpb.0000271310.87997.ae. [DOI] [PubMed] [Google Scholar]
- 19.Stevenson DA, Moyer-Mileur LJ, Carey JC, Maxwell S, Quick JL, Hoff CJ, Viskochil DH. Case-control study of the muscular compartments and osseous strength in neurofibromatosis type 1 using peripheral quantitative computed tomography. J Musculoskel Neuron Interact. 2005;5:145–149. [PubMed] [Google Scholar]
- 20.Caffarelli C, Gonnelli S, Tanzilli L, Vivarelli R, Tamburello S, Balestri P, Nuti R. Quantitative ultrasound and dual energy x-ray absorptiometry in children and adolescents with neurofibromatosis of type 1. J Clin Densitom. 2010;13:77–83. doi: 10.1016/j.jocd.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Tucker T, Schnabel C, Hartmann M, Freidrich RE, Frieling I, Kruse HP, Mautner VF, Friedman JM. Bone health and fracture rate in individuals with neurofibromatosis 1 (NF1) J Med Genet. 2009;46:259–65. doi: 10.1136/jmg.2008.061895. [DOI] [PubMed] [Google Scholar]
- 22.Crew KD, Gammon MD, Steck SE, Hershman DL, Cremers S, Dworakowski E, Shane E, Terry MB, Desai M, Teitelbaum SL, Neugut AI, Santella RM. Association between plasma 25-hydroxyvitamin D and breast cancer risk. Cancer Prev Res. 2009;2:595–604. doi: 10.1158/1940-6207.CAPR-08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali MM, Vaidya V. Vitamin D and cancer. J Cancer Res Ther. 2007;3:225–30. doi: 10.4103/0973-1482.38998. [DOI] [PubMed] [Google Scholar]
- 24.Khazai N, Judd SE, Tangpricha V. Calcium and vitamin D: skeletal and extraskeletal health. Curr Rheumatol Rep. 2008;10:110–7. doi: 10.1007/s11926-008-0020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seitz S, Schnabel C, Busse B, Schmidt HU, Beil FT, Friedrich RE, Schinke T, Mautner VF, Amling M. High bone turnover and accumulation of osteoid in patients with neurofibromatosis 1. Osteoporos Int. 2010;21:119–27. doi: 10.1007/s00198-009-0933-y. [DOI] [PubMed] [Google Scholar]
- 26.Lammert M, Friedman JM, Roth HJ, Friedrich RE, Kluwe L, Atkins D, Schooler T, Mautner VF. Vitamin D deficiency associated with number of neurofibromas in neurofibromatosis 1. J Med Genet. 2006;43:810–3. doi: 10.1136/jmg.2006.041095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brunetti-Pierri N, Doty SB, Hicks J, Phan K, Mendoza-Londono R, Blazo M, Tran A, Carter S, Lewis RA, Plon SE, Phillips WA, O’Brian Smith E, Ellis KJ, Lee B. Generalized metabolic bone disease in Neurofibromatosis type 1. Mol Genet Metab. 2008;94:105–111. doi: 10.1016/j.ymgme.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muram-Zborovski TM, Stevenson DA, Viskochil DH, Dries DC, Willson AR, Mao R. SPRED1 mutations in a neurofibromatosis clinic. J Child Neurol. 2010 doi: 10.1177/0883073809359540. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hintzpeter B, Scheidt-Nave C, Müller MJ, Schenk L, Mensink BG. Higher prevalence of vitamin D deficiency is associated with immigrant background among children and adolescents in Germany. J Nutr. 2008;138:1428–1490. doi: 10.1093/jn/138.8.1482. [DOI] [PubMed] [Google Scholar]
- 30.Priemel M, von Domarus C, Klatte TO, Kessler S, Schlie J, Meier S, Proksch N, Pastor F, Netter C, Streichert T, Püschel K, Amling M. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res. 2010;25:305–12. doi: 10.1359/jbmr.090728. [DOI] [PubMed] [Google Scholar]
- 31.Heaney RP. Bone health. Am J Clin Nutr. 2007;85:300S–303S. doi: 10.1093/ajcn/85.1.300S. [DOI] [PubMed] [Google Scholar]
- 32.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 33.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 34.Stevenson DA, Schwarz EL, Viskochil DH, Moyer-Mileur LJ, Murray M, Firth SD, D’Astous JL, Carey JC, Pasquali M. Evidence of increased bone resorption in neurofibromatosis type 1 using urinary pyridinium crosslink analysis. Pediatr Res. 2008;63:697–701. doi: 10.1203/PDR.0b013e31816fee45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang FC, Chen S, Robling AG, Yu X, Nebesio TD, Yan J, Morgan T, Li X, Yuan J, Hock J, Ingram DA, Clapp DW. Hyperactivation of p21ras and PI3K cooperate to alter murine and human neurofibromatosis type 1-haploinsufficient osteoclast functions. J Clin Invest. 2006;116:2880–2891. doi: 10.1172/JCI29092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakayama J, Kiryu H, Urabe K, Matsuo S, Shibata S, Koga T, Furue M. Vitamin D3 analogues improve café au lait spots in patients with von Recklinghausen’s disease: experimental and clinical studies. Eur J Dermatol. 1999;9:202–6. [PubMed] [Google Scholar]
- 37.Nakayama J, Kokuba H, Terao H, Matsuo S, Ikebe H, Nakagawa H, Hor Y. Inhibitory effects of various vitamin D3 analogues on the growth of cells isolated from neurofibromas in patients with von Recklinghausen neurofibromatosis 1. Eur J Dermatol. 1997;7:169–172. [Google Scholar]
- 38.Yoshida Y, Sato N, Furumura M, Nakayama J. Treatment of pigmented lesions of neurofibromatosis 1 with intense pulsed-radio frequency in combination with topical application of vitamin D3 ointment. J Dermatol. 2007;34:227–30. doi: 10.1111/j.1346-8138.2007.00258.x. [DOI] [PubMed] [Google Scholar]


