Abstract
Protein AMPylation is an emerging posttranslational modification, which plays key roles in bacterial pathogenesis and cell biology. Enzymes with AMPylation activity, referred to as AMPylators, have been identified in several bacterial pathogens and eukaryotes. To facilitate the study of this unique modification, we developed an alkynyl chemical reporter for detection and identification of protein AMPylation substrates. Covalent functionalization of AMPylation substrates with the alkynyl reporter in lieu of adenylyl 5′-monophosphate (AMP) allows their subsequent bioorthogonal ligation with azide-fluorescent dyes or affinity enrichment tags. We show that this chemical reporter is transferred by a range of AMPylators onto their cognate protein substrates and allows rapid detection and identification of AMPylated substrates.
Protein AMPylation refers to the covalent modification of protein side chain hydroxyl groups with adenylyl 5′-monophosphate (AMP) through a phosphodi-ester bond. This posttranslational modification is installed by the enzymatic transfer of AMP from adenosine-5′-triphosphate (ATP) to the substrate hydroxyl group. This catalytic activity was initially described for E. coli glutamine synthetase (GS) adenylyl transferase, which tightly regulates GS activity.1,2 Additional proteins with AMPylation activity (AMPylators) have been identified that contain either the filamentation induced by cAMP (fic) domain or the adenylyl transferase (ATase) domain, which confer AMPylation activity.3,4 Many of these characterized AMPylators serve as bacterial virulence factors that are secreted into the mammalian host cell during infection. There, they AMPylate mammalian host proteins to alter their function for the benefit of the pathogen. In particular, secreted bacterial AMPylators, such as VopS (Vibrio parahaemolyticus), IbpA (Legionella pneumophila), and DrrA (Legionella pneumophila) have been shown to target mammalian small GTPases, like RhoA, Rac1, Cdc42, and Rab1.5–7 Another bacterial effector protein has been identified that selectively deAMPylates a GTPase, indicating the dynamic nature of this modification.8,9 The AMPylation of small GTPases, on threonine or tyrosine residues, interferes with their proper function, either by sterically blocking the interaction with downstream signaling components or GTPase activating proteins (GAPs). Interestingly, fic and ATase domains are not limited to bacterial effector proteins, but have been identified in archaea and eukaryotes as well. Most eukaryotic genomes appear to contain a fic domain protein. AMPylation activity has been observed for the human protein HYPE and the Drosophila protein dFic.4,6 The widespread presence of these domains suggests a ubiquitous role for protein AMPylation as a regulated and reversible posttranslational modification. While radioactive ATP, targeted mass spectrometry, and specific antibodies, can be used to detect AMPylated substrates, more general and efficient analytical tools are still needed for the unbiased identification of new AMPylated substrates and the analysis of their regulation.10,11 We therefore developed an alkynyl chemical reporter for bioorthogonal detection, enrichment and identification of AMPylated proteins (Scheme 1).
SCHEME 1.
Detection and identification of AMPylated substrates with N6-propargyl adenosine-5′-triphosphate (N6pATP)
N6pATP – N6-propargyl adenosine-5′-triphosphate; N6pAMP – N6-propargyl adenosine-5′-monophosphat; PPi – pyrophosphate; CuAAC – Cu(I)-catalyzed azide-alkyne cycloaddition; Tag – rhodamine fluorescence dye or cleavable biotin enrichment tag.18,19
Alkynyl chemical reporters allow Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC) of labeled substrates with azide-functionalized detection and enrichment reagents. This technology has facilitated the analysis of various posttranslational modifications and nucleic acid biogenesis.12 Recent structural studies of AMPylators and previous studies of fluorescent AMP analogs, suggested that a modification of the N6 position of the adenine ring could be tolerated.13–17 Thus, we synthesized the ATP analog N6-propargyl adenosine-5′-triphosphate (N6pATP) as a potential chemical reporter for AMPylation (Supp. Scheme 1).
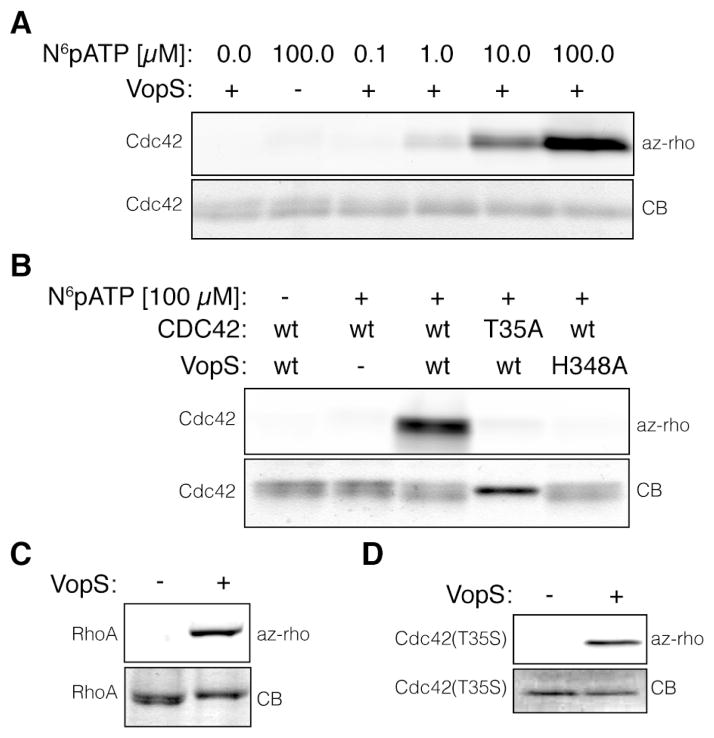
To assess the activity of N6pATP as a possible chemical reporter for AMPylation, we used a well-established in vitro system, based on the recently identified AMPylator VopS and its cognate mammalian target Cdc42.20 Cdc42(Q61L), subsequently referred to as Cdc42, was incubated with VopS in the presence of increasing concentrations of N6pATP under previously reported in vitro AMPylation conditions.21 N6pAMP transfer was analyzed by CuAAC with azido-rhodamine (az-rho) dye and in-gel fluorescence scanning.19 Increasing concentrations of N6pATP yielded dose-dependent increase in fluorescence labeling of Cdc42 (Fig. 1A). The chemical reporter proved to be transferred only in the presence of VopS and appeared to be highly selective for the native substrate Cdc42, as judged by the lack of concomitant BSA labeling (Supp. Fig. 1). To investigate the selectivity of N6pATP, we made use of substrate and AMPylator mutants. When VopS was incubated with the T35A mutant of Cdc42, in which the target threonine residue of VopS was mutated to an alanine, no transfer was observed (Fig. 1B).20 In addition, a catalytic inactive mutant of VopS, H348A, failed to transfer the chemical reporter (Fig. 1B).20 The dependence on this previously described catalytic histidine suggests that N6pAMP is transferred by the same catalytic mechanism as the native AMP group. These data emphasize the distinct selectivity of N6pATP, which was further demonstrated by competitive inhibition with ATP (Supp. Fig. 2). N6pATP appears to be a general cofactor for AMPylation, since it was also utilized by VopS to modify its alternative substrates RhoA (Fig. 1C) and Rac1 (Supp. Fig. 3). Moreover, it served as a chemical reporter for another fic domain AMPylator, IbpA (Fic2, Supp. Fig. 4). Like VopS, Fic2 modifies Cdc42, however, it transfers AMP onto tyrosine residue Y32.22 It should be noted that labeling of Cdc42(T35S) by VopS was also observed (Fig. 1D), supporting the notion that AMPylation may occur on serine residues. We also tested the ATase domain AMPylator DrrA for its ability to utilize N6pATP. Indeed, DrrA accepted N6pATP as cofactor and labeled the reported protein substrate Rab1 (Supp. Fig. 5).7 These experiments demonstrate the versatile nature of N6pATP as a chemical reporter for all known AMPylator families, namely fic domain and ATase domain AMPylators. N6pATP labeling appears to be independent of the target protein substrate and could also allow the identification of serine-modified AMPylation substrates.
Figure 1.
In vitro analysis of VopS activity, using N6-propargyl adenosine-5′-triphosphate (N6pATP) as a chemical reporter, by click-chemistry and in-gel fluorescence scanning. All AMPylation reactions were carried out in 15 μl total volume for 1 h at 30°C. A) Cdc42 (0.5 μg) was incubated with VopS (1 ng) and increasing concentrations of N6pATP. B) Cdc42 (0.5 μg) or Cdc42(T35A) (0.5 μg) was incubated with VopS (1 ng) or VopS(H348A) (1 ng) and N6pATP. C) RhoA (0.5 μg) was incubated with VopS (10 ng). D) Cdc42(T35S) (0.5 μg) was incubated with VopS (10 ng). az-rho - azido-rhodamine fluorescence; CB - coomassie blue.
We next analyzed the ability of VopS, Fic2, and DrrA to modify their substrates with N6pATP in mammalian cell lysates. It has been reported that addition of purified VopS or Fic2 to HeLa cell lysates in the presence of 32P-α-ATP results in the labeling of one distinct band in the molecular weight range of small GTPases, as assessed by autoradiography.6,23 We incubated purified VopS, Fic2, and DrrA with Triton X-100 lysed HeLa cell lysates and N6pATP (100 μM). As previously observed for 32P-α-ATP labeled cell lysates, VopS and Fic2 labeled one distinct protein or protein population at the corresponding molecular weight of small GTPases (Fig. 2A,B). Addition of DrrA also resulted in the modification of one distinct protein or protein population at ~21 kDa (Fig. 2C), although more recombinant DrrA was needed to observe similar levels of labeling. In the absence of recombinant enzyme, the reporter did not significantly label the cell lysate. All three enzymes displayed auto-AMPylation activity as evidenced by a specific fluorescent signal at the corresponding molecular weight (Fig. 2A–C). These results corroborate previous findings, underscoring the distinctive selectivity of these effector proteins for small host GTPases.6,7,23
Figure 2.
Detection of endogenous AMPylation substrates in cell lysates and proteomic substrate identification. AMPylation reactions (A–C) were carried out in 15 μl for 1 h at 30°C A) VopS (100 ng) was added to HeLa cell lysate (10 mg) in the presence of N6pATP (100 μM). B) Fic2 (300 ng) was added to HeLa cell lysate (10 mg) in the presence of N6pATP (100 μM). C) DrrA (4 μg) was added to HeLa cell lysate (10 mg) in the presence of N6pATP (100 μM). D) Proteomic analysis of VopS substrates in HeLa cell lysate revealed two protein IDs above background, VopS and Cdc42. E) Identified Cdc42 peptides. # TS – number of total spectra, # UP – number of unique peptides, XCorr – SEQUEST Xcorr score, DCn – SEQUEST ΔCn score, az-rho - azido-rhodamine fluorescence; CB, coomassie blue; asterisk indicates auto-AMPylation and recombinant AMPylator.
To explore the utility of N6pATP for the unbiased identification of AMPylated substrates, we incubated HeLa cell lysate (1 mg) with N6pATP (100 μM) in the presence or absence of VopS (88μg). The bulk of the samples was then reacted with a cleavable biotin enrichment tag (ortho-hydroxy-azidoethoxy-azo-biotin) and incubated with Streptavidin beads for affinity capture of labeled proteins.18 Captured proteins were digested on beads and the resulting peptides were collected and subjected to protein identification by tandem mass spectrometry. For data analysis, all protein hits of the negative control were subtracted from the VopS containing sample, which revealed two final protein identifications: VopS and Cdc42, with VopS peptides representing the majority of the identified peptides (Fig. 2D,E). The dominant VopS fraction correlates well with the observed fluorescence profiling of the same samples (Supp. Fig. 6). This data demonstrates that N6pATP can be used to identify substrates of individual AMPylators in complex cellular protein samples and confirms Cdc42 as an endogenous VopS target.
In summary, we present an efficient chemical reporter for protein AMPylation, which is utilized by all known classes of protein AMPylators and has minimal non-specific chemical reactivity in cell lysates. N6pATP enables the unbiased detection and proteomic identification of specific AMPylator substrates in complex protein mixtures and should be a powerful chemical tool for the emerging field of protein AMPylation.
Supplementary Material
Acknowledgments
We thank Professor Craig Roy (Yale) for DrrA and Rab1a containing plasmids, Professor Neal Alto for the RhoA construct, as well as Professor Jack Dixon (UCSD) for the Fic2 construct. We thank Paul Dossa, Anuradha Raghavan, and Emma Taylor-Salmon in the Hang Laboratory for technical support. K.O. and P.L. are supported by National Institutes of Health-AID Grants R01-AI056404 and R01-AI087808, and Grant I-1561 from the Welch Research Foundation. K.O. is a Burroughs Wellcome Investigator in Pathogenesis of Infectious Disease and a W.W. Caruth, Jr. Biomedical Scholar. H.C.H acknowledges Irma T. Hirschl/Monique Weill-Caulier Trust, LERNER Trust, and NIH/NIGMS (1R01GM087544).
Footnotes
Supporting Information. Experimental procedures, chemical characterization, supporting figures. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Kingdon HS, Shapiro BM, Stadtman ER. Proc Natl Acad Sci USA. 1967;58:1703–1710. doi: 10.1073/pnas.58.4.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shapiro BM, Stadtman ER. Annu Rev Microbiol. 1970;24:501–524. doi: 10.1146/annurev.mi.24.100170.002441. [DOI] [PubMed] [Google Scholar]
- 3.Woolery AR, Luong P, Broberg CA, Orth K. Front Microbio. 2010;1:113. doi: 10.3389/fmicb.2010.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinch LN, Yarbrough ML, Orth K, Grishin NV. PLoS ONE. 2009;4:e5818. doi: 10.1371/journal.pone.0005818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yarbrough ML, Li Y, Kinch LN, Grishin NV, Ball HL, Orth K. Science. 2009;323:269–272. doi: 10.1126/science.1166382. [DOI] [PubMed] [Google Scholar]
- 6.Worby CA, Mattoo S, Kruger RP, Corbeil LB, Koller A, Mendez JC, Zekarias B, Lazar C, Dixon JE. Mol Cell. 2009;34:93–103. doi: 10.1016/j.molcel.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Müller MP, Peters H, Blümer J, Blankenfeldt W, Goody RS, Itzen A. Science. 2010;329:946–949. doi: 10.1126/science.1192276. [DOI] [PubMed] [Google Scholar]
- 8.Tan Y, Luo ZQ. Nature. 2011;475:506–509. doi: 10.1038/nature10307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neunuebel MR, Chen Y, Gaspar AH, Backlund PS, Jr, Yergey A, Machner MP. Science. 2011;333:453–456. doi: 10.1126/science.1207193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Al-Eryani R, Yarbrough ML, Orth K, Ball HL. J Am Soc Mass Spectrom. 2011;22:752–761. doi: 10.1007/s13361-011-0084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao Y-H, Chuang T, Ball HL, Luong P, Li Y, Flores-Saaib RD, Orth K. J Biotechnol. 2010 doi: 10.1016/j.jbiotec.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sletten EM, Bertozzi CR. Angew Chem Int Ed Engl. 2009;48:6974–6998. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chock PB, Huang CY, Timmons RB, Stadtman ER. Proc Natl Acad Sci USA. 1973;70:3134–3138. doi: 10.1073/pnas.70.11.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhee SG, Ubom GA, Hunt JB, Chock PB. J Biol Chem. 1981;256:6010–6016. [PubMed] [Google Scholar]
- 15.Xiao J, Worby CA, Mattoo S, Sankaran B, Dixon JE. Nat Struct Mol Biol. 2010;17:1004–1010. doi: 10.1038/nsmb.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luong P, Kinch LN, Brautigam CA, Grishin NV, Tomchick DR, Orth K. J Biol Chem. 2010;285:20155–20163. doi: 10.1074/jbc.M110.114884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palanivelu DV, Goepfert A, Meury M, Guye P, Dehio C, Schirmer T. Protein Sci. 2011;20:492–499. doi: 10.1002/pro.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang YY, Grammel M, Raghavan AS, Charron G, Hang HC. Chem Biol. 2010;17:1212–1222. doi: 10.1016/j.chembiol.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charron G, Zhang MM, Yount JS, Wilson J, Raghavan AS, Shamir E, Hang HC. J Am Chem Soc. 2009;131:4967–4975. doi: 10.1021/ja810122f. [DOI] [PubMed] [Google Scholar]
- 20.Yarbrough ML, Li Y, Kinch LN, Grishin NV, Ball HL, Orth K. Science. 2009;323:269–272. doi: 10.1126/science.1166382. [DOI] [PubMed] [Google Scholar]
- 21.Luong P, Kinch LN, Brautigam CA, Grishin NV, Tomchick DR, Orth K. J Biol Chem. 2010;285:20155–20163. doi: 10.1074/jbc.M110.114884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Worby CA, Mattoo S, Kruger RP, Corbeil LB, Koller A, Mendez JC, Zekarias B, Lazar C, Dixon JE. Mol Cell. 2009;34:93–103. doi: 10.1016/j.molcel.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yarbrough ML, Li Y, Kinch LN, Grishin NV, Ball HL, Orth K. Science. 2009;323:269–272. doi: 10.1126/science.1166382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.